Broad-Spectrum Legionaminic Acid-Specific Antibodies in Pooled Human IgGs Revealed by Glycan Microarrays with Chemoenzymatically Synthesized Nonulosonosides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemoenzymatic Synthesis of Leg5,7Ac2-Glycosides
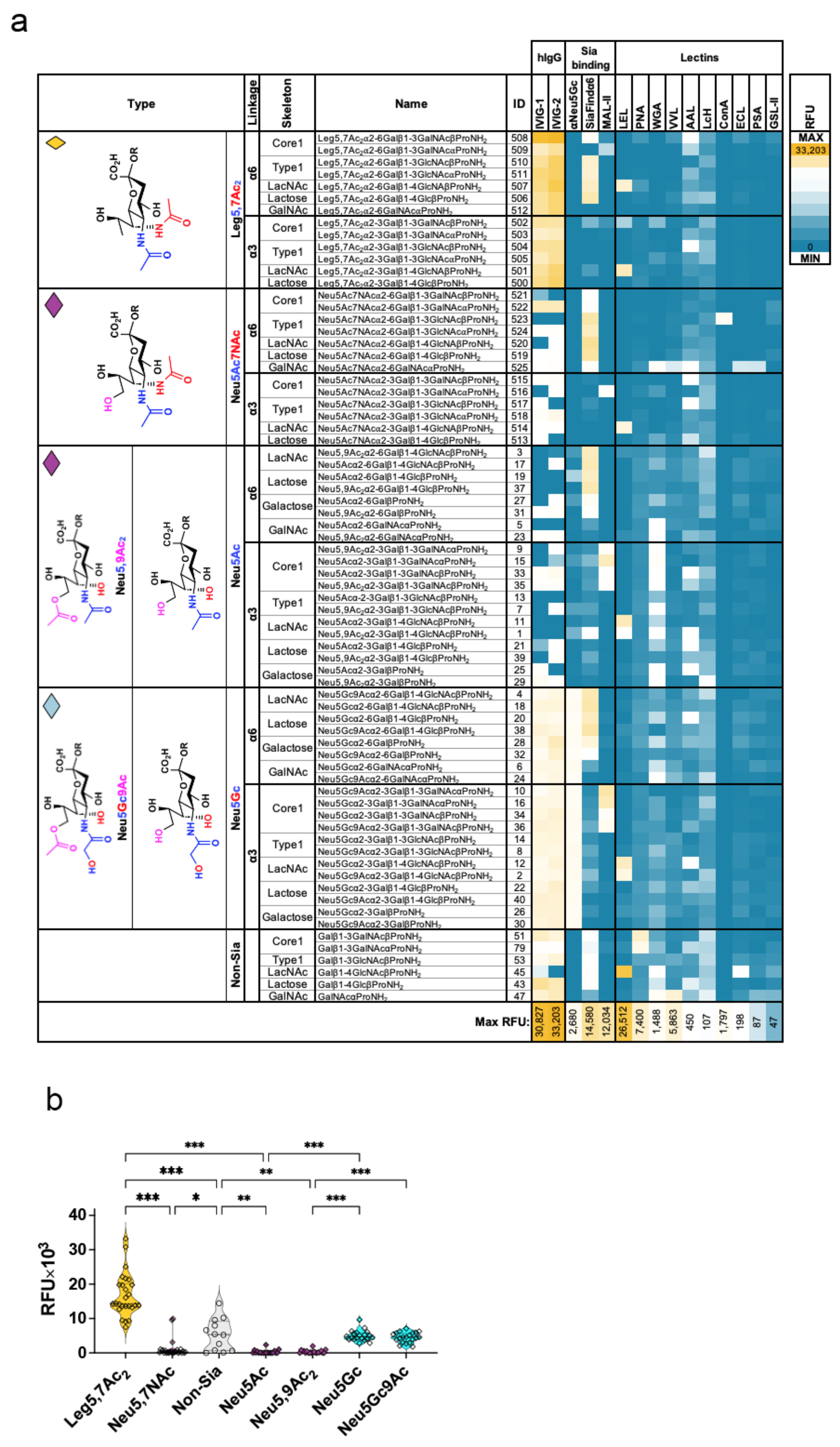
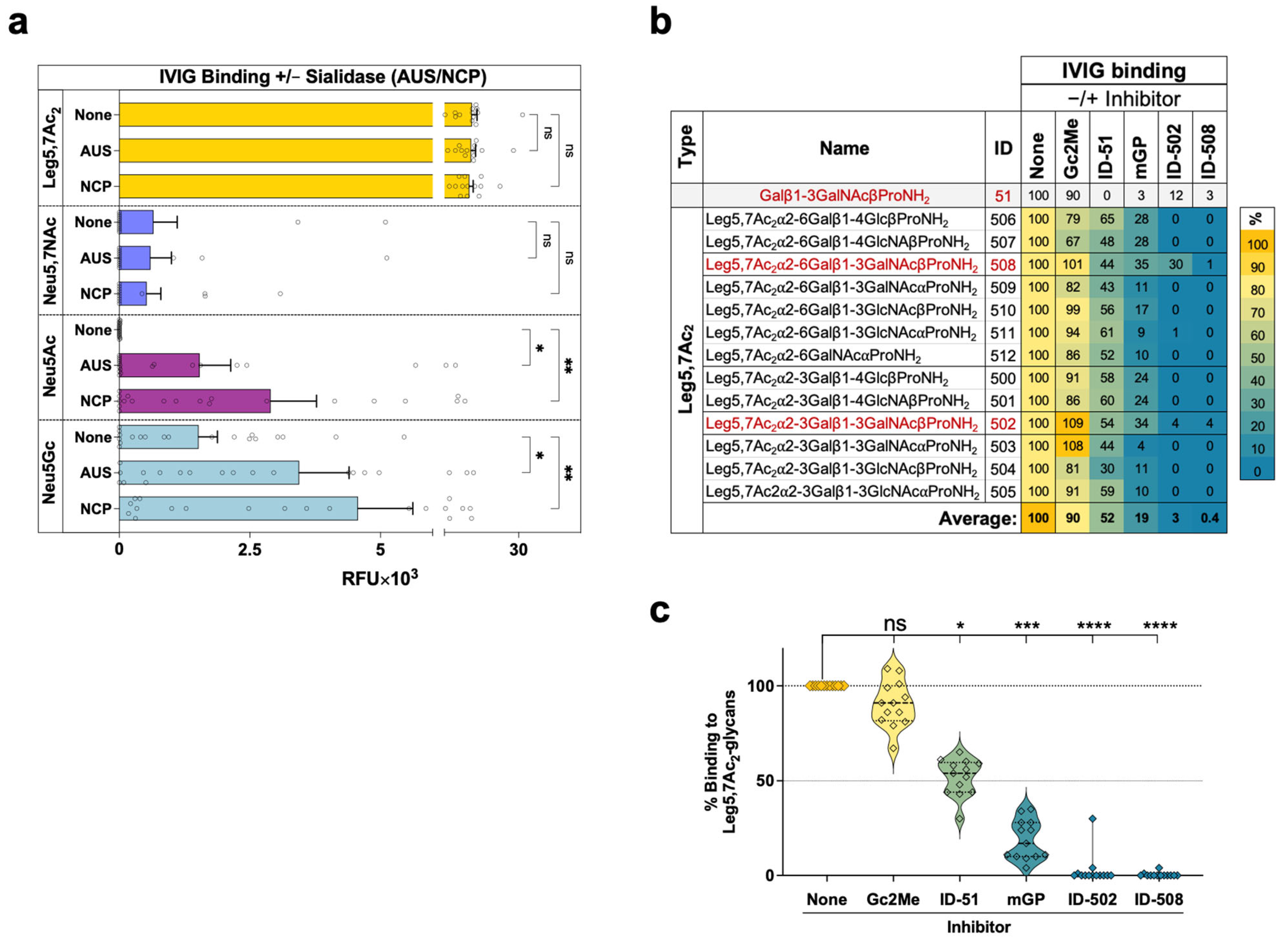
2.2. Glycan Microarray Binding Studies Reveal a Broad Spectrum of Human IgG Antibodies against Leg5,7Ac2-Glycosides
3. Materials and Methods
3.1. Materials and Instruments
3.2. General Procedures for One-Pot Three-Enzyme (OP3E) Preparative-Scale Synthesis of Leg5,7diN3α2–6-Linked Nonulosonosides (12–18)
3.3. General Procedures for One-Pot Three-Enzyme (OP3E) Preparative-Scale Synthesis of Leg5,7diN3α2–3-Linked Nonulosonosides (19–24)
3.4. General Procedures for Synthesizing Leg5,7Ac2-Glycosides (25–37) by Converting the Azido Groups in Leg5,7diN3-Glycosides (12–24) to NHAc Groups
3.5. General Procedures for Converting Cbz-Tagged Glycans to Glycosyl Propylamines
3.6. Glycan Microarray Fabrication
3.7. Glycan Microarray Binding Assays
3.8. Array Slide Processing
3.9. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luetscher, R.N.D.; McKitrick, T.R.; Gao, C.; Mehta, A.Y.; McQuillan, A.M.; Kardish, R.; Boligan, K.F.; Song, X.; Lu, L.; Heimburg-Molinaro, J.; et al. Unique repertoire of anti-carbohydrate antibodies in individual human serum. Sci. Rep. 2020, 10, 15436. [Google Scholar] [CrossRef] [PubMed]
- Geissner, A.; Reinhardt, A.; Rademacher, C.; Johannssen, T.; Monteiro, J.; Lepenies, B.; Thepaut, M.; Fieschi, F.; Mrazkova, J.; Wimmerova, M.; et al. Microbe-focused glycan array screening platform. Proc. Natl. Acad. Sci. USA 2019, 116, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Oyelaran, O.; McShane, L.M.; Dodd, L.; Gildersleeve, J.C. Profiling human serum antibodies with a carbohydrate antigen microarray. J. Proteome Res. 2009, 8, 4301–4310. [Google Scholar] [CrossRef]
- Kappler, K.; Hennet, T. Emergence and significance of carbohydrate-specific antibodies. Genes Immun. 2020, 21, 224–239. [Google Scholar] [CrossRef]
- Marglous, S.; Brown, C.E.; Padler-Karavani, V.; Cummings, R.D.; Gildersleeve, J.C. Serum antibody screening using glycan arrays. Chem. Soc. Rev. 2024, 53, 2603–2642. [Google Scholar] [CrossRef]
- Lewis, A.L.; Chen, X.; Schnaar, R.L.; Varki, A. Sialic acids and other nonulosonic acids. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; pp. 185–204. [Google Scholar]
- Chen, X. Enabling chemoenzymatic strategies and enzymes for synthesizing sialyl glycans and sialyl glycoconjugates. Acc. Chem. Res. 2024, 57, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Knirel, Y.A.; Shashkov, A.S.; Tsvetkov, Y.E.; Jansson, P.E.; Zahringer, U. 5,7-diamino-3,5,7,9-tetradeoxynon-2-ulosonic acids in bacterial glycopolymers: Chemistry and biochemistry. Adv. Carbohydr. Chem. Biochem. 2003, 58, 371–417. [Google Scholar]
- Santra, A.; Xiao, A.; Yu, H.; Li, W.; Li, Y.; Ngo, L.; McArthur, J.B.; Chen, X. A diazido mannose analogue as a chemoenzymatic synthon for synthesizing di-N-acetyllegionaminic acid-containing glycosides. Angew. Chem. Int. Ed. 2018, 57, 2929–2933. [Google Scholar] [CrossRef]
- McArthur, J.B.; Santra, A.; Li, W.; Kooner, A.S.; Liu, Z.; Yu, H.; Chen, X. L. pneumophila CMP-5,7-di-N-acetyllegionaminic acid synthetase (LpCLS)-involved chemoenzymatic synthesis of sialosides and analogues. Org. Biomol. Chem. 2020, 18, 738–744. [Google Scholar] [CrossRef]
- Tsvetkov, Y.E.; Shashkov, A.S.; Knirel, Y.A.; Zahringer, U. Synthesis and identification in bacterial lipopolysaccharides of 5,7-diacetamido-3,5,7,9-tetradeoxy-D-glycero-D-galacto- and -D-glycero-D-talo-non-2-ulosonic acids. Carbohydr. Res. 2001, 331, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, Y.E.; Shashkov, A.S.; Knirel, Y.A.; Zahringer, U. Synthesis and NMR spectroscopy of nine stereoisomeric 5,7-diacetamido-3,5,7,9-tetradeoxynon-2-ulosonic acids. Carbohydr. Res. 2001, 335, 221–243. [Google Scholar] [CrossRef] [PubMed]
- McDonald, N.D.; Boyd, E.F. Structural and biosynthetic diversity of nonulosonic acids (NulOs) that decorate surface structures in bacteria. Trends Microbiol. 2021, 29, 142–157. [Google Scholar] [CrossRef]
- Pradhan, K.; Kulkarni, S.S. Synthesis of nonulosonic acids. Eur. J. Org. Chem. 2020, 2020, 6819–6830. [Google Scholar] [CrossRef]
- Matthies, S.; Stallforth, P.; Seeberger, P.H. Total synthesis of legionaminic acid as basis for serological studies. J. Am. Chem. Soc. 2015, 137, 2848–2851. [Google Scholar] [CrossRef] [PubMed]
- Gintner, M.; Yoneda, Y.; Schmolzer, C.; Denner, C.; Kahlig, H.; Schmid, W. A versatile de novo synthesis of legionaminic acid and 4-epi-legionaminic acid starting from D-serine. Carbohydr. Res. 2019, 474, 34–42. [Google Scholar] [CrossRef]
- Siyabalapitiya Arachchige, S.; Crich, D. Syntheses of legionaminic acid, pseudaminic acid, acetaminic acid, 8-epi-acetaminic acid, and 8-epi-legionaminic acid glycosyl donors from N-acetylneuraminic acid by side chain exchange. Org. Lett. 2022, 24, 2998–3002. [Google Scholar] [CrossRef] [PubMed]
- Popik, O.; Dhakal, B.; Crich, D. Stereoselective synthesis of the equatorial glycosides of legionaminic acid. J. Org. Chem. 2017, 82, 6142–6152. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Buda, S.; Crich, D. Stereoselective synthesis of 5-epi-alpha-sialosides related to the pseudaminic acid glycosides. Reassessment of the stereoselectivity of the 5-azido-5-deacetamidosialyl thioglycosides and use of triflate as nucleophile in the Zbiral deamination of sialic acids. J. Org. Chem. 2016, 81, 10617–10630. [Google Scholar]
- Glaze, P.A.; Watson, D.C.; Young, N.M.; Tanner, M.E. Biosynthesis of CMP-N,N’-diacetyllegionaminic acid from UDP-N,N’-diacetylbacillosamine in Legionella pneumophila. Biochemistry 2008, 47, 3272–3282. [Google Scholar] [CrossRef] [PubMed]
- Schoenhofen, I.C.; Vinogradov, E.; Whitfield, D.M.; Brisson, J.R.; Logan, S.M. The CMP-legionaminic acid pathway in Campylobacter: Biosynthesis involving novel GDP-linked precursors. Glycobiology 2009, 19, 715–725. [Google Scholar] [CrossRef]
- Hassan, M.I.; Lundgren, B.R.; Chaumun, M.; Whitfield, D.M.; Clark, B.; Schoenhofen, I.C.; Boddy, C.N. Total biosynthesis of legionaminic acid, a bacterial sialic acid analogue. Angew. Chem. Int. Ed. 2016, 55, 12018–12021. [Google Scholar] [CrossRef]
- Schoenhofen, I.C.; Young, N.M.; Gilbert, M. Biosynthesis of legionaminic scid and its incorporation into glycoconjugates. Methods Enzymol. 2017, 597, 187–207. [Google Scholar]
- Meng, X.; Boons, G.J.; Wosten, M.; Wennekes, T. Metabolic labeling of legionaminic acid in flagellin glycosylation of Campylobacter jejuni identifies Maf4 as a putative legionaminyl transferase. Angew. Chem. Int. Ed. 2021, 60, 24811–24816. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chokhawala, H.; Karpel, R.; Yu, H.; Wu, B.; Zhang, J.; Zhang, Y.; Jia, Q.; Chen, X. A multifunctional Pasteurella multocida sialyltransferase: A powerful tool for the synthesis of sialoside libraries. J. Am. Chem. Soc. 2005, 127, 17618–17619. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.C.; Leclerc, S.; Wakarchuk, W.W.; Young, N.M. Enzymatic synthesis and properties of glycoconjugates with legionaminic acid as a replacement for neuraminic acid. Glycobiology 2011, 21, 99–108. [Google Scholar] [CrossRef]
- Watson, D.C.; Wakarchuk, W.W.; Leclerc, S.; Schur, M.J.; Schoenhofen, I.C.; Young, N.M.; Gilbert, M. Sialyltransferases with enhanced legionaminic acid transferase activity for the preparation of analogs of sialoglycoconjugates. Glycobiology 2015, 25, 767–773. [Google Scholar] [CrossRef]
- Watson, D.C.; Wakarchuk, W.W.; Gervais, C.; Durocher, Y.; Robotham, A.; Fernandes, S.M.; Schnaar, R.L.; Young, N.M.; Gilbert, M. Preparation of legionaminic acid analogs of sialo-glycoconjugates by means of mammalian sialyltransferases. Glycoconj. J. 2015, 32, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ghosh, T.; Bai, Y.; Santra, A.; Xiao, A.; Chen, X. A substrate tagging and two-step enzymatic reaction strategy for large-scale synthesis of 2,7-anhydro-sialic acid. Carbohydr. Res. 2019, 479, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kooner, A.S.; Diaz, S.; Yu, H.; Santra, A.; Varki, A.; Chen, X. Chemoenzymatic synthesis of sialosides containing 7-N- or 7,9-di-N-acetyl sialic acid as stable O-acetyl analogues for probing sialic acid-binding proteins. J. Org. Chem. 2021, 86, 14381–14397. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fan, Y.; Ye, J.; Wang, F.; Nie, Q.; Wang, L.; Wang, P.G.; Cao, H.; Cheng, J. Successfully engineering a bacterial sialyltransferase for regioselective α2,6-sialylation. ACS Catal. 2018, 8, 7222–7227. [Google Scholar] [CrossRef]
- Sugiarto, G.; Lau, K.; Qu, J.; Li, Y.; Lim, S.; Mu, S.; Ames, J.B.; Fisher, A.J.; Chen, X. A sialyltransferase mutant with decreased donor hydrolysis and reduced sialidase activities for directly sialylating LewisX. ACS Chem. Biol. 2012, 7, 1232–1240. [Google Scholar] [CrossRef]
- Maki, T.; Tsuritani, T.; Yasukata, T. A mild method for the synthesis of carbamate-protected guanidines using the Burgess reagent. Org. Lett. 2014, 16, 1868–1871. [Google Scholar] [CrossRef]
- Song, X.; Xia, B.; Stowell, S.R.; Lasanajak, Y.; Smith, D.F.; Cummings, R.D. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem. Biol. 2009, 16, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Blixt, O.; Head, S.; Mondala, T.; Scanlan, C.; Huflejt, M.E.; Alvarez, R.; Bryan, M.C.; Fazio, F.; Calarese, D.; Stevens, J.; et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 17033–17038. [Google Scholar] [CrossRef]
- Park, S.; Lee, M.R.; Shin, I. Fabrication of carbohydrate chips and their use to probe protein-carbohydrate interactions. Nat. Protoc. 2007, 2, 2747–2758. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, M.R.; Shin, I. Construction of carbohydrate microarrays by using one-step, direct immobilizations of diverse unmodified glycans on solid surfaces. Bioconjug. Chem. 2009, 20, 155–162. [Google Scholar] [CrossRef] [PubMed]
- de Boer, A.R.; Hokke, C.H.; Deelder, A.M.; Wuhrer, M. General microarray technique for immobilization and screening of natural glycans. Anal. Chem. 2007, 79, 8107–8113. [Google Scholar] [CrossRef]
- Song, X.; Xia, B.; Lasanajak, Y.; Smith, D.F.; Cummings, R.D. Quantifiable fluorescent glycan microarrays. Glycoconj. J. 2008, 25, 15–25. [Google Scholar] [CrossRef]
- Yu, H.; Chokhawala, H.A.; Varki, A.; Chen, X. Efficient chemoenzymatic synthesis of biotinylated human serum albumin-sialoglycoside conjugates containing O-acetylated sialic acids. Org. Biomol. Chem. 2007, 5, 2458–2463. [Google Scholar] [CrossRef]
- Bochner, B.S.; Alvarez, R.A.; Mehta, P.; Bovin, N.V.; Blixt, O.; White, J.R.; Schnaar, R.L. Glycan array screening reveals a candidate ligand for Siglec-8. J. Biol. Chem. 2005, 280, 4307–4312. [Google Scholar] [CrossRef]
- Yu, H.; Huang, S.; Chokhawala, H.; Sun, M.; Zheng, H.; Chen, X. Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural alpha-2,6-linked sialosides: A P. damsela alpha-2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew. Chem. Int. Ed. 2006, 45, 3938–3944. [Google Scholar] [CrossRef]
- Kooner, A.S.; Yu, H.; Chen, X. Synthesis of N-glycolylneuraminic scid (Neu5Gc) and its glycosides. Front. Immunol. 2019, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.L.; Padler-Karavani, V.; Ghaderi, D.; Hurtado-Ziola, N.; Yu, H.; Chen, X.; Brinkman-Van der Linden, E.C.; Varki, A.; Varki, N.M. Sensitive and specific detection of the non-human sialic scid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS ONE 2009, 4, e4241. [Google Scholar] [CrossRef] [PubMed]
- Leviatan Ben-Arye, S.; Yu, H.; Chen, X.; Padler-Karavani, V. Profiling anti-Neu5Gc IgG in human sera with a sialoglycan microarray assay. J. Vis. Exp. 2017, 125, e56094. [Google Scholar] [CrossRef]
- Leviatan Ben-Arye, S.; Schneider, C.; Yu, H.; Bashir, S.; Chen, X.; von Gunten, S.; Padler-Karavani, V. Differential recognition of diet-derived Neu5Gc-neoantigens on glycan microarrays by carbohydrate-specific pooled human IgG and IgA antibodies. Bioconjug. Chem. 2019, 30, 1565–1574. [Google Scholar] [CrossRef]
- Varki, A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology 2011, 21, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Kooner, A.S.; Yuan, Y.; Yu, H.; Kang, H.; Klenow, L.; Daniels, R.; Chen, X. Sialosides containing 7-N-acetyl sialic acid are selective substrates for neuraminidases from influenza A viruses. ACS Infect. Dis. 2023, 9, 33–41. [Google Scholar] [CrossRef]
- Zebian, N.; Merkx-Jacques, A.; Pittock, P.P.; Houle, S.; Dozois, C.M.; Lajoie, G.A.; Creuzenet, C. Comprehensive analysis of flagellin glycosylation in Campylobacter jejuni NCTC 11168 reveals incorporation of legionaminic acid and its importance for host colonization. Glycobiology 2016, 26, 386–397. [Google Scholar] [CrossRef]
- Varki, A.; Gagneux, P. Multifarious roles of sialic acids in immunity. Ann. N. Y. Acad. Sci. 2012, 1253, 16–36. [Google Scholar] [CrossRef]
- Gulati, S.; Schoenhofen, I.C.; Lindhout-Djukic, T.; Lewis, L.A.; Moustafa, I.Y.; Saha, S.; Zheng, B.; Nowak, N.; Rice, P.A.; Varki, A.; et al. Efficacy of antigonococcal CMP-nonulosonate therapeutics require cathelicidins. J. Infect. Dis. 2020, 222, 1641–1650. [Google Scholar] [CrossRef]
- Gulati, S.; Schoenhofen, I.C.; Whitfield, D.M.; Cox, A.D.; Li, J.; St Michael, F.; Vinogradov, E.V.; Stupak, J.; Zheng, B.; Ohnishi, M.; et al. Utilizing CMP-sialic acid analogs to unravel Neisseria gonorrhoeae lipooligosaccharide-mediated complement resistance and design novel therapeutics. PLoS Pathog. 2015, 11, e1005290. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Schoenhofen, I.C.; Lindhout-Djukic, T.; Schur, M.J.; Landig, C.S.; Saha, S.; Deng, L.; Lewis, L.A.; Zheng, B.; Varki, A.; et al. Therapeutic CMP-nonulosonates against multidrug-resistant Neisseria gonorrhoeae. J. Immunol. 2020, 204, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Amon, R.; Grant, O.C.; Leviatan Ben-Arye, S.; Makeneni, S.; Nivedha, A.K.; Marshanski, T.; Norn, C.; Yu, H.; Glushka, J.N.; Fleishman, S.J.; et al. A combined computational-experimental approach to define the structural origin of antibody recognition of sialyl-Tn, a tumor-associated carbohydrate antigen. Sci. Rep. 2018, 8, 10786. [Google Scholar] [CrossRef] [PubMed]
- Borenstein-Katz, A.; Warszawski, S.; Amon, R.; Eilon, M.; Cohen-Dvashi, H.; Leviatan Ben-Arye, S.; Tasnima, N.; Yu, H.; Chen, X.; Padler-Karavani, V.; et al. Biomolecular recognition of the glycan neoantigen CA19-9 by distinct antibodies. J. Mol. Biol. 2021, 433, 167099. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, H.; Cao, H.; Lau, K.; Muthana, S.; Tiwari, V.K.; Son, B.; Chen, X. Pasteurella multocida sialic acid aldolase: A promising biocatalyst. Appl. Microbiol. Biotechnol. 2008, 79, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, H.; Karpel, R.; Chen, X. Chemoenzymatic synthesis of CMP-sialic acid derivatives by a one-pot two-enzyme system: Comparison of substrate flexibility of three microbial CMP-sialic acid synthetases. Bioorg. Med. Chem. 2004, 12, 6427–6435. [Google Scholar] [CrossRef]
- Padler-Karavani, V.; Song, X.; Yu, H.; Hurtado-Ziola, N.; Huang, S.; Muthana, S.; Chokhawala, H.A.; Cheng, J.; Verhagen, A.; Langereis, M.A.; et al. Cross-comparison of protein recognition of sialic acid diversity on two novel sialoglycan microarrays. J. Biol. Chem. 2012, 287, 22593–22608. [Google Scholar] [CrossRef]
- Bashir, S.; Leviatan Ben Arye, S.; Reuven, E.M.; Yu, H.; Costa, C.; Galinanes, M.; Bottio, T.; Chen, X.; Padler-Karavani, V. Presentation mode of glycans affect recognition of human serum anti-Neu5Gc IgG antibodies. Bioconjug. Chem. 2019, 30, 161–168. [Google Scholar] [CrossRef]
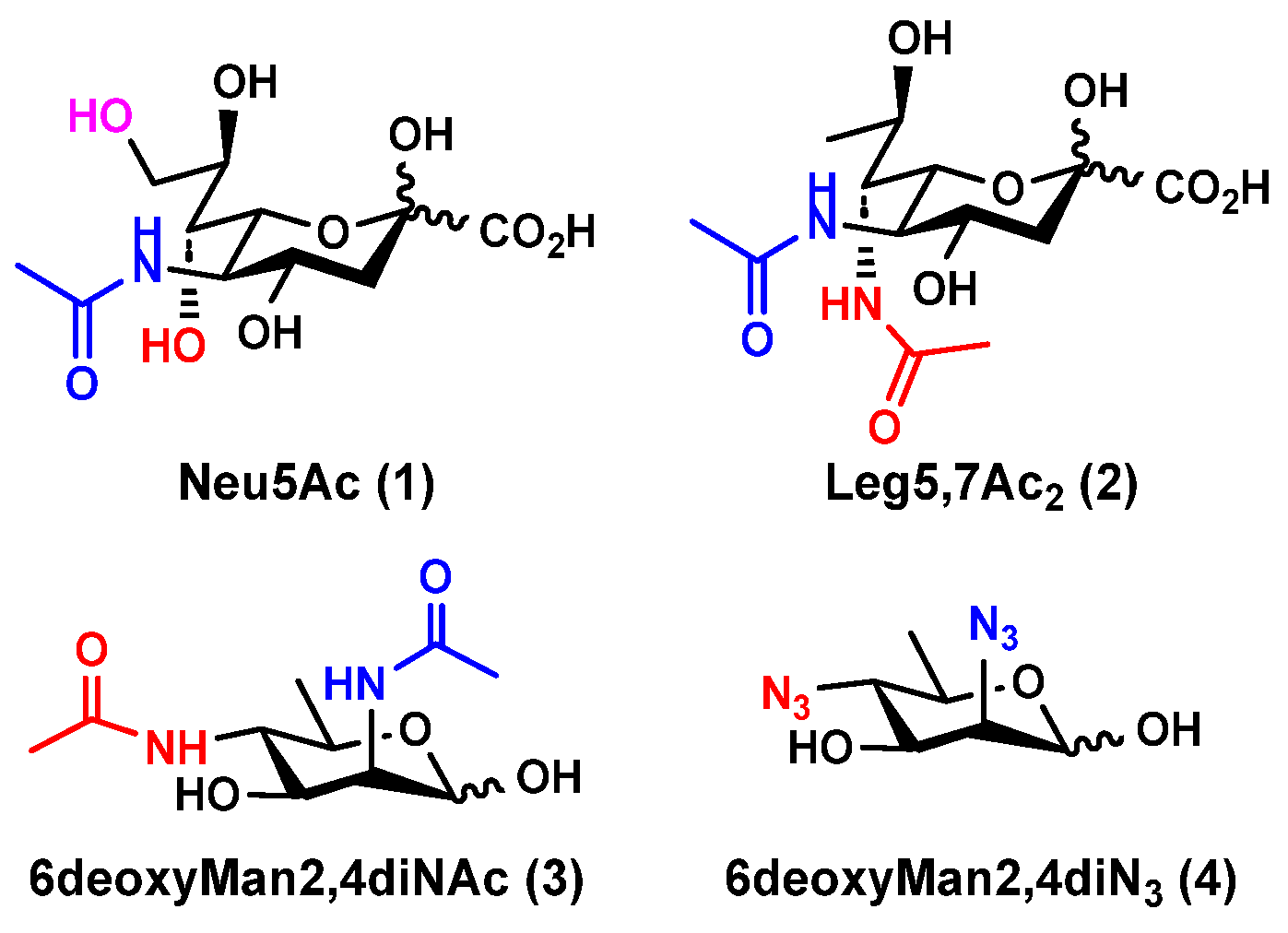
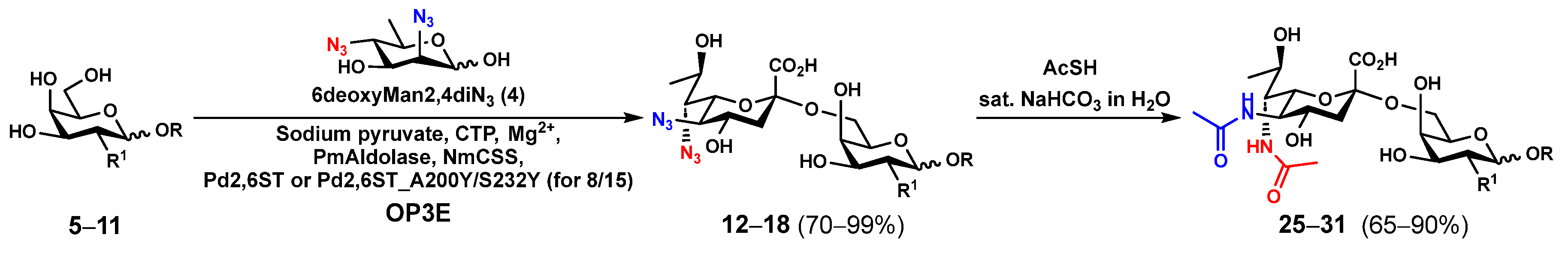
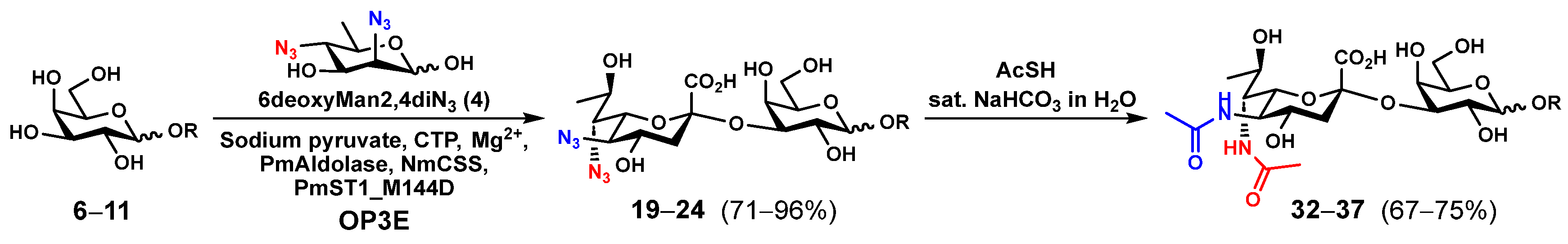
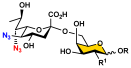
Acceptors (5–11)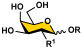 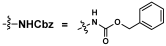 | OP3E Products Leg5,7diN3-glycosides (12–24) | Leg5,7Ac2-glycosides (25–37) | ||
 12–18 (70–99%) |  19–24 (71–96%) |  25–31 (65–90%) |  32–37 (67–75%) | |
 GalNAcαProNHCbz (5) R = αProNHCbz, R1 = NHAc | 12 (99%) | - | 25 (65%) | - |
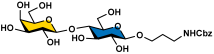 LacβProNHCbz (6) LacβProNHCbz (6)R = β4GlcβProNHCbz, R1 = OH | 13 (75%) | 19 (74%) | 26 (78%) | 32 (68%) |
 LacNAcβProNHCbz (7) LacNAcβProNHCbz (7)R = β4GlcNAcβProNHCbz, R1 = OH | 14 (70%) | 20 (71%) | 27 (68%) | 33 (67%) |
 Galβ3GalNAcβProNHCbz (8) Galβ3GalNAcβProNHCbz (8)R = β3GalNAcβProNHCbz, R1 = OH | 15 (93%) | 21 (96%) | 28 (90%) | 34 (67%) |
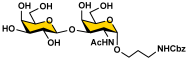 Galβ3GalNAcαProNHCbz (9) Galβ3GalNAcαProNHCbz (9)R = β3GalNAcαProNHCbz, R1 = OH | 16 (77%) | 22 (77%) | 29 (68%) | 35 (69%) |
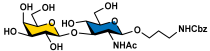 Galβ3GlcNAcβProNHCbz (10) Galβ3GlcNAcβProNHCbz (10)R = β3GlcNAcβProNHCbz, R1 = OH | 17 (81%) | 23 (80%) | 30 (78%) | 36 (75%) |
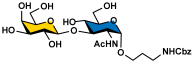 Galβ3GlcNAcαProNHCbz (11) Galβ3GlcNAcαProNHCbz (11)R = β3GlcNAcαProNHCbz, R1 = OH | 18 (99%) | 24 (96%) | 31 (71%) | 37 (72%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kooner, A.S.; Yu, H.; Leviatan Ben-Arye, S.; Padler-Karavani, V.; Chen, X. Broad-Spectrum Legionaminic Acid-Specific Antibodies in Pooled Human IgGs Revealed by Glycan Microarrays with Chemoenzymatically Synthesized Nonulosonosides. Molecules 2024, 29, 3980. https://doi.org/10.3390/molecules29163980
Kooner AS, Yu H, Leviatan Ben-Arye S, Padler-Karavani V, Chen X. Broad-Spectrum Legionaminic Acid-Specific Antibodies in Pooled Human IgGs Revealed by Glycan Microarrays with Chemoenzymatically Synthesized Nonulosonosides. Molecules. 2024; 29(16):3980. https://doi.org/10.3390/molecules29163980
Chicago/Turabian StyleKooner, Anoopjit Singh, Hai Yu, Shani Leviatan Ben-Arye, Vered Padler-Karavani, and Xi Chen. 2024. "Broad-Spectrum Legionaminic Acid-Specific Antibodies in Pooled Human IgGs Revealed by Glycan Microarrays with Chemoenzymatically Synthesized Nonulosonosides" Molecules 29, no. 16: 3980. https://doi.org/10.3390/molecules29163980
APA StyleKooner, A. S., Yu, H., Leviatan Ben-Arye, S., Padler-Karavani, V., & Chen, X. (2024). Broad-Spectrum Legionaminic Acid-Specific Antibodies in Pooled Human IgGs Revealed by Glycan Microarrays with Chemoenzymatically Synthesized Nonulosonosides. Molecules, 29(16), 3980. https://doi.org/10.3390/molecules29163980






