Anti-Biofilm Effect of Hybrid Nanocomposite Functionalized with Erythrosine B on Staphylococcus aureus Due to Photodynamic Inactivation
Abstract
:1. Introduction
2. Results and Discussion
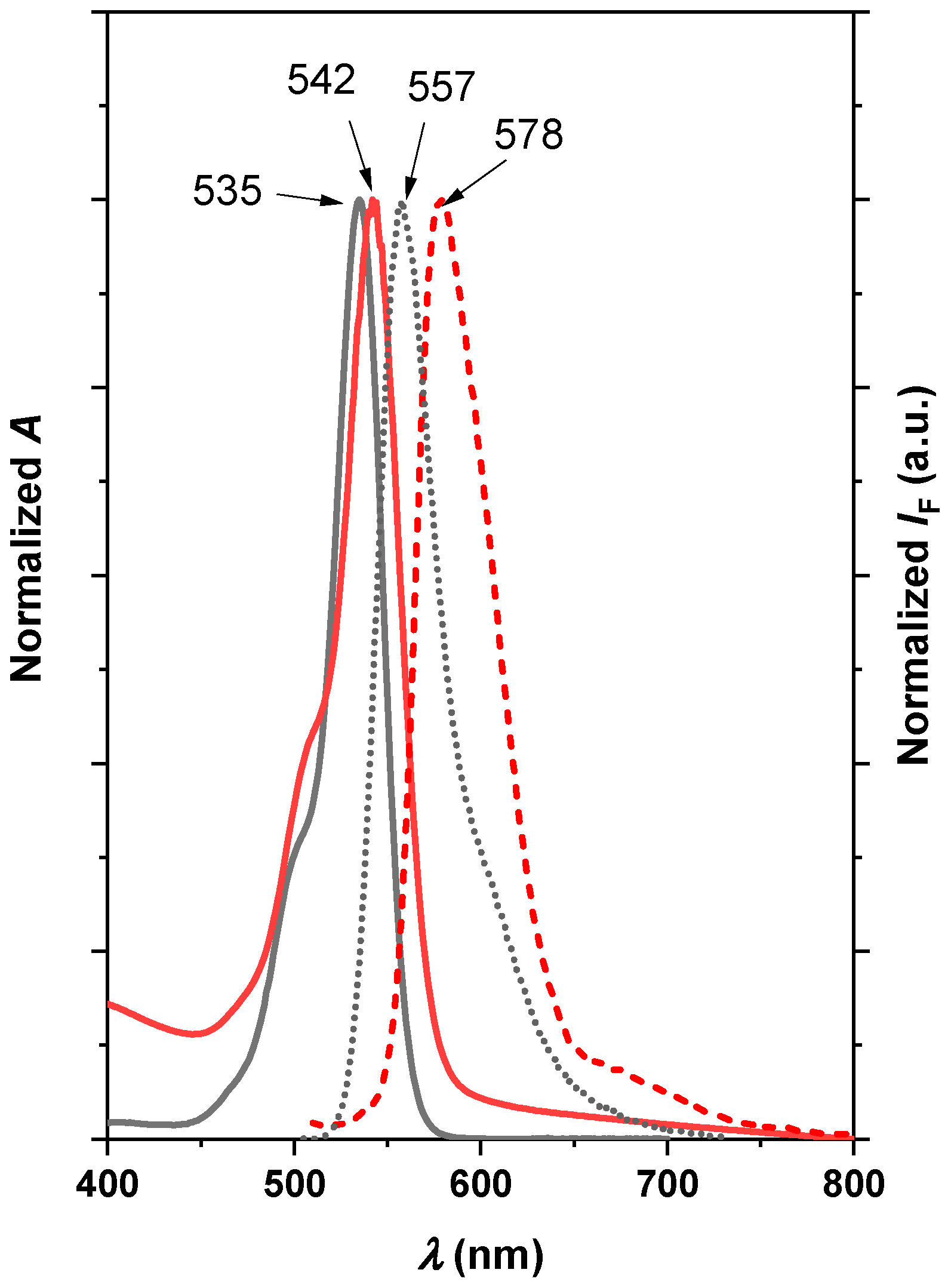
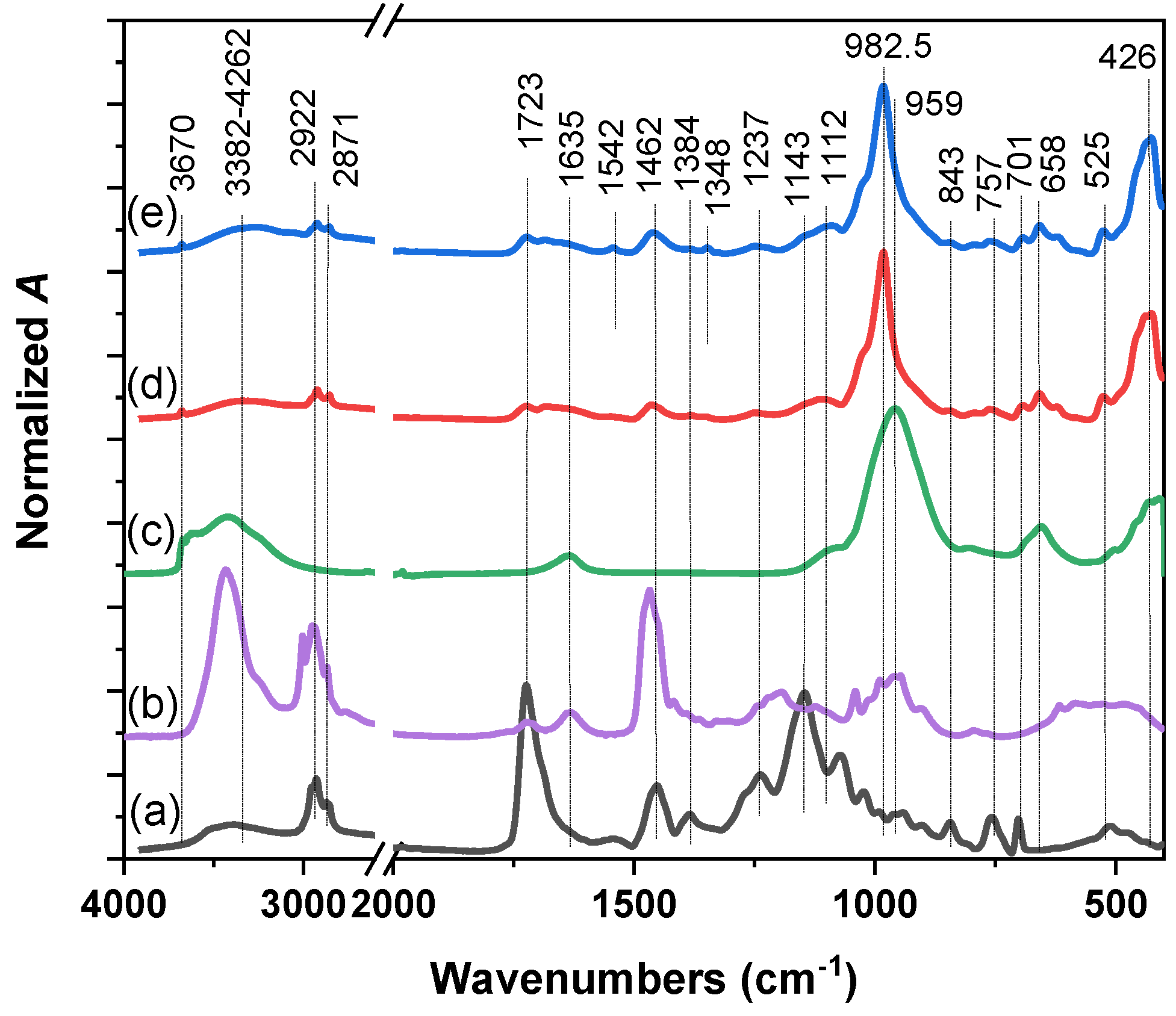
2.1. Physico-Chemical Characterization of Nanocomposite
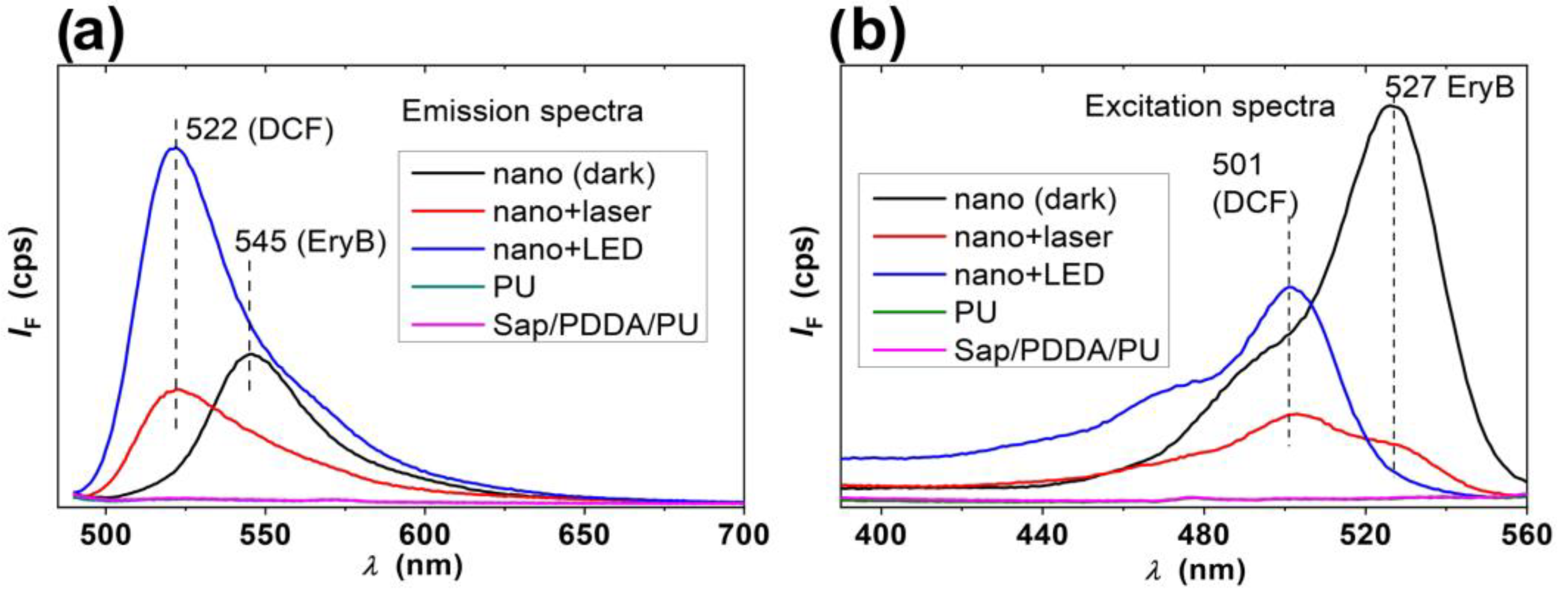
2.2. Photosensitizing Properties of the Nanocomposites
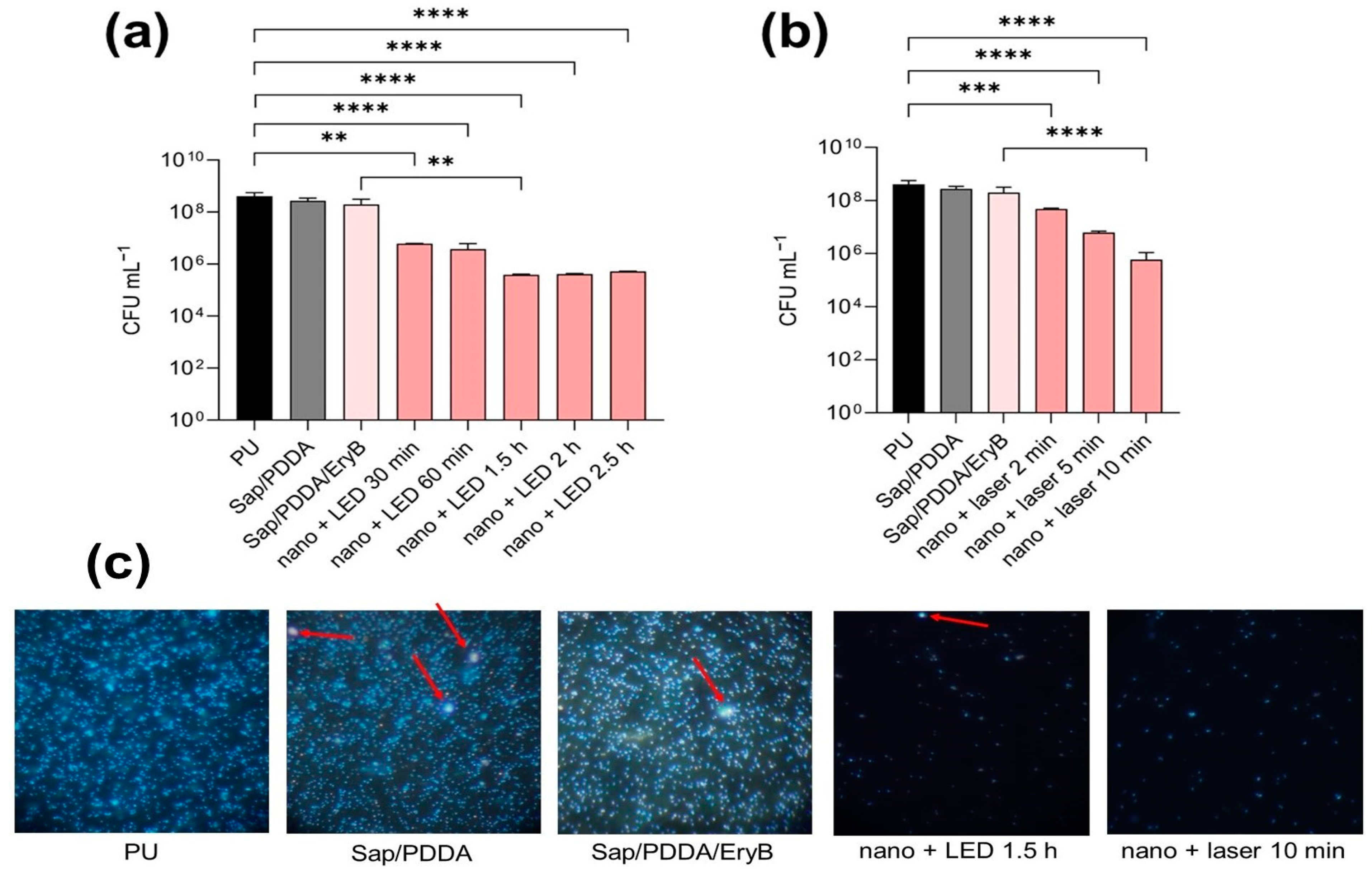
2.3. Antimicrobial and Anti-Biofilm Activity of PDI
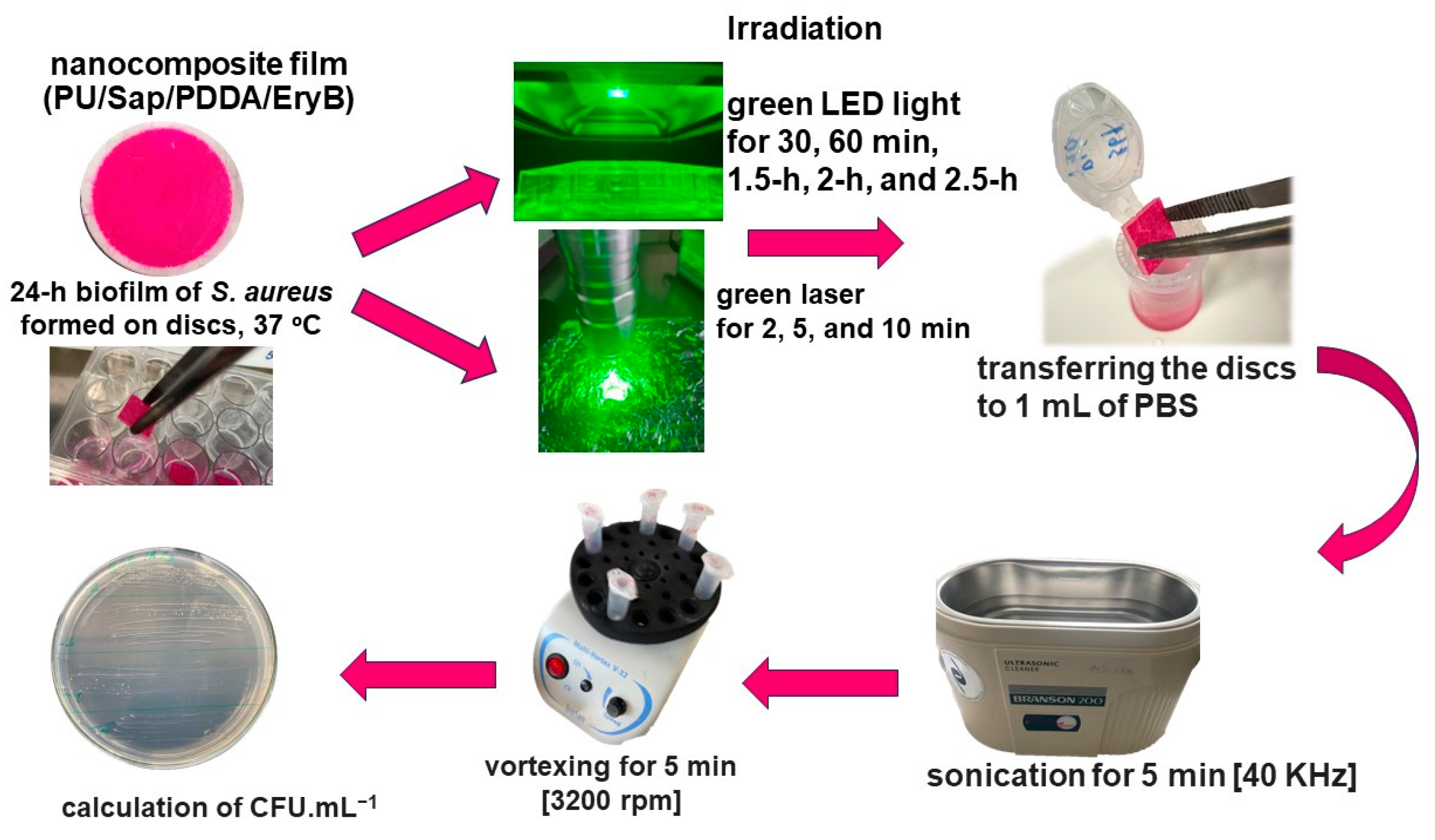
2.4. Anti-Biofilm Effectiveness of PDI with Nanocomposite
3. Materials and Methods
3.1. Bacterial Strains, PS, and Light Source
3.2. Preparation of Nanocomposites
3.3. Characterization of Nanocomposites
3.4. Photosensitizing Properties of the Nanocomposites
3.5. Photodynamic Inactivation of Planktonic and Biofilms Cultures
3.6. PDI of S. aureus Biofilm Formed on Nanocomposite
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus Aureus Host Interactions and Adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hu, Z.; Li, S.; Fang, R.; Ono, H.K.; Hu, D.-L. Molecular Characteristics and Pathogenicity of Staphylococcus Aureus Exotoxins. Int. J. Mol. Sci. 2023, 25, 395. [Google Scholar] [CrossRef] [PubMed]
- Kahl, B.C.; Becker, K.; Löffler, B. Clinical Significance and Pathogenesis of Staphylococcal Small Colony Variants in Persistent Infections. Clin. Microbiol. Rev. 2016, 29, 401–427. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Wang, X.; Koffi, P.F.; English, O.F.; Lee, J.C. Staphylococcus Aureus Extracellular Vesicles: A Story of Toxicity and the Stress of 2020. Toxins 2021, 13, 75. [Google Scholar] [CrossRef]
- Ochoa de Retana, I. Hospital staphylococcal infections. Rev. Clin. Esp. 1983, 168, 77–81. [Google Scholar]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and Virulence of Staphylococcus Aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-Resistant Staphylococcus Aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Stryjewski, M.E.; Corey, G.R. Methicillin-Resistant Staphylococcus Aureus: An Evolving Pathogen. Clin. Infect. Dis. 2014, 58, S10–S19. [Google Scholar] [CrossRef]
- Kayser, F.H. Methicillin-Resistant Staphylococci 1965–1975. Lancet 1975, 306, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Brumfitt, W.; Hamilton-Miller, J. Methicillin-Resistant Staphylococcus Aureus. N. Engl. J. Med. 1989, 320, 1188–1196. [Google Scholar] [CrossRef]
- Ganesan, N.; Mishra, B.; Felix, L.; Mylonakis, E. Antimicrobial Peptides and Small Molecules Targeting the Cell Membrane of Staphylococcus Aureus. Microbiol. Mol. Biol. Rev. 2023, 87, e00037-22. [Google Scholar] [CrossRef]
- Hernández-Cuellar, E.; Tsuchiya, K.; Valle-Ríos, R.; Medina-Contreras, O. Differences in Biofilm Formation by Methicillin-Resistant and Methicillin-Susceptible Staphylococcus Aureus Strains. Diseases 2023, 11, 160. [Google Scholar] [CrossRef]
- Lister, J.L.; Horswill, A.R. Staphylococcus Aureus Biofilms: Recent Developments in Biofilm Dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus Aureus Biofilm: Morphology, Genetics, Pathogenesis and Treatment Strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Tang, X.; Dong, W.; Sun, N.; Yuan, W. A Review of Biofilm Formation of Staphylococcus Aureus and Its Regulation Mechanism. Antibiotics 2022, 12, 12. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Almatroudi, A. Investigating Biofilms: Advanced Methods for Comprehending Microbial Behavior and Antibiotic Resistance. Front. Biosci. Landmark 2024, 29, 133. [Google Scholar] [CrossRef] [PubMed]
- Razdan, K.; Garcia-Lara, J.; Sinha, V.R.; Singh, K.K. Pharmaceutical Strategies for the Treatment of Bacterial Biofilms in Chronic Wounds. Drug Discov. Today 2022, 27, 2137–2150. [Google Scholar] [CrossRef]
- Černáková, L.; Light, C.; Salehi, B.; Rogel-Castillo, C.; Victoriano, M.; Martorell, M.; Sharifi-Rad, J.; Martins, N.; Rodrigues, C.F. Novel Therapies for Biofilm-Based Candida Spp. Infections. Adv. Exp. Med. Biol. 2019, 1214, 93–123. [Google Scholar] [CrossRef] [PubMed]
- Subbiahdoss, G.; Sharifi, S.; Grijpma, D.W.; Laurent, S.; Van Der Mei, H.C.; Mahmoudi, M.; Busscher, H.J. Magnetic Targeting of Surface-Modified Superparamagnetic Iron Oxide Nanoparticles Yields Antibacterial Efficacy against Biofilms of Gentamicin-Resistant Staphylococci. Acta Biomater. 2012, 8, 2047–2055. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Chakrabartty, I.; Mishra, A.K.; Chopra, H.; Mahanta, S.; Avula, S.K.; Patowary, K.; Ahmed, R.; Mishra, B.; Mohanta, T.K.; et al. Nanotechnology in Combating Biofilm: A Smart and Promising Therapeutic Strategy. Front. Microbiol. 2023, 13, 1028086. [Google Scholar] [CrossRef]
- Tran, H.M.; Tran, H.; Booth, M.A.; Fox, K.E.; Nguyen, T.H.; Tran, N.; Tran, P.A. Nanomaterials for Treating Bacterial Biofilms on Implantable Medical Devices. Nanomaterials 2020, 10, 2253. [Google Scholar] [CrossRef]
- Damyanova, T.; Dimitrova, P.D.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention. Pharmaceutics 2024, 16, 162. [Google Scholar] [CrossRef]
- Al-Wrafy, F.A.; Al-Gheethi, A.A.; Ponnusamy, S.K.; Noman, E.A.; Fattah, S.A. Nanoparticles Approach to Eradicate Bacterial Biofilm-Related Infections: A Critical Review. Chemosphere 2022, 288, 132603. [Google Scholar] [CrossRef]
- Kauser, A.; Parisini, E.; Suarato, G.; Castagna, R. Light-Based Anti-Biofilm and Antibacterial Strategies. Pharmaceutics 2023, 15, 2106. [Google Scholar] [CrossRef] [PubMed]
- Lineback, C.B.; Nkemngong, C.A.; Wu, S.T.; Li, X.; Teska, P.J.; Oliver, H.F. Hydrogen Peroxide and Sodium Hypochlorite Disinfectants Are More Effective against Staphylococcus Aureus and Pseudomonas Aeruginosa Biofilms than Quaternary Ammonium Compounds. Antimicrob. Resist. Infect. Control 2018, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T. A New Strategy to Destroy Antibiotic Resistant Microorganisms: Antimicrobial Photodynamic Treatment. Mini Rev. Med. Chem. 2009, 9, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Mi, G.; Shi, D.; Wang, M.; Webster, T.J. Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces. Adv. Healthc. Mater. 2018, 7, 1800103. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, R.; Feng, Y.; Ouyang, H.; Zhou, N.; Zhang, X.; Wei, Y. Recent Development and Prospects of Surface Modification and Biomedical Applications of MXenes. Nanoscale 2020, 12, 1325–1338. [Google Scholar] [CrossRef]
- Ahmadabadi, H.Y.; Yu, K.; Kizhakkedathu, J.N. Surface Modification Approaches for Prevention of Implant Associated Infections. Colloids Surf. B Biointerfaces 2020, 193, 111116. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Caserta, S.; Pasquino, R.; Jones, S.A.; Prokopovich, P. Prolonged Antimicrobial Activity of PMMA Bone Cement with Embedded Gentamicin-Releasing Silica Nanocarriers. ACS Appl. Bio Mater. 2019, 2, 1850–1861. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Kim, W.; Hendricks, G.L.; Tori, K.; Fuchs, B.B.; Mylonakis, E. Strategies against Methicillin-Resistant Staphylococcus Aureus Persisters. Future Med. Chem. 2018, 10, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Hall, L.; Halton, K.; Graves, N. Improving Hospital Environmental Hygiene with the Use of a Targeted Multi-Modal Bundle Strategy. Infect. Dis. Health 2018, 23, 107–113. [Google Scholar] [CrossRef]
- Dadi, N.C.T.; Bujdák, J.; Medvecká, V.; Pálková, H.; Barlog, M.; Bujdáková, H. Surface Characterization and Anti-Biofilm Effectiveness of Hybrid Films of Polyurethane Functionalized with Saponite and Phloxine B. Materials 2021, 14, 7583. [Google Scholar] [CrossRef]
- Dadi, N.C.T.; Dohál, M.; Medvecká, V.; Bujdák, J.; Koči, K.; Zahoranová, A.; Bujdáková, H. Physico-Chemical Characterization and Antimicrobial Properties of Hybrid Film Based on Saponite and Phloxine B. Molecules 2021, 26, 325. [Google Scholar] [CrossRef]
- Galdino, D.Y.T.; Da Rocha Leódido, G.; Pavani, C.; Gonçalves, L.M.; Bussadori, S.K.; Paschoal, M.A.B. Photodynamic Optimization by Combination of Xanthene Dyes on Different Forms of Streptococcus Mutans: An in Vitro Study. Photodiagnosis Photodyn. Ther. 2021, 33, 102191. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, M.A.B.; Gonçalves, L.M.; Cavalcante, S.I.A.; Andrade-Maia, G.; Duarte, S. Morphological Changes and Viability of Streptococcus Mutans Biofilm Treated with Erythrosine: A Confocal Laser Scanning Microscopy Analysis. Microsc. Res. Tech. 2024, 87, 888–895. [Google Scholar] [CrossRef]
- Silva, A.; Borges, A.; Freitas, C.; Hioka, N.; Mikcha, J.; Simões, M. Antimicrobial Photodynamic Inactivation Mediated by Rose Bengal and Erythrosine Is Effective in the Control of Food-Related Bacteria in Planktonic and Biofilm States. Molecules 2018, 23, 2288. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Mone, N.S.; Kamble, E.E.; Pardesi, K.R.; Satpute, S.K. Antibacterial and Antibiofilm Potency of Menadione against Multidrug-Resistant S. Aureus. Curr. Microbiol. 2022, 79, 282. [Google Scholar] [CrossRef]
- Bilská, K.; Bujdák, J.; Bujdáková, H. Nanocomposite System with Photoactive Phloxine B Eradicates Resistant Staphylococcus Aureus. Heliyon 2024, 10, e33660. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Antimicrobial Photodynamic Inactivation: A Bright New Technique to Kill Resistant Microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Almeida, A. Photodynamic Therapy in the Inactivation of Microorganisms. Antibiotics 2020, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Benthall, G.; Touzel, R.E.; Hind, C.K.; Titball, R.W.; Sutton, J.M.; Thomas, R.J.; Wand, M.E. Evaluation of Antibiotic Efficacy against Infections Caused by Planktonic or Biofilm Cultures of Pseudomonas Aeruginosa and Klebsiella Pneumoniae in Galleria Mellonella. Int. J. Antimicrob. Agents 2015, 46, 538–545. [Google Scholar] [CrossRef]
- De Carvalho, B.M.D.F.; Garcia, B.A.; Gomes, A.K.P.; Alcantara, D.D.; De Freitas Pontes, K.M. Antimicrobial Photodynamic Therapy as a Technique for Decontamination of Acrylic Resin Devices Provided by Different Dental Laboratories. J. Lasers Med. Sci. 2023, 14, e8. [Google Scholar] [CrossRef]
- Erythrosine—CAMEO. Available online: https://cameo.mfa.org/wiki/Erythrosine (accessed on 27 May 2024).
- Gonçalves, M.L.L.; Sobral, A.P.T.; Gallo, J.M.A.S.; Gimenez, T.; Ferri, E.P.; Ianello, S.; Motta, P.D.B.; Motta, L.J.; Horliana, A.C.R.T.; Santos, E.M.; et al. Antimicrobial Photodynamic Therapy with Erythrosine and Blue Light on Dental Biofilm Bacteria: Study Protocol for Randomised Clinical Trial. BMJ Open 2023, 13, e075084. [Google Scholar] [CrossRef]
- Borba, A.S.M.; Da Silva Pereira, S.M.; Borba, M.C.M.; Paschoal, M.A.B.; De Jesus Tavarez, R.R.; De Castro Rizzi, C.; Ferreira, M.C.; Maia Filho, E.M. Photodynamic Therapy with High-Power LED Mediated by Erythrosine Eliminates Enterococcus Faecalis in Planktonic Forms. Photodiagnosis Photodyn. Ther. 2017, 19, 348–351. [Google Scholar] [CrossRef]
- Rolim, J.P.M.L.; de-Melo, M.A.S.; Guedes, S.F.; Albuquerque-Filho, F.B.; De Souza, J.R.; Nogueira, N.A.P.; Zanin, I.C.J.; Rodrigues, L.K.A. The Antimicrobial Activity of Photodynamic Therapy against Streptococcus Mutans Using Different Photosensitizers. J. Photochem. Photobiol. B Biol. 2012, 106, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.L.L.; Santos, E.M.; Renno, A.C.M.; Horliana, A.C.R.T.; Cruz, M.D.A.; Parisi, J.R.; Prates, R.A.; Leal-Rossi, A.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; et al. Erythrosine as a Photosensitizer for Antimicrobial Photodynamic Therapy with Blue Light-Emitting Diodes—An in Vitro Study. Photodiagnosis Photodyn. Ther. 2021, 35, 102445. [Google Scholar] [CrossRef]
- Garapati, C.; Clarke, B.; Zadora, S.; Burney, C.; Cameron, B.D.; Fournier, R.; Baugh, R.F.; Boddu, S.H.S. Development and Characterization of Erythrosine Nanoparticles with Potential for Treating Sinusitis Using Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2015, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.C.; Santos, A.R.D.; Bona, E.; Freitas, C.F.; Silva, J.V.D.O.; Malacarne, L.C.; Machinski Junior, M.; Abreu Filho, B.A.D.; Mikcha, J.M.G. Optimization of the Erythrosine-Mediated Photodynamic Therapy against Escherichia Coli Using Response Surface Methodology. Photodiagnosis Photodyn. Ther. 2024, 45, 103916. [Google Scholar] [CrossRef]
- Costa, A.C.B.P.; Campos Rasteiro, V.M.; Da Silva Hashimoto, E.S.H.; Araújo, C.F.; Pereira, C.A.; Junqueira, J.C.; Jorge, A.O.C. Effect of Erythrosine- and LED-Mediated Photodynamic Therapy on Buccal Candidiasis Infection of Immunosuppressed Mice and Candida Albicans Adherence to Buccal Epithelial Cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 67–74. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Mota, H.C.; Sibata, C.H. Clinical PD/PDT in North America: An Historical Review. Photodiagn. Photodyn. Ther. 2004, 1, 263–277. [Google Scholar] [CrossRef]
- Mahdi, Z.; Habiboallh, G.; Mahbobeh, N.N.; Mina, Z.J.; Majid, Z.; Nooshin, A. Lethal Effect of Blue Light-Activated Hydrogen Peroxide, Curcumin and Erythrosine as Potential Oral Photosensitizers on the Viability of Porphyromonas Gingivalis and Fusobacterium Nucleatum. Laser Ther. 2015, 24, 103–111. [Google Scholar] [CrossRef]
- Wood, S.; Metcalf, D.; Devine, D.; Robinson, C. Erythrosine Is a Potential Photosensitizer for the Photodynamic Therapy of Oral Plaque Biofilms. J. Antimicrob. Chemother. 2006, 57, 680–684. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.-H.; Childs, C.J.; Sibata, C.H. Photosensitizers in Clinical PDT. Photodiagn. Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.; Dos Santos, A.R.; Trevisan, D.A.C.; Bonin, E.; Freitas, C.F.; Batista, A.F.P.; Hioka, N.; Simões, M.; Graton Mikcha, J.M. Xanthene Dyes and Green LED for the Inactivation of Foodborne Pathogens in Planktonic and Biofilm States. Photochem. Photobiol. 2019, 95, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Yassunaka, N.N.; De Freitas, C.F.; Rabello, B.R.; Santos, P.R.; Caetano, W.; Hioka, N.; Nakamura, T.U.; De Abreu Filho, B.A.; Mikcha, J.M.G. Photodynamic Inactivation Mediated by Erythrosine and Its Derivatives on Foodborne Pathogens and Spoilage Bacteria. Curr. Microbiol. 2015, 71, 243–251. [Google Scholar] [CrossRef]
- Hochma, E.; Hovor, I.; Nakonechny, F.; Nisnevitch, M. Photo- and Sono-Active Food Colorants Inactivating Bacteria. Int. J. Mol. Sci. 2023, 24, 15126. [Google Scholar] [CrossRef] [PubMed]
- Fekrirad, Z.; Darabpour, E.; Kashef, N. Eradication of Acinetobacter Baumannii Planktonic and Biofilm Cells Through Erythrosine-Mediated Photodynamic Inactivation Augmented by Acetic Acid and Chitosan. Curr. Microbiol. 2021, 78, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.; Cardoso, G.; Maciel, R.R.G.; de Souza, N.C. Morphological Alterations on Citrobacter Freundii Bacteria Induced by Erythrosine Dye and Laser Light. Lasers Med. Sci. 2015, 30, 469–473. [Google Scholar] [CrossRef]
- Bergmann, E.V.; Capeloto, O.A.; Catanio, A.T.S.; Flizikowski, G.A.S.; Kimura, N.M.; Freitas, C.F.; Herculano, L.S.; Astrath, N.G.C.; Malacarne, L.C. Photoactivation of Erythrosine in Simulated Body Fluids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 259, 119867. [Google Scholar] [CrossRef]
- Aerssens, D.; Cadoni, E.; Tack, L.; Madder, A. A Photosensitized Singlet Oxygen (1O2) Toolbox for Bio-Organic Applications: Tailoring 1O2 Generation for DNA and Protein Labelling, Targeting and Biosensing. Molecules 2022, 27, 778. [Google Scholar] [CrossRef]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular Mechanisms of Singlet Oxygen in Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 16890. [Google Scholar] [CrossRef]
- Salleres, S.; Arbeloa, F.L.; Martínez, V.; Arbeloa, T.; Arbeloa, I.L. Adsorption of Fluorescent R6G Dye into Organophilic C12TMA Laponite Films. J. Colloid Interface Sci. 2008, 321, 212–219. [Google Scholar] [CrossRef]
- Bujdák, J.; Baranyaiová, T.Š.; Boháč, P.; Mészáros, R. Adsorption of Dye Molecules and Its Potential for the Development of Photoactive Hybrid Materials Based on Layered Silicates. J. Phys. Chem. B 2023, 127, 1063–1073. [Google Scholar] [CrossRef]
- Madejová, J.; Barlog, M.; Jankovič, Ľ.; Slaný, M.; Pálková, H. Comparative Study of Alkylammonium- and Alkylphosphonium-Based Analogues of Organo-Montmorillonites. Appl. Clay Sci. 2021, 200, 105894. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline Studies of the Clay Minerals Society Source Clays: Infrared Methods. Clays Clay Min. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Lyu, J.S.; Lee, J.-S.; Han, J. Development of a Biodegradable Polycaprolactone Film Incorporated with an Antimicrobial Agent via an Extrusion Process. Sci. Rep. 2019, 9, 20236. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, S.; Baino, F.; Ferreira, A.M.; Sartori, S.; Novajra, G.; Ciardelli, G.; Vitale-Brovarone, C. Collagen/Polyurethane-Coated Bioactive Glass: Early Achievements towards the Modelling of Healthy and Osteoporotic Bone. KEM 2014, 631, 184–189. [Google Scholar] [CrossRef]
- Reiniers, M.J.; van Golen, R.F.; Bonnet, S.; Broekgaarden, M.; van Gulik, T.M.; Egmond, M.R.; Heger, M. Preparation and Practical Applications of 2’,7’-Dichlorodihydrofluorescein in Redox Assays. Anal. Chem. 2017, 89, 3853–3857. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Use of Spectroscopic Probes for Detection of Reactive Oxygen Species. Clin. Chim. Acta 2006, 368, 53–76. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a Fluorescent Probe for Reactive Oxygen Species Measurement: Forty Years of Application and Controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- Sanchez Ferrer, A.; Santema, J.S.; Hilhorst, R.; Visser, A.J. Fluorescence Detection of Enzymatically Formed Hydrogen Peroxide in Aqueous Solution and in Reversed Micelles. Anal. Biochem. 1990, 187, 129–132. [Google Scholar] [CrossRef]
- Hsieh, B.-C.; Ni, Y.-H.; Zhang, G.-M.; Chiu, Y.-C.; Hou, Y.-T. Development of Erythrosine-Based Photodynamic Therapy with a Targeted Drug Delivery System to Induce HepG2 Cell Apoptosis in Vitro. Biochem. Eng. J. 2022, 177, 108267. [Google Scholar] [CrossRef]
- de Carvalho Goulart, R.; Thedei, G.; Souza, S.L.S.; Tedesco, A.C.; Ciancaglini, P. Comparative Study of Methylene Blue and Erythrosine Dyes Employed in Photodynamic Therapy for Inactivation of Planktonic and Biofilm-Cultivated Aggregatibacter Actinomycetemcomitans. Photomed. Laser Surg. 2010, 28 (Suppl. S1), S85–S90. [Google Scholar] [CrossRef]
- Romão, I.Q.; Cavalcante, S.I.A.; Leite, H.L.A.; Gonçalves, L.M.; Branco-de-Almeida, L.S.; Paschoal, M.A.B. Effect of Combining Erythrosine with a High-Power Dental Curing Light Appliance on the Viability of a Planktonic Culture of Streptococcus Mutans. Photomed. Laser Surg. 2018, 36, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Park, H.-W.; Lee, J.-H.; Seo, H.-W.; Lee, S.-Y. The Photodynamic Therapy on Streptococcus Mutans Biofilms Using Erythrosine and Dental Halogen Curing Unit. Int. J. Oral Sci. 2012, 4, 196–201. [Google Scholar] [CrossRef]
- Skoura, E.; Boháč, P.; Barlog, M.; Pálková, H.; Mautner, A.; Bugyna, L.; Bujdáková, H.; Bujdák, J. Structure, Photoactivity, and Antimicrobial Properties of Phloxine B / Poly(Caprolactone) Nanocomposite Thin Films. Appl. Clay Sci. 2023, 242, 107037. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Aoki, A.; Hiratsuka, K.; Chui, C.; Ichinose, A.; Aung, N.; Kitanaka, Y.; Hayashi, S.; Toyoshima, K.; Iwata, T.; et al. Application of Different Wavelengths of LED Lights in Antimicrobial Photodynamic Therapy for the Treatment of Periodontal Disease. Antibiotics 2023, 12, 1676. [Google Scholar] [CrossRef]
- Hasenleitner, M.; Plaetzer, K. In the Right Light: Photodynamic Inactivation of Microorganisms Using a LED-Based Illumination Device Tailored for the Antimicrobial Application. Antibiotics 2019, 9, 13. [Google Scholar] [CrossRef]
- Piksa, M.; Lian, C.; Samuel, I.C.; Pawlik, K.J.; Samuel, I.D.W.; Matczyszyn, K. The Role of the Light Source in Antimicrobial Photodynamic Therapy. Chem. Soc. Rev. 2023, 52, 1697–1722. [Google Scholar] [CrossRef] [PubMed]
- Zmejkoski, D.Z.; Zdravković, N.M.; Budimir Filimonović, M.D.; Pavlović, V.B.; Butulija, S.V.; Milivojević, D.D.; Marković, Z.M.; Todorović Marković, B.M. Reduction in Pathogenic Biofilms by the Photoactive Composite of Bacterial Cellulose and Nanochitosan Dots under Blue and Green Light. J. Funct. Biomater. 2024, 15, 72. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Yu, Y.; Li, W.; Ma, Z.; Wang, J.; Zhang, X.; Gao, H.; Liu, D. Photoactive Silver Nanoagents for Backgroundless Monitoring and Precision Killing of Multidrug-Resistant Bacteria. Nanotheranostics 2021, 5, 472–487. [Google Scholar] [CrossRef]
- Asnaashari, M.; Mojahedi, S.M.; Asadi, Z.; Azari-Marhabi, S.; Maleki, A. A Comparison of the Antibacterial Activity of the Two Methods of Photodynamic Therapy (Using Diode Laser 810 Nm and LED Lamp 630 Nm) against Enterococcus Faecalis in Extracted Human Anterior Teeth. Photodiagnosis Photodyn. Ther. 2016, 13, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Ricatto, L.G.O.; Conrado, L.A.L.; Turssi, C.P.; França, F.M.G.; Basting, R.T.; Amaral, F.L.B. Comparative Evaluation of Photodynamic Therapy Using LASER or Light Emitting Diode on Cariogenic Bacteria: An in Vitro Study. Eur. J. Dent. 2014, 8, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Czímerová, A.; Iyi, N.; Bujdák, J. Energy Transfer between Rhodamine 3B and Oxazine 4 in Synthetic-Saponite Dispersions and Films. J. Colloid Interface Sci. 2007, 306, 316–322. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugyna, L.; Bilská, K.; Boháč, P.; Pribus, M.; Bujdák, J.; Bujdáková, H. Anti-Biofilm Effect of Hybrid Nanocomposite Functionalized with Erythrosine B on Staphylococcus aureus Due to Photodynamic Inactivation. Molecules 2024, 29, 3917. https://doi.org/10.3390/molecules29163917
Bugyna L, Bilská K, Boháč P, Pribus M, Bujdák J, Bujdáková H. Anti-Biofilm Effect of Hybrid Nanocomposite Functionalized with Erythrosine B on Staphylococcus aureus Due to Photodynamic Inactivation. Molecules. 2024; 29(16):3917. https://doi.org/10.3390/molecules29163917
Chicago/Turabian StyleBugyna, Larysa, Katarína Bilská, Peter Boháč, Marek Pribus, Juraj Bujdák, and Helena Bujdáková. 2024. "Anti-Biofilm Effect of Hybrid Nanocomposite Functionalized with Erythrosine B on Staphylococcus aureus Due to Photodynamic Inactivation" Molecules 29, no. 16: 3917. https://doi.org/10.3390/molecules29163917







