Evaluation of Saffron Quality Using Rapid Quantitative Inspection Technology with Near-Infrared Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Statistical Data Used for Predictive Modeling
2.2. Original Near-Infrared Spectra of Saffron
2.3. Division of Sample Set
2.4. Spectral Data Preprocessing
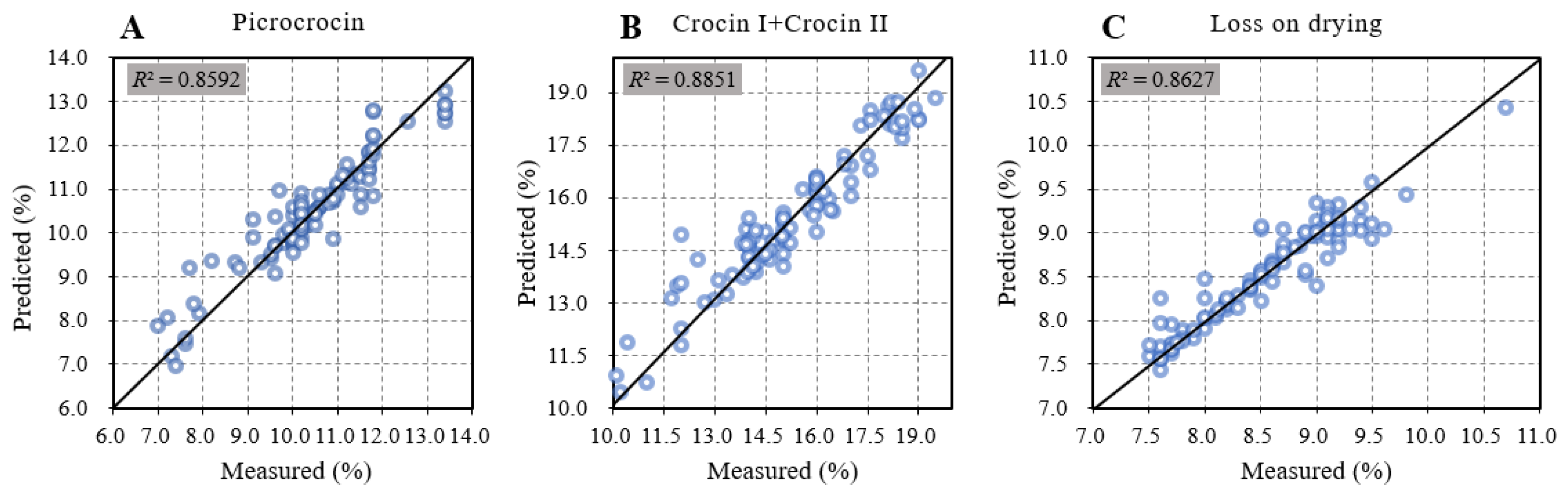
2.5. Model Verification and Evaluation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Screening of the Chromatographic Elution Program
3.3. Saffron Sample Collection and Processing
3.4. LD Experiment with Saffron Samples
3.5. TCCC and CP Determination
3.6. Statistical Analysis
3.7. Near-Infrared Spectra Preprocessing Methods
3.8. Selection of Characteristic Spectral Variables
3.9. Modeling Methods and Model Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Pharmacopoeia Committee of People’s Republic of China. Pharmacopeia of Pharmacopeia of People’s Republic of China, 11th ed.; China Medical Science Press: Beijing, China, 2020; Volume I, p. 134.
- European Directorate for the Quality of Medicines and HealthCare. European Pharmacopoeia, 8th ed.; European Directorate for the Quality of Medicines and HealthCare: Strasbourg, France, 2014. [Google Scholar]
- ISO 3632-1; Technical Specification. Saffron (Crocus sativus L.), Part 1 (Specification) and Part 2 (Test Methods). International Organization for Standardization (ISO): Geneva, Switzerland, 2011.
- The Editorial Committee of the Japanese Pharmacopoeia Japanese Pharmacopoeia; Ministry of Welfare: Tokyo, Japan, 2011.
- Ruan, Z.G.; Li, B. The Principle and Application of Near-Infrared Spectroscopy for the Chinese Herbal Medicines. China Tradit. Pat. Med. 2011, 31, 408–417. [Google Scholar]
- Huang, Z.W.; Guo, T.; Huang, W.J.; Li, B.; Xu, H.R.; Ye, C.X.; Yan, S.K.; Xiao, X.; Luo, G.A. Research Progress of Near-Infrared Spectroscopy in Quality Evaluation of Valuable Chinese Medicinal Materials. Chin. Tradit. Herb. Drugs 2022, 53, 6328–6336. [Google Scholar]
- Chu, X.L.; Chen, P.; Li, J.Y.; Liu, D.; Xu, Y.P. Progresses and Perspectives of Near Infrared Spectroscopy Analytical Technology. J. Instrum. Anal. 2020, 39, 1181–1188. [Google Scholar]
- Liu, X.; Han, L.J. Feasibility of Using Near-Infrared Reflectance Spectroscopy for the Analysis of Compositions of Straw Silage. Spectrosc. Spectr. Anal. 2006, 26, 2016–2020. [Google Scholar]
- Raina, A.; Kaul, S.; Dhar, M.K. Sniffing Out Adulteration in Saffron: Detection Methods and Health Risks. Food Control 2024, 155, 110042. [Google Scholar] [CrossRef]
- Yang, W.H.; Hao, J.W.; Huang, H.P.; Chen, N.D.; Qin, C.F.; Liu, D.; Zhu, F.C.; Chen, N.F. Rapid Determination of Polysaccharide in Pteridium aquilinum by Near Infrared Diffuse Reflectance Spectroscopy. Chin. J. Mod. Appl. Pharm. 2023, 40, 597–602. [Google Scholar]
- Qiu, J.; Zhang, A.H.; Li, J.L.; Xiao, Z.H.; Li, C.Z.; Lai, P.Y. Quantitative Models Study on Rapid Detection of Main Quality Indexes of Litsea cubeba Fruits by Near-infrared Spectroscopy. J. Chin. Cereal Oil 2023, 38, 165–170. [Google Scholar]
- Zhang, Y.Z.; Zhang, L.B.; Wang, J.; Tang, X.X.; Wu, H.; Wang, M.H.; Zeng, W.; Zeng, W.; Mo, Q.H.; Li, Y.Q.; et al. Rapid Determination of the Oil and Moisture Contents in Camellia gauchowensis Chang and Camellia semiserrata Chi Seeds Kernels by Near-Infrared Reflectance Spectroscopy. Molecules 2018, 23, 2332. [Google Scholar] [CrossRef]
- Yang, N.L.; Cheng, Y.Y.; Qu, H.B. Quantitative Determination of Mannitol in Cordyceps inensis Using Near Infrared Spectroscopy and Artificial Neural Networks. Chin. J. Anal. Chem. 2003, 31, 664–668. [Google Scholar]
- Hu, X.; Zhang, C.; Zhang, Y.H. Study Crocin-II and Total Glucosidenear in Croci Stigma by Near Infrared Spectroscopy. Chin. J. Anal. Chem. 2003, 221, 664–668. [Google Scholar]
- Liu, J.; Yang, Y.J.; Wang, W.Y.; Lv, X.N.; Li, M.; Dai, D. Determination of Loureirin B, Loureirin A in China Domestic Resina Draconis Using Near Infrared Diffuse Reflectance Spectroscopy. Tradit. Chin. Drug Res. Clin. Phar. 2015, 26, 102–105. [Google Scholar]
- Wang, C.Y.; Qi, J.W.; An, X.P.; Liu, N.; Wang, Y.; Wang, B.Y. Rapid Prediction of Reducing Sugar and Soluble Protein Content in Fermented Bran Based on Nir Quantitative Analysis Model. J. Chin. Agr. Univ. 2024, 29, 190–203. [Google Scholar]
- Zhao, X.; Meng, X.M.; Fan, W.X.; Li, Y.R.; Song, Y.H.; Wang, J. A Quantitative Model for Rapid Detection of Main Quality Indicators of Peanut Kernels Using Near-Infrared Spectroscopy. J. Hebei Agric. Univ. 2024, 47, 25–30+65. [Google Scholar]
- Nie, P.C.; Wu, D.; Sun, D.W.; Cao, F.; Bao, Y.D.; He, Y. Potential of Visible and Near Infrared Spectroscopy and Pattern Recognition for Rapid Quantification of Notoginseng Powder with Adulterants. Sensors 2013, 13, 13820–13834. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.L.; Chen, R.Z.; Cheng, K.; Lu, X.Y. Rapid Quantitative Determination of Fritillaria thunbergii Miq Maxed into Fritillaria cirrhosa D. Don by Near-Infrared Diffuse Reflectance Spectroscopy. Chin. J. Pharm. Anal. 2005, 25, 150–152. [Google Scholar]
- Hao, J.W.; Chen, Y.; Chen, N.D.; Qin, C.F. Rapid Detection of Adulterationin Dendrobium huoshanense Using Nir Spectroscopy Coupled with Chemometric Methods. J. AOAC Int. 2021, 104, 854–859. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Qin, X.; Wei, J.B.; Wei, H.Y.; Ye, S.Y.; Su, Z.H.; Liang, Y.H. Consistency Evaluation and Quantitative Analysis of Smilax glabra Formula Granules Based on Portable Near Infrared Spectrometer. J. Guangxi Med. Univ. 2024, 41, 590–597. [Google Scholar]
- Wang, Y.F.; Wei, L.G.; Wang, H.L.; Zhu, Z.Y.; Yang, J.L. Research on Rapid Determination of Moisture Content in Honey-Processed Asteris Radix et Rhizoma. Mod. Chin. Med. 2024, 26, 1053–1058. [Google Scholar]
- An, S.Y.; Zhang, L.; Shang, X.Z.; Yue, H.S.; Liu, W.Y.; Ju, A.C. Variable Selection Method in the NIR Quantitative Analysis Model of Total Saponins in Red Ginseng Extract. Spectrosc. Spect. Anal. 2021, 41, 206–209. [Google Scholar]
- Gupta, V.; Jamwal, G.; Rai, G.K.; Gupta, S.K.; Shukla, R.M.; Dadrwal, B.K.; Prabhakar, P.; Tripathy, S.; Rajpoot, S.K.; Singh, A.K.; et al. Biosynthesis of Biomolecules from Saffron as An Industrial Crop and Their Regulation, with Emphasis on the Chemistry, Extraction Methods, Identification Techniques, and Potential Applications in Human Health and Food: A Critical Comprehensive Review. Biocatal. Agric. Biotechnol. 2024, 59, 103260. [Google Scholar] [CrossRef]
- Sheng, X.H.; Li, Z.M.; Li, Z.W.; Zhu, T.T.; Wang, J. Quantification of Fruit Juice Content in Fruity Beer by Near-Infrared Spectroscopy. Food Ferment. 2020, 46, 247–252. [Google Scholar]

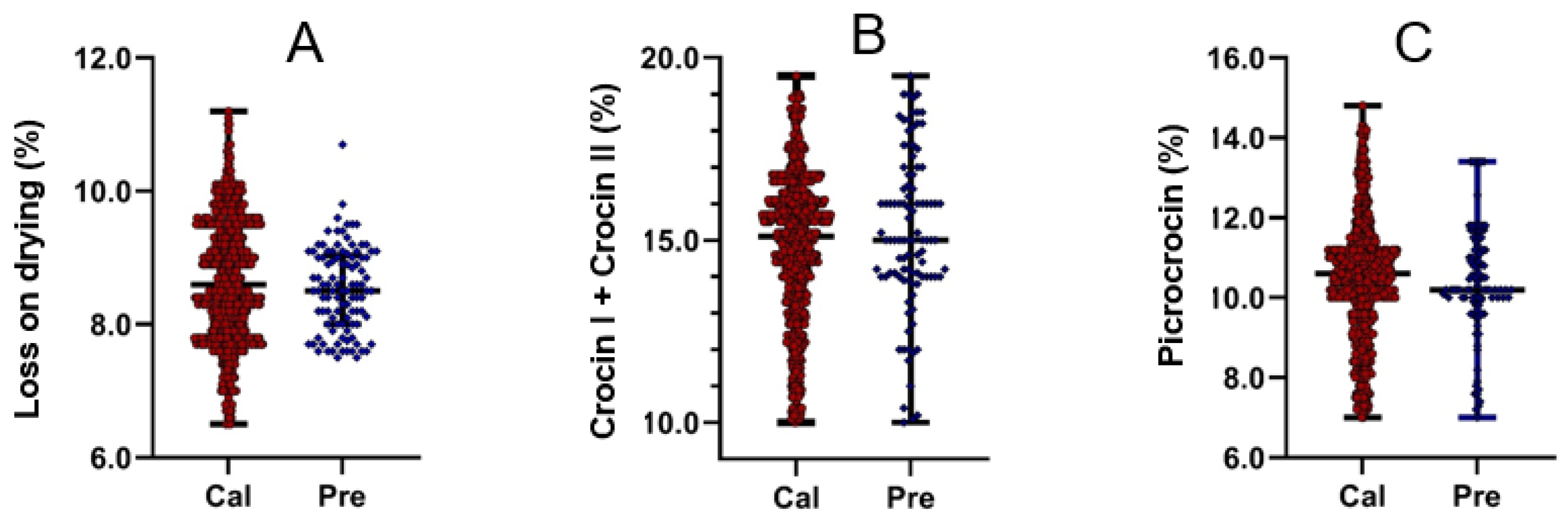

| Parameter | LD | TCCC | CP | |||
|---|---|---|---|---|---|---|
| Calibration Set | Prediction Set | Calibration Set | Prediction Set | Calibration Set | Prediction Set | |
| Max (%) | 11.2 | 10.7 | 19.5 | 19.5 | 14.8 | 13.4 |
| Min (%) | 6.4 | 7.5 | 10.0 | 10.0 | 7.0 | 7.0 |
| Average (%) | 8.65 | 8.53 | 14.86 | 15.2 | 10.54 | 10.41 |
| Standard deviation | 0.91 | 0.63 | 2.00 | 2.17 | 1.46 | 1.39 |
| Variance | 0.84 | 0.4 | 4.00 | 4.75 | 2.13 | 1.94 |
| Pretreatment a | Index | R | SEC | SECV | RPD |
|---|---|---|---|---|---|
| Method 1 | LD | 0.95 | 0.2994 | 0.3201 | 3.203 |
| TCCC | 0.87 | 1.0289 | 1.1149 | 2.028 | |
| CP | 0.89 | 0.6924 | 0.7627 | 2.193 | |
| Method 2 | LD | 0.96 | 0.2542 | 0.2763 | 3.571 |
| TCCC | 0.90 | 0.8687 | 0.9859 | 2.294 | |
| CP | 0.91 | 0.6213 | 0.6836 | 2.412 | |
| Method 3 | LD | 0.95 | 0.2962 | 0.3250 | 3.203 |
| TCCC | 0.86 | 1.0299 | 1.1030 | 3.203 | |
| CP | 0.86 | 0.7617 | 0.8186 | 1.960 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, H.; Sheng, X.; Wang, R.; Yao, Y.; Zhu, Q.; Yi, Z.; Xu, Z.; Wang, Y.; Zheng, C.; et al. Evaluation of Saffron Quality Using Rapid Quantitative Inspection Technology with Near-Infrared Spectroscopy. Molecules 2024, 29, 3983. https://doi.org/10.3390/molecules29173983
Zhou Y, Zhang H, Sheng X, Wang R, Yao Y, Zhu Q, Yi Z, Xu Z, Wang Y, Zheng C, et al. Evaluation of Saffron Quality Using Rapid Quantitative Inspection Technology with Near-Infrared Spectroscopy. Molecules. 2024; 29(17):3983. https://doi.org/10.3390/molecules29173983
Chicago/Turabian StyleZhou, Ying, Han Zhang, Xiaohui Sheng, Rong Wang, Yao Yao, Qinglan Zhu, Ze Yi, Zhe Xu, Yi Wang, Cheng Zheng, and et al. 2024. "Evaluation of Saffron Quality Using Rapid Quantitative Inspection Technology with Near-Infrared Spectroscopy" Molecules 29, no. 17: 3983. https://doi.org/10.3390/molecules29173983






