Phytochemical Analysis, Biological Activities, and Docking of Phenolics from Shoot Cultures of Hypericum perforatum L. Transformed by Agrobacterium rhizogenes
Abstract
1. Introduction
- (1)
- phenolic profile (phenolic acids, flavan-3-ols, flavonols, anthocyanins, naphthodianthrones, acyl-phloroglucinols, and xanthones) in H. perforatum TSL and NTS extracts using HPLC/DAD/ESI-MSn methodology;
- (2)
- enzyme-inhibitory activity against monoamine oxidase-A (MAO-A), acetylcholinesterase (AChE), butyrylcholinesterase (BChE), tyrosinase (TYR), α-amylase (α-AM), α-glucosidase (α-GL), pancreatic lipase (PL) and cholesterol esterase (CHE) by in vitro assays; and
- (3)
- potential interactions between representative phenolic compounds and target enzymes using molecular docking studies.
2. Results
2.1. HPLC/DAD/ESI-MSn Analysis of Phenolic Compounds in H. perforatum Transformed Shoots
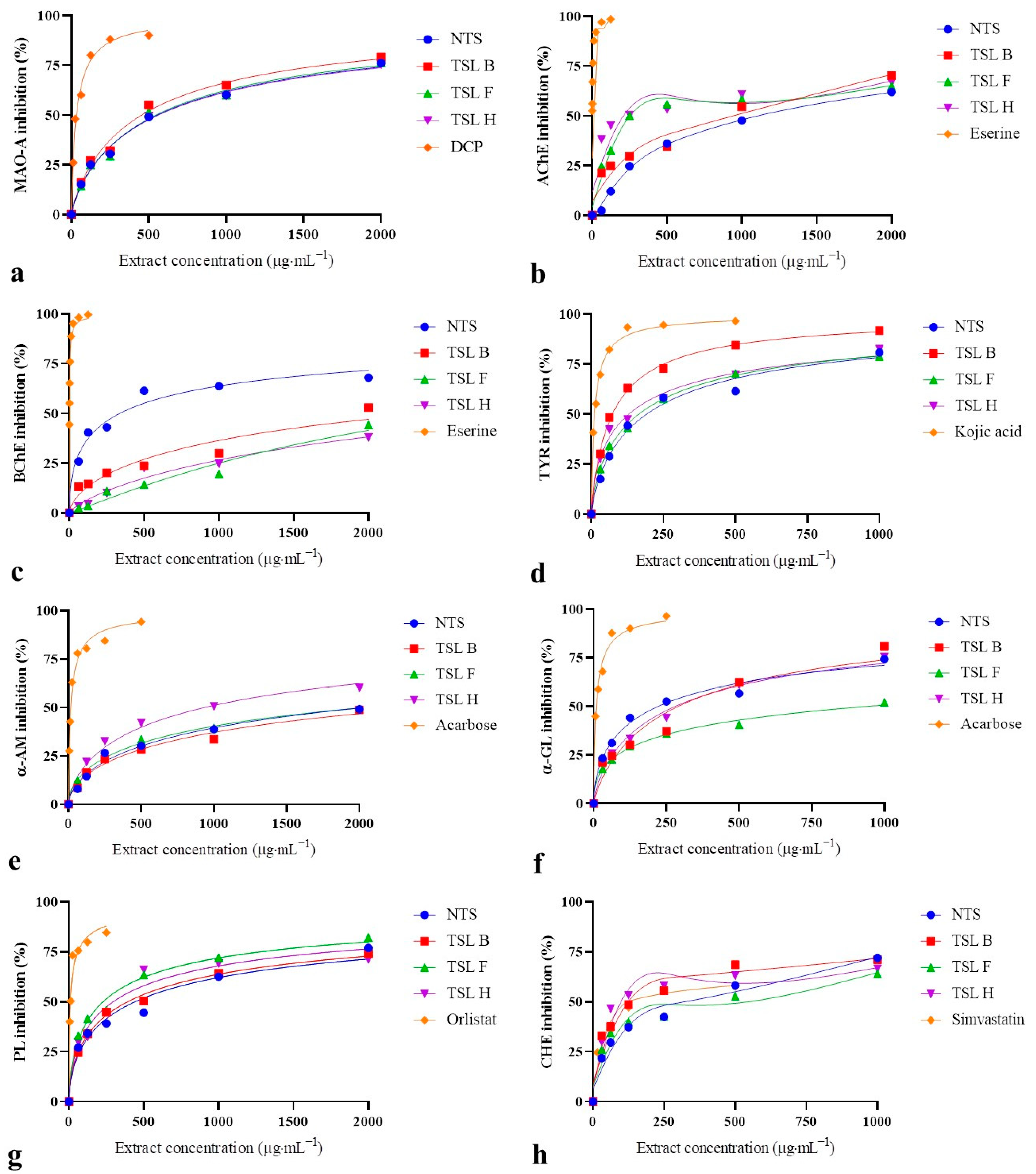
2.2. In Vitro Biological Activity of H. perforatum Transformed Shoots
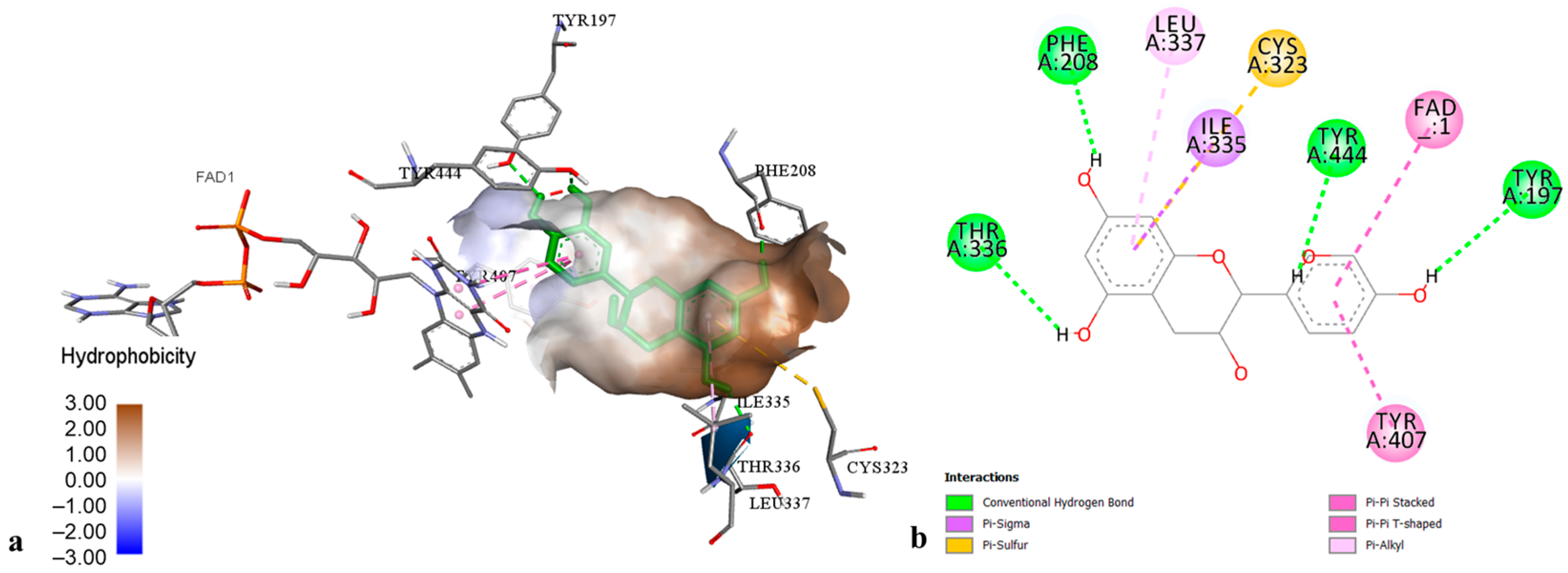
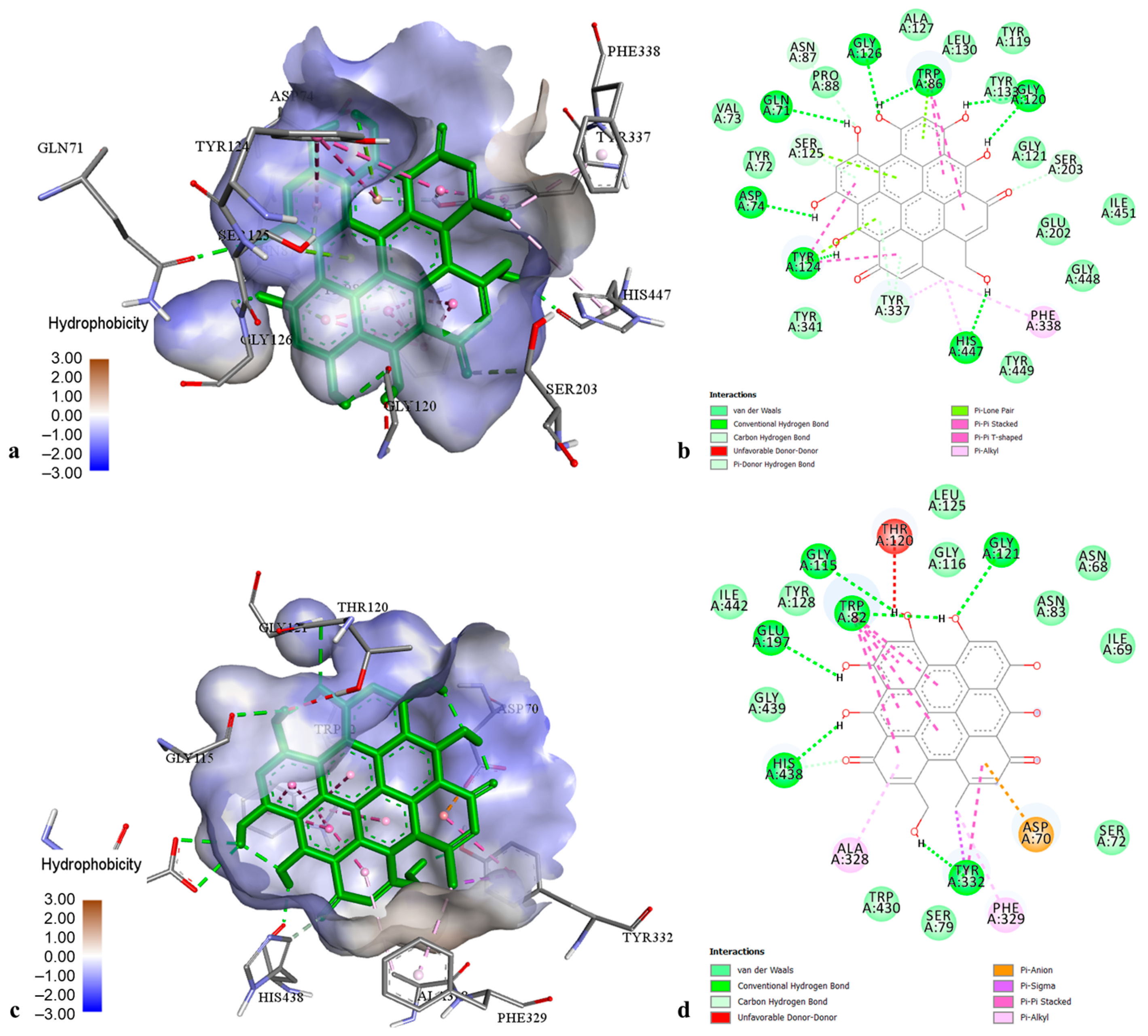
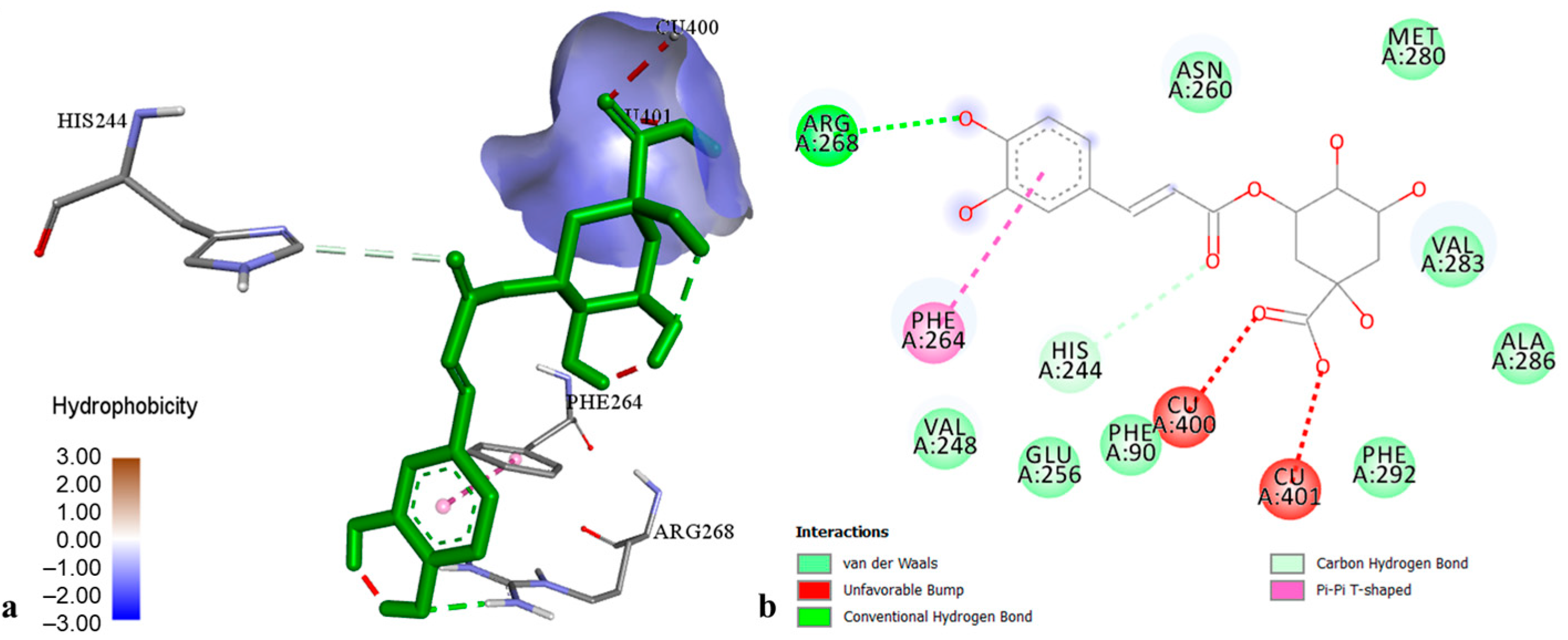
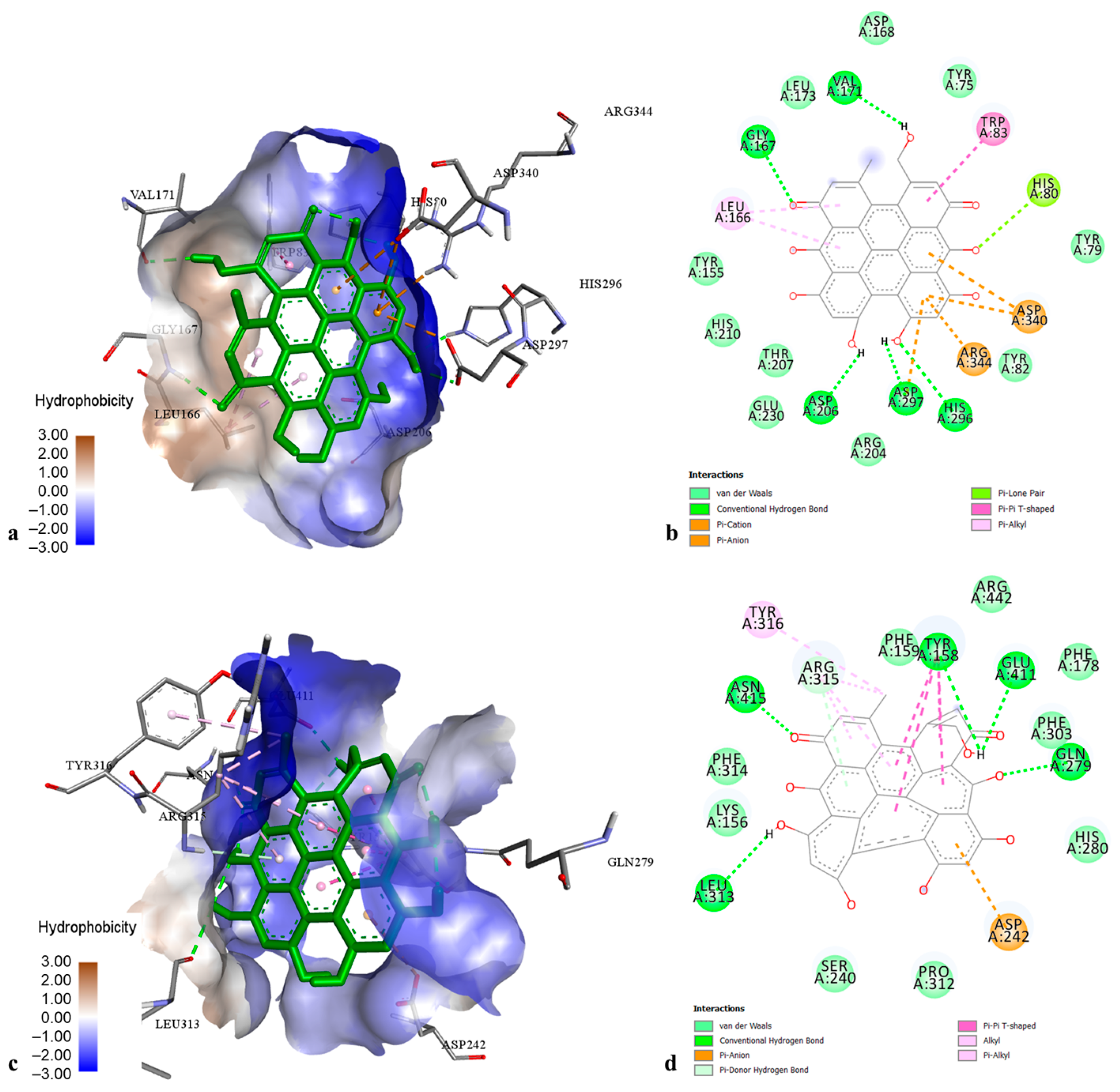
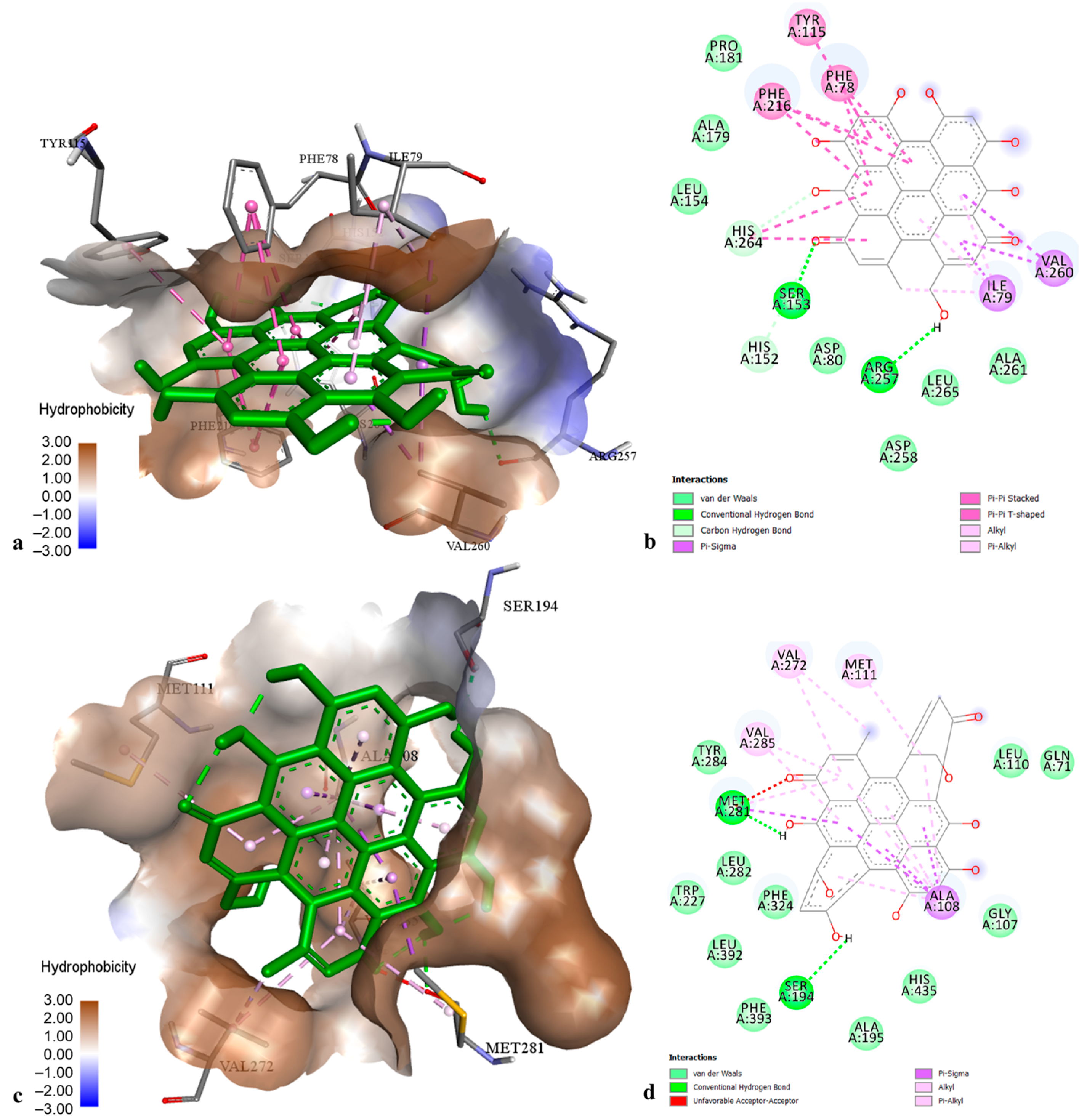
2.3. Molecular Modelling of Phenolic Compounds from H. perforatum Transformed Shoots
3. Discussion
3.1. Analysis of Phenolic Compounds in H. perforatum Transformed Shoots
3.2. Biological Activity of H. perforatum Transformed Shoots
4. Materials and Methods
4.1. Plant Material and Culture Conditions
4.2. Chromatographic Identification and Quantification of Phenolic Compounds
4.3. In Vitro Biological Activities
4.4. Molecular Modelling
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rafailovska, E.; Tushevski, O.; Shijakova, K.; Simic, S.G.; Kjovkarovska, S.D.; Miova, B. Hypericum perforatum L. extract exerts insulinotropic effects and inhibits gluconeogenesis in diabetic rats by regulating AMPK expression and PKCε concentration. J. Ethnopharmacol. 2023, 302, 115899. [Google Scholar] [CrossRef]
- Kapoor, S.; Chandel, R.; Kaur, R.; Kumar, S.; Kumar, R.; Janghu, S.; Kaur, A.; Kumar, V. The Flower of Hypericum perforatum L.: A traditional source of bioactives for new food and pharmaceutical applications. Biochem. Syst. Ecol. 2023, 110, 104702. [Google Scholar] [CrossRef]
- Shasmita Behera, S.; Mishra, P.; Samal, M.; Mohapatra, D.; Monalisa, K.; Naik, S.K. Recent advances in tissue culture and secondary metabolite production in Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2023, 154, 13–28. [Google Scholar] [CrossRef]
- Ion, V.; Ielciu, I.; Cârje, A.G.; Muntean, D.L.; Crişan, G.; Păltinean, R. Hypericum spp.—An Overview of the Extraction Methods and Analysis of Compounds. Separations 2022, 9, 17. [Google Scholar] [CrossRef]
- Silva, A.R.; Taofiq, O.; Ferreira, I.C.; Barros, L. Hypericum genus cosmeceutical application–A decade comprehensive review on its multifunctional biological properties. Ind. Crops Prod. 2021, 159, 113053. [Google Scholar] [CrossRef]
- Tusevski, O.; Krstikj, M.; Stanoeva, J.P.; Stefova, M.; Simic, S.G. Phenolic profile and biological activity of Hypericum perforatum L.: Can roots be considered as a new source of natural compounds? S. Afr. J. Bot. 2018, 117, 301–310. [Google Scholar] [CrossRef]
- Bruni, R.; Sacchetti, G. Factors affecting polyphenol biosynthesis in wild and field grown St. John’s Wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 2009, 14, 682–725. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Ounnar, S.; Righezza, M.; Kascakova, S.; Refregiers, M.; Spasenoski, M.; Joseph, C.; Hagège, D. Identification and quantification of hypericin and pseudohypericin in different Hypericum perforatum L. in vitro cultures. Plant Physiol. Biochem. 2005, 43, 591–601. [Google Scholar] [CrossRef]
- Cui, X.H.; Chakrabarty, D.; Lee, E.J.; Paek, K.Y. Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresour. Technol. 2010, 101, 4708–4716. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, G.; Avato, P.; Monacelli, B.; Santamaria, A.R.; Argentieri, M.P. Metabolites in cell suspension cultures, calli, and in vitro regenerated organs of Hypericum perforatum cv. Topas. Plant Sci. 2003, 165, 977–982. [Google Scholar] [CrossRef]
- Mulinacci, N.; Giaccherini, C.; Santamaria, A.R.; Caniato, R.; Ferrari, F.; Valletta, A.; Vincieri, F.F.; Pasqua, G. Anthocyanins and xanthones in the calli and regenerated shoots of Hypericum perforatum var. angustifolium (sin. Fröhlich) Borkh. Plant Physiol. Biochem. 2008, 46, 414–420. [Google Scholar] [CrossRef]
- Murthy, H.N.; Kim, Y.S.; Park, S.Y.; Paek, K.Y. Hypericins: Biotechnological production from cell and organ cultures. Appl. Microbiol. Biotechnol. 2014, 98, 9187–9198. [Google Scholar] [CrossRef]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 2019, 71, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Shakya, P.; Franklin, G. A perspective on Hypericum perforatum genetic transformation. Front. Plant Sci. 2016, 7, 197427. [Google Scholar] [CrossRef] [PubMed]
- Vinterhalter, B.; Ninković, S.; Cingel, A.; Vinterhalter, D. Shoot and root culture of Hypericum perforatum L. transformed with Agrobacterium rhizogenes A4M70GUS. Biol. Plant. 2006, 50, 767–770. [Google Scholar] [CrossRef]
- Franklin, G.; Oliveira, M.; Dias, A.C.P. Production of transgenic Hypericum perforatum plants via particle bombardment-mediated transformation of novel organogenic cell suspension cultures. Plant Sci. 2007, 172, 1193–1203. [Google Scholar] [CrossRef]
- Komarovská, H.; Giovannini, A.; Košuth, J.; Čellárová, E. Agrobacterium rhizogenes-mediated transformation of Hypericum tomentosum L. and Hypericum tetrapterum Fries. Z. Naturforsch. C 2009, 64, 864–868. [Google Scholar] [CrossRef]
- Tusevski, O.; Stanoeva, J.P.; Stefova, M.; Kungulovski, D.; Pancevska, N.A.; Sekulovski, N.; Panov, S.; Simic, S.G. Hairy roots of Hypericum perforatum L.: A promising system for xanthone production. Open Life Sci. 2013, 8, 1010–1022. [Google Scholar] [CrossRef]
- Zubrická, D.; Mišianiková, A.; Henzelyová, J.; Valletta, A.; De Angelis, G.; D’Auria, F.D.; Simonetti, G.; Pasqua, G.; Čellárová, E. Xanthones from roots, hairy roots and cell suspension cultures of selected Hypericum species and their antifungal activity against Candida albicans. Plant Cell Rep. 2015, 34, 1953–1962. [Google Scholar] [CrossRef]
- Franklin, G.; Conceição, L.F.; Kombrink, E.; Dias, A.C. Xanthone biosynthesis in Hypericum perforatum cells provides antioxidant and antimicrobial protection upon biotic stress. Phytochemistry 2009, 70, 60–68. [Google Scholar] [CrossRef]
- Bulgakov, V.P. Functions of rol genes in plant secondary metabolism. Biotechnol. Adv. 2008, 26, 318–324. [Google Scholar] [CrossRef]
- Tusevski, O.; Todorovska, M.; Stanoeva, J.P.; Stefova, M.; Simic, S.G. In Vitro and in Silico Insights on the Biological Activities, Phenolic Compounds Composition of Hypericum perforatum L. Hairy Root Cultures. Phyton-Int. J. Exp. Bot. 2023, 92, 921–941. [Google Scholar] [CrossRef]
- Tusevski, O.; Vinterhalter, B.; Krstić Milošević, D.; Soković, M.; Ćirić, A.; Vinterhalter, D.; Zdravković Korać, S.; Petreska Stanoeva, J.; Stefova, M.; Gadzovska Simic, S. Production of phenolic compounds, antioxidant and antimicrobial activities in hairy root and shoot cultures of Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2017, 128, 589–605. [Google Scholar] [CrossRef]
- Tusevski, O.; Petreska Stanoeva, J.; Stefova, M.; Spasenoski, M.; Gadzovska Simic, S. State of antioxidant systems and phenolic compounds’ production in Hypericum perforatum L. hairy roots. Acta Physiol. Plant. 2019, 41, 132. [Google Scholar] [CrossRef]
- Rafailovska, E.; Tushevski, O.; Gadzovska-Simic, S.; Dinevska-Kjovkarovska, S.; Miova, B. Hypericum perforatum L. Hairy Root Extracts–Regulation of Glycemic, Metabolic, Serum Enzyme and Lipid Profile in STZ-Induced Diabetic Rats. Maced. Vet. Rev. 2022, 45, 5–15. [Google Scholar] [CrossRef]
- Tusevski, O.; Gadzovska Simic, S. Non-Enzymatic and Enzymatic Antioxidant Responses of Hypericum perforatum L. Hairy Roots upon Photooxidative Stress. Horticulturae 2023, 9, 581. [Google Scholar] [CrossRef]
- Tusevski, O.; Todorovska, M.; Todorovska, I.; Stanoeva, J.P.; Simic, S.G. Photoperiod modulates the production of biologically active compounds in Hypericum perforatum L. hairy roots: An in vitro and in silico approach. Plant Cell Tissue Organ Cult. 2024, 156, 96. [Google Scholar] [CrossRef]
- Tusevski, O.; Todorovska, M.; Todorovska, I.; Petreska Stanoeva, J.; Gadzovska Simic, S. Production of Phenylpropanoids, Naphthodianthrones and Antioxidant Status of Hypericum perforatum L. Transgenic Shoots. Horticulturae 2024, 10, 59. [Google Scholar] [CrossRef]
- Tusevski, O.; Petreska Stanoeva, J.; Stefova, M.; Pavokovic, D.; Gadzovska Simic, S. Identification and quantification of phenolic compounds in Hypericum perforatum L. transgenic shoots. Acta Physiol. Plant. 2014, 36, 2555–2569. [Google Scholar] [CrossRef]
- Bertoli, A.; Giovannini, A.; Ruffoni, B.; Guardo, A.D.; Spinelli, G.; Mazzetti, M.; Pistelli, L. Bioactive constituent production in St. John’s wort in vitro hairy roots. Regenerated plant lines. J. Agric. Food Chem. 2008, 56, 5078–5082. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Kwiecień, I.; Szydłowska, A.; Kawka, B.; Beerhues, L.; Ekiert, H. Accumulation of biologically active phenolic acids in agitated shoot cultures of three Hypericum perforatum cultivars: ‘Elixir’, ‘Helos’ and ‘Topas’. Plant Cell Tissue Organ Cult. 2015, 123, 273–281. [Google Scholar] [CrossRef]
- Liang, H.; Liang, Y.; Dong, J.; Lu, J. Tea extraction methods in relation to control of epimerization of tea catechins. J. Sci. Food Agric. 2007, 87, 1748–1752. [Google Scholar] [CrossRef]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive procyanidins from dietary sources: The relationship between bioactivity and polymerization degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Nigutová, K.; Kusari, S.; Sezgin, S.; Petijová, L.; Henzelyová, J.; Bálintová, M.; Spiteller, M.; Čellárová, E. Chemometric evaluation of hypericin and related phytochemicals in 17 in vitro cultured Hypericum species, hairy root cultures and hairy root-derived transgenic plants. J. Pharm. Pharmacol. 2019, 71, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Guglielmi, G. Determination of major constituents in St. John’s Wort under different extraction conditions. Pharm. Biol. 2004, 42, 83–89. [Google Scholar] [CrossRef]
- Filippini, R.; Piovan, A.; Borsarini, A.; Caniato, R. Study of dynamic accumulation of secondary metabolites in three subspecies of Hypericum perforatum. Fitoterapia 2010, 81, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Soelberg, J.; Jørgensen, L.B.; Jäger, A.K. Hyperforin accumulates in the translucent glands of Hypericum perforatum. Ann. Bot. 2007, 99, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Charchoglyan, A.; Abrahamyan, A.; Fujii, I.; Boubakir, Z.; Gulder, T.A.; Kutchan, T.M.; Vardapetyan, H.; Bringmann, G.; Ebizuka, Y.; Beerhues, L. Differential accumulation of hyperforin and secohyperforin in Hypericum perforatum tissue cultures. Phytochemistry 2007, 68, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Tocci, N.; Gaid, M.; Kaftan, F.; Belkheir, A.K.; Belhadj, I.; Liu, B.; Svatoš, A.; Hänsch, R.; Pasqua, G.; Beerhues, L. Exodermis and endodermis are the sites of xanthone biosynthesis in Hypericum perforatum roots. New Phytol. 2018, 217, 1099–1112. [Google Scholar] [CrossRef]
- Brasili, E.; Miccheli, A.; Marini, F.; Pratico, G.; Sciubba, F.; Di Cocco, M.E.; Cechinel, V.F.; Tocci, N.; Valletta, A.; Pasqua, G. Metabolic profile and root development of Hypericum perforatum L. in vitro roots under stress conditions due to chitosan treatment and culture time. Front. Plant Sci. 2016, 7, 507. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Sobarzo-Sánchez, E.; Uriarte, E.; Khatkar, A. Quercetin and related chromenone derivatives as monoamine oxidase inhibitors: Targeting neurological and mental disorders. Molecules 2019, 24, 418. [Google Scholar] [CrossRef] [PubMed]
- Bandaruk, Y.; Mukai, R.; Kawamura, T.; Nemoto, H.; Terao, J. Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase-A reaction in mouse brain mitochondria. J. Agric. Food Chem. 2012, 60, 10270–10277. [Google Scholar] [CrossRef] [PubMed]
- Stringer, T.P.; Guerrieri, D.; Vivar, C.; Van Praag, H. Plant-derived flavanol (−) epicatechin mitigates anxiety in association with elevated hippocampal monoamine and BDNF levels, but does not influence pattern separation in mice. Transl. Psychiatry 2015, 5, e493. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Zhang, H.Y. Theoretical evaluation of flavonoids as multipotent agents to combat Alzheimer’s disease. J. Mol. Struc-THEOCHEM 2006, 767, 3–9. [Google Scholar] [CrossRef]
- Orhan, G.; Orhan, I.; Sener, B. Recent developments in natural and synthetic drug research for Alzheimer’s disease. Lett. Drug Des. Disc. 2006, 3, 268–274. [Google Scholar] [CrossRef]
- Altun, M.L.; Yılmaz, B.S.; Orhan, I.E.; Citoglu, G.S. Assessment of cholinesterase and tyrosinase inhibitory and antioxidant effects of Hypericum perforatum L.(St. John’s wort). Ind. Crops Prod. 2013, 43, 87–92. [Google Scholar] [CrossRef]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002, 14, 77–91. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Choi, S.B.; Tan, S.C.; Wahab, H.A.; Chan, K.L.; Murugaiyah, V. Prenylated xanthones from mangosteen as promising cholinesterase inhibitors and their molecular docking studies. Phytomedicine 2014, 21, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.A.; Dias, A.C.; Ferreres, F.; Malva, J.O.; Oliveira, C.R. Neuroprotective effect of H. perforatum extracts on β-amyloid-induced neurotoxicity. Neurotox. Res. 2004, 6, 119–130. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C.P. Neuroprotective activity of Hypericum perforatum and its major components. Front. Plant Sci. 2016, 7, 197445. [Google Scholar] [CrossRef]
- Orhan, I.E.; Senol Deniz, F.S.; Trædal-Henden, S.; Cerón-Carrasco, J.P.; den Haan, H.; Peña-García, J.; Pérez-Sánchez, H.; Emerce, E.; Skalicka-Wozniak, K. Profiling auspicious butyrylcholinesterase inhibitory activity of two herbal molecules: Hyperforin and hyuganin C. Chem. Biodivers. 2019, 16, e1900017. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Sharma, B.; Parashar, A.; Sharma, V.; Kumar, A.; Mehta, V. 2D-QSAR, molecular docking and MD simulation based virtual screening of the herbal molecules against Alzheimer’s disorder: An approach to predict CNS activity. J. Biomol. Struct. Dyn. 2024, 42, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Zengin, G.; Lobine, D.; Molina-García, L.; Mollica, A.; Mahomoodally, M.F. Phytochemical characterization, in vitro and in silico approaches for three Hypericum species. New J. Chem. 2018, 42, 5204–5214. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, P.; Wang, Y.; Wang, Y.; Hochstetter, D. Combined effects of green tea extracts, green tea polyphenols or epigallocatechin gallate with acarbose on inhibition against α-amylase and α-glucosidase in vitro. Molecules 2013, 18, 11614–11623. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Hu, N.; Yue, H.; Wang, H. Inhibitory activity and mechanism investigation of hypericin as a novel α-glucosidase inhibitor. Molecules 2021, 26, 4566. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, J.; Tian, J.; Chen, S.; Ye, X.; Hu, Y.; Chen, J. Inhibitory mechanism of novel allosteric inhibitor, Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves proanthocyanidins against α-glucosidase. J. Funct. Foods 2019, 56, 286–294. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [CrossRef] [PubMed]
- Algethami, F.K.; Saidi, I.; Abdelhamid, H.N.; Elamin, M.R.; Abdulkhair, B.Y.; Chrouda, A.; Ben Jannet, H. Trifluoromethylated Flavonoid-Based Isoxazoles as Antidiabetic and Anti-Obesity Agents: Synthesis, In Vitro α-Amylase Inhibitory Activity, Molecular Docking and Structure–Activity Relationship Analysis. Molecules 2021, 26, 5214. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Davies, G.J. Structure of the Aspergillus oryzae α-amylase complexed with the inhibitor acarbose at 2.0 Å resolution. Biochemistry 1997, 36, 10837–10845. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Chen, W.H.; Park, H.; Ke, Z.; Wang, B. Binding mechanism and synergetic effects of xanthone derivatives as noncompetitive α-glucosidase inhibitors: A theoretical and experimental study. J. Phys. Chem. B 2013, 117, 13464–13471. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khayat, M.T.A.; Ahmed, S.; Abo-Haded, H. α-Amylase inhibition of xanthones from Garcinia mangostana pericarps and their possible use for the treatment of diabetes with molecular docking studies. J. Food Biochem. 2019, 43, e12844. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Intrawangso, J.; Hemrid, A.; Chanathong, B.; Mäkynen, K. Extracts of edible plants inhibit pancreatic lipase, cholesterol esterase and cholesterol micellization, and bind bile acids. Food Technol. Biotechnol. 2012, 50, 11–16. Available online: https://hrcak.srce.hr/78897 (accessed on 22 July 2024).
- Tian, J.Y.; Tao, R.Y.; Zhang, X.L.; Liu, Q.; He, Y.B.; Su, Y.L.; Ji, T.F.; Ye, F. Effect of Hypericum perforatum L. extract on insulin resistance and lipid metabolic disorder in high-fat-diet induced obese mice. Phytother. Res. 2015, 29, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.D.; Guan, X.Q.; Cao, Y.F.; Weng, Z.M.; Hu, Q.; Liu, H.B.; Jia, S.N.; Zang, S.Z.; Zhou, Q.; Yang, L.; et al. Inhibition of pancreatic lipase by the constituents in St. John’s Wort: In vitro and in silico investigations. Int. J. Biol. Macromol. 2020, 145, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Villa, L.; Vistoli, G. VEGA–an open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J. Comput. Aided Mol. Des. 2004, 18, 167–173. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
| Peak | Phenolic Compounds | NTS | TSL B | TSL F | TSL H |
|---|---|---|---|---|---|
| Phenolic acids | |||||
| F2 | Chlorogenic acid | 8.75 ± 1.01 c | 0.77 ± 0.06 a | 12.44 ± 1.63 d | 4.04 ± 0.89 b |
| F3 | 3-p-Coumaroylquinic acid | 1.97 ± 0.43 b | 0.46 ± 0.27 a | 0.21 ± 0.16 a | 0.97 ± 0.52 ab |
| F5 | 3-Feruloylquinic acid | 0.86 ± 0.07 b | n.d. | 0.29 ± 0.02 a | 1.23 ± 0.16 c |
| Flavan-3-ols | |||||
| F1 | (epi)catechin-(epi)gallocatechin dimer | n.d. | n.d. | 4.17 ± 0.53 | n.d. |
| F4 | Procyanidin B2 | n.d. | 1.54 ± 0.17 a | 4.14 ± 0.38 b | 1.40 ± 0.11 a |
| F6 | Procyanidin trimer | n.d. | n.d. | 3.71 ± 0.55 | n.d. |
| F7 | (epi)catechin | 5.64 ± 0.41 b | 4.97 ± 0.25 ab | 6.05 ± 0.82 b | 4.62 ± 0.17 a |
| Flavonol glycosides and aglycons | |||||
| F9 | Quercetin 6-C-glucoside | 0.91 ± 0.05 a | n.d. | 0.95 ± 0.09 a | n.d. |
| F11 | Kaempferol 6-C-glucoside | n.d. | 0.81 ± 0.04 b | 0.46 ± 0.07 a | 0.97 ± 0.11 b |
| F12 | Hyperoside (quercetin 3-O-galactoside) | 2.22 ± 0.20 b | 1.47 ± 0.23 a | 2.41 ± 0.15 b | 1.66 ± 0.09 a |
| F13 | Rutin (quercetin 3-O-rutinoside) | n.d. | 0.45 ± 0.02 | n.d. | n.d. |
| F14 | Quercitrin (quercetin 3-O-rhamnoside) | 3.89 ± 0.52 ab | 4.15 ± 0.39 b | 3.06 ± 0.67 a | 3.00 ± 0.23 a |
| F15 | Quercetin | 0.31 ± 0.02 a | 0.53 ± 0.07 b | 0.44 ± 0.05 b | 0.42 ± 0.05 b |
| Anthocyanins | |||||
| F8 | Cyanidin 3-O-glycoside | 0.08 ± 0.01 a | 0.13 ± 0.02 b | 0.13 ± 0.01 b | 0.11 ± 0.02 ab |
| F10 | Cyanidin 3-O-rhamnoside | 1.42 ± 0.15 ab | 2.08 ± 0.13 c | 1.25 ± 0.10 a | 1.75 ± 0.21 bc |
| Naphthodianthrones | |||||
| F16 | Pseudohypericin | 0.66 ± 0.03 a | 1.91 ± 0.06 c | 0.76 ± 0.06 a | 1.18 ± 0.06 b |
| F17 | Hypericin | 0.03 ± 0.00 b | 0.07 ± 0.00 d | 0.02 ± 0.00 a | 0.04 ± 0.00 c |
| F18 | Protopseudohypericin | 0.09 ± 0.01 a | 0.48 ± 0.03 c | 0.35 ± 0.03 b | 0.58 ± 0.02 d |
| Acyl-phloroglucinols | |||||
| F19 | Hyperforin | 1.70 ± 0.13 b | 3.31 ± 0.27 c | 1.60 ± 0.10 b | 1.07 ± 0.08 a |
| F20 | Adhyperforin | 0.40 ± 0.03 b | 0.35 ± 0.03 b | 0.19 ± 0.02 a | 0.38 ± 0.05 b |
| Xanthones | |||||
| X1 | Mangiferin | 11.26 ± 1.98 b | 4.47 ± 0.41 a | 12.97 ± 2.63 b | 4.81 ± 0.62 a |
| X2 | Brasilixanthone B | n.d. | 1.85 ± 0.23 a | 1.72 ± 0.10 a | n.d. |
| X3 | Trihydroxyxanthone-sulfonate | 7.88 ± 0.97 b | 5.97 ± 0.34 a | 8.37 ± 0.66 b | 6.04 ± 0.40 a |
| X4 | Dimethylmangiferin | 1.08 ± 0.12 b | 1.52 ± 0.21 b | 0.30 ± 0.02 a | 0.99 ± 0.19 b |
| X5 | Dihydroxy-metoxyxanthone-sulfonate | 4.73 ± 0.38 b | 4.49 ± 0.44 ab | 3.50 ± 0.32 a | 3.94 ± 0.58 ab |
| X6 | Mangiferin C-prenyl isomer | 0.18 ± 0.01 a | n.d. | 0.16 ± 0.05 a | n.d. |
| X7 | 1,3,6,7-Tetrahydroxyxanthone 2-prenyl xanthone | n.d. | 0.05 ± 0.00 a | 0.14 ± 0.02 b | n.d. |
| X8 | 1,3,6,7-Tetrahydroxyxanthone 8-prenyl xanthone | n.d. | n.d. | 0.14 ± 0.01 | n.d. |
| X9 | γ-Mangostin | 0.62 ± 0.04 b | 0.47 ± 0.06 a | 0.48 ± 0.04 a | 0.78 ± 0.10 b |
| X10 | 5-O-Methyl-2-deprenylrheediaxanthone B | n.d. | n.d. | 0.21 ± 0.03 a | 0.15 ± 0.02 a |
| X11 | Cadensin G | 6.83 ± 0.81 ab | 5.59 ± 0.77 a | 7.41 ± 0.46 b | 6.77 ± 0.61 ab |
| MAO-A (IC50 µg·mL−1) | AChE (IC50 µg·mL−1) | BChE (IC25 µg·mL−1) | TYR (IC50 µg·mL−1) | α-AM (IC25 µg·mL−1) | α-GL (IC50 µg·mL−1) | PL (IC50 µg·mL−1) | CHE (IC50 µg·mL−1) | |
|---|---|---|---|---|---|---|---|---|
| NTS | 511.99 ± 51.70 bc | 1107.23 ± 78.76 d | 75.12 ± 10.56 b | 150.44 ± 10.12 d | 277.04 ± 30.15 d | 156.99 ± 9.78 b | 406.95 ± 49.64 c | 302.17 ± 1.91 c |
| TSL B | 433.90 ± 30.03 b | 944.91 ± 59.11 c | 480.03 ± 14.34 c | 80.66 ± 2.25 b | 261.98 ± 12.96 d | 345.69 ± 37.48 c | 352.68 ± 26.34 c | 125.93 ± 12.70 b |
| TSL F | 546.77 ± 47.03 c | 233.32 ± 25.82 b | 1257.49 ± 42.04 e | 147.59 ± 14.29 d | 214.43 ± 27.40 c | 879.90 ± 23.66 d | 230.39 ± 21.58 b | 572.04 ± 40.96 d |
| TSL H | 566.16 ± 41.01 c | 217.90 ± 17.12 b | 850.49 ± 51.92 d | 119.16 ± 9.77 c | 169.91 ± 7.52 b | 298.86 ± 30.79 c | 254.80 ± 24.79 b | 102.50 ± 6.50 a |
| DCP | 36.86 ± 0.70 a | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. |
| Eserine | n.t. | 11.97 ± 0.64 a | 0.10 ± 0.02 a | n.t. | n.t. | n.t. | n.t. | n.t. |
| Kojic acid | n.t. | n.t. | n.t. | 19.39 ± 1.77 a | n.t. | n.t. | n.t. | n.t. |
| Acarbose | n.t. | n.t. | n.t. | n.t. | 6.77 ± 0.76 a | 15.43 ± 1.15 a | n.t. | n.t. |
| Orlistat | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | 13.88 ± 0.77 a | n.t. |
| Simvastatin | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | 160.47 ± 28.29 b |
| Ligands | Enzymes | Binding Energy (kcal·mol−1) | Inhibition Constant (Ki) |
|---|---|---|---|
| Chlorogenic acid | MAO-A | −7.31 | 4.4 µM |
| AChE | −7.30 | 4.49 µM | |
| BChE | −5.84 | 52.07 µM | |
| TYR | −8.09 | 1.17 µM | |
| α-AM | −4.30 | 703.86 µM | |
| α-GL | −5.09 | 156.36 µM | |
| PL | −6.14 | 31.74 µM | |
| CHE | −5.02 | 208.55 µM | |
| Epicatechin | MAO-A | −8.52 | 564.15 nM |
| AChE | −8.19 | 995.35 nM | |
| BChE | −8.45 | 635.22 nM | |
| TYR | −4.37 | 622.42 µM | |
| α-AM | −7.76 | 2.04 µM | |
| α-GL | −7.44 | 3.51 µM | |
| PL | −8.38 | 722.92 nM | |
| CHE | −5.64 | 73.02 µM | |
| Pseudohypericin | MAO-A | −6.50 | 17.15 µM |
| AChE | −12.00 | 1.59 nM | |
| BChE | −14.56 | 21.14 pM | |
| TYR | −7.21 | 5.20 µM | |
| α-AM | −11.58 | 3.27 nM | |
| α-GL | −11.65 | 2.89 nM | |
| PL | −12.70 | 489.00 pM | |
| CHE | −10.51 | 19.91 nM | |
| Hyperforin | MAO-A | −4.99 | 270.76 µM |
| AChE | −8.49 | 600.06 nM | |
| BChE | −11.06 | 7.75 nM | |
| TYR | −6.37 | 21.33 µM | |
| α-AM | −8.02 | 1.33 µM | |
| α-GL | −10.30 | 28.32 nM | |
| PL | −8.42 | 673.63 nM | |
| CHE | −4.48 | 518.91 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tusevski, O.; Todorovska, M.; Petreska Stanoeva, J.; Gadzovska Simic, S. Phytochemical Analysis, Biological Activities, and Docking of Phenolics from Shoot Cultures of Hypericum perforatum L. Transformed by Agrobacterium rhizogenes. Molecules 2024, 29, 3893. https://doi.org/10.3390/molecules29163893
Tusevski O, Todorovska M, Petreska Stanoeva J, Gadzovska Simic S. Phytochemical Analysis, Biological Activities, and Docking of Phenolics from Shoot Cultures of Hypericum perforatum L. Transformed by Agrobacterium rhizogenes. Molecules. 2024; 29(16):3893. https://doi.org/10.3390/molecules29163893
Chicago/Turabian StyleTusevski, Oliver, Marija Todorovska, Jasmina Petreska Stanoeva, and Sonja Gadzovska Simic. 2024. "Phytochemical Analysis, Biological Activities, and Docking of Phenolics from Shoot Cultures of Hypericum perforatum L. Transformed by Agrobacterium rhizogenes" Molecules 29, no. 16: 3893. https://doi.org/10.3390/molecules29163893
APA StyleTusevski, O., Todorovska, M., Petreska Stanoeva, J., & Gadzovska Simic, S. (2024). Phytochemical Analysis, Biological Activities, and Docking of Phenolics from Shoot Cultures of Hypericum perforatum L. Transformed by Agrobacterium rhizogenes. Molecules, 29(16), 3893. https://doi.org/10.3390/molecules29163893







