Abstract
Photoactive artificial nanocatalysts that mimic natural photoenergy systems can yield clean and renewable energy. However, their poor photoabsorption capability and disfavored photogenic electron–hole recombination hinder their production. Herein, we designed two nanocatalysts with various microstructures by combining the tailored self-assembly of the meso-tetra(p-hydroxyphenyl) porphine photosensitizer with the growth of titanium dioxide (TiO2). The porphyrin photoabsorption antenna efficiently extended the absorption range of TiO2 in the visible region, while anatase TiO2 promoted the efficient electron–hole separation of porphyrin. The photo-induced electrons were transferred to the surface of the Pt co-catalyst for the generation of hydrogen via water splitting, and the hole was utilized for the decomposition of methyl orange dye. The hybrid structure showed greatly increased photocatalytic performance compared to the core@shell structure due to massive active sites and increased photo-generated electron output. This controlled assembly regulation provides a new approach for the fabrication of advanced, structure-dependent photocatalysts.
1. Introduction
Energy sustainability is crucial for anthropogenic activity and the development of societies [1]. Recent technological advancements, economic development, and population growth have exerted increasing pressure on the global energy supply. However, with the decline of fossil fuel reserves and climate change, the development of green sustainable alternative energy has become a major challenge [2,3]. To address this energy shortage while reducing the dependency on fossil fuels, many new forms of energy, including wind energy, solar energy, geothermal energy, hydropower, hydrogen energy, and bioenergy have been explored [4,5,6,7]. Renewable, sustainable, and green hydrogen energy is considered an attractive alternative to fossil fuels [8,9]. Furthermore, the generation of hydrogen fuel from water and sunlight, two of the most abundant natural resources on Earth [10], offers a promising pathway for acquiring hydrogen. Solar water splitting transforms sunlight into hydrogen through photocatalytic, photoelectrochemical, and photovoltaic electrolysis routes [11,12,13]. The photocatalytic approach works without a corrosive electrolyte or external power, providing a more competitive pathway to hydrogen production.
The catalyst is the key to photocatalysis, and among various photocatalysts [14,15,16,17], titanium dioxide (TiO2)-based nanomaterials are the most widely used. TiO2 crystals were divided into anatase, rutile, and brookite phases [18,19,20], which have good photophysical properties and chemical stability, an appropriate band gap matching the redox level of water [21,22], and an adjustable microstructure regulated by hydrothermal, sol–gel, or redox methods [23,24]. Specifically, TiO2-related catalysts have wide applications in photocatalytic water decomposition and pollutant degradation [25]. The photocatalytic performance of TiO2 is affected by its microstructural characteristics. N-doping, photosensitizer modification, and composite strategy are important approaches to increasing the light absorption, carrier concentration, and photocatalytic activity of TiO2 crystals [26,27,28]. The final catalytic efficiency of different structures suggests microstructure-dependent catalytic activity. However, the wide band gap of TiO2 only responds to ultraviolet (UV) light (about 4% of the solar spectrum) instead of visible light (about 40% of the solar spectrum) [29,30,31]. Moreover, the harmful crystal defects and low specific surface area of TiO2 significantly hinder its photocatalytic performance [32,33]. Therefore, it is urgent to develop a nanocatalyst with a unique structure to enhance the photocatalytic performance of TiO2 in visible light. Although a variety of artificial TiO2 microstructures that mimic natural light-capturing behavior have been studied over the past decades, the precise control of TiO2 microstructures on the nanoscale in photocatalysis remains a major challenge.
Self-assembly is a common phenomenon in nature. For instance, self-assembly controls the chloroplast complex by programmatically regulating the size and aggregated spatial structure of functional motifs to drive natural photosynthesis. Moreover, self-assembly is a powerful strategy used to fabricate nanomaterials with unique microstructures and new functions [34,35,36,37]. Among numerous photoactive motifs, porphyrins are superior assembly modules with a rigid planar structure and strong visible light absorption capability [38,39,40]. However, they are hindered by the face recombination of photoelectrons and holes after irradiation and the risk of photobleaching without a protective external shell. The synergistic effect of porphyrin/TiO2 with microstructural regulation offers a promising strategy to address these issues.
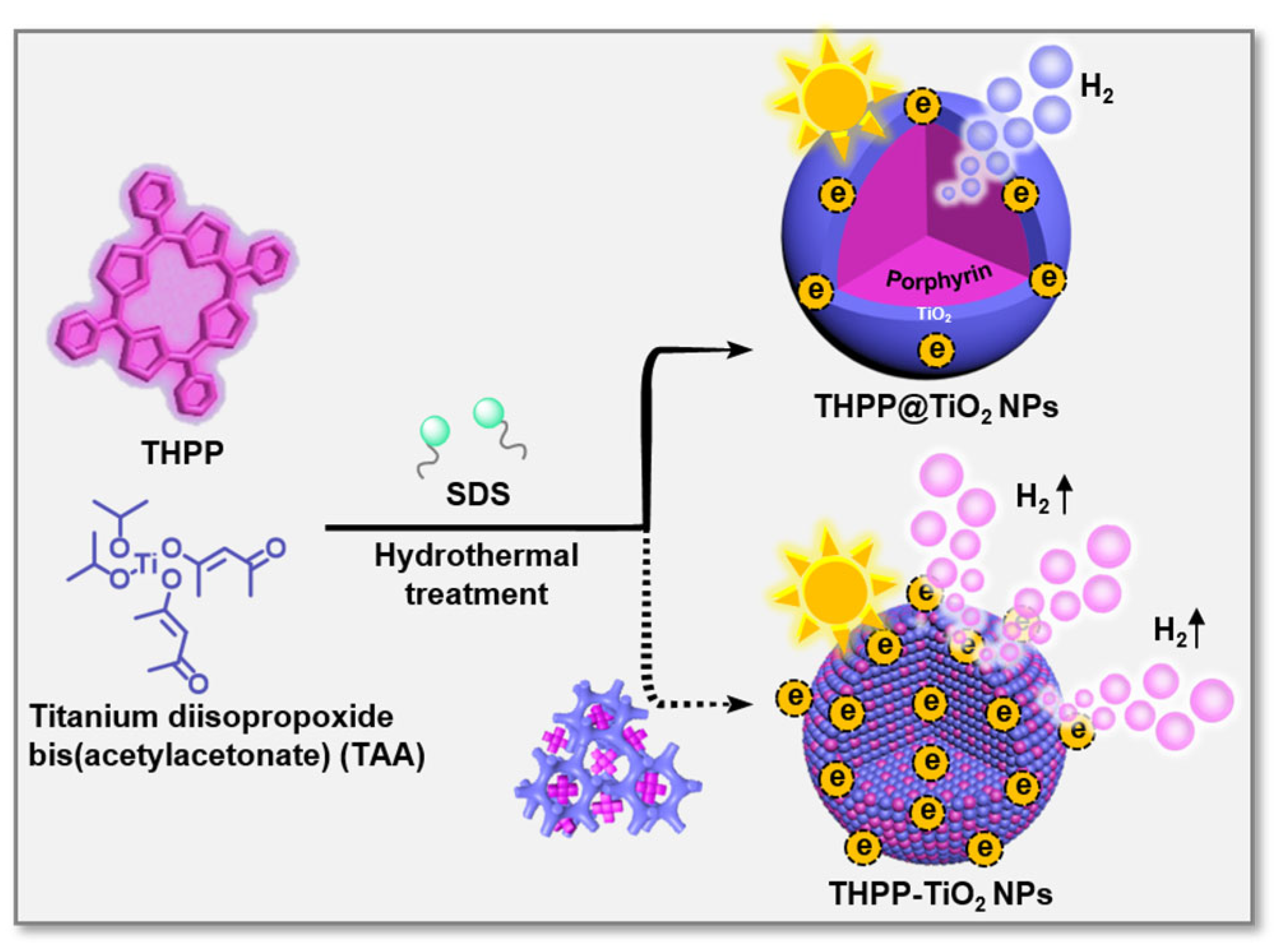
Herein, we developed two meso-tetra(4-hydroxyphenyl) porphyrin (THPP)/TiO2 nanocomposites with various assembled microstructures (Figure 1), i.e., hybrid-type nanoparticles (THPP-TiO2 NPs) and core@shell type (THPP@TiO2 NPs), through the tailored self-assembly of porphyrin and the hydrolysis/condensation of the TiO2 precursor, titanium diisopropoxide bis(acetylacetonate) (TAA). The controlled co-assembly can be regulated due to the stronger hydrogen bonding interactions between the hydroxyl group of the TiO2 scaffold and THPP than those of meso-tetra(4-benzenesulfonic acid)porphyrin (TSPP) and meso-tetra(4-carboxyphenyl)porphyrin (TCPP). The sodium dodecyl sulfate (SDS) surfactant and hydrothermal treatment further limited the size of the obtained nanocomposite and increased the crystallization degree of TiO2, respectively. Acting as a light-capturing antenna, porphyrin assisted the THPP-TiO2 NPs in harvesting and transferring visible light into a higher level of hydrogen using its large specific surface area, abundant active sites, superior light-capturing capability, and high crystallinity. Our advanced photocatalytic system provides a technically simple method for the microstructural regulation of catalysts to greatly improve photoenergy utilization and visible-light-responsive photocatalytic hydrogen evolution.
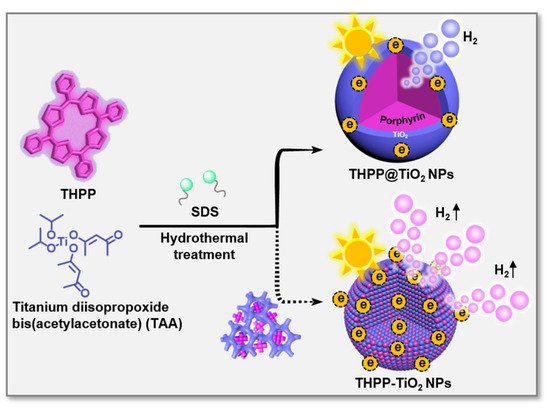
Figure 1.
Schematic representation of the co-assembly process and two nanocomposites (THPP-TiO2 NPs and THPP@TiO2 NPs) with various microstructures. The blue and purple represent TiO2 and THPP species.
2. Results
2.1. The Optimization of Light-Capturing Blocks
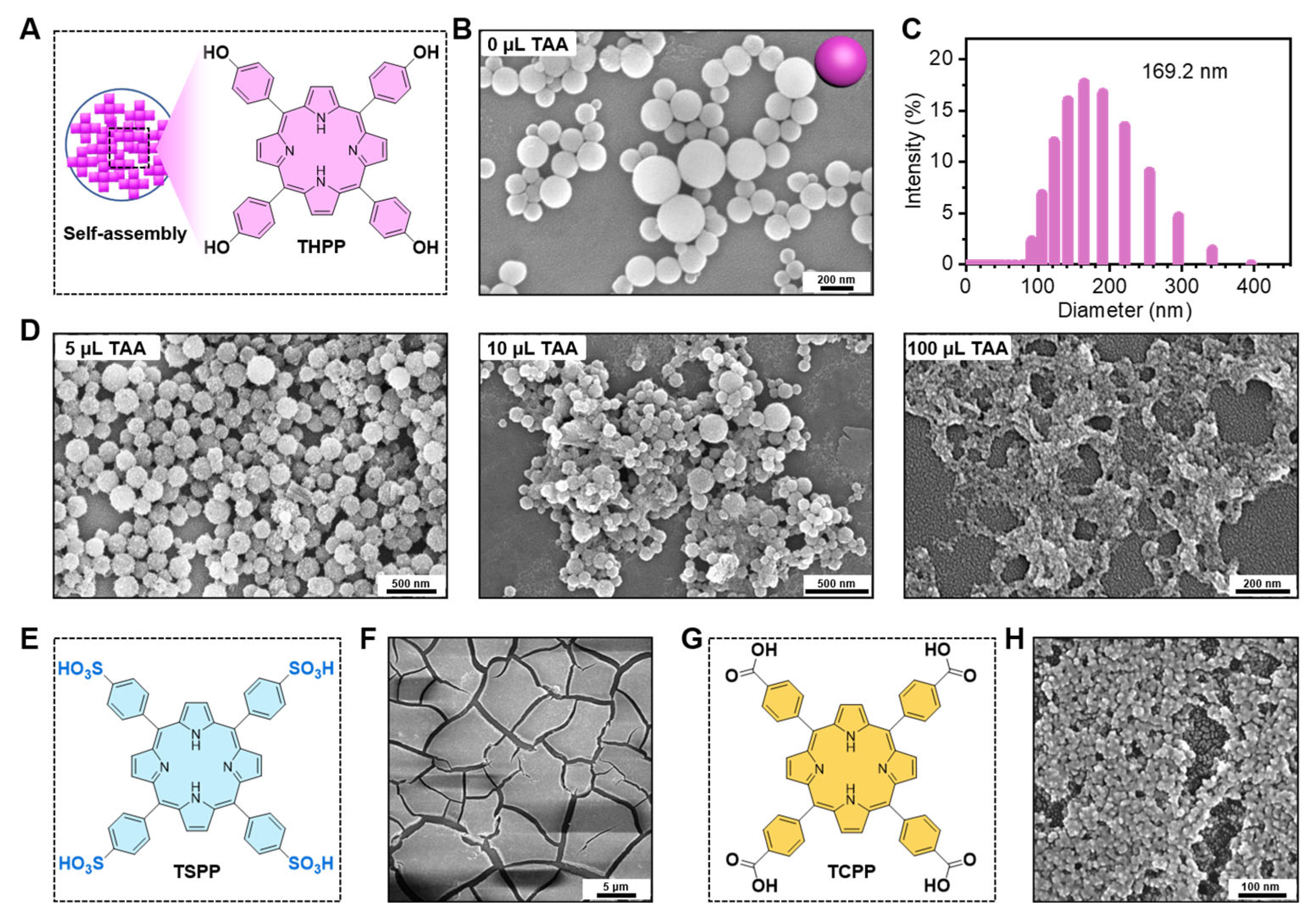
The THPP building blocks were self-assembled into 169.2 nm smooth nanospheres in a 25 mL reaction system (Figure 2A–C). The smooth surface of the THPP NPs gradually became rough with the increasing ratio of TiO2 precursor (TAA). The proportion of TAA has a crucial role in the co-assembly process. The optimal ratio of TAA was determined according to the morphologies of the nanomaterials. The scanning electron microscopy (SEM) images of the THPP-TiO2 nanocomposites indicated that 5 μL of TAA could acquire a monodispersed and homogeneous distribution of TiO2 in nanoparticle than 10 μL or 100 μL (Figure 2D). The THPP in THPP-TiO2 NPs was replaced by TSPP (Figure 2E,F) or TCPP (Figure 2G,H) but both were unable to yield a homogeneous morphology similar to that of the THPP-TiO2 NPs. These results suggested that the benzene hydroxyl group in THPP had a stronger binding force with the hydroxyl group of TiO2 in the sol–gel preparation process, yielding a homogeneous incorporation.
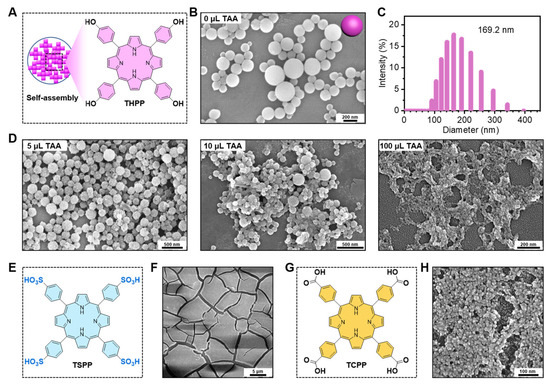
Figure 2.
(A) Description of the self-assembly of THPP NPs; (B) scanning electron microscopy (SEM) image of THPP NPs; (C) diameter statistic of THPP NPs; (D) SEM images of THPP-TiO2 NPs prepared with 1 mg of THPP and various volumes of TAA; (E) chemical structure of TSPP; (F) SEM image of TSPP-TiO2 NPs; (G) chemical structure of TCPP; (H) SEM image of TCPP-TiO2 NPs.
2.2. The Regulation of Assembled Microstructure
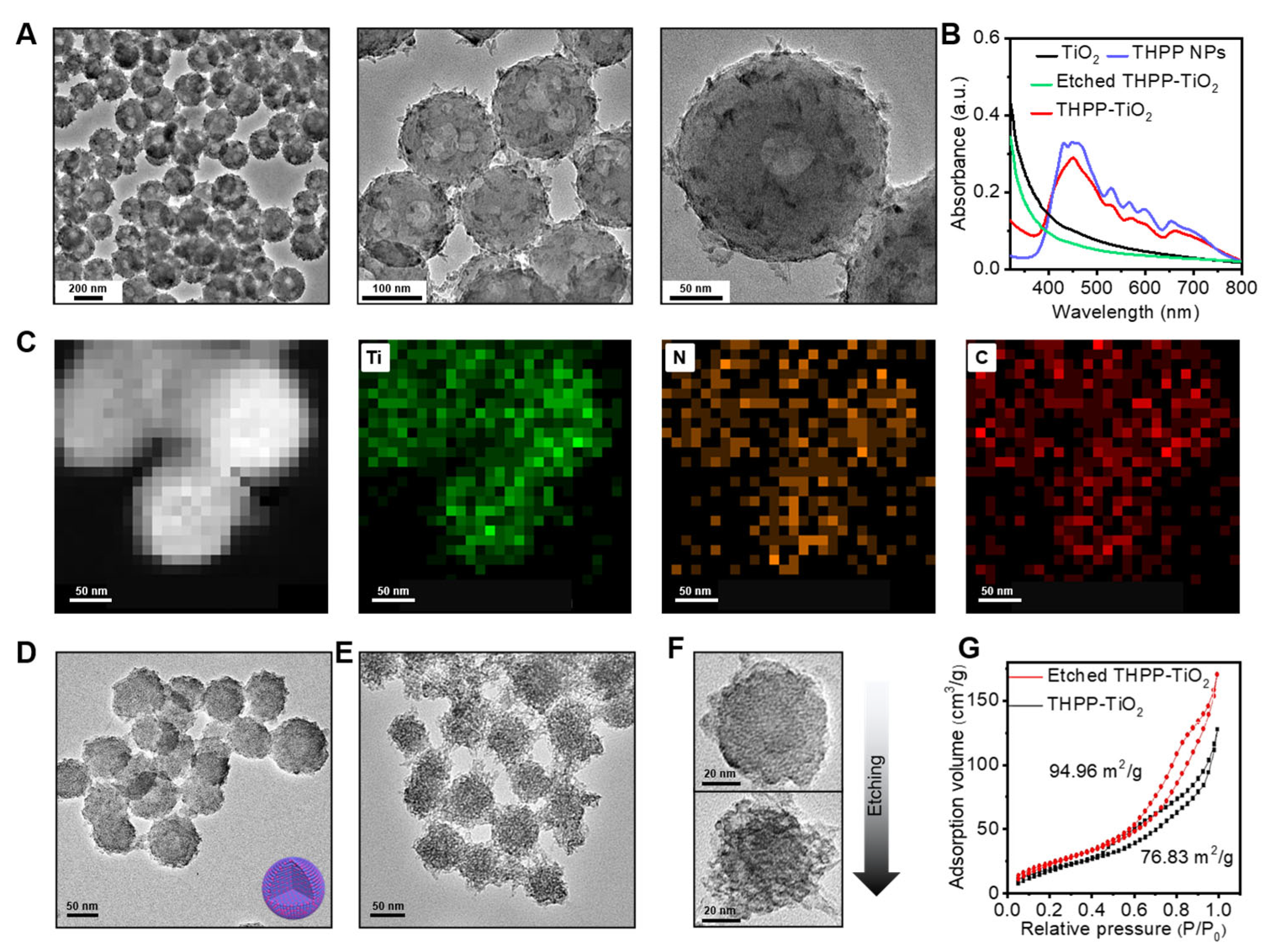
The TEM images of THPP-TiO2 NPs revealed a rough morphology (Figure 3A). The UV–vis spectra indicated successful THPP loading (Figure 3B), which disappeared after etching by sodium hydroxide (NaOH). The elemental mapping further suggested a homogeneous distribution of Ti, N, and C in the nanoparticles (Figure 3C). To further decrease the nanoparticle size to improve the catalytic performance, we used an interfacial self-assembly microemulsion process driven by SDS micelle. As a result, the THPP-TiO2 NPs greatly decreased in size around the 50–100 nm range (Figure 3D). Moreover, the TEM images of the etched THPP-TiO2 NPs revealed an obvious pore structure after etching (Figure 3E,F). The specific surface area is an important factor of the catalyst in the photocatalysis process. The N2 adsorption–desorption isotherms were collected to detect the pore change. The results indicated that the specific surface area of the THPP-TiO2 NPs increased from 76.83 to 94.96 m2/g after etching (Figure 3G).
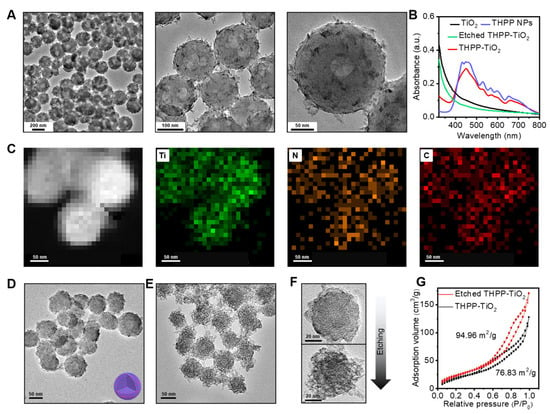
Figure 3.
(A) Transmission electron microscopy (TEM) images of THPP-TiO2 NPs prepared without SDS at different magnifications; (B) UV–vis absorption spectra of the indicated samples; (C) element mapping of large-size THPP-TiO2 NPs; (D,E) TEM images of small-size THPP-TiO2 NPs before and after etching with NaOH (1 M), which was prepared assisted by SDS; (F) magnifying TEM images of THPP-TiO2 NPs before (top) and after (down) etching with NaOH; (G) N2 adsorption–desorption isotherms of small-size THPP-TiO2 NPs before and after etching.
2.3. The Optimization for the Crystallinity of TiO2
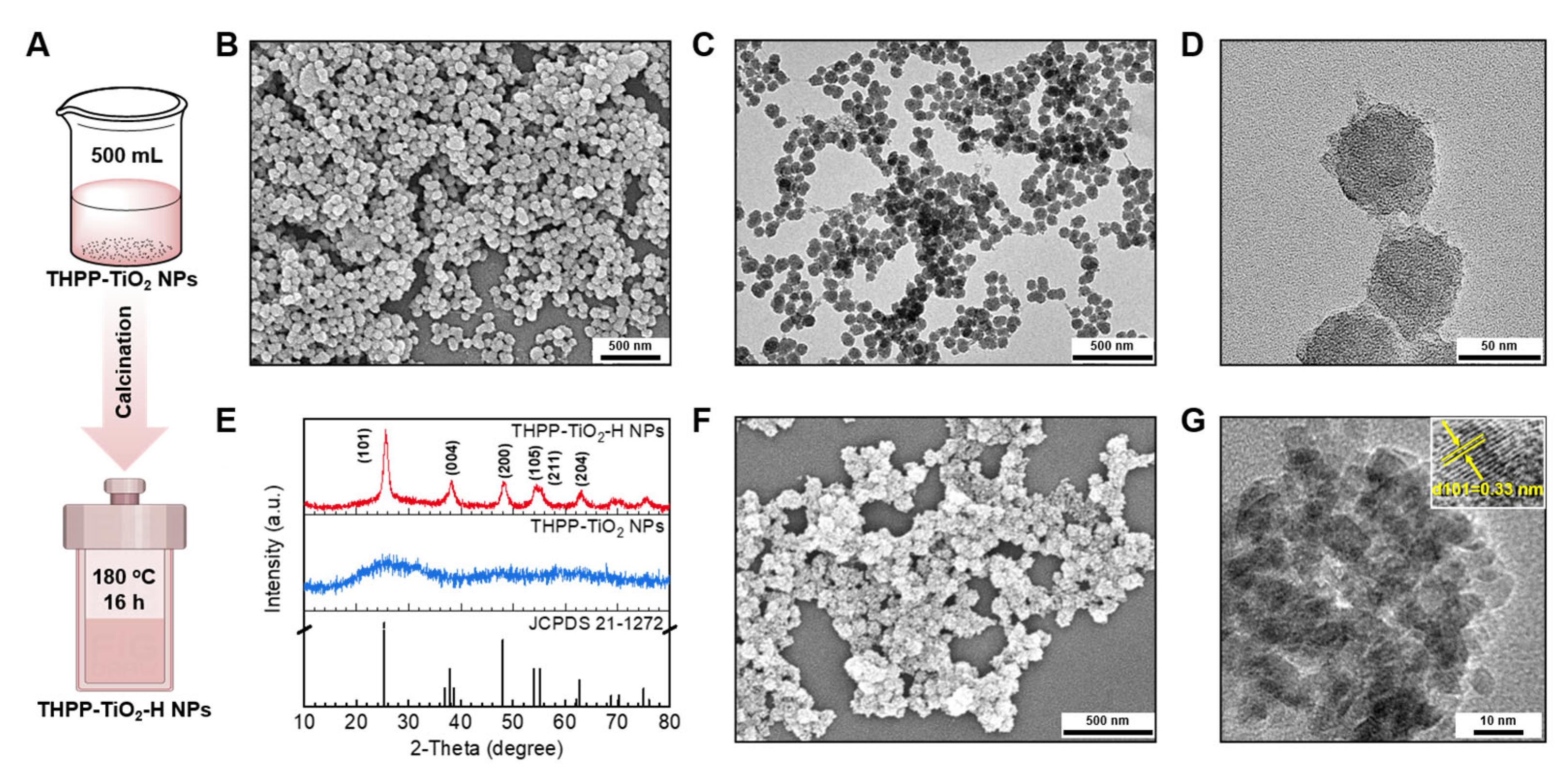
Anatase generates a higher oxidation capacity due to a higher band gap (3.2 eV), oxygen vacancies to capture electrons, and catalytic activity compared to those of rutile. Therefore, the amorphous TiO2 in THPP-TiO2 NPs were further changed to anatase-type TiO2 by hydrothermal treatment. To expand their application, the volume of the reaction system was increased from 25 to 500 mL (Figure 4A). The SEM and TEM images indicated that the products remained stable in size and morphology after scaled-up preparation (Figure 4B–D). The hydrolysis condensation product of TAA is amorphous TiO2 in THPP-TiO2, exhibiting no prominent peaks in the X-ray diffraction (XRD) pattern (Figure 4E) and changed to the TiO2 crystal of THPP-TiO2-H NPs after hydrothermal treatment. The characteristic peaks of THPP-TiO2-H NPs matched the JCPDS pattern (JCPDS 21-1272) of anatase. The SEM image of the THPP-TiO2-H NPs maintained a morphology similar to that before hydrothermal treatment (Figure 4F). The magnified TEM image indicated a 0.33 nm lattice spacing assigned to the (101) crystal face (Figure 4G, inset: lattice spacing) [41]. These results indicated that the THPP-TiO2-H NPs had superior anatase crystal structure.
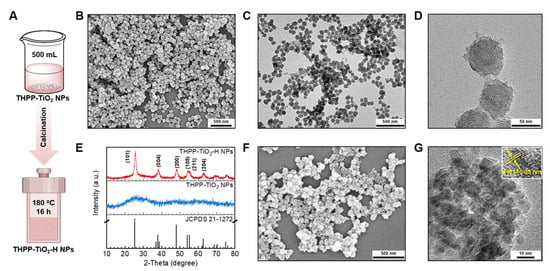
Figure 4.
(A) Description of the scaled-up (500 mL) preparation and following hydrothermal treatment of THPP-TiO2 NPs; (B–D) SEM and TEM images of the obtained THPP-TiO2 NPs; (E) XRD patterns of THPP-TiO2 NPs, THPP-TiO2-H NPs, and the standard card (JCPDS 21-1272); (F,G) SEM and magnified TEM images of THPP-TiO2-H NPs (inset: detailed lattice fringe). The hydrothermal treatment is expressed as the -H suffix.
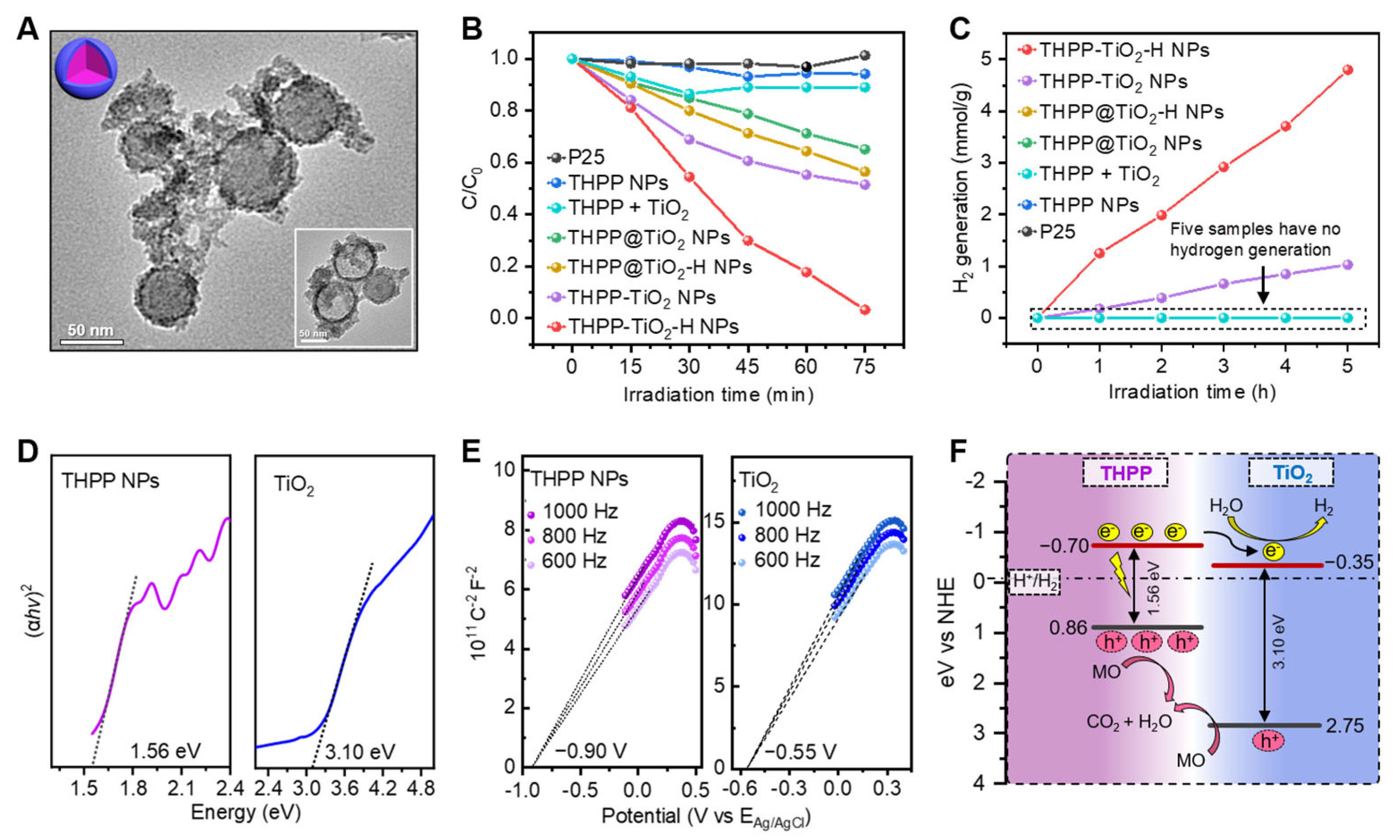
2.4. Structure-Dependent Photocatalysis
Lastly, we investigated the photocatalytic performance and verified the catalytic mechanism of the nanocomposites. The hybrid microstructure of the THPP-TiO2 NPs was adjusted to the core@shell control samples (THPP@TiO2 NPs) through a two-step method, i.e., the self-assembly of THPP combined with a TiO2 shell coating. The TEM image of the etched THPP@TiO2 NPs showed a hollow TiO2 shell structure (inset, Figure 5A). Methyl orange (MO) is a representative pollutant often used to investigate the photocatalytic performance of catalysts. Compared to the negligible photocatalytic activity of the commercial titanium dioxide photocatalyst P25, the THPP-TiO2-H NPs revealed a higher degradation output, exceeding 96.7% after 75 min (Figure 5B). The photocatalytic hydrogen production capabilities of both THPP-TiO2-H NPs and THPP-TiO2 NPs indicated irradiated time-dependent hydrogen generation, respectively reaching 4.80 and 1.03 mmol/g, which was much higher than that of P25, THPP NPs, THPP + TiO2, THPP@TiO2-H NPs, and THPP@TiO2 NPs (Figure 5C). These results indicated that the commercial P25 control sample was unable to efficiently respond to visible light (>388 nm) [42,43] due to the intrinsic wide band gap (band gap 3.2 eV) of TiO2. Moreover, hybrid structure and hydrothermal treatment were essential in the exhibited photocatalytic activity, suggesting that the assembly could greatly increase the microstructure-dependent photocatalytic activity.
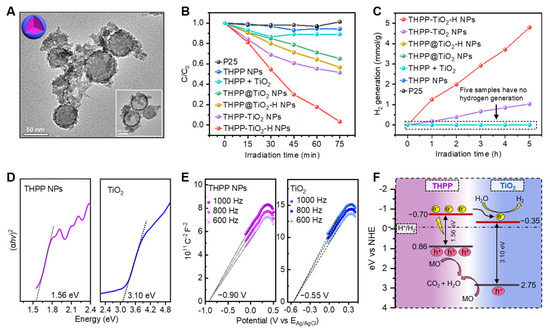
Figure 5.
(A) TEM images of THPP@TiO2 nanospheres before and after (inset image) etching with NaOH (1 M); (B) relative degradation rate of MO by the indicated treatments. The mass of the catalysts was 6 mg in each catalytic system. The THPP + TiO2 is a mixture of THPP NPs (3.75 mg) of TiO2 NPs (2.25 mg). C0 is the initial concentration of MO; (C) photocatalytic hydrogen production of the indicated samples (2 mg) as a function of irradiation time. The THPP + TiO2 is the mixture of THPP NPs (1.25 mg) of TiO2 NPs (0.75 mg). The volume fraction of the triethanolamine sacrificial reagent (triethanolamine, TEOA) and the mass percent of the Pt co-catalyst were 10 vol % and 2%, respectively. (D) Tauc plots of (αhv)2 vs. Eg; (E) Mott–Schottky plots of THPP NPs and TiO2 NPs; (F) description of photocatalytic mechanism in THPP-TiO2-H NPs.
To further explore the photogenerated electron-transport process of catalysts in the photocatalytic process, the band energies of TiO2 and THPP NPs were investigated. According to the UV–Vis diffuse reflectance spectra, the energy gaps (Eg) of the THPP assemblies and TiO2 NPs were calculated to be 1.56 and 3.10 eV (Figure 5D). The Mott–Schottky curve showed that the conduction band (ECB) positions of the THPP assemblies and TiO2 NPs were −0.70 and −0.35 eV vs. NHE (Figure 5E). According to the equation Eg = EVB − ECB, the EVB values of the THPP assemblies and TiO2 NPs were calculated to be 0.86 and 2.75 eV. The staggered band structures between THPP and TiO2 facilitate charge separation in the THPP-TiO2-H NPs. Under visible light irradiation, the electron–hole pairs are simultaneously generated at both THPP and TiO2. The photogenerated electrons at the conduction band of THPP would spontaneously transfer to the TiO2, with a lower conduction band potential. The transfer of the photogenerated carriers effectively accelerates the separation of photogenerated electron–hole pairs of THPP [44], yielding higher hydrogen production. Meanwhile, the surface Pt cocatalyst [38] facilitated photoelectron transport from TiO2 to H2O by inhibiting the undesired recombination of photo-generated electron–hole pairs on TiO2, improving the utilization efficiency of visible light. Additionally, the holes of THPP and TiO2 NPs were used for MO degradation (Figure 5F). The hybrid structure in THPP-TiO2-H NPs is beneficial to the above process. However, the core@shell structure in THPP@TiO2-H NPs is unable to efficiently transfer the electron to TiO2 due to the harmful recombination of electrons and holes, inducing low catalytic efficiency.
3. Materials and Methods
3.1. Materials
THPP, TSPP, TCPP, sodium dodecyl sulfate (SDS), and titanium diisopropoxide bis(acetylacetonate) (TAA, 75 wt.% in isopropanol) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium hydroxide (NaOH, 1 M) standard solution was obtained from Acros Organics (Geel, Belgium). Tetrahydrofuran (THF) was obtained from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, China). Commercial titanium dioxide (P25) was purchased from Degussa Co., Essen, Germany. All chemicals were used as received without further purification. X-ray powder diffraction (XRD) patterns were obtained using Bruker D8 Advanced (Bremen, Germany). The surface area of powders was measured using an ASAP2020 adsorption analyzer (Norcross, GA, USA). The photocatalytic system was purchased from PerfectLight Co., Ltd. (Beijing, China). The morphological observation was performed on the transmission electron microscopy (JEOL JEM-2010, Tokyo, Japan) operated at 200 kV accelerating voltage and scanning electron microscope (JEOL JSM-6710F). Ultraviolet and visible absorption spectroscopy was collected on a Xidi UV-5400 spectrophotometer (Shanghai, China).
3.2. Preparation of THPP Nanoparticles
The self-assembly method was used to prepare THPP NPs. Briefly, 1 mg of THPP powder was dissolved in 1 mL of tetrahydrofuran (THF) solvent under sonication for 5 min. Then, the mixture was injected into 25 mL of water and further grown at 25 °C for 24 h.
3.3. Preparation of THPP-TiO2 Nanocomposites
To decrease the size of the nanocomposites, the SDS surfactant was introduced into the above system. Briefly, 50 mg of THPP powder and 250 μL of TAA were dissolved in 50 mL of THF and stirred for 0.5 h at 25 °C. Subsequently, the solution was quickly added to 500 mL of SDS aqueous solution (0.01 M) under magnetic stirring, followed by magnetic stirring for 24 h. The TiO2 sample was prepared according to the same procedure but with the removal of THPP. The THPP-TiO2 NPs with large sizes were prepared according to the same procedure but lack of SDS. The pure TiO2 sample was prepared following the same procedure without adding THPP.
3.4. Preparation of THPP@TiO2 Nanocomposites
The THPP@TiO2 nanocomposites were prepared using a two-step method. Briefly, 1 mg of THPP power was dissolved in 1 mL of THF solvent under sonication for 5 min. The mixture was injected into 25 mL of SDS aqueous solution (0.01 M) and further grown at 25 °C for 24 h. Subsequently, 5 μL of TAA dispersed in 10 μL of THF solvent was added to the solution. The reaction system was allowed to further grow for 24 h and acquire the THPP@TiO2 NPs after centrifugation at 8000 rpm for 10 min and washing two times with water.
3.5. Hydrothermal Treatment
To increase the crystallinity, the TiO2 NPs, THPP@TiO2, and small-size THPP-TiO2 nanocomposites required a hydrothermal process. First, the resulting NPs solution was centrifuged at 8000 rpm for 10 min to remove excess reactants. Then, the precipitate was redispersed in water and transferred to a reactor containing a Teflon liner. Lastly, the reactors were sent to the oven and carried a hydrothermal treatment at 180 °C for 16 h. The ultimate products were collected using a centrifugation procedure at 8000 rpm for 10 min.
3.6. Photocatalytic Methyl Orange (MO) Decomposition
To test the photocatalytic performance, 6 mg of various nanocomposites or P25 were added to 3 mL of methyl orange (MO, 20 mg/L) dye aqueous solution, which was further stirred for 30 min in a dark environment. Then, the mixture was sent to the irradiation of visible light (PLS-SXE300/300UV, Beijing Perfectlight Technology Co., Ltd., 300 W, wavelength: UV cutoff filter > 400 nm). Subsequently, 400 μL of solution was taken out every 15 min and performed five times. These tested solutions were centrifuged at 8000 rpm for 10 min. The absorption of the supernatant was measured.
3.7. Photocatalytic Hydrogen Generation
The photocatalytic hydrogen generation assay was administrated in 50 mL of aqueous solution (pH = 8.1–8.2 adjusted with HCl) containing 5 mL of triethanolamine (TEOA, 10 vol%) sacrificial reagent and 41 μL of potassium chloroplatinate (5 mM). Then, 2 mg of tested samples were respectively added to various parallel solutions to harvest the hydrogen generation under visible light (UV cutoff filter > 400 nm) irradiation and tested using gas chromatography.
3.8. Mott–Schottky Tests
The Mott–Schottky plots and photocurrent response were measured on an electrochemical workstation (Autolab, Utrecht, The Netherlands) equipped with a standard three-electrode system. Briefly, the electrolyte solution is Na2SO4 (0.5 M) aqueous solution, which was purged with N2 to remove air before utilization. The working electrode was prepared by coating the catalysts onto the Pt/C electrode. The reference and counter electrode respectively adopted the Ag/AgCl electrode and platinum plate.
4. Conclusions
In this paper, we controllably established two microstructural regulation modes to explore the structure-dependent photocatalytic performance. In the co-assembly process, the benzene hydroxyl group acquired optimal morphology and hybrid uniformity via the strong interaction between the hydroxy groups of THPP and TiO2. The introduction of the SDS template efficiently limited the growth of the resulting nanocomposites even in large-scale applications. Compared to the core@shell structure of the THPP@TiO2 nanocomposites, the THPP-TiO2 nanocomposites with hybrid microstructure exhibited the close contact of porphyrin with TiO2 and abundant catalytic sites, which promoted photogenerated electron–hole separation. Anatase TiO2 accelerated the transport efficiency of photoelectrons to Pt on the surface, leading to high MO decomposition efficiency and photocatalytic hydrogen production. The ingenious assembly of structure-regulated nanocatalysts provides insight into the fabrication of advanced photocatalysts and photocatalytic activity modulation.
Author Contributions
Conceptualization, J.W. and F.B.; methodology, Y.L., X.L. (Xinpeng Lv) and Y.Z.; validation, Y.L., G.W., S.L., S.C. and C.Q.; data analysis, Y.L., M.H., P.S., Z.L., X.L. (Xi Li) and J.G.; writing—original draft preparation, Y.L. and X.L. (Xinpeng Lv); writing—review and editing, J.S., J.W. and F.B.; supervision, F.B.; project administration, J.S. and F.B.; funding acquisition, G.W., J.W. and F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhongyuan High-Level Talents Special Support Plan (No. 204200510009) from Feng Bai, Postdoctral Fellowship Program of CPSF (GZC20230673) from Gaoyang Wang, National Natural Science Foundation of China (NSFC 22275050) from Jiefei Wang, Excellent Young Scholars of Henan Province (242300421132) from Jiefei Wang, Joint Training Funds for Science and Technology R&D of Henan Province (222301420059) from Jiefei Wang, Program for Science and Technology Innovation Talents in Universities of Henan Province (22HASTIT004) from Jiefei Wang.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, J.H.; Hansora, D.; Sharma, P.; Jang, J.W.; Lee, J.S. Toward practical solar hydrogen production-an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 2019, 48, 1908–1971. [Google Scholar] [CrossRef]
- Cho, J.H.; Ma, J.; Kim, S.Y. Toward high-efficiency photovoltaics-assisted electrochemical and photoelectrochemical CO2 reduction: Strategy and challenge. Exploration 2023, 3, 20230001. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.J.; Tournet, J.; Dastafkan, K.; Liu, Y.; Hau Ng, Y.; Karuturi, S.K.; Zhao, C.; Yin, Z.Y. Noble-metal-free multicomponent nanointegration for sustainable energy conversion. Chem. Rev. 2021, 121, 10271–10366. [Google Scholar] [CrossRef]
- Ang, T.Z.; Salem, M.; Kamarol, M.; Das, H.S.; Nazari, M.A.; Prabaharan, N. A comprehensive study of renewable energy sources: Classifications, challenges and suggestions. Energy Strategy Rev. 2022, 43, 100939. [Google Scholar] [CrossRef]
- Wu, G.C.; Deshmukh, R.; Trainor, A.; Uppal, A.; Chowdhury, A.F.M.K.; Baez, C.; Martin, E.; Higgins, J.; Mileva, A.; Ndhlukula, K. Avoiding ecosystem and social impacts of hydropower, wind, and solar in southern africa’s low-carbon electricity system. Nat. Commun. 2024, 15, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Ricks, W.; Voller, K.; Galban, G.; Norbeck, J.H.; Jenkins, J.D. The role of flexible geothermal power in decarbonized electricity systems. Nat. Energy 2024. [Google Scholar] [CrossRef]
- Cherp, A.; Vinichenko, V.; Tosun, J.; Gordon, J.A.; Jewell, J. National growth dynamics of wind and solar power compared to the growth required for global climate targets. Nat. Energy 2021, 6, 742–754. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, C.M.; Zhu, J.; Qin, W.; Tao, X.P.; Fan, F.T.; Li, R.G.; Li, C. A hydrogen farm strategy for scalable solar hydrogen production with particulate photocatalysts. Angew. Chem. Int. Ed. 2020, 59, 9653–9658. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, Y.Z.; Xin, X.; Yang, J.B.; Wang, M.H.; Wang, R.L.; Guo, P.; Huang, W.J.; Sobrido, A.J.; Wei, B.Q.; et al. In situ photocatalytically enhanced thermogalvanic cells for electricity and hydrogen production. Science 2023, 381, 291–296. [Google Scholar] [CrossRef]
- Zhou, P.; Navid, I.A.; Ma, Y.J.; Xiao, Y.X.; Wang, P.; Ye, Z.W.; Zhou, B.W.; Sun, K.; Mi, Z.T. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef]
- Jia, J.Y.; Seitz, L.C.; Benck, J.D.; Huo, Y.J.; Chen, Y.S.; Ng, J.W.D.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogenefficiency over 30%. Nat. Commun. 2016, 7, 13237–13242. [Google Scholar] [CrossRef]
- Wang, K.H.; Tao, Y.; Tang, Z.K.; Xu, X.; Benetti, D.; Vidal, F.; Zhao, H.G.; Rosei, F.; Sun, X.H. Efficient photoelectrochemical hydrogen generation based on core size effect of heterostructured quantum dots. Small 2024, 20, 2306453. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.J.; Zhang, W.J.; Dong, L.; Ma, Z.; Xu, J.S.; Gu, X.L.; Chen, Z.P. Progress on quantum dot photocatalysts for biomass valorization. Exploration 2023, 3, 20220169. [Google Scholar] [CrossRef]
- Chen, Y.T.; Hong, C.X.; Xu, Q.; Zheng, H.H.; Wang, C.; Lu, H.S.; Zhang, S.; Du, M.M.; Zeng, G.N. Visible light enhancement of biocarbon quantum-dot-decorated TiO2 for naphthalene removal. Molecules 2024, 29, 2708. [Google Scholar] [CrossRef]
- Vadivel, D.; Suryakumar, S.; Casella, C.; Speltini, A.; Dondi, D. Advancements in materials science and photocatalysts for sustainable development. Catalysts 2024, 14, 378. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, S.N.; Zang, S.Q. Programmable kernel structures of atomically precise metal nanoclusters for tailoring catalytic properties. Exploration 2023, 3, 20220005. [Google Scholar] [CrossRef]
- Du, X.Z.; Fan, H.J.; Liu, S.L.; Zhang, Z.C. Selective nucleophilic α-C alkylation of phenols with alcohols via Ti=Cα intermediate on anatase TiO2 surface. Nat. Commun. 2023, 14, 4479–4488. [Google Scholar] [CrossRef]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase polymorph of TiO2 (anatase, rutile, brookite, TiO2 (B)) for efficient photocatalyst: Fabrication and activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Mansfeldova, V.; Zlamalova, M.; Tarabkova, H.; Janda, P.; Vorokhta, M.; Piliai, L.; Kavan, L. Work function of TiO2 (anatase, rutile, and brookite) single crystals: Effects of the environment. J. Phys. Chem. C 2021, 125, 1902–1912. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Tang, X.; Liu, P.L.; Huang, Q.; Li, T.X.; Ju, L. Recent progress of ion-modified TiO2 for enhanced photocatalytic hydrogen production. Molecules 2024, 29, 2347. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Xiao, L.Q.; Chen, Y.; Yang, F.; Meng, H.Y.; Zhang, W.Y.; Zhang, Y.F.; Wu, Y. TiO2-based catalysts with various structures for photocatalytic application: A review. Catalysts 2024, 14, 366. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.Q.; Yan, J.H.; Yu, J.Y.; Sun, G.; Ding, B. Soft Zr-doped TiO2 nanofibrous membranes with enhanced photocatalytic activity for water purification. Sci. Rep. 2017, 7, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Prathan, A.; Sanglao, J.; Wang, T.; Bhoomanee, C.; Ruankham, P.; Gardchareon, A.; Wongratanaphisan, D. Controlled structure and growth mechanism behind hydrothermal growth of TiO2 nanorods. Sci. Rep. 2020, 10, 8065–8074. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Soler, L.; Cazorla, C.; Oliveras, J.; Bastús, N.G.; Puntes, V.F.; Llorca, J. Facet-engineered TiO2 drives photocatalytic activity and stability of supported noble metal clusters during H2 evolution. Nat. Commun. 2023, 14, 6165–6174. [Google Scholar] [CrossRef] [PubMed]
- Alsharaeh, E.H.; Bora, T.; Soliman, A.; Ahmed, F.; Bharath, G.; Ghoniem, M.G.; Abu-Salah, K.M.; Dutta, J. Sol-gel-assisted microwave-derived synthesis of anatase Ag/TiO2/GO nanohybrids toward efficient visible light phenol degradation. Catalysts 2017, 7, 133. [Google Scholar] [CrossRef]
- Sacco, N.; Iguini, A.; Gamba, I.; Marchesini, F.A.; García, G. Pd:in-doped TiO2 as a bifunctional catalyst for the photoelectrochemical oxidation of paracetamol and simultaneous green hydrogen production. Molecules 2024, 29, 1073. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.Y.; Zhang, L.Y.; Cheng, B.; Yu, J.G. Dual cocatalysts in TiO2 photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Li, L.D.; Yan, J.Q.; Wang, T.; Zhao, Z.J.; Zhang, J.; Gong, J.L.; Guan, N.J. Sub-10nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 5881–5890. [Google Scholar] [CrossRef]
- Guo, Z.R.; Zhang, X.; Li, X.Y.; Cui, C.; Zhang, Z.L.; Li, H.S.; Zhang, D.X.; Li, J.Y.; Xu, X.Y.; Zhang, J.T. Enhanced charge separation by incomplete calcination modified co-doped TiO2 nanoparticle for isothiazolinone photocatalytic degradation. Nano Res. 2024, 17, 4834–4843. [Google Scholar] [CrossRef]
- Wang, P.; Guo, S.; Wang, H.J.; Chen, K.K.; Zhang, N.; Zhang, Z.M.; Lu, T.B. A broadband and strong visible-light-absorbing photosensitizer boosts hydrogen evolution. Nat. Commun. 2019, 10, 3155–3166. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Li, J.G.; Kamiyama, H.; Katada, M.; Ohashi, N.; Moriyoshi, Y.; Ishigaki, T. Pyrogenic Iron(III)-doped TiO2 nanopowders synthesized in RF thermal plasma: Phase formation, defect structure, band gap, and magnetic properties. J. Am. Chem. Soc. 2005, 127, 10982–10990. [Google Scholar] [CrossRef]
- Ning, F.D.; Qin, J.Q.; Dan, X.; Pan, S.F.; Bai, C.; Shen, M.; Li, Y.L.; Fu, X.W.; Zhou, S.; Shen, Y.B.; et al. Nanosized proton conductor array with high specific surface area improves fuel cell performance at low Pt loading. ACS Nano 2023, 17, 9487–9500. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.Y.; Xu, W.; Ren, C.H.; Zhang, L.P.; Zhang, C.R.; Liu, J.F.; Yang, C.H. Recent progress in quantitative analysis of self-assembled peptides. Exploration 2024. [Google Scholar] [CrossRef]
- McDowall, D.; Greeves, B.J.; Clowes, R.; McAulay, K.; Fuentes-Caparrós, A.M.; Thomson, L.; Khunti, N.; Cowieson, N.; Nolan, M.C.; Wallace, M.; et al. Controlling photocatalytic activity by self-assembly-tuning perylene bisimide photocatalysts for the hydrogen evolution reaction. Adv. Energy Mater. 2020, 10, 2002469. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, Z.W.; Ravaine, S.; He, M.X.; Song, Y.L.; Yin, Y.D.; Zheng, H.B.; Teng, J.H.; Zhang, A. From colloidal particles to photonic crystals: Advances in self-assembly and their emerging applications. Chem. Soc. Rev. 2021, 50, 5898–5951. [Google Scholar] [CrossRef]
- Hu, Q.Q.; Li, H.; Wang, L.H.; Gu, H.Z.; Fan, C.H. DNA nanotechnology-enabled drug delivery systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Zhong, Y.; Wang, L.; Zhang, N.; Cao, R.H.; Bian, K.F.; Alarid, L.; Haddad, R.E.; Bai, F.; Fan, H.Y. Morphology-controlled synthesis and metalation of porphyrin nanoparticles with enhanced photocatalytic performance. Nano Lett. 2016, 16, 6523–6528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, L.; Wang, H.M.; Cao, R.H.; Wang, J.F.; Bai, F.; Fan, H.Y. Self-assembled one-dimensional porphyrin nanostructures with enhanced photocatalytic hydrogen generation. Nano Lett. 2017, 18, 560–566. [Google Scholar] [CrossRef]
- Tang, Q.X.; Han, Y.F.; Chen, L.X.; Qi, Q.Y.; Yu, J.L.; Yu, S.B.; Yang, B.; Wang, H.Y.; Zhang, J.S.; Xie, S.H.; et al. Bioinspired self-assembly of metalloporphyrins and polyelectrolytes into hierarchical supramolecular nanostructures for enhanced photocatalytic H2 production in Water. Angew. Chem. Int. Edit. 2024, 63, e202315599. [Google Scholar] [CrossRef]
- Mi, Y.; Weng, Y.X. Band alignment and controllable electron migration between rutile and anatase TiO2. Sci. Rep. 2015, 5, 11482–11491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Sun, P.F.; Fei, X.Z.; Wu, X.J.; Huang, Z.Y.; Zhong, W.F.; Gong, Q.B.; Zheng, Y.P.; Zhang, Q.H.; Xie, S.J.; et al. Unusual facet and co-catalyst effects in TiO2-based photocatalytic coupling of methane. Nat. Commun. 2024, 15, 4453–4462. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Bi, W.T.; Zhang, L.; Tao, S.; Chu, W.S.; Zhang, Q.; Luo, Y.; Wu, C.Z.; Xie, Y. Single-atom Pt as co-catalyst for enhanced photocatalytic H2 evolution. Adv. Mater. 2016, 28, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Xia, S.Y.; Wang, J.F.; Ren, X.T.; Chen, S.D.; Zhong, Y.; Bai, F. Synthesis of the ZnTPyP/WO3 nanorod-on-nanorod heterojunction direct Z-scheme with spatial charge separation ability for enhanced photocatalytic hydrogen generation. Nanoscale 2023, 15, 2871–2881. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).