Investigating the Electrochemical Performance of MnFe2O4@xC Nanocomposites as Anode Materials for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Morphology Analysis
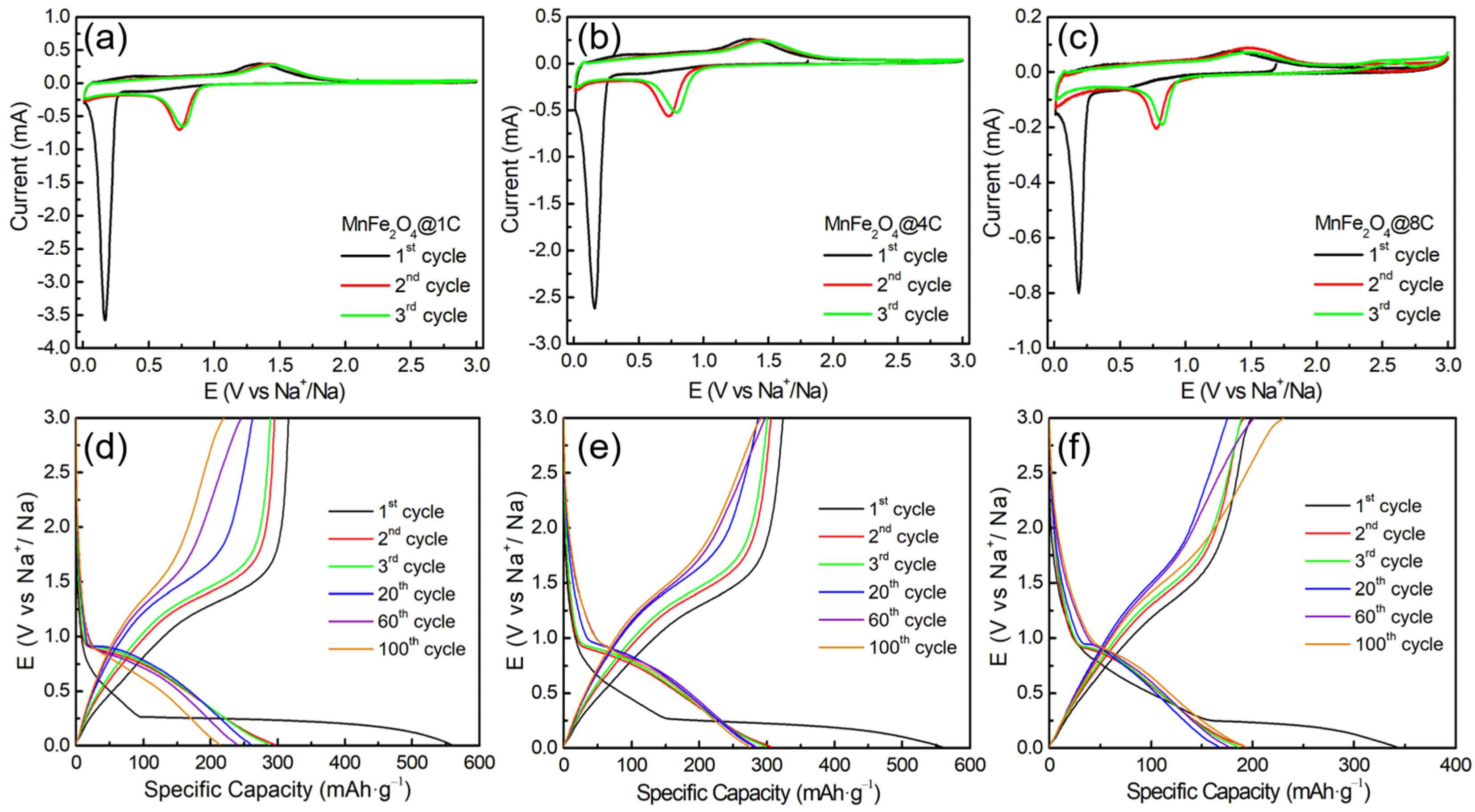
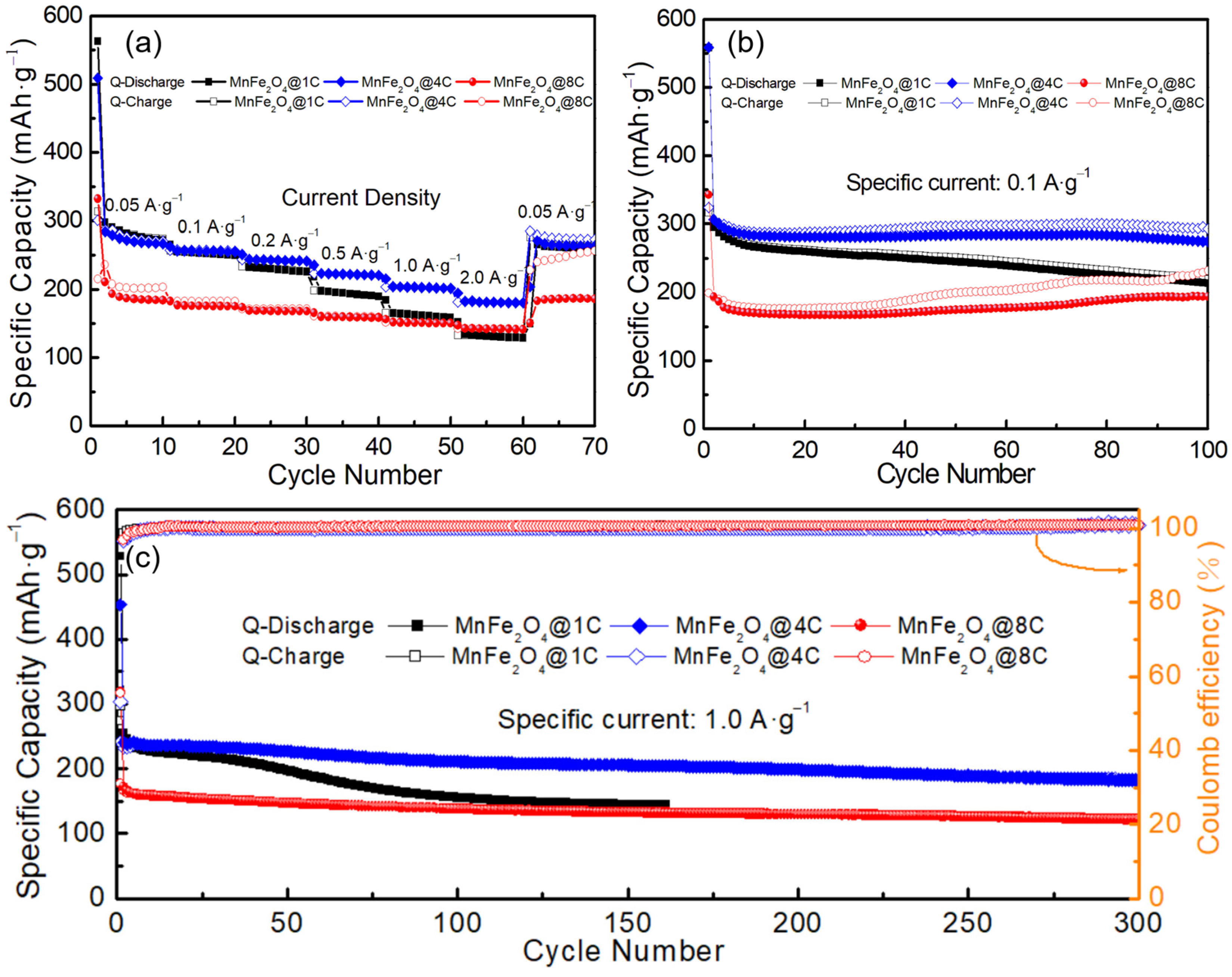
2.2. Sodium-Ion Storage Performance of the MnFe2O4@xC Nanocomposites
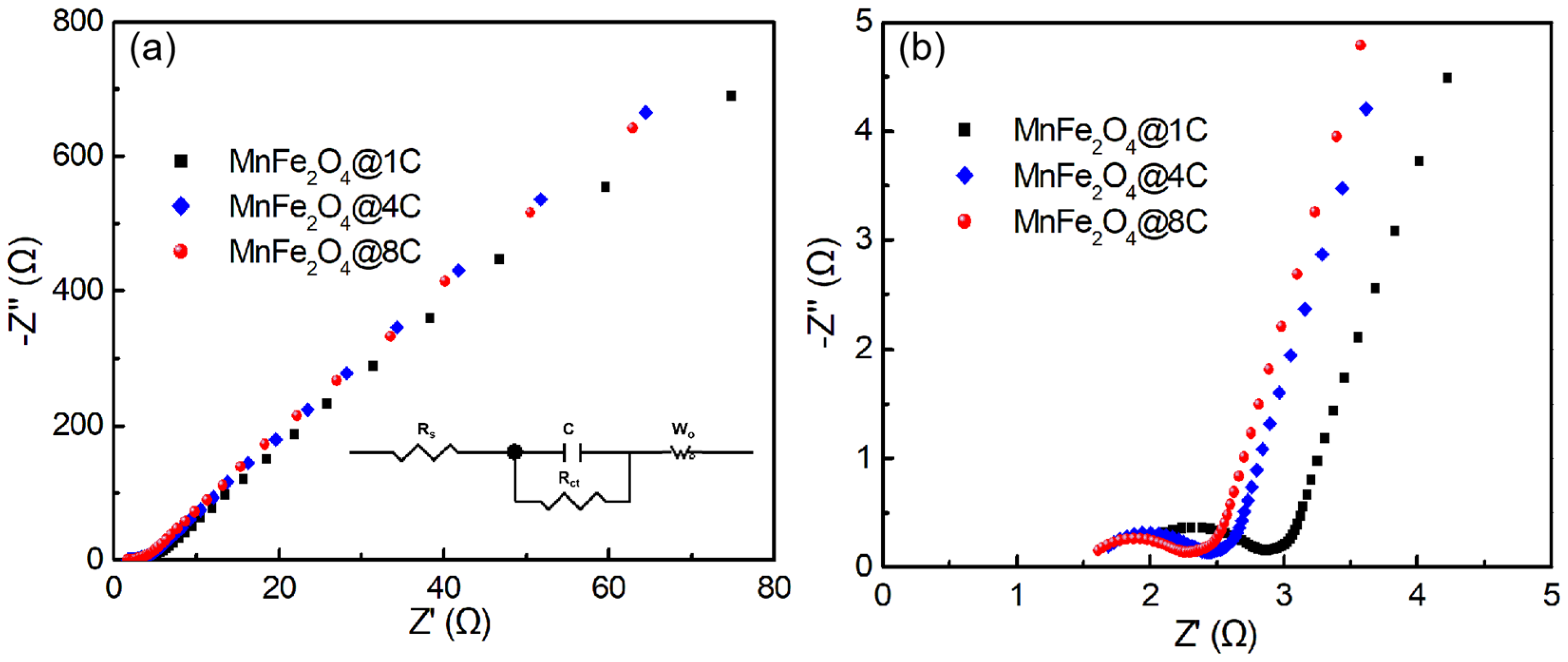
2.3. Exploring the Origin of the Improvement in the Sodium-Ion Storage of the MnFe2O4@xC Nanocomposites
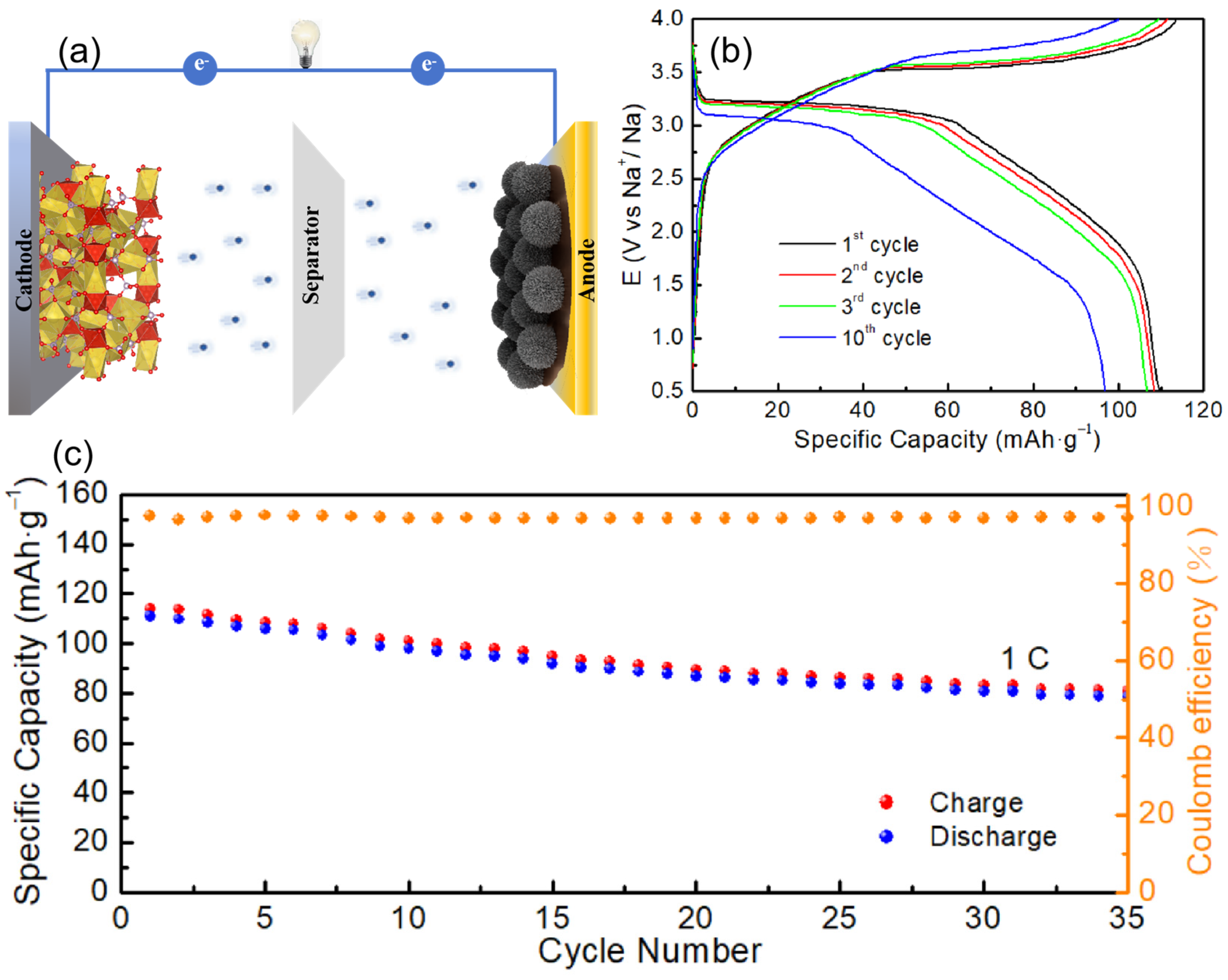
2.4. Full-Cell Performance
3. Materials and Methods
3.1. Synthesis of the MnFe2O4 Spherical Nanoparticles
3.2. Synthesis of the MnFe2O4@xC Spherical Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Zhang, H.; Peng, J.; Li, L.; Xiao, Y.; Li, L.; Liu, Y.; Qiao, Y.; Chou, S.-L. A 30-year overview of sodium-ion batteries. Carbon Energy 2024, 6, e464. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Liao, H.; Zheng, Y.; Fu, X.; Lu, J.; Cheng, S.; Gao, Y. Progress and Prospect of Bimetallic Oxides for Sodium-Ion Batteries: Synthesis, Mechanism, and Optimization Strategy. ACS Nano 2024, 18, 7796–7824. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ye, L.; Wei, A.; Xu, G.; Cao, Z.; Zhu, P.; Chen, Y. Constructing SnS/Fe2O3 heterostructure anchored on few-layered graphene as an ion-adsorption/diffusion enhancer for ultrafast and cycle-stable sodium storage. Chem. Eng. J. 2023, 457, 141243. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Gu, Z.-Y.; Heng, Y.-L.; Ang, E.-H.; Geng, H.-B.; Wu, X.-L. Advanced layered oxide cathodes for sodium/potassium-ion batteries: Development, challenges, and prospects. Chem. Eng. J. 2023, 452, 139438. [Google Scholar] [CrossRef]
- Deng, X.; Chen, Z.; Cao, Y. Transition metal oxides based on conversion reaction for sodium-ion battery anodes. Mater. Today Chem. 2018, 9, 114–132. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Q.; Zhong, W.; Deng, Q.; Yang, C.; Liu, Y.; Hu, J.; Yang, C. Construction of defective MoxW1-xS2/Cu7.2S4 polyhedral heterostructures for fast sodium storage. Chem. Eng. J. 2023, 451, 138645. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Y.; Lu, X.; Qi, F.; Liang, Y.; Trukhanov, A.; Wu, Y.; Sun, Z.; Lu, X. Fully Active Bimetallic Phosphide Zn0.5Ge0.5P: A Novel High-Performance Anode for Na-Ion Batteries Coupled with Diglyme-Based Electrolyte. ACS Appl. Mater. Interfaces 2022, 14, 31803–31813. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, R.; Zhang, P.; Dun, C.; Urban, J.J.; Yang, S.; Wang, T.; Ma, Y.; Zhong, Y.; He, J.; et al. Are sodiation/de-sodiation reactions reversible in two-dimensional metallic NbSe2? Energy Environ. Sci. 2024, 17, 2173–2181. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, K.; Feng, P.; Zhang, Z.; Cheng, S.; Jiang, K. Surface-dominated storage of heteroatoms-doping hard carbon for sodium-ion batteries. Energy Storage Mater. 2020, 27, 43–50. [Google Scholar] [CrossRef]
- Guo, S.; Li, H.; Lu, Y.; Liu, Z.; Hu, X. Lattice softening enables highly reversible sodium storage in anti-pulverization Bi–Sb alloy/carbon nanofibers. Energy Storage Mater. 2020, 27, 270–278. [Google Scholar] [CrossRef]
- Huang, J.; Callender, K.I.E.; Qin, K.; Girgis, M.; Paige, M.; Yang, Z.; Clayborne, A.Z.; Luo, C. Halogenated Carboxylates as Organic Anodes for Stable and Sustainable Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 40784–40792. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Xu, B.; Tiong, V.T.; Hu, J.; Ma, J. Progress on iron oxides and chalcogenides as anodes for sodium-ion batteries. Chem. Eng. J. 2020, 379, 122261. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Yu, C.; Jiao, L.; Chen, J. MnFe2O4@C Nanofibers as High-Performance Anode for Sodium-Ion Batteries. Nano Lett. 2016, 16, 3321–3328. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.K.N.; Babu, G.N.S.; Sathish, M. CuMn2O4 anchored on graphene sheets as high-performance electrodes for sodium-ion batteries. J. Energy Storage 2023, 65, 107346. [Google Scholar] [CrossRef]
- He, Q.; Gu, S.; Wu, T.; Zhang, S.; Ao, X.; Yang, J.; Wen, Z. Self-supported mesoporous FeCo2O4 nanosheets as high capacity anode material for sodium-ion battery. Chem. Eng. J. 2017, 330, 764–773. [Google Scholar] [CrossRef]
- Maggay, I.V.B.; De Juan, L.M.Z.; Lu, J.-S.; Nguyen, M.T.; Yonezawa, T.; Chan, T.-S.; Liu, W.-R. Electrochemical properties of novel FeV2O4 as an anode for Na-ion batteries. Sci. Rep. 2018, 8, 8839. [Google Scholar] [CrossRef]
- Yang, X.-T.; Huang, T.-Y.; Wang, Y.-H.; Dong, J.-C.; Wei, Q.-L.; Zhang, H.; Lin, X.-M.; Li, J.-F. Understanding the origin of the improved sodium ion storage performance of the transition metal oxide@carbon nanocomposite anodes. J. Chem. Phys. 2023, 158, 174708. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, P.; Tang, Y.; Peng, C.; Lai, Y.; Li, J.; Zhang, Z. Bimetallic ZIF–derived polyhedron ZnCo2O4 anchored on the reduced graphene oxide as an anode for sodium-ion battery. Ionics 2019, 25, 2945–2950. [Google Scholar] [CrossRef]
- Chandra Sekhar, B.; Packiyalakshmi, P.; Kalaiselvi, N. Custom-designed ZnMn2O4/nitrogen-doped graphene composite anode validated for sodium ion battery application. RSC Adv. 2017, 7, 20057–20061. [Google Scholar] [CrossRef]
- Ghosh, S.; de Donder, T.; Gunnarsson, K.; Kumar, V.K.; Martha, S.K.; Svedlindh, P.; Kessler, V.G.; Seisenbaeva, G.A.; Pol, V.G. Investigating the stable operating voltage for the MnFe2O4 Li-ion battery anode. Sustain. Energy Fuels 2021, 5, 1904–1913. [Google Scholar] [CrossRef]
- Du, Y.; Liu, C.; Sun, S.; Wu, S. Core-shell MnO@MnFe2O4 Anchored by Reduced Graphene Oxide as Anode of Li-Ion Batteries Operated under Ultrawide Temperature Range. ChemElectroChem 2017, 4, 2500–2506. [Google Scholar] [CrossRef]
- Man, Y.; Sun, J.; Zhao, X.; Duan, L.; Fei, Y.; Bao, J.; Mo, X.; Zhou, X. An ultrastable sodium-ion battery anode enabled by carbon-coated porous NaTi2(PO4)3 olive-like nanospheres. J. Colloid Interface Sci. 2023, 635, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, Q.; Zhu, K.; Wang, G.; Cao, D.; Yan, J. N-doped carbon coated MoO3/MoS2 integrated MXene nanosheets with ultra-long cycle stability for sodium-ion batteries. Appl. Surf. Sci. 2024, 652, 159294. [Google Scholar] [CrossRef]
- Deng, Z.; He, M.; Feng, Y.; Xiong, D. A fluorine-doped MnFe2O4 nanorod/carbon composite as an anode material for high-performance lithium-ion batteries. Int. J. Electrochem. Sci. 2020, 15, 4203–4217. [Google Scholar] [CrossRef]
- Han, J.; Chen, Y.; Xiang, X.; Wang, T.; Shen, J.; Zhang, N.; Liang, C.; Liu, X.; Ma, X. A Comparative Analysis of the Antibacterial Spectrum of Ultrasmall Manganese Ferrite Nanozymes with Varied Surface Modifications. ACS Appl. Mater. Interfaces 2024, 16, 14385–14404. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.; Bhosale, S.; Sankannavar, R.; Chavan, V.; Jambhale, C.; Kim, H.; Kolekar, S. Bimetallic MnFe2-MOF and Its Derived MnFe2O4 Nanostructures for Supercapacitive Applications. ACS Appl. Nano Mater. 2024, 7, 4078–4091. [Google Scholar] [CrossRef]
- Dieu Cam, N.T.; Pham, H.-D.; Pham, T.-D.; Thu Phuong, T.T.; Van Hoang, C.; Thanh Tung, M.H.; Trung, N.T.; Huong, N.T.; Thu Hien, T.T. Novel photocatalytic performance of magnetically recoverable MnFe2O4/BiVO4 for polluted antibiotics degradation. Ceram. Int. 2021, 47, 1686–1692. [Google Scholar] [CrossRef]
- Qin, H.; Yang, Y.; Shi, W.; She, Y. Heterogeneous Fenton degradation of ofloxacin catalyzed by magnetic nanostructured MnFe2O4 with different morphologies. Environ. Sci. Pollut. Res. 2021, 28, 26558–26570. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, J.; Su, Q.; Du, G.; Zhang, S.; Cao, G.; Zhu, T.; Zhao, X. Understanding Li-storage mechanism and performance of MnFe2O4 by in situ TEM observation on its electrochemical process in nano lithium battery. Nano Energy 2014, 8, 84–94. [Google Scholar] [CrossRef]
- Liu, B.; Hu, X.; Xu, H.; Luo, W.; Sun, Y.; Huang, Y. Encapsulation of MnO Nanocrystals in Electrospun Carbon Nanofibers as High-Performance Anode Materials for Lithium-Ion Batteries. Sci. Rep. 2014, 4, 4229. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 Nanotubes in Gas Sensor and Lithium-Ion Battery Applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.-W.; Byun, D.; Choi, W. Coaxial-nanostructured MnFe2O4 nanoparticles on polydopamine-coated MWCNT for anode materials in rechargeable batteries. Nanoscale 2018, 10, 18949–18960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bai, J.; Gao, F.; Li, S.; Liu, Y.; Guo, S. Tailoring of hierarchical MnMoO4/C nanocauliflowers for high-performance lithium/sodium ion half/full batteries. J. Alloys Compd. 2022, 906, 164394. [Google Scholar] [CrossRef]
- Vincent, M.; Kumar, S.S.; Kowalski, D. Pseudocapacitive vs. diffusion controlled charge storage in Fe2O3 nanosheet Na-ion battery. Electrochim. Acta 2023, 469, 143161. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, C.; Ma, T.; Zhu, J.; Liu, J.; Yang, G.; Yang, Y. A novel CoO hierarchical morphologies on carbon nanofiber for improved reversibility as binder-free anodes in lithium/sodium ion batteries. J. Alloys Compd. 2019, 794, 385–395. [Google Scholar] [CrossRef]
- Yusoff, N.F.M.; Idris, N.H.; Din, M.F.M.; Majid, S.R.; Harun, N.A. Three-dimensional network of Mn3O4/reduced graphene oxide aerogel with improved electrochemical performances of sodium-ion batteries. J. Mater. Sci. 2023, 58, 8924–8939. [Google Scholar] [CrossRef]
- Zhang, S.; Ai, Y.; Wu, S.-C.; Liao, H.-J.; Su, T.-Y.; Chen, J.-H.; Wang, C.-H.; Lee, L.; Chen, Y.-Z.; Xu, B.; et al. 3D CoMoSe4 Nanosheet Arrays Converted Directly from Hydrothermally Processed CoMoO4 Nanosheet Arrays by Plasma-Assisted Selenization Process Toward Excellent Anode Material in Sodium-Ion Battery. Nanoscale Res. Lett. 2019, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, W.-B.; Li, Y.; Liu, H.-B.; Zhao, Z.-Y.; Li, K.; Wang, Y.-K.; Li, X.; Ma, T.-L.; Kong, L.-B. Construction of ultra-stable and high-performance sodium-ion hybrid capacitors via CoMoO4 nanorods anode. J. Power Sources 2024, 592, 233791. [Google Scholar] [CrossRef]
- Yu, K.; Chang, M.; Yue, L.; Wang, X.; Chen, J.; Liang, C. Submicron cubic ZnMn2O4 loaded on biomass porous carbon used as high-performance bifunctional electrode for lithium-ion and sodium-ion batteries. J. Alloys Compd. 2024, 971, 172769. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Zhou, X.; Wang, C.; Pan, Z.; Xu, X.; Liu, X.; Wang, Z.; Wu, Y.; Jiang, S.; et al. Graphene oxide-supported MnV2O6 nanoribbons with enhanced electrochemical performance for sodium-ion batteries. J. Power Sources 2024, 597, 234117. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, W.; Zhou, C.; Yang, L.; Wang, H.; Gao, Q.; Zhu, M. N-doped carbon encapsulated CoMoO4 nanorods as long-cycle life anode for sodium-ion batteries. J. Colloid Interface Sci. 2020, 576, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Chang, X.; Butts, D.; DeBlock, R.; Lan, K.; Li, J.; Chao, D.; Peng, D.-L.; Dunn, B. Surface-redox sodium-ion storage in anatase titanium oxide. Nat. Commun. 2023, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, Y.; Kim, G.T.; Diemant, T.; Behm, R.J.; Geiger, D.; Kaiser, U.; Varzi, A.; Passerini, S. Superior Lithium Storage Capacity of α-MnS Nanoparticles Embedded in S-Doped Carbonaceous Mesoporous Frameworks. Adv. Energy Mater. 2019, 9, 1902077. [Google Scholar] [CrossRef]
- An, C.; Yuan, Y.; Zhang, B.; Tang, L.; Xiao, B.; He, Z.; Zheng, J.; Lu, J. Graphene Wrapped FeSe2 Nano-Microspheres with High Pseudocapacitive Contribution for Enhanced Na-Ion Storage. Adv. Energy Mater. 2019, 9, 1900356. [Google Scholar] [CrossRef]
- Chen, X.; Feng, X.; Ren, B.; Jiang, L.; Shu, H.; Yang, X.; Chen, Z.; Sun, X.; Liu, E.; Gao, P. High Rate and Long Lifespan Sodium-Organic Batteries Using Pseudocapacitive Porphyrin Complexes-Based Cathode. Nano-Micro Lett. 2021, 13, 71. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Ma, Y.; He, Y.; Hou, J.; Che, H.; Zhang, X.; Han, S.; Wang, Z.; Li, Z.; et al. Fabrication, characterisation and magneto-responsive performance of manganese ferrite nanospheres for fast separation of oil slicks from water surfaces. Ceram. Int. 2023, 49, 32962–32970. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, G.; Ma, Y.; Zuo, P.; Cheng, X. Nanosized core/shell silicon@carbon anode material for lithium ion batteries with polyvinylidene fluoride as carbon source. J. Mater. Chem. 2010, 20, 3216–3220. [Google Scholar] [CrossRef]
- Hong, S.-M.; Etacheri, V.; Hong, C.N.; Choi, S.W.; Lee, K.B.; Pol, V.G. Enhanced Lithium- and Sodium-Ion Storage in an Interconnected Carbon Network Comprising Electronegative Fluorine. ACS Appl. Mater. Interfaces 2017, 9, 18790–18798. [Google Scholar] [CrossRef]



| Materials | Voltage Range (V vs. Na+/Na) | Specific Capacity (mAh·g−1)/Cycles/ Current Density (A·g−1) | Rate Performance (mAh·g−1)/Current Density (A·g−1) | Ref. |
|---|---|---|---|---|
| CuMn2O4/ graphene | 0.01–3.0 | 313/50th/0.1 | 145/2.0 | [14] |
| Fe2O3-600 nanosheets | 0.01–3.0 | ~100/250th/0.5 | 100/0.5 | [35] |
| CNF/CoO-4 | 0.01–3.0 | 138/100th/0.1 | — | [36] |
| Mn3O4/rGO aerogels | 0.01–3.0 | 283/100th/0.1 | 121/1.0 | [37] |
| CoMoO4@C | 0.5–3.0 | 46/50th/0.5 | 45/2.0 | [38] |
| CoMoO4 nanorod | 0.01–3.0 | 200/60th/0.1 | 160/0.5 | [39] |
| ZnMn2O4/jute porous carbon | 0.01–3.0 | 392.4/200th/0.1 | 244.7/1.0 | [40] |
| MnV2O6/GO | 0.01–3.0 | 323.8/100th/0.1 | 213.8/2.0 | [41] |
| CoMoO4@NC | 0.01–3.0 | 281/80th/0.1 | 221/2.0 | [42] |
| MnFe2O4@4C | 0.01–3.0 | 250/100th/0.1 | 182/2.0 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-W.; Niu, B.-T.; Lin, B.-L.; Lin, Y.-T.; Chen, X.-P.; Guo, H.-X.; Chen, Y.-X.; Lin, X.-M. Investigating the Electrochemical Performance of MnFe2O4@xC Nanocomposites as Anode Materials for Sodium-Ion Batteries. Molecules 2024, 29, 3912. https://doi.org/10.3390/molecules29163912
Liu S-W, Niu B-T, Lin B-L, Lin Y-T, Chen X-P, Guo H-X, Chen Y-X, Lin X-M. Investigating the Electrochemical Performance of MnFe2O4@xC Nanocomposites as Anode Materials for Sodium-Ion Batteries. Molecules. 2024; 29(16):3912. https://doi.org/10.3390/molecules29163912
Chicago/Turabian StyleLiu, Shi-Wei, Bai-Tong Niu, Bi-Li Lin, Yuan-Ting Lin, Xiao-Ping Chen, Hong-Xu Guo, Yan-Xin Chen, and Xiu-Mei Lin. 2024. "Investigating the Electrochemical Performance of MnFe2O4@xC Nanocomposites as Anode Materials for Sodium-Ion Batteries" Molecules 29, no. 16: 3912. https://doi.org/10.3390/molecules29163912





