The n-Butanol Extract Obtained from the Inner Bark of Tabebuia rosea (Bertol.) DC, Specioside, and Catalposide Induce Leukemia Cell Apoptosis in the Presence of Apicidin
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis
2.2. Effect of the n-Butanol Extract on Cell Viability
2.3. Selectivity Index
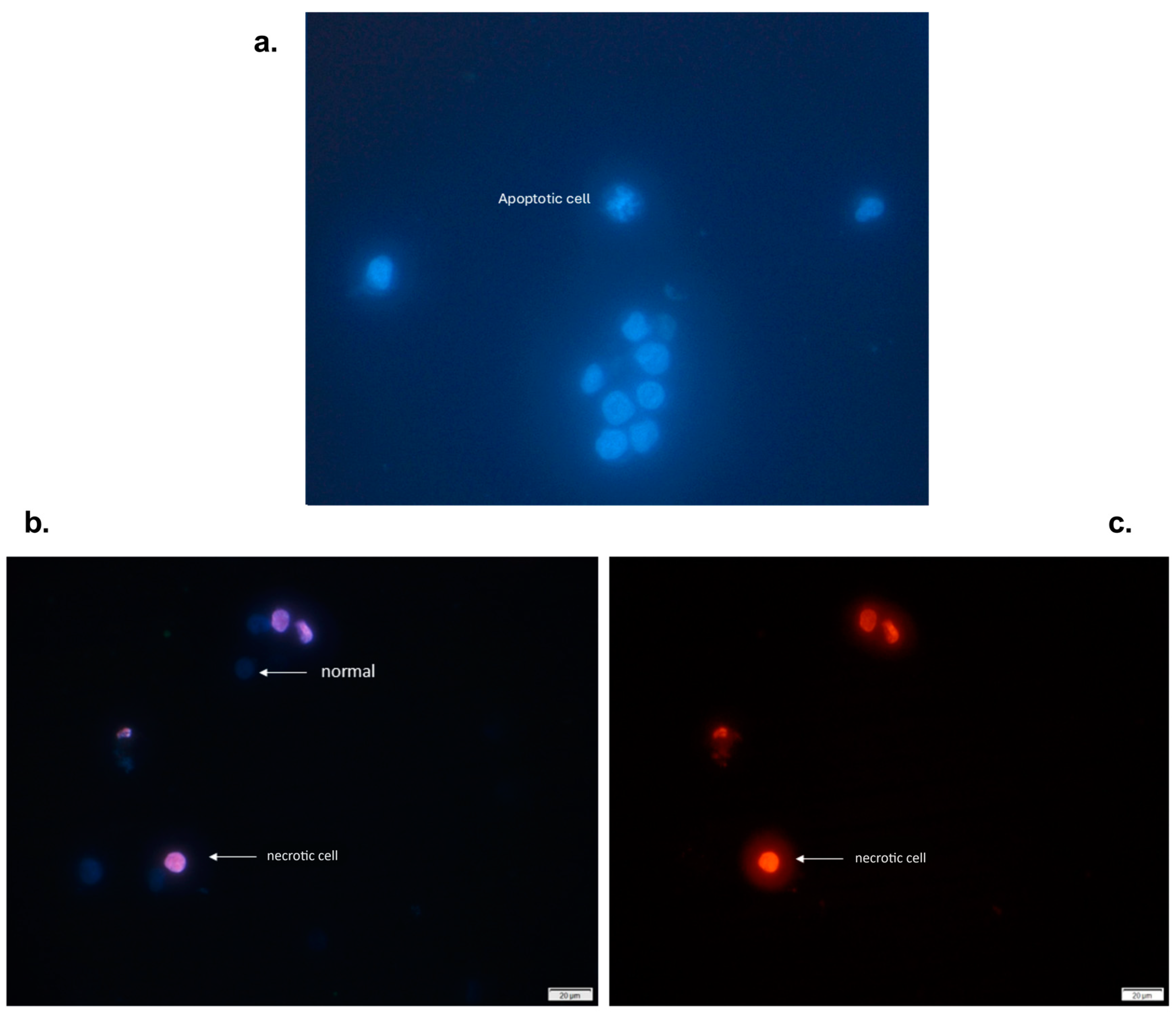
2.4. Nuclear Morphology of Cells Treated with an n-Butanol Extract from the Inner Bark of Tabebuia rosea and Pretreated with Apicidin
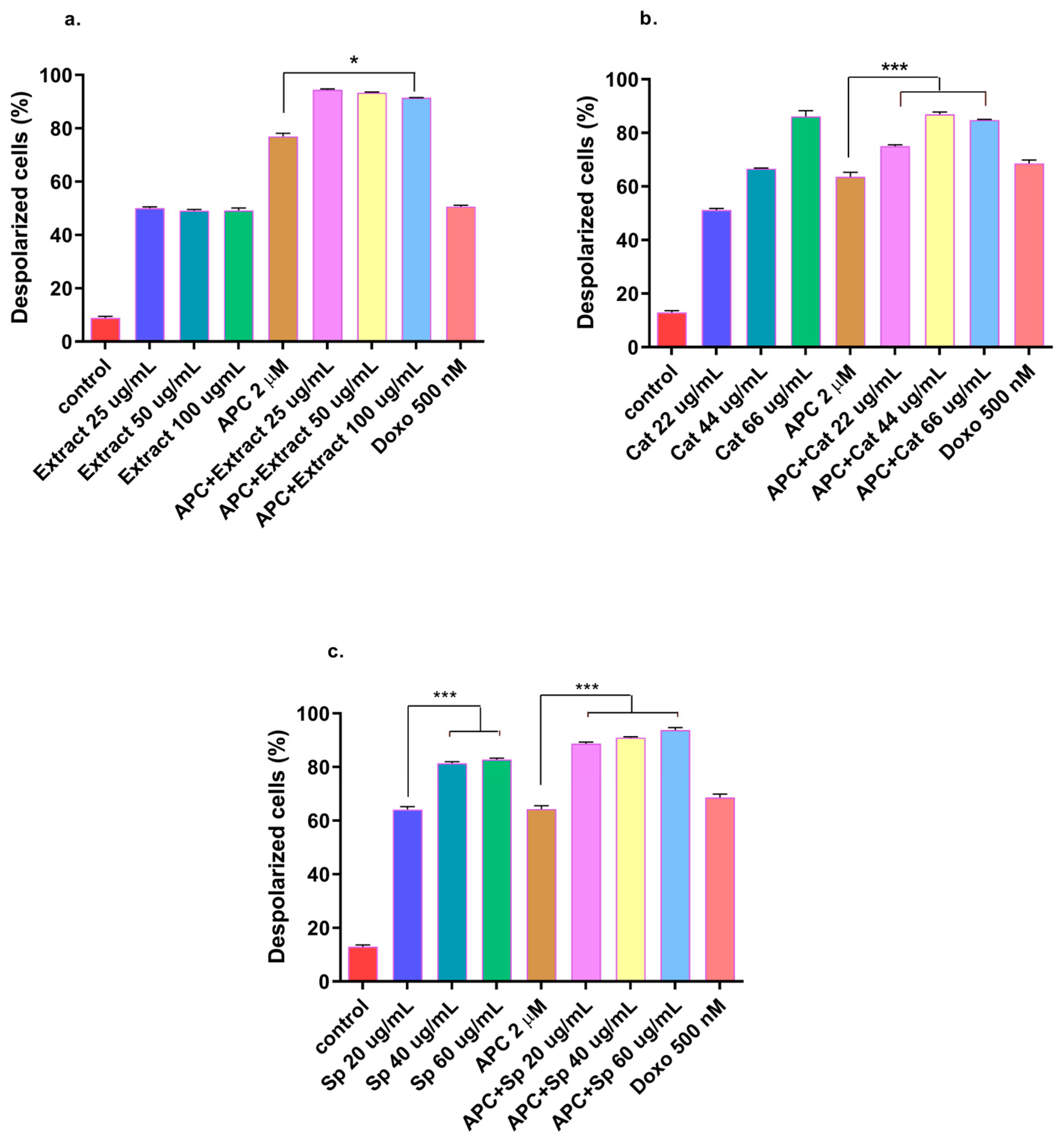
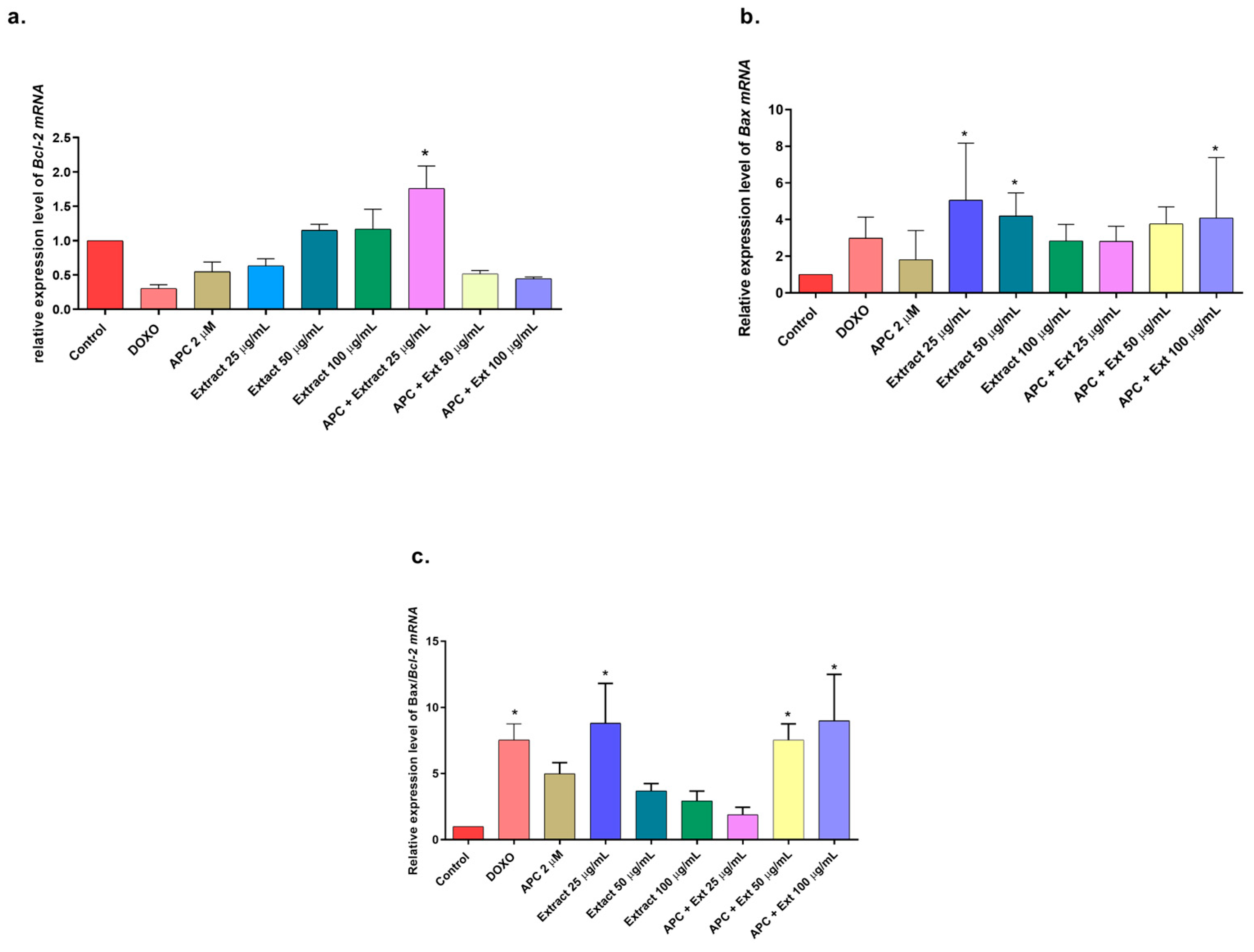
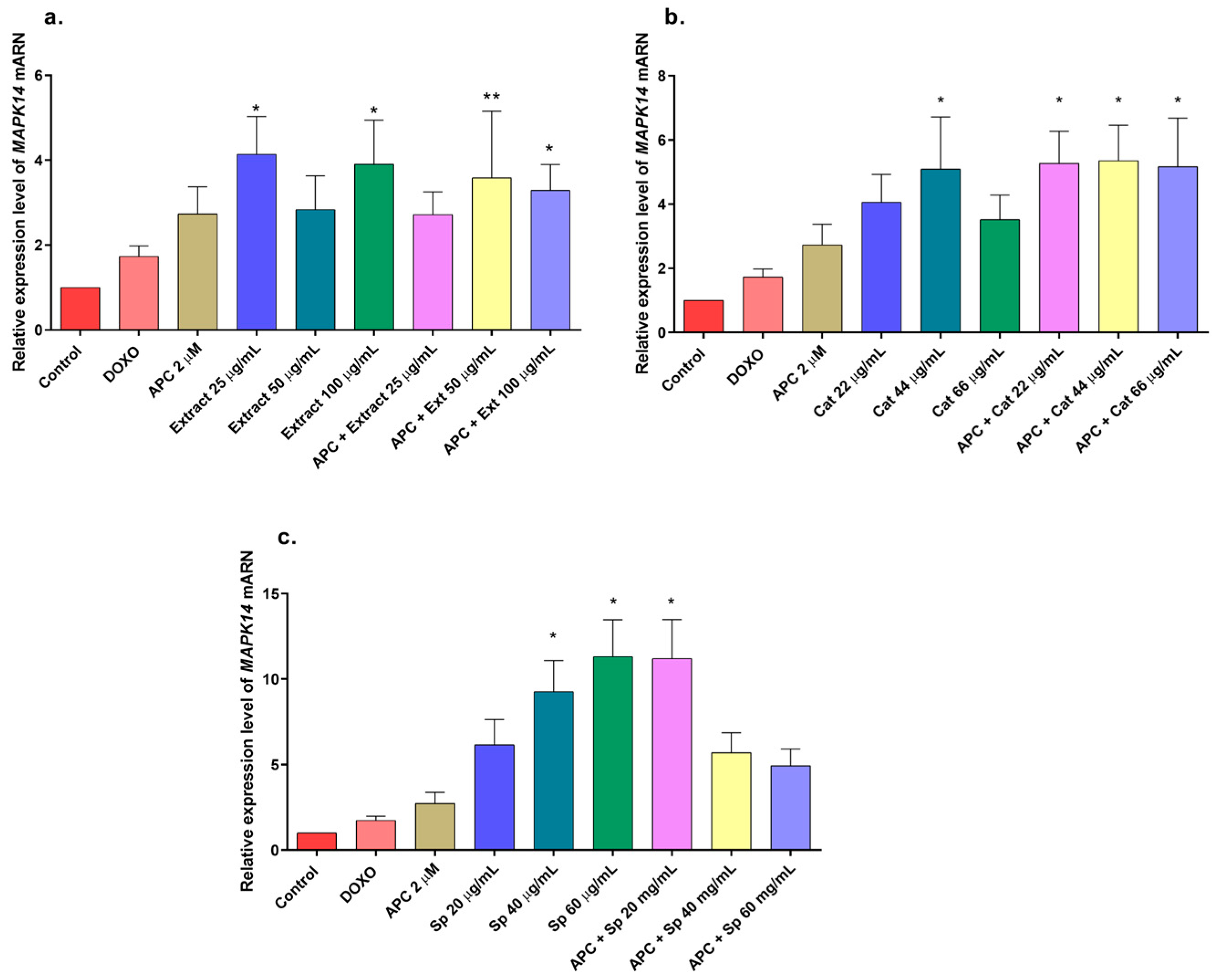
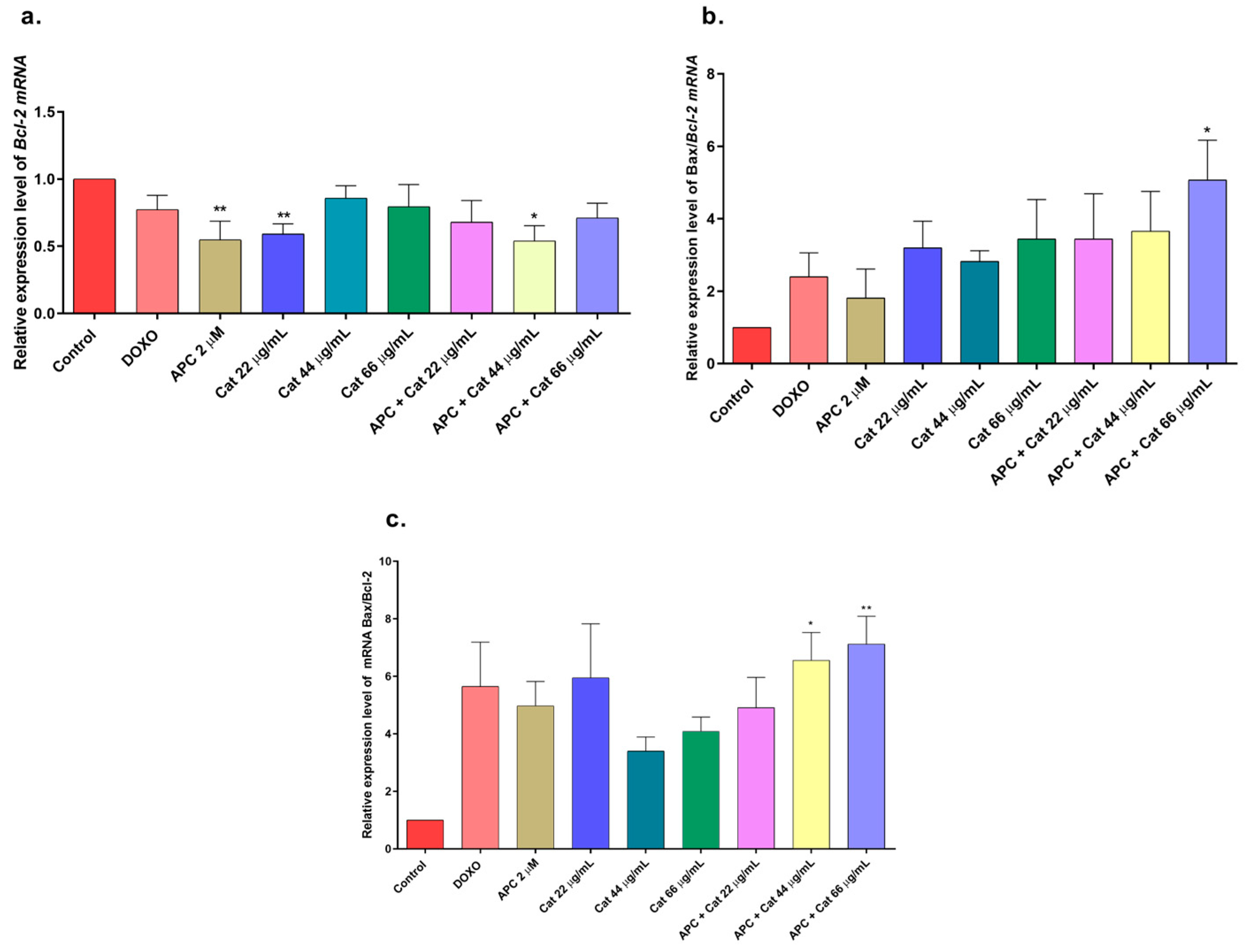
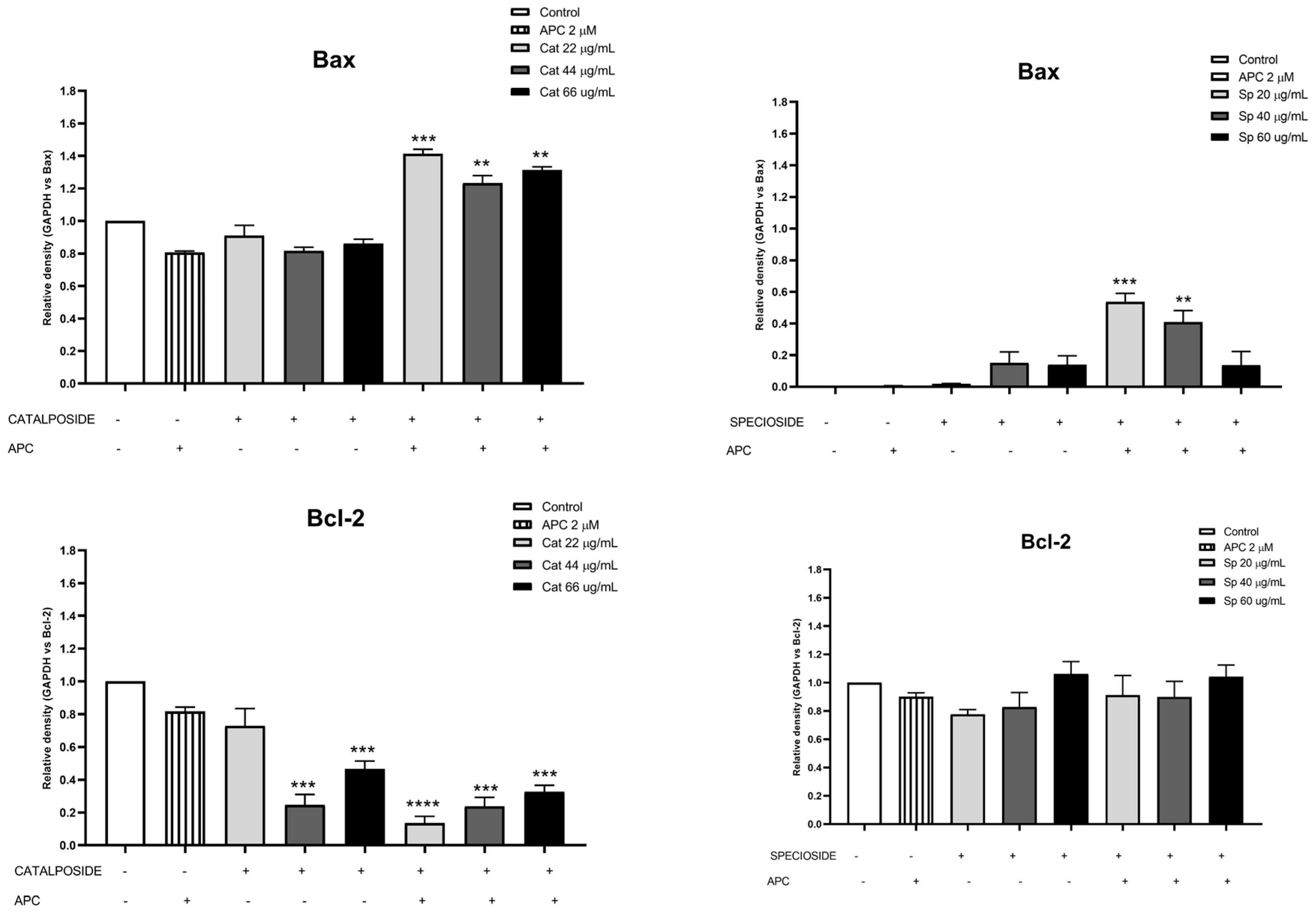
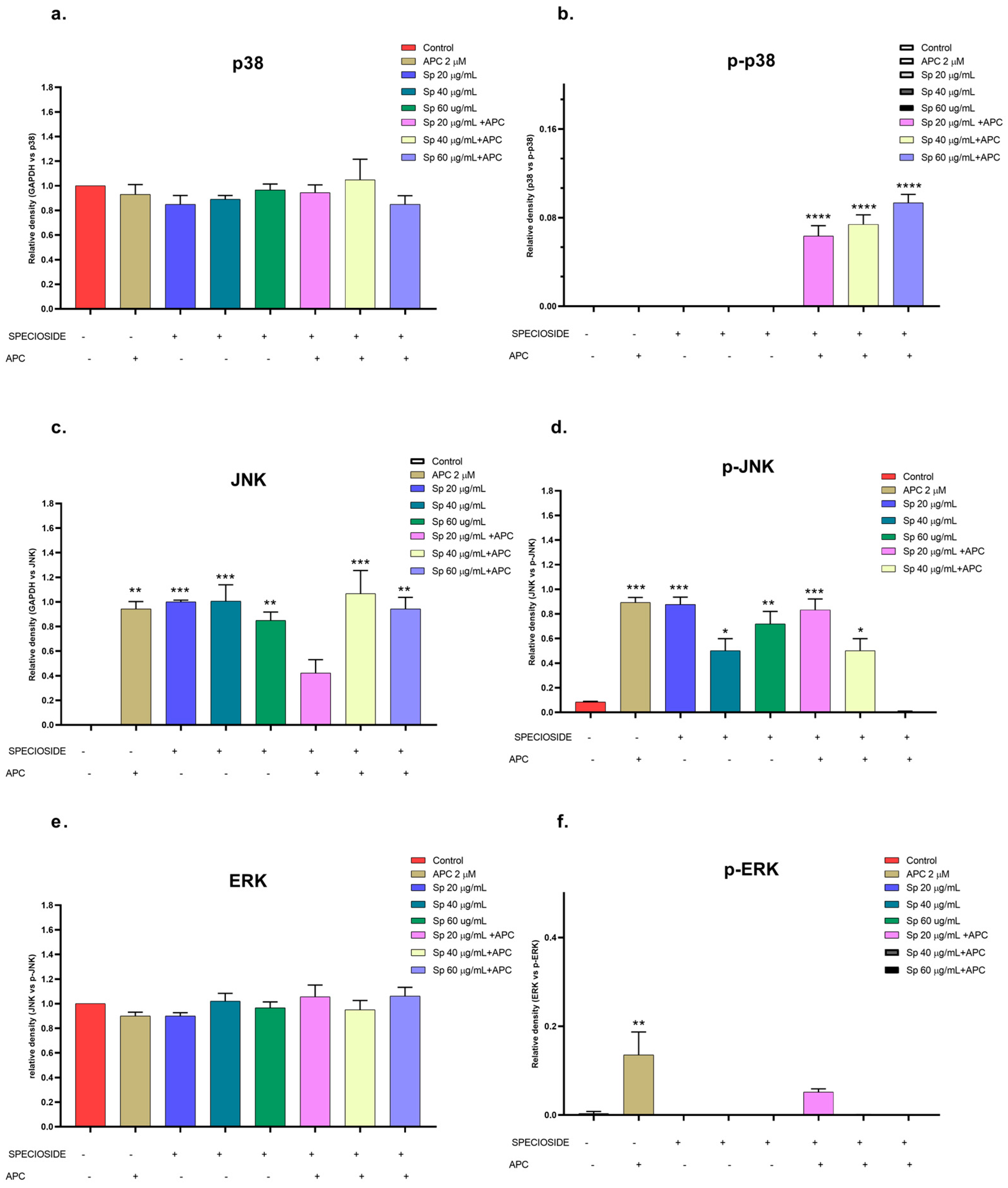
2.5. Effects of Extract, Specioside, and Catalposide on Cell Apoptosis
3. Materials and Methods
3.1. Plant Material and Preparation of Extract
3.2. Preliminary Phytochemical Analysis
3.3. Fractionation and Isolation of Specioside
3.4. Cell Culture
3.5. Cell Viability Assay (MTT)
3.6. Estimation of the Selectivity Index (SI)
3.7. Fluorescence Microscopic Analysis of Cell Death (Propidium Iodide and Hoechst Staining)
3.8. Flow Cytometric Analysis of Apoptosis via the ANNEXIN V-CF647/7-AAD Double-Staining Assay
3.9. Determination of Mitochondrial Membrane Potential (ΔΨm)
3.10. Cell Cycle Analysis
3.11. Western Blot Analysis
3.12. RT–qPCR
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Stacy, C.P.; Brown, A. Treatment of Pediatric Acute Lymphoblastic Leukemia. Pediatr. Clin. N. Am. 2015, 62, 13. [Google Scholar]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2016, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef]
- Murray, V.; Shaw, D. WHO Monographs on Selected Medicinal Plants, Volume 1. Health Hyg. 2000, 21, 129. [Google Scholar]
- Vogl, S.; Picker, P.; Mihaly Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Gentry, A.H. A Synopsis of Bignoniaceae Ethnobotany and Economic Botany. Ann. Mo. Bot. Gard. 1992, 79, 53–64. [Google Scholar] [CrossRef]
- Hamed, A.N.E.; Mahmoud, B.K.; Samy, M.N.; Kamel, M.S. An extensive review on genus “Tabebuia”, family bignoniaceae: Phytochemistry and biological activities (1967 to 2018). J. Herb. Med. 2020, 24, 100410. [Google Scholar] [CrossRef]
- Olmstead, R.G.; Zjhra, M.L.; Lohmann, L.G.; Grose, S.O.; Eckert, A.J. A molecular phylogeny and classification of Bignoniaceae. Am. J. Bot. 2009, 96, 1731–1743. [Google Scholar] [CrossRef]
- Michel, A.F.R.M.; Melo, M.M.; Campos, P.P.; Oliveira, M.S.; Oliveira, F.A.S.; Cassali, G.D.; Ferraz, V.P.; Cota, B.B.; Andrade, S.P.; Souza-Fagundes, E.M. Evaluation of anti-inflammatory, antiangiogenic and antiproliferative activities of Arrabidaea chica crude extracts. J. Ethnopharmacol. 2015, 165, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, H.; Matos, F. Medicinal Plants in Brazil: Native and Exotic; Instituto Plantarum de Estudosda Flora, Editora Plantarum: Nova Odessa, Brazil, 2008; p. 576. [Google Scholar]
- Mafioleti, L.; da Silva Junior, I.F.; Colodel, E.M.; Flach, A.; de Oliveira Martins, D.T. Evaluation of the toxicity and antimicrobial activity of hydroethanolic extract of Arrabidaea chica (Humb. & Bonpl.) B. Verl. J. Ethnopharmacol. 2013, 150, 576–582. [Google Scholar] [PubMed]
- Moya, E.; Brea, M. First Pleistocene record of fossil wood of Bignoniaceae in the Americas and a comparison with the extant Tabebuia alliance and Tecomeae. Bot. J. Linn. Soc. 2018, 187, 303–318. [Google Scholar] [CrossRef]
- Gómez-Estrada, H.; Díaz-Castillo, F.; Franco-Ospina, L.; Mercado-Camargo, J.; Guzmán-Ledezma, J.; Medina, J.D.; Gaitán-Ibarra, R. Folk medicine in the northern coast of Colombia: An overview. J. Ethnobiol. Ethnomed 2011, 7, 27. [Google Scholar] [CrossRef]
- Hajdu, Z.; Hohmann, J. An ethnopharmacological survey of the traditional medicine utilized in the community of Porvenir, Bajo Paraguá Indian Reservation, Bolivia. J. Ethnopharmacol. 2012, 139, 838–857. [Google Scholar] [CrossRef]
- Lima Bezerra, J.J.; Johanes, I.; Vieira Pinheiro, A.A. Anticancer potential and toxicity of the genus Handroanthus Mattos (Bignoniaceae): A systematic review. Toxicon 2022, 217, 131–142. [Google Scholar] [CrossRef]
- Garzón-Castaño, S.C.; Lopera-Castrillón, I.A.; Jiménez-González, F.J.; Siller-López, F.; Veloza, L.A.; Sepúlveda-Arias, J.C. Nrf2-Mediated Antioxidant Activity of the inner bark extracts obtained from Tabebuia rosea (Bertol) DC and Tabebuia chrysantha (JACQ) G. Nicholson. F1000Research 2018, 7, 1937. [Google Scholar] [CrossRef]
- Queiroz, M.L.; Valadares, M.C.; Torello, C.O.; Ramos, A.L.; Oliveira, A.B.; Rocha, F.D.; Arruda, V.A.; Accorci, W.R. Comparative studies of the effects of Tabebuia avellanedae bark extract and β-lapachone on the hematopoietic response of tumor-bearing mice. J. Ethnopharmacol. 2008, 117, 228–235. [Google Scholar] [CrossRef]
- Cardona-Trujillo, M.C.; Jiménez-González, F.J.; Veloza, L.A.; Sepúlveda-Arias, J.C. In Vitro Anti-Toxoplasma Activity of Extracts Obtained from Tabebuia rosea and Tabebuia chrysantha: The Role of β-Amyrin. Molecules 2024, 29, 920. [Google Scholar] [CrossRef]
- Garzón-Castaño, S.C.; Jiménez-González, F.J.; Veloza, L.A.; Sepúlveda-Arias, J.C. Activation of the Keap1-Nrf2 pathway by specioside and the n-butanol extract from the inner bark of Tabebuia rosea (Bertol) DC. F1000Research 2020, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gonzalez, F.J.; Vélez-Gómez, J.M.; Melchor-Moncada, J.J.; Veloza, L.A.; Sepúlveda-Arias, J.C. Antioxidant, anti-inflammatory, and antiproliferative activity of extracts obtained from Tabebuia rosea (Bertol.) DC. Pharmacogn. Mag. 2018, 14, 25. [Google Scholar]
- Compadre, C.; Jáuregui, J.; Nathan, P.J.; Enríquez, R. Isolation of 6-O-(p-Coumaroyl)-Catalpol from Tabebuia rosea. Planta Medica 1982, 46, 42–44. [Google Scholar] [CrossRef]
- Inagaki, R.; Ninomiya, M.; Tanaka, K.; Watanabe, K.; Koketsu, M. Synthesis and cytotoxicity on human leukemia cells of furonaphthoquinones isolated from Tabebuia plants. Chem. Pharm. Bull. 2013, 61, 670–673. [Google Scholar] [CrossRef]
- Khandelwal, P.; Singh, P. Tabebuin and tecomaquinone-III-dimeric quinones from Tabebuia rosea. J. Indian Chem. Soc. 2008, 85, 310–312. [Google Scholar]
- Kang, N.; Wang, M.-M.; Wang, Y.-H.; Zhang, Z.-N.; Cao, H.-R.; Lv, Y.-H.; Yang, Y.; Fan, P.-H.; Qiu, F.; Gao, X.-M. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem. Toxicol. 2014, 67, 193–200. [Google Scholar] [CrossRef]
- Dias, R.B.; de Araújo, T.B.S.; de Freitas, R.D.; da Cruz Rodrigues, A.C.B.; Sousa, L.P.; Sales, C.B.S.; de Faro Valverde, L.; Soares, M.B.P.; Dos Reis, M.G.; Della Coletta, R. β-Lapachone and its iodine derivatives cause cell cycle arrest at G2/M phase and reactive oxygen species-mediated apoptosis in human oral squamous cell carcinoma cells. Free Radic. Biol. Med. 2018, 126, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, N.-J. Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules 2017, 22, 1287. [Google Scholar] [CrossRef] [PubMed]
- Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef]
- Bauden, M.; Tassidis, H.; Ansari, D. In vitro cytotoxicity evaluation of HDAC inhibitor Apicidin in pancreatic carcinoma cells subsequent time and dose dependent treatment. Toxicol. Lett. 2015, 236, 8–15. [Google Scholar] [CrossRef]
- Kumar, S.; Chaudhary, A.K.; Kumar, R.; O’Malley, J.; Dubrovska, A.; Wang, X.; Yadav, N.; Goodrich, D.W.; Chandra, D. Combination therapy induces unfolded protein response and cytoskeletal rearrangement leading to mitochondrial apoptosis in prostate cancer. Mol. Oncol. 2016, 10, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Al-Lazikani, B.; Banerji, U.; Workman, P. Combinatorial drug therapy for cancer in the postgenomic era. Nat. Biotechnol. 2012, 30, 679. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Singh, J.; Hatcher, S.; Ku, A.; Ding, Z.; Feng, F.; Sharma, R.; Pfister, S. Model selection for the preclinical development of new drug–radiotherapy combinations. Clin. Oncol. 2021, 33, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Reza, A.S.M.A.; Khan, M.A.; Sujon, K.M.; Sharmin, R.; Rashid, M.; Sadik, M.G.; Reza, M.A.; Tsukahara, T.; Capasso, R.; et al. Unfolding the apoptotic mechanism of antioxidant enriched-leaves of Tabebuia pallida (lindl.) Miers in EAC cells and mouse model. J. Ethnopharmacol. 2021, 278, 114297. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Efferth, T. African flora has the potential to fight multidrug resistance of cancer. BioMed Res. Int. 2015, 2015, 914813. [Google Scholar] [CrossRef]
- Huertas-Martínez, J.; Rello-Varona, S.; Herrero-Martín, D.; Barrau, I.; García-Monclús, S.; Sáinz-Jaspeado, M.; Lagares-Tena, L.; Núñez-Álvarez, Y.; Mateo-Lozano, S.; Mora, J.; et al. Caveolin-1 is downregulated in alveolar rhabdomyosarcomas and negatively regulates tumor growth. Oncotarget 2014, 5, 9744. [Google Scholar] [CrossRef]
- Feldman, R.; Martinez, J.D. Growth suppression by ursodeoxycholic acid involves caveolin-1 enhanced degradation of EGFR. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2009, 1793, 1387–1394. [Google Scholar] [CrossRef][Green Version]
- Oliveira, P.F.d.; Alves, J.M.; Damasceno, J.L.; Oliveira, R.A.M.; Dias, H.; Crotti, A.E.M.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Ohlsson, R.; Henikoff, S. The epigenetic progenitor origin of human cancer. Nat. Rev. Genet. 2006, 7, 21–33. [Google Scholar] [CrossRef]
- Kwon, S.H.; Ahn, S.H.; Kim, Y.K.; Bae, G.-U.; Yoon, J.W.; Hong, S.; Lee, H.Y.; Lee, Y.-W.; Lee, H.-W.; Han, J.-W. Apicidin, a Histone Deacetylase Inhibitor, Induces Apoptosis and Fas/Fas Ligand Expression in Human Acute Promyelocytic Leukemia Cells. J. Biol. Chem. 2002, 277, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-Y.; Ahn, S.-G.; Yoon, J.-H. Apicidin, a histone deaceylase inhibitor, induces both apoptosis and autophagy in human oral squamous carcinoma cells. Oral. Oncol. 2011, 47, 1032–1038. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Lee, J.; Na, Y.J.; Choi, W.S.; Lee, B.M.; Kang, K.W.; Kim, H.S. Mechanism of apicidin-induced cell cycle arrest and apoptosis in Ishikawa human endometrial cancer cells. Chem. Biol. Interact. 2009, 179, 169–177. [Google Scholar] [CrossRef]
- Cai, X.; Guo, L.; Pei, F.; Chang, X.; Zhang, R. Polyphyllin G exhibits antimicrobial activity and exerts anticancer effects on human oral cancer OECM-1 cells by triggering G2/M cell cycle arrest by inactivating cdc25C-cdc2. Arch. Biochem. Biophys. 2018, 644, 93–99. [Google Scholar] [CrossRef]
- Telang, N.; Nair, H.B.; Wong, G.Y.C. Growth inhibitory efficacy and anti-aromatase activity of Tabebuia avellanedae in a model for postmenopausal luminal a breast cancer. Biomed. Rep. 2019, 11, 222–229. [Google Scholar] [PubMed]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.A.; Ishqi, H.M.; Sarwar, T.; Rehman, S.U.; Tabish, M. Identification and expression analysis of alternatively spliced new transcript isoform of Bax gene in mouse. Gene 2017, 621, 21–31. [Google Scholar] [CrossRef]
- Saracoglu, I.; Harput, U.S. In vitro cytotoxic activity and structure-activity relationships of iridoid glucosides derived from Veronica species. Phytother. Res. 2012, 26, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance toward death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Gupta, R.; Ambasta, R.K.; Kumar, P. Pharmacological intervention of histone deacetylase enzymes in the neurodegenerative disorders. Life Sci. 2020, 243, 1–26. [Google Scholar] [CrossRef]
- Zhang, L.; Tatsuno, T.; Hasegawa, I.; Tadano, T.; Ohta, T. Furanonaphthoquinones from Tabebuia avellanedae induce cell cycle arrest and apoptosis in the human non-small cell lung cancer cell line A549. Phytochem. Lett. 2015, 11, 9–17. [Google Scholar] [CrossRef]
- Cruz, V.S.; Rodrigues, F.A.; Braga, K.; Machado, P.A.; Bianchi Filho, C.; Prado, Y.C.; Araújo, E.G. β Lapachone blocks the cell cycle and induces apoptosis in canine osteosarcoma cells. Pesqui. Veter Bras. 2018, 38, 2224–2232. [Google Scholar] [CrossRef]
- Dinda, B. Pharmacology of Iridoids. In Pharmacology and Applications of Naturally, Occurring Iridoids; Dinda, B., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 145–254. [Google Scholar]
- Cao, S.; Zhu, X.; Du, L. P38 MAP kinase is involved in oleuropein-induced apoptosis in A549 cells by a mitochondrial apoptotic cascade. Biomed. Pharmacother. 2017, 95, 1425–1435. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Huang, Y.; Wang, Y.; Xiang, L.; He, X. A spirostanol saponin isolated from Tupistra chinensis Baker simultaneously induces apoptosis and autophagy by regulating the JNK pathway in human gastric cancer cells. Steroids 2017, 164, 108737. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, K.; Liu, F.; Chen, H.; Wang, X.; Zu, X.; Ma, X.; Wang, T.; Wu, Q.; Zheng, Y.; et al. Gossypetin is a novel MKK3 and MKK6 inhibitor that suppresses esophageal cancer growth in vitro and in vivo. Cancer Lett. 2019, 442, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhu, R.; Tian, S.; Wang, Y.; Lou, S.; Zhao, H. Jatamanvaltrate P induces cell cycle arrest, apoptosis and autophagy in human breast cancer cells in vitro and in vivo. Biomed. Pharmacother. 2017, 89, 1027–1036. [Google Scholar] [CrossRef]
- Atmaca, H.; Bozkurt, E.; Cittan, M.; Dilek Tepe, H. Effects of Gallium aparine extract on the cell viability, cell cycle and cell death in breast cancer cell lines. J. Ethnopharmacol. 2016, 186, 305–310. [Google Scholar] [CrossRef]
- Bokhari, J.; Khan, M.R.; Shabbir, M.; Rashid, U.; Jan, S.; Zai, J.A. Evaluation of diverse antioxidant activities of Gallium aparine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 24–29. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, X.; Chen, C.; Huijuan, W.; Zhao, H.; Liu, W.; Xiang, Z.; Wang, Q. Apigenin, a flavonoid constituent derived from P. villosa, inhibits hepatocellular carcinoma cell growth by CyclinD1/CDK4 regulation via p38 MAPK-p21 signaling. Pathol. Res. Pract. 2020, 216, 152701. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Castro, A.; Stärkel, P.; Galle, M.; Crespo, R. 1,8-Cineole promotes G0/G1 cell cycle arrest and oxidative stress-induced senescence in HepG2 cells and sensitizes cells to anti-senescence drugs. Life Sci. 2020, 243, 117271. [Google Scholar] [CrossRef]
- Jiménez-González, F.J.; Veloza, L.A.; Sepúlveda-Arias, J.C. Anti-infectious activity in plants of the genus Tabebuia. Univ. Sci. 2013, 18, 257–267. [Google Scholar] [CrossRef]
- Melchor Moncada, J.J.; Socarrás Cárdenas, A.; Mantilla Muriel, L.E.; Sepúlveda Arias, J.C.; Mancilla Estacio, L.I.; Felipe Villalba, M.F.; Caballero, L.C. Actividad Biológica de Plantas de la Familia Bignoniaceae. In Biotecnología y sus Aplicaciones en el Sector Salud; Editorial Universidad Tecnológica de Pereira: Pereira, Colombia, 2020; pp. 358–386. [Google Scholar]
- Dal Piaz, F.; Vassallo, A.; Temraz, A.; Cotugno, R.; Belisario, M.A.; Bifulco, G.; Chini, M.G.; Pisano, C.; De Tommasi, N.; Braca, A. A chemical-biological study reveals C9-type iridoids as novel heat shock protein 90 (Hsp90) inhibitors. J. Med. Chem. 2013, 56, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer. Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
- Khanam, R.; Ahmad, K.; Hejazi, I.I.; Siddique, I.A.; Kumar, V.; Bhat, A.R.; Azam, A.; Athar, F. Inhibitory growth evaluation and apoptosis induction in MCF-7 cancer cells by new 5-aryl-2-butylthio-1,3,4-oxadiazole derivatives. Cancer Chemother. Pharmacol. 2017, 80, 1027–1042. [Google Scholar] [CrossRef]





| Time | 12 h | 24 h | 48 h | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell Line | Jurkat | THP-1 | PBMC | Jurkat | THP-1 | PBMC | Jurkat | THP-1 | PBMC |
| Extract | 314.8 ± 2.2 | 35.3 ± 3.2 | 340.5 ± 2.22 | 189.5 ± 0.5 | 44.7 ± 4.082 | 123.64 ± 5.2 | 354.3 ± 0.1 | 36.44 ± 1.4 | 99.4 ± 4.7 |
| SI | 1.08 | 9.64 | 0.65 | 2.77 | 0.28 | 2.72 | |||
| Catalposide | 106.74 ± 5.3 | 121.6 ± 3.3 | 125.67 ± 2.8 | 92.77 ± 1.5 | 43.86 ± 2.2 | 87.7 ± 3.1 | 71.16 ± 2.8 | 30.16 ± 1.67 | 43.4 ± 3.4 |
| SI | 1.18 | 1.03 | 0.94 | 1.99 | 0.61 | 1.44 | |||
| Specioside | 137.66 ± 1.3 | 47.25 ± 2.3 | 165.25 ± 3.1 | 81.71 ± 3.5 | 40.32 ± 1.3 | 70.66 ± 3.7 | 68.86 ± 2.1 | 26.33 ± 6.8 | 54.22 ± 3.7 |
| SI | 1.20 | 3.49 | 0.86 | 1.75 | 0.79 | 2.06 | |||
| Doxorubicin (µg/mL) | 0.339 ± 1.2 | 1.268 ± 0.2 | 1.364 ± 0.15 | 0.258 ± 0.1 | 0.189 ± 0.1 | 0.224 ± 0.1 | 0.050 ± 0.0 | 0.005 ± 0.01 | 0.018 ± 0.1 |
| SI | 4.02 | 1.07 | 0.86 | 1.183 | 0.36 | 3.6 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Pepinosa, N.Y.; Veloza, L.A.; Sepúlveda-Arias, J.C. The n-Butanol Extract Obtained from the Inner Bark of Tabebuia rosea (Bertol.) DC, Specioside, and Catalposide Induce Leukemia Cell Apoptosis in the Presence of Apicidin. Molecules 2024, 29, 3986. https://doi.org/10.3390/molecules29173986
Guerrero-Pepinosa NY, Veloza LA, Sepúlveda-Arias JC. The n-Butanol Extract Obtained from the Inner Bark of Tabebuia rosea (Bertol.) DC, Specioside, and Catalposide Induce Leukemia Cell Apoptosis in the Presence of Apicidin. Molecules. 2024; 29(17):3986. https://doi.org/10.3390/molecules29173986
Chicago/Turabian StyleGuerrero-Pepinosa, Nancy Yadira, Luz Angela Veloza, and Juan Carlos Sepúlveda-Arias. 2024. "The n-Butanol Extract Obtained from the Inner Bark of Tabebuia rosea (Bertol.) DC, Specioside, and Catalposide Induce Leukemia Cell Apoptosis in the Presence of Apicidin" Molecules 29, no. 17: 3986. https://doi.org/10.3390/molecules29173986
APA StyleGuerrero-Pepinosa, N. Y., Veloza, L. A., & Sepúlveda-Arias, J. C. (2024). The n-Butanol Extract Obtained from the Inner Bark of Tabebuia rosea (Bertol.) DC, Specioside, and Catalposide Induce Leukemia Cell Apoptosis in the Presence of Apicidin. Molecules, 29(17), 3986. https://doi.org/10.3390/molecules29173986






