Enhanced Photocatalytic Performance under Ultraviolet and Visible Light Illumination of ZnO Thin Films Prepared by Modified Sol-Gel Method
Abstract
:1. Introduction
2. Results and Discussion
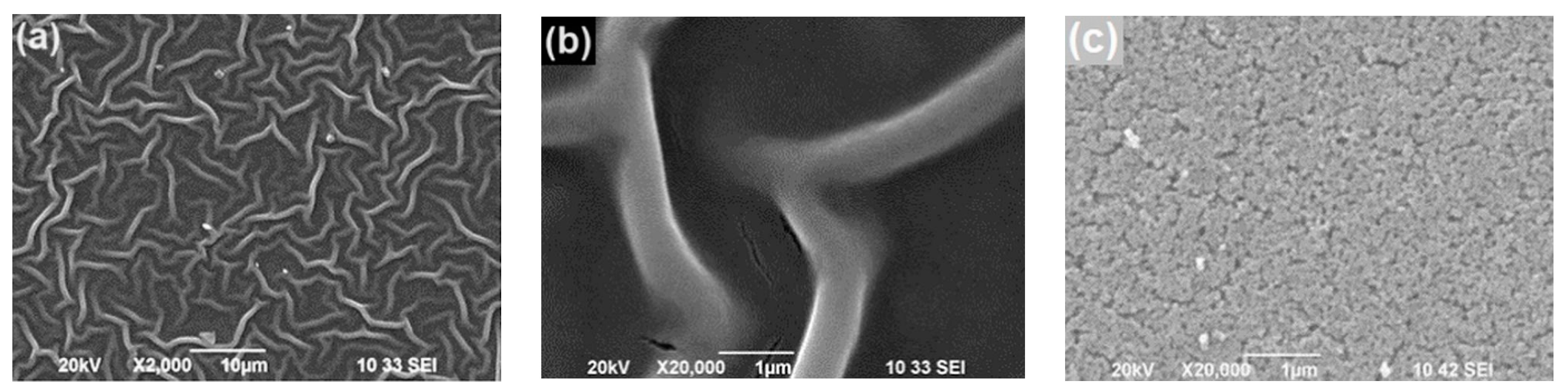
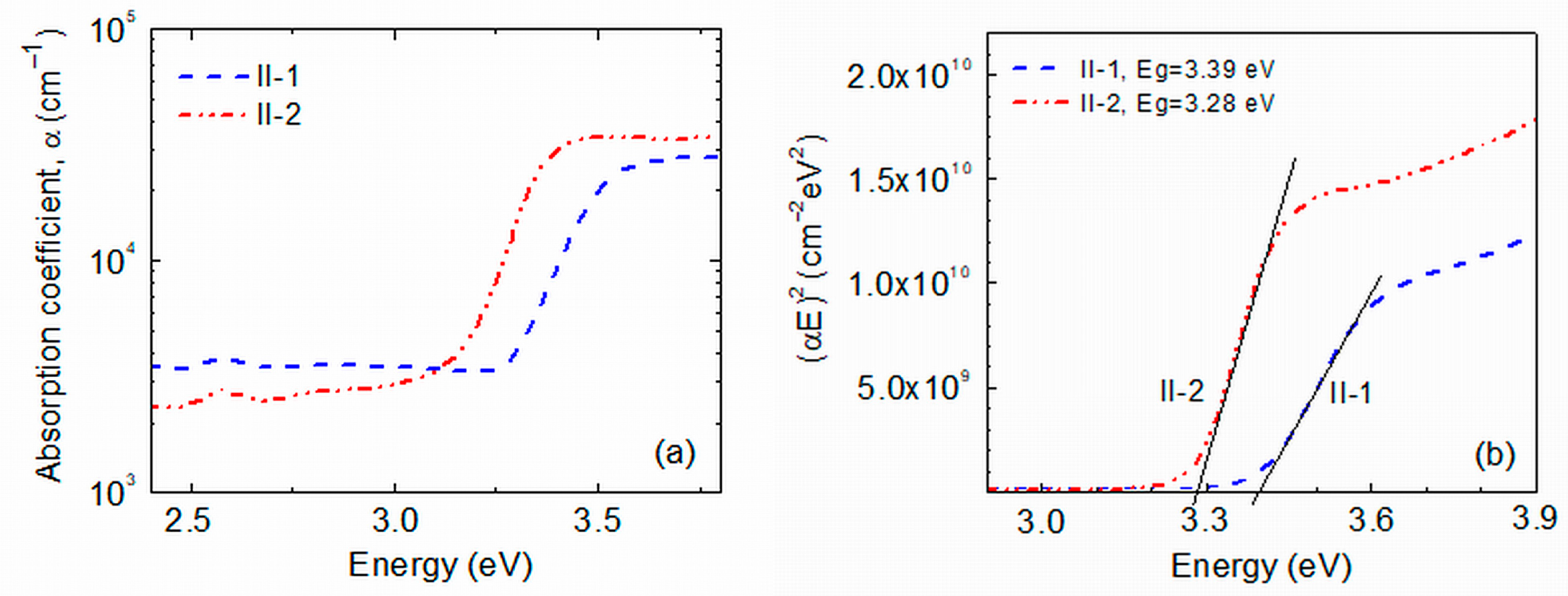
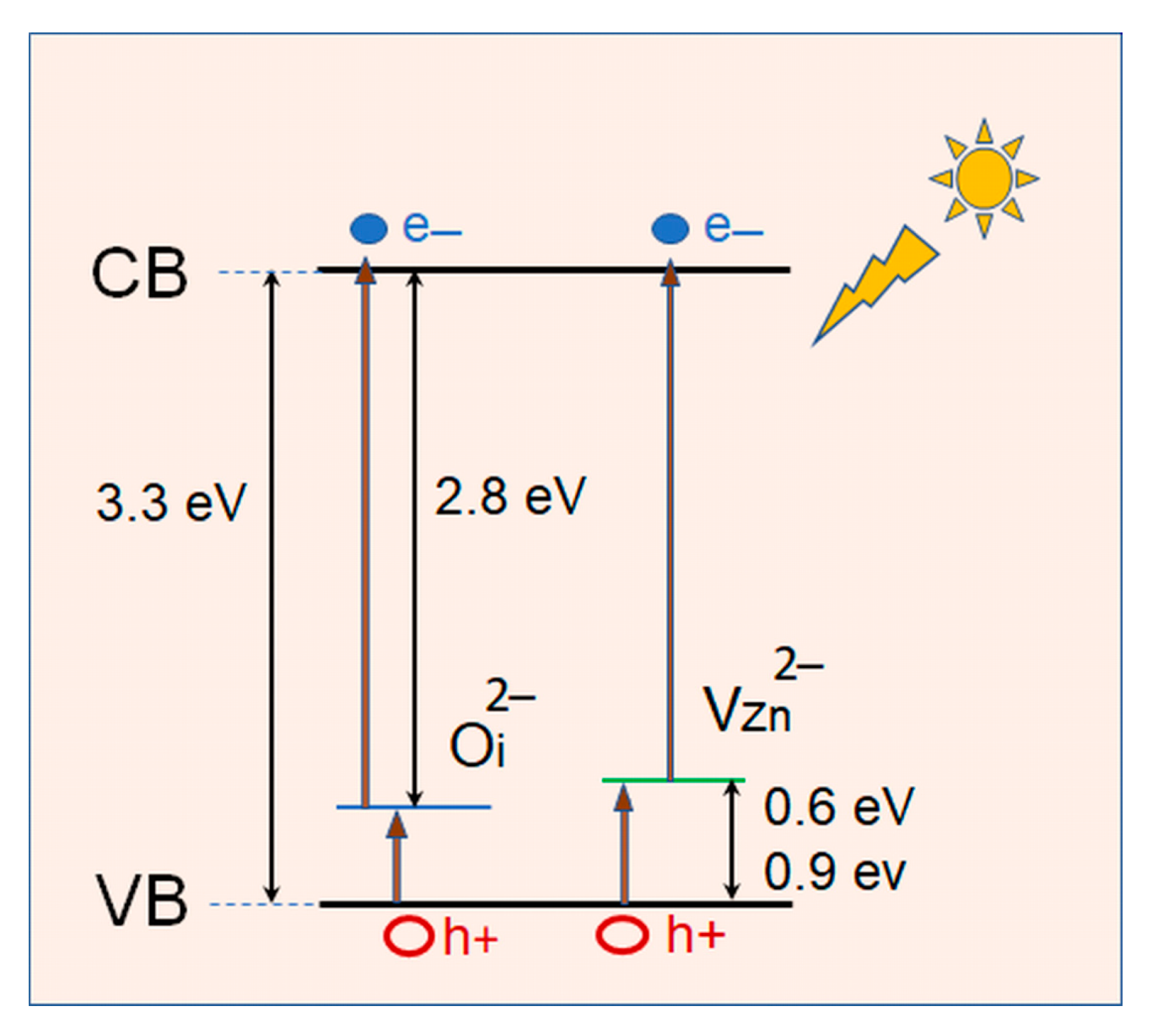
2.1. Surface Morphology, Lattice Structure and Optical Properties
2.2. Photocatalytic Activity, Recyclability Tests
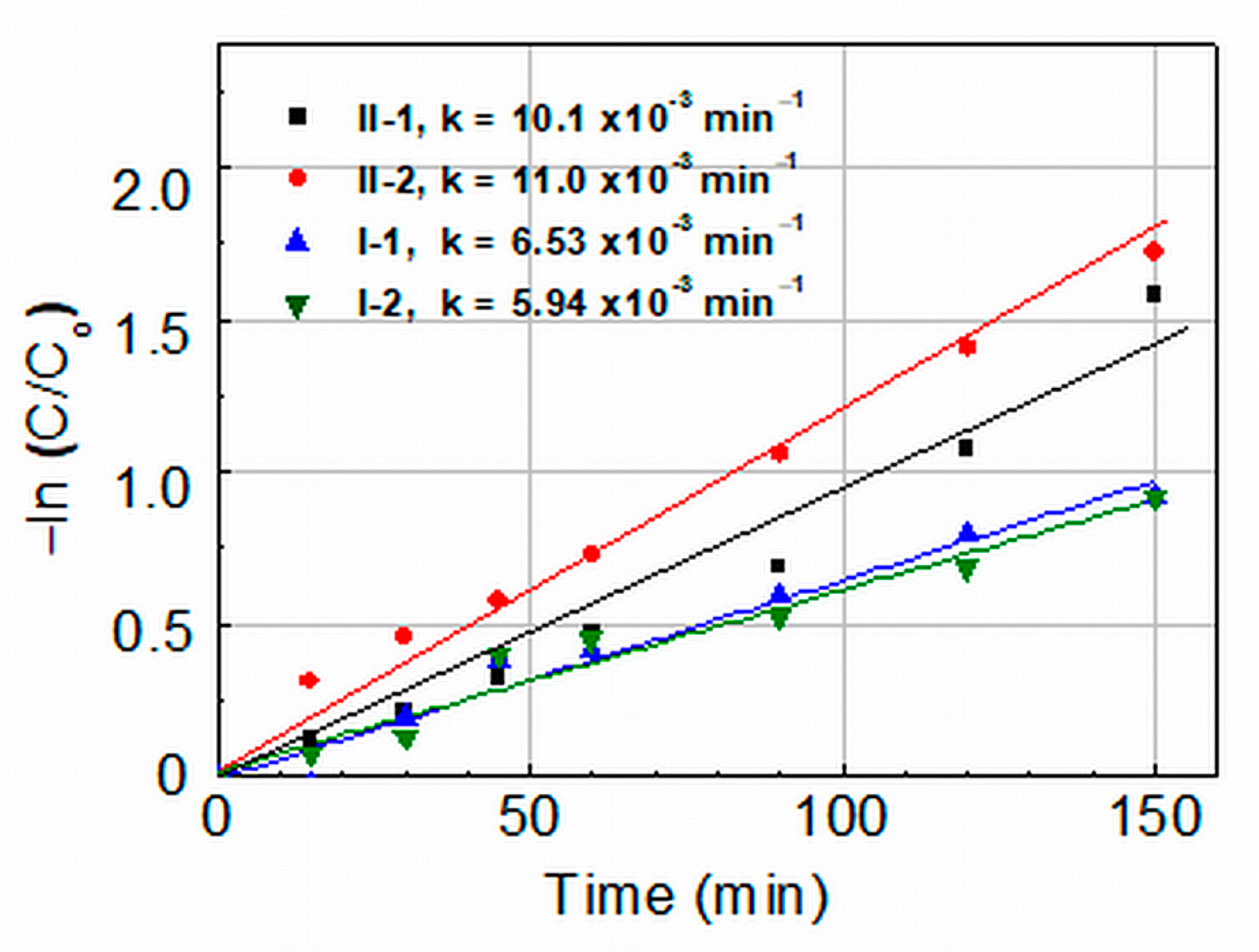
2.2.1. Photocatalytic Activity under UV Light Illumination
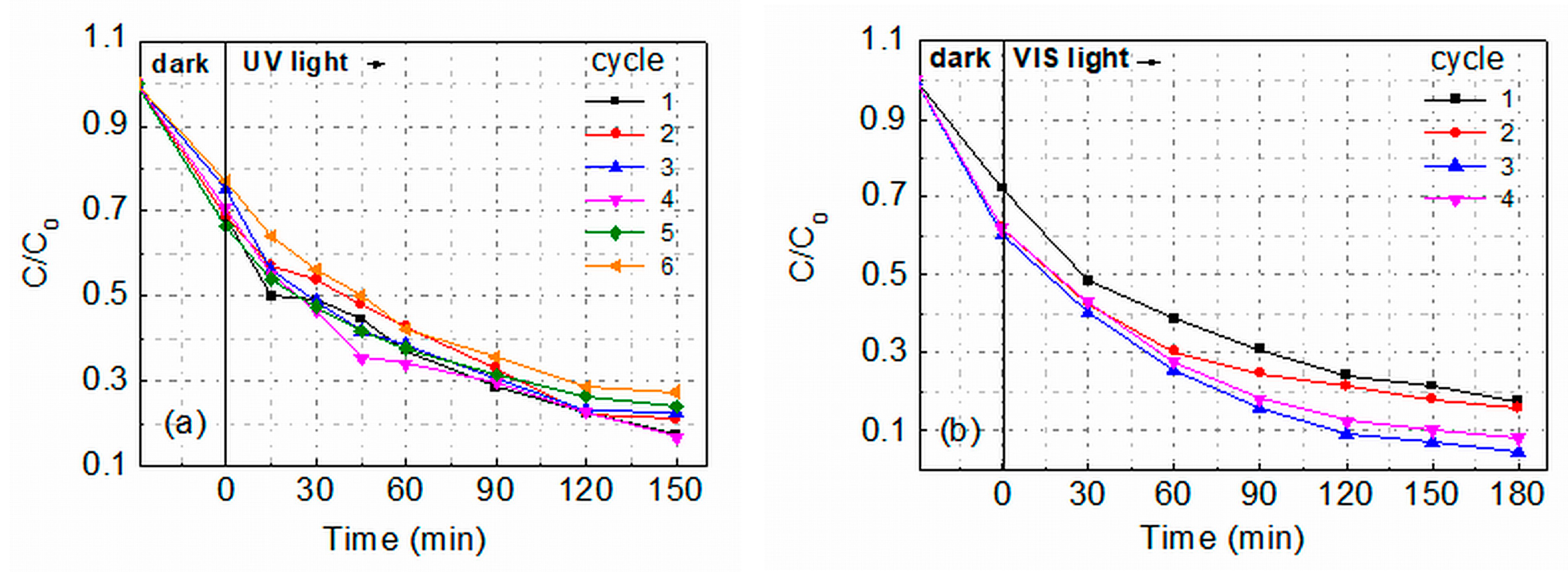
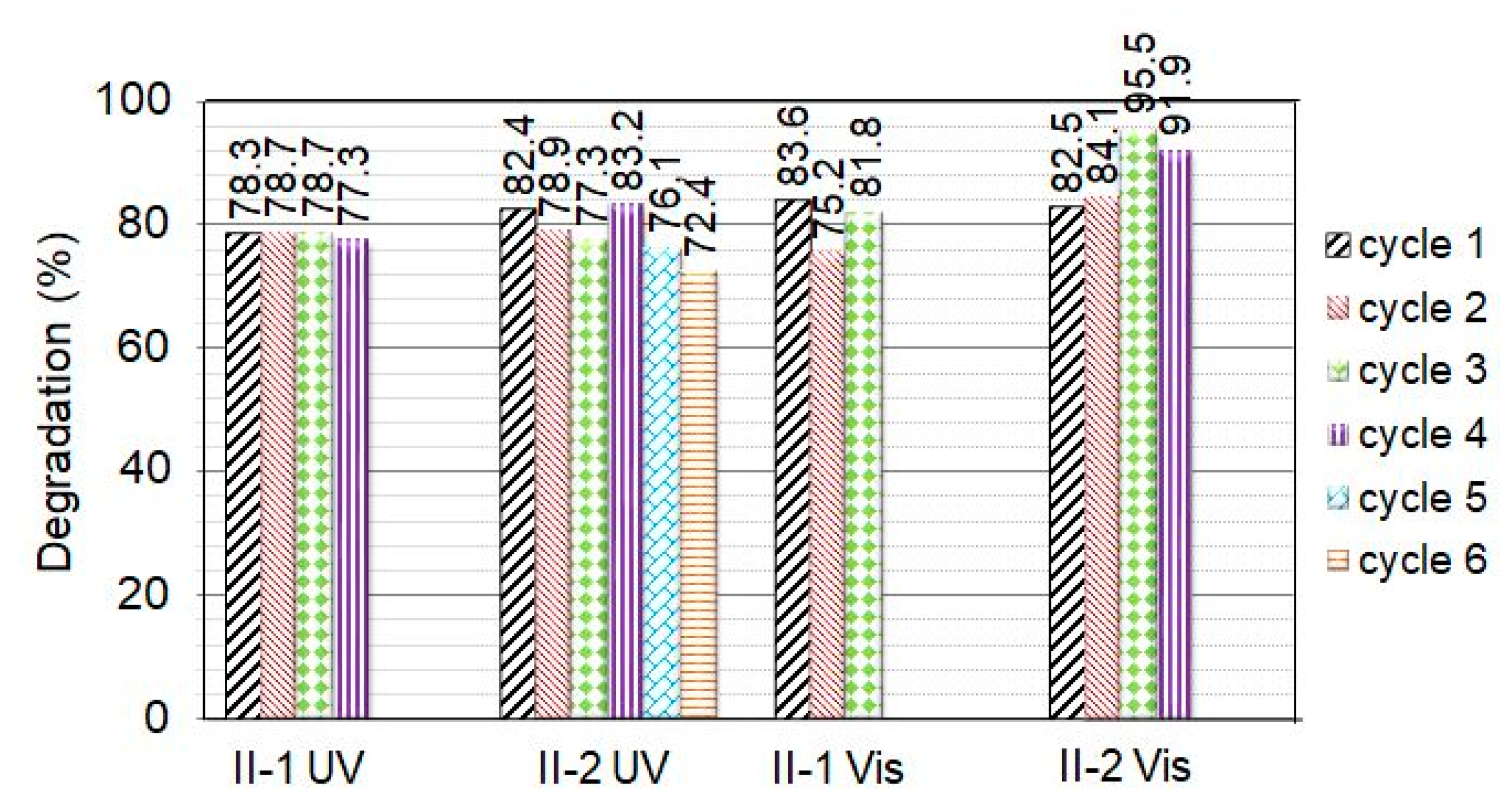
2.2.2. Recyclability Tests under UV and VIS Light Irradiation
3. Materials and Methods
3.1. Films Preparation
3.2. Sample Characterization
3.3. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Umar, E.; Ikram, M.; Haider, J.; Nabgan, W.; Imran, M.; Nazir, G. A state-of-art review of the metal oxide-based nanomaterials effect on photocatalytic degradation of malachite green dyes and a bibliometric analysis. Glob. Chall. 2023, 7, 2300001. [Google Scholar] [CrossRef] [PubMed]
- Podasca, V.-E.; Damaceanu, M.-D. Photopolymerized films with ZnO and doped ZnO particles used as efficient photocatalysts in Malachite Green dye decomposition. Appl. Sci. 2020, 10, 1954. [Google Scholar] [CrossRef]
- Johar, M.A.; Afzal, R.A.; Alazba, A.A.; Manzoor, U. Photocatalysis and bandgap engineering using ZnO nanocomposites. Adv. Mater. Sci. Eng. 2015, 2015, 934587. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Han, J.; Qiu, W.; Gao, W. Potential dissolution and photo-dissolution of ZnO thin films. J. Hazard. Mater. 2010, 178, 115–122. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Teh, S.J.; Lai, C.W. Photocatalytic aqueous oxidation on ZnO: A review. Catalysts 2017, 7, 93. [Google Scholar] [CrossRef]
- Din, M.I.; Najeeb, J.; Ahmad, G. Recent advancements in the architecting schemes of Zinc oxide based photocatalytic assemblies. Sep. Purif. Rev. 2017, 47, 267–287. [Google Scholar] [CrossRef]
- Fu, H.; Xu, T.; Zhu, S.; Zhu, Y. Photo-corrosion inhibition and enhancement of photocatalytic activity for ZnO via hybridization with C60. Environ. Sci. Technol. 2008, 42, 8064–8069. [Google Scholar] [CrossRef]
- Dimitropoulos, M.; Aggelopoulos, C.A.; Sygellou, L.; Tsantis, S.T.; Koutsoukos, P.G.; Yannopoulos, S.N. Unveiling the photo-corrosion mechanism of zinc oxide photocatalyst: Interplay between surface corrosion and regeneration. J. Environ. Chem. Eng. 2024, 12, 112102. [Google Scholar] [CrossRef]
- Ishioka, J.; Kogure, K.; Ofuji, K.; Kawaguchi, K.; Jeem, M.; Kato, T.; Shibayama, T.; Watanabe, S. In situ direct observation of photo-corrosion in ZnO crystals in ionic liquid using a laser-equipped high-voltage electron microscope. AIP Adv. 2017, 7, 035220. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Djurišić, A.B.; He, Y.; Ng, A.M.C. VIS-light photocatalysts: Prospects and challenges. APL Mater. 2020, 8, 030903. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, H.; Yang, G.; Chen, L.; Guo, P.; Zhang, L. A facile synthesis of ZnO/CNT hierarchical microsphere composites with enhanced photocatalytic degradation of methylene blue. RSC Adv. 2015, 5, 72476–72481. [Google Scholar] [CrossRef]
- Saikia, L.; Bhuyan, D.; Saikia, M.; Malakar, B.; Dutta, D.K.; Sengupta, P.; Babajani, N.; Jamshidi, S. Photocatalytic performance of ZnO nanomaterials for self-sensitized degradation of malachite green dye under solar light. Appl. Catal. A Gen. 2015, 490, 42–49. [Google Scholar] [CrossRef]
- Babajani, N.; Jamshidi, S. Investigation of photocatalytic malachite green degradation by iridium doped zinc oxide nanoparticles: Application of response surface methodology. J. Alloys Compd. 2019, 782, 533–544. [Google Scholar] [CrossRef]
- Neto, N.F.A.; Carvalho, R.G.; Garcia, L.M.P.; Nascimento, R.M.; Paskocimas, C.A.; Longo, E.; Delmonte, M.R.B.; Motta, F.V. Influence of doping with Sm3+ on photocatalytic reuse of ZnO thin films obtained by spin coating. Rev. Matér. 2019, 24, n4-10. [Google Scholar] [CrossRef]
- Andia-Huaracha, S.C.; Zapana-Cayo, L.M.; Aragón, F.F.H.; Aquino, J.C.R.; Coaquira, J.A.H.; Gonzales-Lorenzo, C.D.; Ayala-Arenas, J.S.; Solis, J.L.; Morais, P.C.; Pacheco-Salazar, D.G. Tuning the photocatalytic activity of ZnO nanoparticles by the annihilation of intrinsic defects provoked by the thermal annealing. J. Nanoparticle Res. 2022, 24, 50. [Google Scholar] [CrossRef]
- Yadav, R.; Chundawat, T.S.; Rawat, P.; Rao, G.K.; Vaya, D. Photocatalytic degradation of malachite green dye by ZnO and ZnO–b-cyclodextrin nanocomposite. Bull. Mater. Sci. 2021, 44, 250. [Google Scholar] [CrossRef]
- Gegova-Dzhurkova, R.; Nesheva, D.; Dzhurkov, V.; Šćepanović, M.; Grujić-Brojčin, M.; Bineva, I.; Mihailov, V.; Levi, Z.; Manolov, E.; Popović, Z.V. Modification of surface morphology and lattice order in nanocrystalline ZnO thin films prepared by spin-coating sol-gel method. J. Sol Gel Sci. Technol. 2021, 100, 56–67. [Google Scholar] [CrossRef]
- Kwon, S.J.; Park, J.-H.; Park, J.-G. Wrinkling of a sol-gel-derived thin film. Phys. Rev. E 2005, 71, 011604. [Google Scholar] [CrossRef]
- Kim, H.T.; Lee, S.-Y.; Park, C. Controls of surface morphology on sol-gel derived ZnO films under isothermal treatment conditions. Vacuum 2017, 143, 312–315. [Google Scholar] [CrossRef]
- Moussa, N.B.; Lajnef, M.; Jebari, N.; Villebasse, C.; Bayle, F.; Chaste, J.; Madouri, A.; Chtourou, R.; Herth, E. Synthesis of ZnO sol-gel thin-films CMOS Compatible. RSC Adv. 2021, 11, 22723–22733. [Google Scholar] [CrossRef] [PubMed]
- Musavi, E.; Khanlary, M.; Khakpour, Z. Red-orange photoluminescence emission of sol-gel dip-coated prepared ZnO and ZnO: Al nano-crystalline films. J. Lumin. 2019, 216, 116696. [Google Scholar] [CrossRef]
- Rodrigues, J.; Sedrine, N.B.; Correia, M.R.; Monteiro, T. Photoluminescence investigations of ZnO micro/nanostructures. Mater. Today Chem. 2020, 16, 100243. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Lin, K.-F.; Cheng, H.-M.; Hsu, H.-C.; Lin, L.-J.; Hsieh, W.-F. Band gap variation of size-controlled ZnO quantum dots synthesized by sol-gel method, Chem. Phys. Lett. 2005, 409, 208–211. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures via a sol-gel synthesis of solvent driven shapecontrolled crystal growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef]
- Kaneva, N.; Stambolova, I.; Blaskov, V.; Dimitriev, Y.; Vassilev, S.; Dushkin, C. Photocatalytic activity of nanostructured ZnO films prepared by two different methods for the photoinitiated decolorization of malachite green. J. Alloys Compd. 2010, 500, 252–258. [Google Scholar] [CrossRef]
- Kaneva, N.V.; Yordanov, G.G.; Dushkin, C.D. Manufacturing of patterned ZnO films with application for photoinitiated decolorization of malachite green in aqueous solutions. Bull. Mater. Sci. 2010, 33, 111–117. [Google Scholar] [CrossRef]
- Baseri, H.; Alizadeh, E. Photocatalytic degradation of Malachite Green using ZnO and ZnO-TiO2 nanoparticles from aqueous solution. J. Nanoanal. 2018, 5, 232–240. [Google Scholar] [CrossRef]
- Arsalani, N.; Bazazi, S.; Abuali, M.; Jodeyri, S. A new method for preparing ZnO/CNT nanocomposites with enhanced photocatalytic degradation of malachite green under VIS light. J. Photochem. Photobiol. A Chem. 2020, 389, 112207. [Google Scholar] [CrossRef]
- Kaneva, N.; Stambolova, I.; Blaskov, V.; Eliyas, A.; Vassilev, S. Microwave-assisted and conventional sol-gel preparation of photocatalytically active ZnO/TiO2/glass multilayers. Cent. Eur. J. Chem. 2013, 11, 1055–1065. [Google Scholar] [CrossRef]
- Stambolova, I.; Blaskov, V.; Kaneva, N.; Shipochka, M.; Vassilev, S.; Dimitrov, O.; Eliyas, A. Effect of titanium dopant on the surface features and on the photocatalytic characteristics of ZnO films. Mat. Sci. Semicond. Process. 2014, 25, 244–250. [Google Scholar] [CrossRef]
- Meena, S.; Vaya, D.; Das, B.K. Photocatalytic degradation of Malachite Green dye by modified ZnO nanomaterial. Bull. Mater. Sci. 2016, 39, 1735–1743. [Google Scholar] [CrossRef]
- Mort, J. Geminate and non-geminate recombination in amorphous semiconductors. J. Phys. Colloq. 1981, 42, C4-433–C4-441. [Google Scholar] [CrossRef]
- Gegova-Dzhurkova, R.; Nesheva, D.; Mihailov, V.; Dzhurkov, V.; Terziyska, P.; Manolov, E. Effect of infrared laser irradiation on electrical conductivity and ethanol sensitivity of sol-gel ZnO thin films. J. Phys. Conf. Ser. 2021, 1762, 012037. [Google Scholar] [CrossRef]
- Kamış, H.; Karakuş, N.D.; Taymaz, B.H. Electrochemical production of ZnO and ZnO:Ag core-shell nanorods on ITO substrate and their photocatalytic and photoelectrochemical performance. Bilge Int. J. Sci. Technol. Res. 2019, 3, 161–177. [Google Scholar] [CrossRef]
- Saad, A.M.; Abukhadra, M.R.; Ahmed, S.A.-K.; Elzanaty, A.M.; Mady, A.H.; Betiha, M.A.; Shim, J.-J.; Rabie, A.M. Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce-ZnO nano-flowers under visible light. J. Environ. Manag. 2020, 258, 110043. [Google Scholar] [CrossRef]
- Taylorb, C.M.; Ramirez-Canon, A.; Wenk, J.; Mattia, D. Enhancing the photo-corrosion resistance of ZnO nanowire photocatalysts. J. Hazard. Mater. 2019, 378, 120799. [Google Scholar] [CrossRef]
- Liu, M.; Kim, H.K. Ultraviolet detection with ultrathin ZnO epitaxial films treated; with oxygen plasma. Appl. Phys. Lett. 2004, 84, 173–175. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, X.; Chen, X.; Yu, Y.; Dai, W.; Fu, X.; Anpo, M. Correlation between photo-corrosion of ZnO and lattice relaxation induced by its surface vacancies. Phys. Chem. C 2021, 125, 3242–3255. [Google Scholar] [CrossRef]
- Gsiea, A.; Goss, J.P.; Briddon, P.R.; Ramadan, M.; Al-Habashi, R.; Etmimi, K.; Khaled, M. Native Point Defects in ZnO. Int. J. Mater. Metall. Eng. 2014, 8, 127–132. [Google Scholar]
- Gurylev, V.; Perng, T.P. Defect engineering of ZnO: Review on oxygen and zinc vacancies. J. Eur. Ceram. Soc. 2021, 412, 4977–4996. [Google Scholar] [CrossRef]



| Film Group | Sample Name | Preparation Steps 1st Step | 2nd Step | 3rd Step |
|---|---|---|---|---|
| Group I | I-1 | Furnace drying at 140 °C for 15 min | ||
| I-2 | Furnace drying at 140 °C for 15 min | Furnace annealing at 400 °C for 60 min | ||
| Group II | II-1 | Hot air drying at ~95 °C for 5 min | Furnace drying at 140 °C for 15 min | |
| II-2 | Hot air drying at ~95 °C for 5 min | Furnace drying at 140 °C for 15 min | Furnace annealing at 400 °C for 60 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gegova-Dzhurkova, R.; Nesheva, D.; Stambolova, I.; Zaharieva, K.; Dzhurkov, V.; Miloushev, I. Enhanced Photocatalytic Performance under Ultraviolet and Visible Light Illumination of ZnO Thin Films Prepared by Modified Sol-Gel Method. Molecules 2024, 29, 4005. https://doi.org/10.3390/molecules29174005
Gegova-Dzhurkova R, Nesheva D, Stambolova I, Zaharieva K, Dzhurkov V, Miloushev I. Enhanced Photocatalytic Performance under Ultraviolet and Visible Light Illumination of ZnO Thin Films Prepared by Modified Sol-Gel Method. Molecules. 2024; 29(17):4005. https://doi.org/10.3390/molecules29174005
Chicago/Turabian StyleGegova-Dzhurkova, Radka, Diana Nesheva, Irina Stambolova, Katerina Zaharieva, Valeri Dzhurkov, and Ilko Miloushev. 2024. "Enhanced Photocatalytic Performance under Ultraviolet and Visible Light Illumination of ZnO Thin Films Prepared by Modified Sol-Gel Method" Molecules 29, no. 17: 4005. https://doi.org/10.3390/molecules29174005





