Advances in Aptamer-Based Biosensors for the Detection of Foodborne Mycotoxins
Abstract
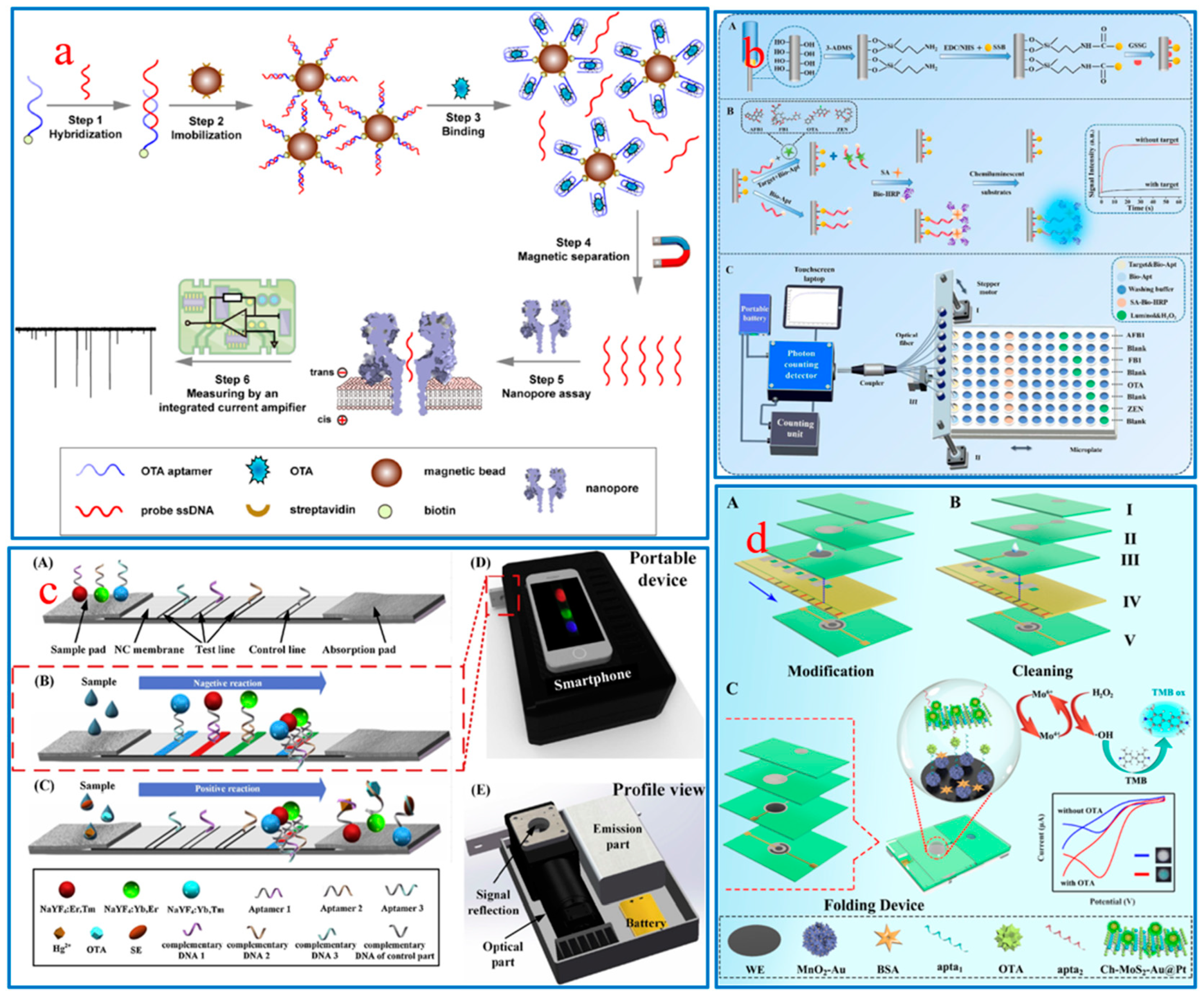
:1. Introduction
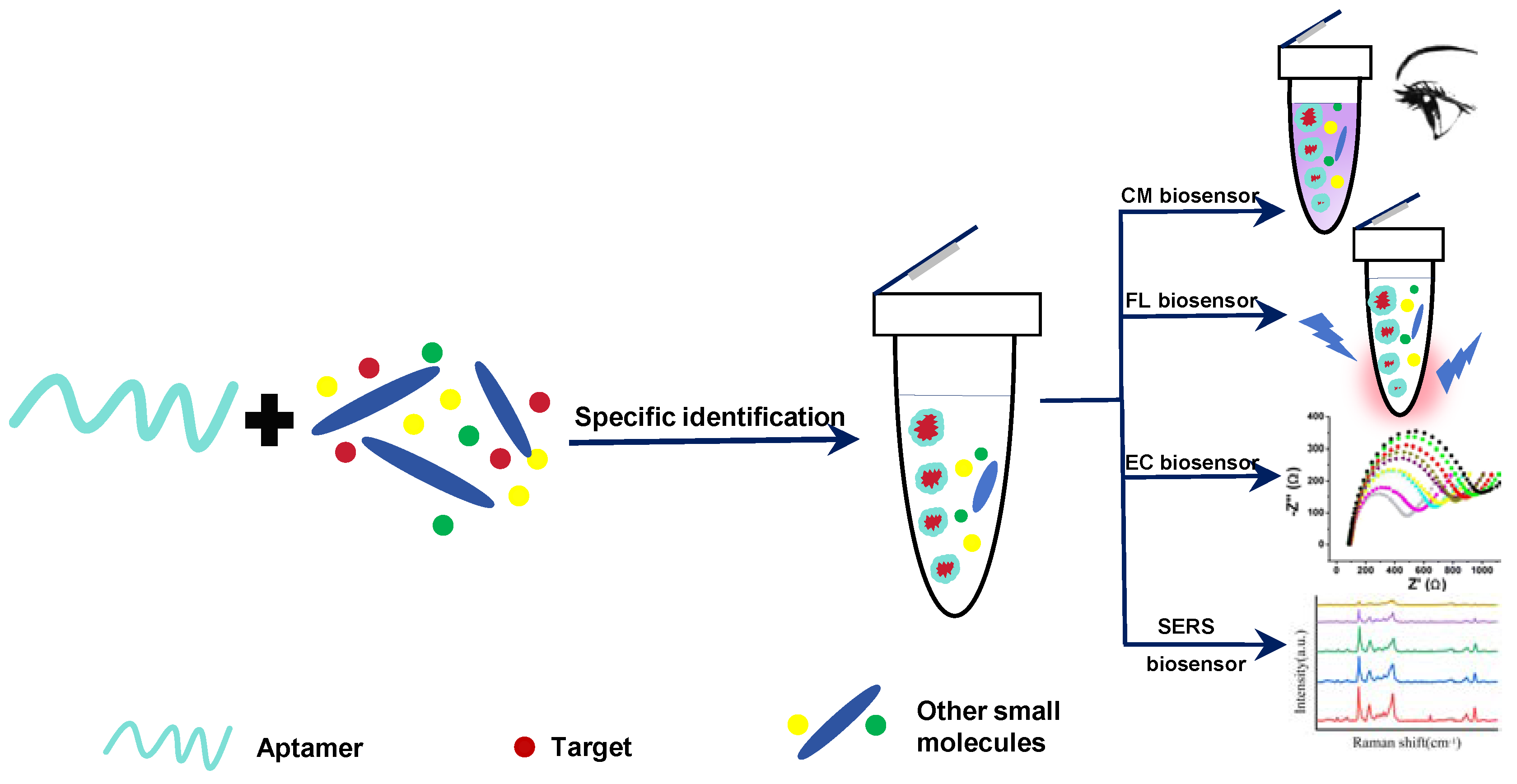
2. Detection Strategies on Aptamer Sensors for Foodborne Mycotoxins
2.1. Single-Mode Aptamer Sensors
2.2. Dual-Mode Aptamer Sensors
2.2.1. CM-FL Aptamer Sensors
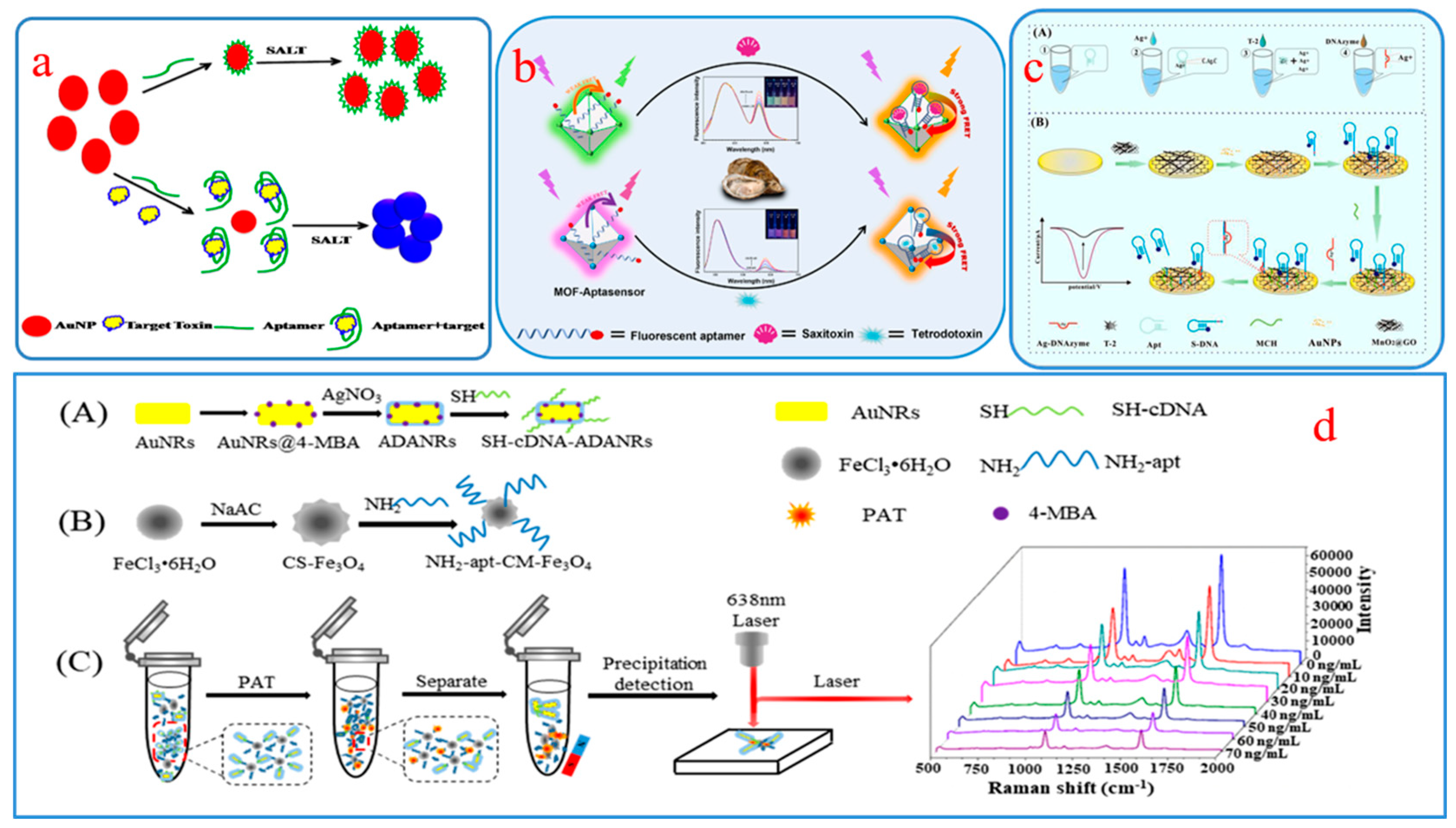
2.2.2. SERS-FL Aptamer Sensors
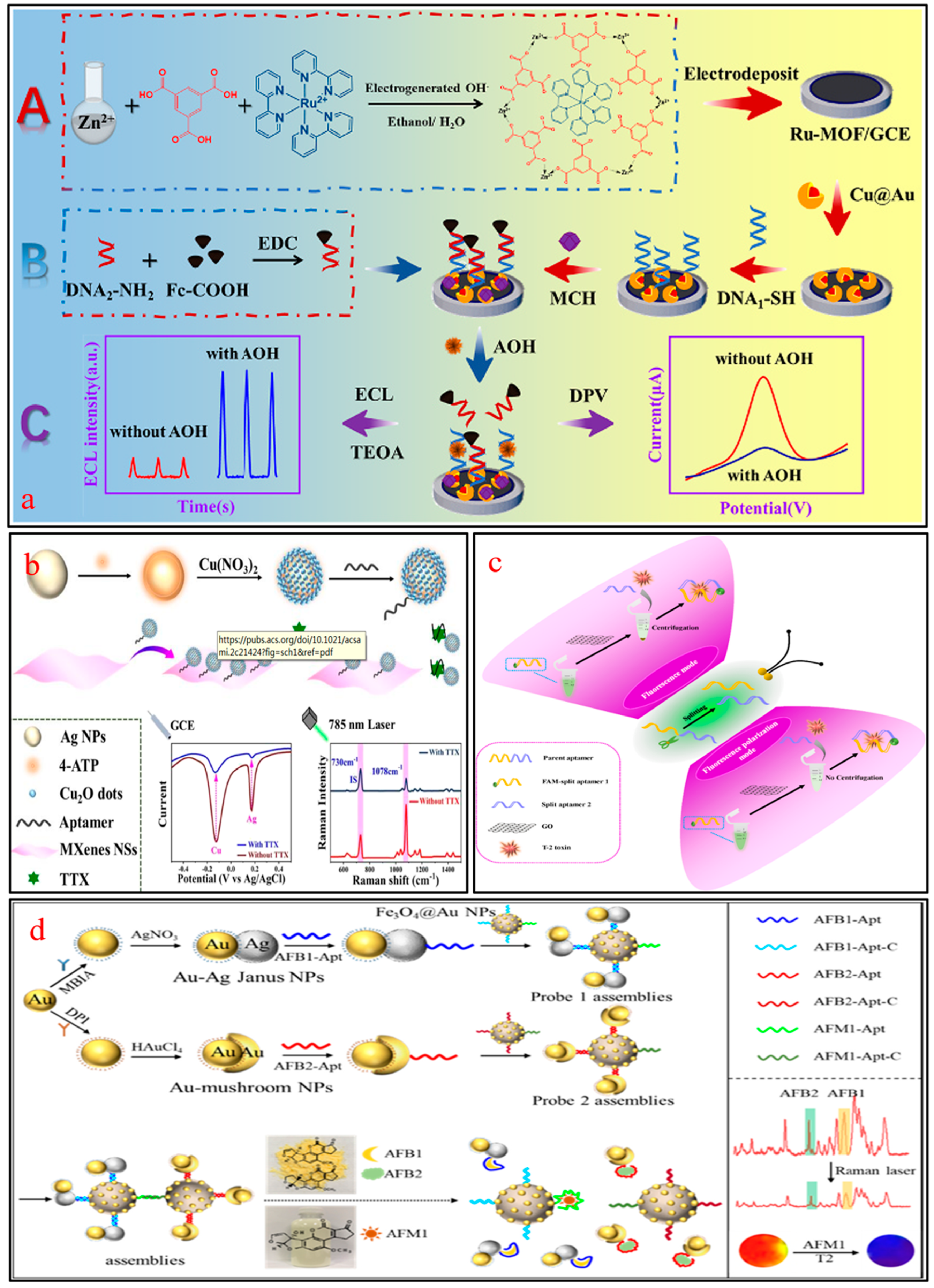
2.2.3. EC-PEC Aptamer Sensors
2.2.4. Other Dual-Mode Aptamer Sensors
3. Aptamer Sensors for In-Field Detection
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Jia, Y.; Zhao, S.; Li, D.; Yang, J.; Yang, L. Portable chemiluminescence optical fiber aptamer-based biosensors for analysis of multiple mycotoxins. Food Control 2023, 144, 109361. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, J.; Liu, N.; Yang, M.; Zhao, Q.; Li, C.; Liu, M. Structure-guided post-SELEX optimization of an ochratoxin A aptamer. Nucleic Acids Res. 2019, 47, 5963–5972. [Google Scholar] [CrossRef]
- Goud, K.Y.; Reddy, K.K.; Satyanarayana, M.; Kummari, S.; Gobi, K.V. A review on recent developments in optical and electrochemical aptamer-based assays for mycotoxins using advanced nanomaterials. Microchim. Acta 2019, 187, 29. [Google Scholar] [CrossRef]
- Chen, M.; Qileng, A.; Liang, H.; Lei, H.; Liu, W.; Liu, Y. Advances in immunoassay-based strategies for mycotoxin detection in food: From single-mode immunosensors to dual-mode immunosensors. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1285–1311. [Google Scholar] [CrossRef]
- Dong, F.; Ni, T.; Chen, Y.; Sun, Y.; Zheng, Z.; Li, Y.; Gong, C.; Ren, L.; Yan, X.; Wang, G. Foodborne disease outbreaks caused by biotoxins in Yantai city: A 10-year spatiotemporal monitoring study. Foodborne Pathog. Dis. 2023, 21, 194–202. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H.; Tsim, K.W.K.; Shen, X.; Li, X.; Li, X.; Lei, H.; Liu, Y. Aflatoxin B1 induces inflammatory liver injury via gut microbiota in mice. J. Agric. Food Chem. 2023, 71, 10787–10797. [Google Scholar] [CrossRef]
- Garofalo, M.; Payros, D.; Taieb, F.; Oswald, E.; Nougayrède, J.-P.; Oswald, I.P. From ribosome to ribotoxins: Understanding the toxicity of deoxynivalenol and shiga toxin, two food borne toxins. Crit. Rev. Food Sci. 2023, 1–13. [Google Scholar] [CrossRef]
- Stoev, S.D. Foodborne diseases due to underestimated hazard of joint mycotoxin exposure at low levels and possible risk assessment. Toxins 2023, 15, 464. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J.; Wang, P.; Wang, Y.; Chen, J. Thin-layer chromatography of mycotoxins and comparison with other chromatographic methods. J. Chromatogr. A 1998, 815, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Nao, S.-C.; Wu, K.-J.; Wang, W.; Leung, C.-H.; Ma, D.-L. Recent progress and development of G-Quadruplex-based luminescent assays for ochratoxin A detection. Front. Chem. 2020, 8, 767. [Google Scholar] [CrossRef]
- Zhang, K.; Banerjee, K. A review: Sample preparation and chromatographic technologies for detection of aflatoxins in foods. Toxins 2020, 12, 539. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; Bassolli Borsatto, J.V.; Maciel, E.V.S.; Lanças, F.M. Current role of modern chromatography and mass spectrometry in the analysis of mycotoxins in food. TrAC-Trends Anal. Chem. 2021, 135, 116156. [Google Scholar] [CrossRef]
- Smith, L.L.; Francis, K.A.; Johnson, J.T.; Gaskill, C.L. Quantitation of fumonisin B1 and B2 in feed using FMOC pre-column derivatization with HPLC and fluorescence detection. Food Chem. 2017, 234, 174–179. [Google Scholar] [CrossRef]
- Hidalgo-Ruiz, J.L.; Romero-González, R.; Martínez Vidal, J.L.; Garrido Frenich, A. A rapid method for the determination of mycotoxins in edible vegetable oils by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2019, 288, 22–28. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, M.; Ma, H.; Xuan, Z.; Tian, W.; Liu, H.; Wang, S.; Zhang, Y. Development and validation of an automated magneto-controlled pretreatment for chromatography-free detection of aflatoxin B1 in cereals and oils through atomic absorption spectroscopy. Toxins 2022, 14, 454. [Google Scholar] [CrossRef]
- Kocot, K.; Pytlakowska, K.; Talik, E.; Krafft, C.; Sitko, R. Sensitive determination of uranium using β-cyclodextrin modified graphene oxide and X-ray fluorescence techniques: EDXRF and TXRF. Talanta 2022, 246, 123501. [Google Scholar] [CrossRef]
- Aydin, M.; Aydin, E.B.; Sezgintürk, M.K. Chapter one-Advances in immunosensor technology. Adv. Clin. Chem. 2021, 102, 1–62. [Google Scholar]
- Jia, M.; Liao, X.; Fang, L.; Jia, B.; Liu, M.; Li, D.; Zhou, L.; Kong, W. Recent advances on immunosensors for mycotoxins in foods and other commodities. TrAC-Trend. Anal. Chem. 2021, 136, 116193. [Google Scholar] [CrossRef]
- Kholafazad-Kordasht, H.; Hasanzadeh, M.; Seidi, F. Smartphone based immunosensors as next generation of healthcare tools: Technical and analytical overview towards improvement of personalized medicine. TrAC-Trend. Anal. Chem. 2021, 145, 116455. [Google Scholar] [CrossRef]
- Zhang, H.; Miller, B.L. Immunosensor-based label-free and multiplex detection of influenza viruses: State of the art. Biosens. Bioelectron. 2019, 141, 111476. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Kuang, H.; Xu, C. Visible and eco-friendly immunoassays for the detection of cyclopiazonic acid in maize and rice. J. Food Sci. 2020, 85, 105–113. [Google Scholar] [CrossRef]
- Siva, S.; Jin, J.-O.; Choi, I.; Kim, M. Nanoliposome based biosensors for probing mycotoxins and their applications for food: A review. Biosens. Bioelectron. 2023, 219, 114845. [Google Scholar] [CrossRef]
- Jiang, C.; Lan, L.; Yao, Y.; Zhao, F.; Ping, J. Recent progress in application of nanomaterial-enabled biosensors for ochratoxin A detection. TrAC-Trend. Anal. Chem. 2018, 102, 236–249. [Google Scholar] [CrossRef]
- Küçük, N.; Kaya, Ş.; Şahin, S.; Çağlayan, M.O. Structural switching aptamer-based electrochemical sensor for mycotoxin patulin detection. Toxicon 2024, 239, 107583. [Google Scholar] [CrossRef]
- Bashir, A.; Yang, Q.; Wang, J.; Hoyer, S.; Chou, W.; McLean, C.; Davis, G.; Gong, Q.; Armstrong, Z.; Jang, J.; et al. Machine learning guided aptamer refinement and discovery. Nat. Commun. 2021, 12, 2366. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; Huang, Q.; Tong, T.; Zhou, Y.; Li, Z.; Bai, Q.; Liang, H.; Chen, L. Recent advances on aptamer-based biosensors for detection of pathogenic bacteria. World J. Microb. Biot. 2021, 37, 45. [Google Scholar] [CrossRef]
- Zhao, Y.; Yavari, K.; Liu, J. Critical evaluation of aptamer binding for biosensor designs. TrAC-Trends Anal. Chem. 2022, 146, 116480. [Google Scholar] [CrossRef]
- Yu, H.; Alkhamis, O.; Canoura, J.; Liu, Y.; Xiao, Y. Advances and challenges in small-molecule DNA aptamer isolation, characterization, and sensor development. Angew. Chem. Int. Ed. 2021, 60, 16800–16823. [Google Scholar] [CrossRef]
- Lyu, C.; Khan, I.M.; Wang, Z. Capture-SELEX for aptamer selection: A short review. Talanta 2021, 229, 122274. [Google Scholar] [CrossRef]
- Liu, M.; Yue, F.; Kong, Q.; Liu, Z.; Guo, Y.; Sun, X. Aptamers against pathogenic bacteria: Selection strategies and apta-assay/aptasensor application for food safety. J. Agric. Food Chem. 2022, 70, 5477–5498. [Google Scholar] [CrossRef]
- Li, Y.-K.; Li, W.-T.; Liu, X.; Yang, T.; Chen, M.-L.; Wang, J.-H. Functionalized magnetic composites based on the aptamer serve as novel bio-adsorbent for the separation and preconcentration of trace lead. Talanta 2019, 203, 210–219. [Google Scholar] [CrossRef]
- Idili, A.; Gerson, J.; Parolo, C.; Kippin, T.; Plaxco, K.W. An electrochemical aptamer-based sensor for the rapid and convenient measurement of l-tryptophan. Anal. Bioanal. Chem. 2019, 411, 4629–4635. [Google Scholar] [CrossRef]
- Kovačič, M.; Podbevšek, P.; Tateishi-Karimata, H.; Takahashi, S.; Sugimoto, N.; Plavec, J. Thrombin binding aptamer G-quadruplex stabilized by pyrene-modified nucleotides. Nucleic Acids Res. 2020, 48, 3975–3986. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L.; Zhao, Y.; Zhu, C.; Yang, R.; Fang, M.; Luan, Y. Aptamer-based point-of-care-testing for small molecule targets: From aptamers to aptasensors, devices and applications. TrAC-Trends Anal. Chem. 2023, 169, 117408. [Google Scholar] [CrossRef]
- Frohnmeyer, E.; Tuschel, N.; Sitz, T.; Hermann, C.; Dahl, G.T.; Schulz, F.; Baeumner, A.J.; Fischer, M. Aptamer lateral flow assays for rapid and sensitive detection of cholera toxin. Analyst 2019, 144, 1840–1849. [Google Scholar] [CrossRef]
- Xing, K.-Y.; Peng, J.; Shan, S.; Liu, D.-F.; Huang, Y.-N.; Lai, W.-H. Green enzyme-linked immunosorbent assay based on the single-stranded binding protein-assisted aptamer for the detection of mycotoxin. Anal. Chem. 2020, 92, 8422–8426. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Fadeev, M.; Zhang, P.; Carmieli, R.; Li, J.; Sohn, Y.S.; Karmi, O.; Nechushtai, R.; Pikarsky, E.; Fan, C.; et al. Aptamer-modified Au nanoparticles: Functional nanozyme bioreactors for cascaded catalysis and catalysts for chemodynamic treatment of cancer cells. ACS Nano 2022, 16, 18232–18243. [Google Scholar] [CrossRef]
- Geng, Z.; Cao, Z.; Liu, R.; Liu, K.; Liu, J.; Tan, W. Aptamer-assisted tumor localization of bacteria for enhanced biotherapy. Nat. Commun. 2021, 12, 6584. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, X.; Ran, C.; Li, L.; Fu, T.; Su, D.; Xie, S.; Tan, W. Aptamer-based targeted protein degradation. ACS Nano 2023, 17, 6150–6164. [Google Scholar] [CrossRef]
- Lu, L.; Yu, R.; Zhang, L. AFB1 colorimetric aptamer sensor for the detection of AFB1 in ten different kinds of miscellaneous beans based on gold nanoparticles and smartphone imaging. Food Chem. 2023, 421, 136205. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, G.; Wang, X.; Zhang, Y.; Li, Z.; Liu, P.; Yu, B.; Zhang, J. A metal-organic framework/aptamer system as a fluorescent biosensor for determination of aflatoxin B1 in food samples. Talanta 2020, 219, 121342. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, L.; Zhao, Q. Low temperature greatly enhancing responses of aptamer electrochemical sensor for aflatoxin B1 using aptamer with short stem. ACS Sens. 2020, 5, 3246–3253. [Google Scholar] [CrossRef]
- Bakhshandeh, F.; Saha, S.; Sen, P.; Sakib, S.; MacLachlan, R.; Kanji, F.; Osman, E.; Soleymani, L. A universal bacterial sensor created by integrating a light modulating aptamer complex with photoelectrochemical signal readout. Biosens. Bioelectron. 2023, 235, 115359. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.-W.; Pu, H.; Wei, Q. Ultrasensitive analysis of kanamycin residue in milk by SERS-based aptasensor. Talanta 2019, 197, 151–158. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Xue, Z.; Peng, B.; Kou, X.; Gao, Z. Research progress of dual-mode sensing technology strategy based on SERS and its application in the detection of harmful substances in foods. Trends Food Sci. Tech. 2024, 148, 104487. [Google Scholar] [CrossRef]
- Schmitz, F.R.W.; Cesca, K.; Valério, A.; de Oliveira, D.; Hotza, D. Colorimetric detection of pseudomonas aeruginosa by aptamer-functionalized gold nanoparticles. Appl. Microbiol. Biot. 2023, 107, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Mahjub, R.; Shayesteh, O.H.; Derakhshandeh, K.; Ranjbar, A.; Mehri, F.; Heshmati, A. A novel label-free colorimetric polyA aptasensing approach based on cationic polymer and silver nanoparticles for detection of tobramycin in milk. Food Chem. 2022, 382, 132580. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, Q.-G.; Xiao, S.-J.; Yang, G.-P.; Liu, X.; Zheng, Q.-Q.; Fan, J.-Q.; Liang, R.-P.; Qiu, J.-D. DNAzyme-derived aptamer reversely regulates the two types of enzymatic activities of covalent-organic frameworks for the colorimetric analysis of uranium. Anal. Chem. 2023, 95, 4703–4711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, Y.; Huang, X.; Hu, X.; Huang, X.; Yin, L.; Huang, Q.; Wen, Y.; Li, B.; Shi, J.; et al. Switchable aptamer-fueled colorimetric sensing toward agricultural fipronil exposure sensitized with affiliative metal-organic framework. Food Chem. 2023, 407, 135115. [Google Scholar] [CrossRef]
- Mondal, B.; Ramlal, S.; Lavu, P.S.; N, B.; Kingston, J. Highly sensitive colorimetric biosensor for staphylococcal enterotoxin B by a label-free aptamer and gold nanoparticles. Front. Microbiol. 2018, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Saraji, M. Optical aptasensor based on silver nanoparticles for the colorimetric detection of adenosine. Spectrochim. Acta A 2019, 213, 1–5. [Google Scholar] [CrossRef]
- He, Y.; Tian, F.; Zhou, J.; Zhao, Q.; Fu, R.; Jiao, B. Colorimetric aptasensor for ochratoxin A detection based on enzyme-induced gold nanoparticle aggregation. J. Hazard. Mater. 2020, 388, 121758. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, J.; Hur, H.; Oh, S.; Lee, H.H. Highly sensitive colorimetric assay of cortisol using cortisol antibody and aptamer sandwich assay. Biosensors 2021, 11, 163. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Z.; Li, B.; Du, J. Aptamer biorecognition-triggered hairpin switch and nicking enzyme assisted signal amplification for ultrasensitive colorimetric bioassay of kanamycin in milk. Food Chem. 2021, 339, 128059. [Google Scholar] [CrossRef]
- McKeague, M.; Velu, R.; Hill, K.; Bardóczy, V.; Mészáros, T.; DeRosa, M.C. Selection and characterization of a novel DNA aptamer for label-free fluorescence biosensing of ochratoxin A. Toxins 2014, 6, 2435–2452. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Yu, M.; Fu, F.; Han, W.; Li, G.; Xie, J.; Song, Y.; Swihart, M.T.; Song, E. Dual recognition strategy for specific and sensitive detection of bacteria using aptamer-coated magnetic beads and antibiotic-capped gold nanoclusters. Anal. Chem. 2016, 88, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Lee, W.-I.; Lee, N.-E. Culture-free, highly sensitive, quantitative detection of bacteria from minimally processed samples using fluorescence imaging by smartphone. Biosens. Bioelectron. 2018, 109, 90–97. [Google Scholar] [CrossRef]
- Zhu, W.; Ji, G.; Chen, R.; Xiang, Y.; Ji, S.; Zhang, S.; Gao, Z.; Liu, H.; Wang, Y.; Han, T. A fluorescence aptasensor based on hybridization chain reaction for simultaneous detection of T-2 toxins and zearalenone1. Talanta 2023, 255, 124249. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Xu, S.; Jiang, Y.; Ding, Z.; Xie, J. Aptamers-functionalized nanoscale MOFs for saxitoxin and tetrodotoxin sensing in sea foods through FRET. Spectrochim. Acta A 2023, 284, 121827. [Google Scholar] [CrossRef]
- Ikebukuro, K.; Kiyohara, C.; Sode, K. Electrochemical detection of protein using a double aptamer sandwich. Anal. Lett. 2004, 37, 2901–2909. [Google Scholar] [CrossRef]
- Jiang, K.; Nie, D.; Huang, Q.; Fan, K.; Tang, Z.; Wu, Y.; Han, Z. Thin-layer MoS2 and thionin composite-based electrochemical sensing platform for rapid and sensitive detection of zearalenone in human biofluids. Biosens. Bioelectron. 2019, 130, 322–329. [Google Scholar] [CrossRef]
- Mao, Y.; Dang, M.; Zhang, J.; Huang, X.; Qiao, M.; Song, L.; Zhao, Q.; Ding, M.; Wang, Y.; Li, Z.; et al. Peptide amphiphile inspired self-assembled, ordered gold nanocomposites for improved sensitivity of electrochemical immunosensor: Applications in determining the total aflatoxin amount in food stuffs. Talanta 2022, 247, 123532. [Google Scholar] [CrossRef]
- Wang, L.; Jin, H.; Wei, M.; Ren, W.; Zhang, Y.; Jiang, L.; Wei, T.; He, B. A DNAzyme-assisted triple-amplified electrochemical aptasensor for ultra-sensitive detection of T-2 toxin. Sensor. Actuat. B-Chem. 2021, 328, 129063. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface Raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Inter. Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, H.; Sun, D.-W. Fingerprinting and tagging detection of mycotoxins in agri-food products by surface-enhanced Raman spectroscopy: Principles and recent applications. Trends Food Sci. Technol. 2021, 110, 393–404. [Google Scholar] [CrossRef]
- Zhu, A.; Jiao, T.; Ali, S.; Xu, Y.; Ouyang, Q.; Chen, Q. SERS sensors based on aptamer-gated mesoporous silica nanoparticles for quantitative detection of staphylococcus aureus with signal molecular release. Anal. Chem. 2021, 93, 9788–9796. [Google Scholar] [CrossRef]
- Song, L.; Li, J.; Li, H.; Chang, Y.; Dai, S.; Xu, R.; Dou, M.; Li, Q.; lv, G.; Zheng, T. Highly sensitive SERS detection for aflatoxin B1 and ochratoxin A based on aptamer-functionalized photonic crystal microsphere array. Sensor. Actuat. B Chem. 2022, 364, 131778. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, L.; Jiang, S.; El-Seedi, H.R.; El-Garawani, I.M.; Zou, X. Sensitive determination of patulin by aptamer functionalized magnetic surface enhanced Raman spectroscopy (SERS) sensor. J. Food Compos. Anal. 2023, 115, 104985. [Google Scholar] [CrossRef]
- Dong, X.; Shi, Z.; Xu, C.; Yang, C.; Chen, F.; Lei, M.; Wang, J.; Cui, Q. CdS quantum dots/Au nanoparticles/ZnO nanowire array for self-powered photoelectrochemical detection of escherichia coli O157:H7. Biosens. Bioelectron. 2020, 149, 111843. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.; Teo, A.J.T.; Li, K.H. Acoustic biosensors and microfluidic devices in the decennium: Principles and applications. Micromachines 2022, 13, 24. [Google Scholar] [CrossRef]
- Maldonado, J.; Estévez, M.C.; Fernández-Gavela, A.; González-López, J.J.; González-Guerrero, A.B.; Lechuga, L.M. Label-free detection of nosocomial bacteria using a nanophotonic interferometric biosensor. Analyst 2020, 145, 497–506. [Google Scholar] [CrossRef]
- Jiao, F.; Cai, Z. A smartphone-based nanoenzyme-modulated aptasensor using an infrared camera for rapid detection of kanamycin. Chem. Eng. J. 2024, 481, 148699. [Google Scholar] [CrossRef]
- Sayad, A.; Skafidas, E.; Kwan, P. Magneto-impedance biosensor sensitivity: Effect and enhancement. Sensors 2020, 20, 5213. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, R.; Shi, H.; Tang, B.; Xiao, H.; Zhao, G. A simple highly sensitive and selective aptamer-based colorimetric sensor for environmental toxins microcystin-LR in water samples. J. Hazard. Mater. 2016, 304, 474–480. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Y.; Yan, X.; Qi, X.; Wang, L.; Ma, R.; Wang, S.; Mao, X. Development of a terminal-fixed aptamer and a label-free colorimetric aptasensor for highly sensitive detection of saxitoxin. Sensor. Actuat. B Chem. 2021, 344, 130320. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Nan, M.; Li, Y.; Yun, J.; Wang, Y.; Bi, Y. Novel colorimetric aptasensor based on unmodified gold nanoparticle and ssDNA for rapid and sensitive detection of T-2 toxin. Food Chem. 2021, 348, 129128. [Google Scholar] [CrossRef]
- Luan, Y.; Chen, J.; Li, C.; Xie, G.; Fu, H.; Ma, Z.; Lu, A. Highly sensitive colorimetric detection of ochratoxin A by a label-free aptamer and gold nanoparticles. Toxins 2015, 7, 5377–5385. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Ma, W.; Liu, L.; Ma, W.; Zhao, Y.; Zhu, Y.; Xu, L.; Kuang, H.; Xu, C. Fluorescent strip sensor for rapid determination of toxins. Chem. Commun. 2011, 47, 1574–1576. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, J.; Huo, B.; Qin, Y.; Zhang, J.; Chen, M.; Peng, Y.; Bai, J.; Li, S.; et al. A fluorescence aptasensor based on controlled zirconium-based MOFs for the highly sensitive detection of T-2 toxin. Spectrochim. Acta A 2021, 259, 119893. [Google Scholar] [CrossRef]
- Kuang, H.; Chen, W.; Xu, D.; Xu, L.; Zhu, Y.; Liu, L.; Chu, H.; Peng, C.; Xu, C.; Zhu, S. Fabricated aptamer-based electrochemical “signal-off” sensor of ochratoxin A. Biosens. Bioelectron. 2010, 26, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Y.; Ma, X.; Jia, F.; Guo, X.; Wang, Z. Impedimetric aptamer-based determination of the mold toxin fumonisin B1. Microchim. Acta 2015, 182, 1709–1714. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Abnous, K.; Taghdisi, S.M.; Verdian, A.; Sameiyan, E.; Ramezani, M.; Alibolandi, M. An ultra-sensitive dual-responsive aptasensor with combination of liquid crystal and intercalating dye molecules: A food toxin case study. Food Chem. 2022, 381, 132265. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Zhang, Y.; Chen, M.; Lin, X.; Zhang, J.; Han, J.; Luo, X. Dual-mode aptasensor based on a coumarin-benzothiazole fluorescent and colorimetric probe for label-free and visual detection of ochratoxin A. Sensor. Actuat. B Chem. 2024, 408, 135529. [Google Scholar] [CrossRef]
- Lin, X.; Fang, Y.; Chen, Q.; Guo, Z.; Chen, X.; Chen, X. Magnetically actuated microfluidic chip combined with a G-quadruplex DNAzyme-based fluorescent/colorimetric sensor for the dual-mode detection of ochratoxin A in wheat. Talanta 2024, 267, 125273. [Google Scholar] [CrossRef] [PubMed]
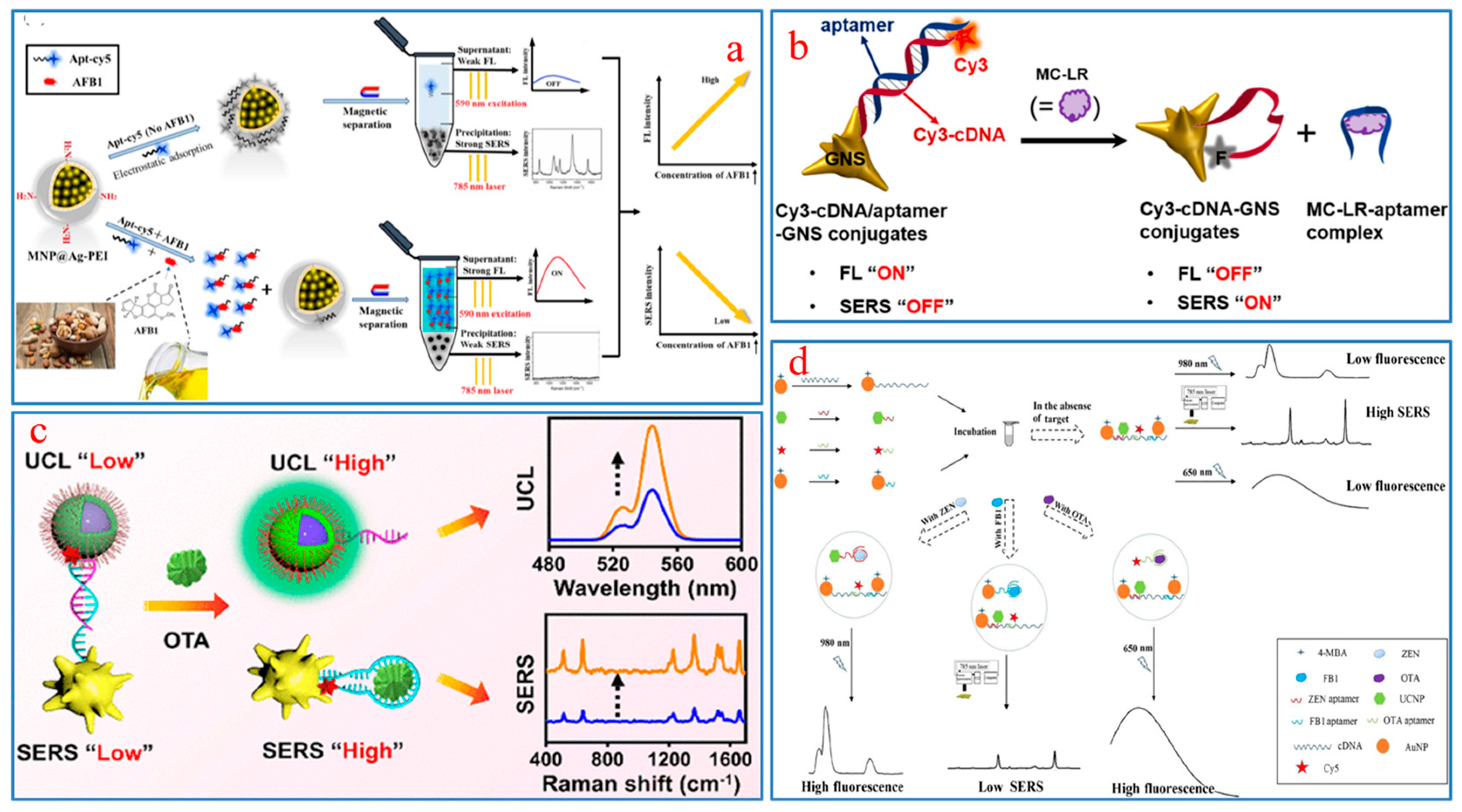
- He, H.; Sun, D.-W.; Pu, H.; Wu, Z. A SERS-fluorescence dual-signal aptasensor for sensitive and robust determination of AFB1 in nut samples based on Apt-Cy5 and MNP@Ag-PEI. Talanta 2023, 253, 123962. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, C.; He, C.; Zhou, Y.; Wang, Z.; Duan, N.; Wu, S. Upconversion Nanoparticles assembled with gold nanourchins as luminescence and surface-enhanced Raman scattering dual-mode aptasensors for detection of ochratoxin A. ACS Appl. Nano Mater. 2021, 4, 8231–8240. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, B.; Ye, Y.; Qi, X.; Zhang, Y.; Xia, X.; Wang, X.; Zhou, N. A fluorescence and surface-enhanced Raman scattering dual-mode aptasensor for rapid and sensitive detection of ochratoxin A. Biosens. Bioelectron. 2022, 207, 114164. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Duan, M.; Su, T.; Ying, D.; Wu, S.; Wang, Z.; Duan, N. A colorimetric and SERS dual-mode aptasensor for the detection of Shiga toxin type II based on Mn/Fe-MIL(53)@AuNSs. Talanta 2024, 270, 125636. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Shi, Y.; Hu, B.; Zheng, Q.; Piao, Y.; Feng, L.; Cao, J. Electrochemical/photoelectrochemical dual-mode aptasensor for sensitive aflatoxin B1 assay based on distance-modulation strategy using Au NPs/PCZIF-8-ZnO as sensing substrate. Food Chem. 2024, 441, 138382. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Meng, S.; Wang, M.; Li, W.; Dong, N.; Liu, D.; Li, Y.; You, T. In-depth interpretation of aptamer-based sensing on electrode: Dual-mode electrochemical-photoelectrochemical sensor for the ratiometric detection of patulin. Food Chem. 2023, 410, 135450. [Google Scholar] [CrossRef]
- Zou, Y.; Xia, T.; Zuo, Y.; Gu, Y.; Zhang, J.; Wei, J.; Qian, J.; Hao, N.; Wang, K. Dual-mode sensing chip for photoelectrochemical and electrochromic visual determination of deoxynivalenol mycotoxin. Microchim. Acta 2023, 190, 466. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Jin, Z.; Zhao, Y. Electroactive and SERS-active Ag@Cu2O NP-programed aptasensor for dual-mode detection of tetrodotoxin. ACS Appl. Mater. Interfaces 2023, 15, 10240–10249. [Google Scholar] [CrossRef]
- Wang, X.; Yang, F.; Deng, C.; Zhang, Y.; Yang, X.; Chen, X.; Huang, Y.; Ye, H.; Zhong, J.; Wang, Z. A dual-mode method based on aptamer recognition and time-resolved fluorescence resonance energy transfer for histamine detection in fish. Molecules 2022, 27, 8711. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, M.; Zhu, H.; Zhang, M.; Li, X.; Wang, M.; Liu, B.; Pan, J.; Niu, X. Dual-mode fluorescence and colorimetric detection of pesticides realized by integrating stimulus-responsive luminescence with oxidase-mimetic activity into cerium-based coordination polymer nanoparticles. J. Hazard. Mater. 2022, 423, 127077. [Google Scholar] [CrossRef]
- Chen, J.-W.; Liu, X.-P.; Feng, K.-J.; Liang, Y.; Jiang, J.-H.; Shen, G.-L.; Yu, R.-Q. Detection of adenosine using surface-enhanced Raman scattering based on structure-switching signaling aptamer. Biosens. Bioelectron. 2008, 24, 66–71. [Google Scholar] [CrossRef]
- He, D.; Wu, Z.; Cui, B.; Xu, E. Aptamer and gold nanorod-based fumonisin B1 assay using both fluorometry and SERS. Microchim. Acta 2020, 187, 215. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, H.; Paidi, S.K.; Mesyngier, N.; Preheim, S.; Barman, I. A fluorescence and surface-enhanced Raman spectroscopic dual-modal aptasensor for sensitive detection of cyanotoxins. ACS Sens. 2020, 5, 1419–1426. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, J.; Liu, J.; Zhang, Y. Recent progress of rare-earth doped upconversion nanoparticles: Synthesis, optimization, and applications. Adv. Sci. 2019, 6, 1901358. [Google Scholar] [CrossRef]
- Ma, W.; Fu, P.; Sun, M.; Xu, L.; Kuang, H.; Xu, C. Dual quantification of microRNAs and telomerase in living cells. J. Am. Chem. Soc. 2017, 139, 11752–11759. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, D.; Cui, B.; Jin, Z.; Xu, E.; Yuan, C.; Liu, P.; Fang, Y.; Chai, Q. Trimer-based aptasensor for simultaneous determination of multiple mycotoxins using SERS and fluorimetry. Microchim. Acta 2020, 187, 495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fu, Y.; Xiao, K.; Du, C.; Zhang, X.; Chen, J. Sensitive dual-mode biosensors for CYFRA21-1 assay based on the dual-signaling electrochemical ratiometric strategy and “on-off-on” PEC method. Anal. Chem. 2021, 93, 6801–6807. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, K.; Zhu, L.; Zhang, S.; Wang, M.; He, L.; Zhang, Z.; Du, M. MOF@COF heterostructure hybrid for dual-mode photoelectrochemical-electrochemical HIV-1 DNA sensing. Langmuir 2021, 37, 13479–13492. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Chai, Y.; Yuan, R.; Yuan, Y. In situ formation of multifunctional DNA nanospheres for a sensitive and accurate dual-mode biosensor for photoelectrochemical and electrochemical assay. Anal. Chem. 2020, 92, 8364–8370. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.M.; Cai, L.; Liu, C.; Cho, I.S.; Lee, C.H.; Weisse, J.M.; Yang, P.; Zheng, X. Simultaneously efficient light absorption and charge separation in WO3/BiVO4 core/shell nanowire photoanode for photoelectrochemical water oxidation. Nano Lett. 2014, 14, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Meng, S.; Shen, X.; Li, Y.; Yan, X.; You, T. Dual-ratiometric aptasensor for streptomycin detection based on the in-situ coupling of photoelectrochemical and electrochemical assay with a bifunctional probe of methylene blue. Sens. Actuators B Chem. 2021, 332, 129529. [Google Scholar] [CrossRef]
- Hao, N.; Dai, Z.; Meng, X.; Hua, R.; Lu, J.; Wang, K. A portable solar-driven ratiometric photo-electrochromic visualization biosensor for detection of ochratoxin A. Sens. Actuators B Chem. 2020, 306, 127594. [Google Scholar] [CrossRef]
- Meng, S.; Li, Y.; Dong, N.; Liu, S.; Liu, C.; Gong, Q.; Chen, Z.; Jiang, K.; Li, X.; Liu, D.; et al. Portable visual photoelectrochemical biosensor based on a MgTi2O5/CdSe heterojunction and reversible electrochromic supercapacitor for dual-modal Cry1Ab protein detection. Anal. Chem. 2023, 95, 18224–18232. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Ozalp, V.C.; Oztekin, M.; Arica, M.Y. Rapid and label-free detection of brucella melitensis in milk and milk products using an aptasensor. Talanta 2019, 200, 263–271. [Google Scholar] [CrossRef]
- Shan, X.; Kuang, D.; Feng, Q.; Wu, M.; Yang, J. A dual-mode ratiometric aptasensor for accurate detection of pathogenic bacteria based on recycling of DNAzyme activation. Food Chem. 2023, 423, 136287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, M.; Song, X.; Lai, W.; Zhao, C.; Li, J.; Wei, Z.; Hong, C. Dual-functional nanomaterials polyo-phenylenediamine and Ru-Au complement each other to construct an electrochemical and electrochemiluminescent dual-mode aptamer sensor for sensitive detection of alternariol. Anal. Chem. 2023, 95, 12459–12469. [Google Scholar] [CrossRef]
- Zhu, P.; Asumadu, P.; Zhou, S.; Wang, M.; Liu, C.; Zhang, Q.; Zhong, J.; Guan, H.; Ye, H. Recognition mechanism of split T-2 toxin aptamer coupled with reliable dual-mode detection in peanut and beer. Food Biosci. 2024, 60, 104268. [Google Scholar] [CrossRef]
- Cao, H.; Liang, D.; Tang, K.; Sun, Y.; Xu, Y.; Miao, M.; Zhao, Y. SERS and MRS signals engineered dual-mode aptasensor for simultaneous distinguishment of aflatoxin subtypes. J. Hazard. Mater. 2024, 462, 132810. [Google Scholar] [CrossRef]
- Goyal, S.; Singh, P.; Sengupta, S.; Muthukrishnan, A.B.; Jayaraman, G. DNA-aptamer-based qPCR using light-up dyes for the detection of nucleic acids. ACS Omega 2023, 8, 47277–47282. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, J.; Yao, K.; Yin, Y.; Gong, M.M.; Yang, C.; Lin, F. Paper-based microfluidic device (DON-chip) for rapid and low-cost deoxynivalenol quantification in food, feed, and feed ingredients. ACS Sens. 2019, 4, 3072–3079. [Google Scholar] [CrossRef] [PubMed]
- Epifania, R.; Soares, R.R.G.; Pinto, I.F.; Chu, V.; Conde, J.P. Capillary-driven microfluidic device with integrated nanoporous microbeads for ultrarapid biosensing assays. Sens. Actuators B Chem. 2018, 265, 452–458. [Google Scholar] [CrossRef]
- Machado, J.M.D.; Soares, R.R.G.; Chu, V.; Conde, J.P. Multiplexed capillary microfluidic immunoassay with smartphone data acquisition for parallel mycotoxin detection. Biosens. Bioelectron. 2018, 99, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Su, Z.; Li, Y.; Xi, L.; Li, G. An aptamer-assisted biological nanopore biosensor for ultra-sensitive detection of ochratoxin A with a portable single-molecule measuring instrument. Talanta 2022, 248, 123619. [Google Scholar] [CrossRef]
- Jin, B.; Yang, Y.; He, R.; Park, Y.I.; Lee, A.; Bai, D.; Li, F.; Lu, T.J.; Xu, F.; Lin, M. Lateral flow aptamer assay integrated smartphone-based portable device for simultaneous detection of multiple targets using upconversion nanoparticles. Sens. Actuators B Chem. 2018, 276, 48–56. [Google Scholar] [CrossRef]
- Zhang, X.; Zhi, H.; Zhu, M.; Wang, F.; Meng, H.; Feng, L. Electrochemical/visual dual-readout aptasensor for ochratoxin A detection integrated into a miniaturized paper-based analytical device. Biosens. Bioelectron. 2021, 180, 113146. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, R.; Ramadan, Q.; Zourob, M. An integrated lab-on-a-chip platform for pre-concentration and detection of colorectal cancer exosomes using anti-CD63 aptamer as a recognition element. Biosens. Bioelectron. 2023, 220, 114856. [Google Scholar] [CrossRef]
- Nandimandalam, M.; Costantini, F.; Lovecchio, N.; Iannascoli, L.; Nascetti, A.; de Cesare, G.; Caputo, D.; Manetti, C. Split aptamers immobilized on polymer brushes integrated in a lab-on-chip system based on an array of amorphous silicon photosensors: A novel sensor assay. Materials 2021, 14, 7210. [Google Scholar] [CrossRef]
- Lonchamps, P.-L.; He, Y.; Wang, K.; Lu, X. Detection of pathogens in foods using microfluidic “lab-on-chip”: A mini review. J. Agric. Food Res. 2022, 10, 100430. [Google Scholar] [CrossRef]


| Method | Analyte | Actual Samples | Linear Range | LOD | Reference | |
|---|---|---|---|---|---|---|
| Single-mode | CM | SEB | Milk | 0.5 ng/mL–50 µg/mL | 0.5 ng/mL | [50] |
| MC-LR | Water | 0.5 ng/mL–7.5 µg/mL | 0.37 ng/mL | [76] | ||
| STX | Scallop | 0.044–11.241 ng/mL | 43 pg/mL | [77] | ||
| T-2 | Wheat, corn | 0.1–5000 ng/mL | 57.8 pg/mL | [78] | ||
| OTA | Liquor | 0.05–50 ng/mL | 9 pg/mL | [79] | ||
| FL | TTX | Shellfish | 0–0.149 µg/mL | 0.979 ng/mL | [59] | |
| OTA | 3.63–40.30 ng/mL | 3.63 ng/mL | [55] | |||
| OTA | 0–10 ng/mL | 1.9 ng/mL | [80] | |||
| T-2 | Beer | 0.5–100 ng/mL | 0.239 ng/mL | [81] | ||
| EC | T-2 | 2 fg/mL–20 ng/mL | 0.107 fg/mL | [63] | ||
| OTA | Wine | 0.1–20 ng/mL | 30 pg/mL | [82] | ||
| FB1 | Maize | 0.072 ng/mL–7.22 µg/mL | 1.44 pg/mL | [83] | ||
| SERS | PAT | 0–70 ng/mL | 0.038 ng/mL | [70] | ||
| AFB1 | Chinese herbs | 0.01–100 ng/mL | 0.36 pg/mL | [69] | ||
| OTA | Chinese herbs | 0.001–10 ng/mL | 0.034 pg/mL | [69] | ||
| Dual-mode | CM-FL | OTA | 40.38 fg/mL–40.38 pg/mL | 13.79 fg/mL | [84] | |
| OTA | 40.38 ng/mL–10.09 µg/mL | 40.38 ng/mL | [85] | |||
| OTA | Wheat | 0.05–10 μg/kg | 0.008 μg/kg | [86] | ||
| SERS-FL | AFB1 | Peanut | 0.001–1000 ng/mL | 0.45 pg/mL | [87] | |
| OTA | Beer | 0.01–100 ng/mL | 3.2 pg/mL | [88] | ||
| OTA | Coffee | 5–250 pg/mL | 1.03 pg/mL | [89] | ||
| SERS-CM | STX2 | Milk | 0.05–500 ng/mL | 26 pg/mL | [90] | |
| EC-PEC | AFB1 | Peanut, corn | 0.02 pg/mL–100 ng/mL | 9.3 fg/mL | [91] | |
| PAT | Juice | 50 fg/mL–500 ng/mL | 30 fg/mL | [92] | ||
| DON | Beer | 1 fg/mL–100 pg/mL | 0.37 fg/mL | [93] | ||
| EC-SERS | TTX | Fish | 100 pg/mL–10 μg/mL | 31.6 pg/mL | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, D.; Zeng, X.; Liu, C.; Wu, Y.; Fu, C. Advances in Aptamer-Based Biosensors for the Detection of Foodborne Mycotoxins. Molecules 2024, 29, 3974. https://doi.org/10.3390/molecules29163974
Li Y, Zhang D, Zeng X, Liu C, Wu Y, Fu C. Advances in Aptamer-Based Biosensors for the Detection of Foodborne Mycotoxins. Molecules. 2024; 29(16):3974. https://doi.org/10.3390/molecules29163974
Chicago/Turabian StyleLi, Yangyang, Dan Zhang, Xiaoyuan Zeng, Cheng Liu, Yan Wu, and Cuicui Fu. 2024. "Advances in Aptamer-Based Biosensors for the Detection of Foodborne Mycotoxins" Molecules 29, no. 16: 3974. https://doi.org/10.3390/molecules29163974
APA StyleLi, Y., Zhang, D., Zeng, X., Liu, C., Wu, Y., & Fu, C. (2024). Advances in Aptamer-Based Biosensors for the Detection of Foodborne Mycotoxins. Molecules, 29(16), 3974. https://doi.org/10.3390/molecules29163974






