Unveiling the Influence of Water Molecules for NF3 Removal by the Reaction of NF3 with OH: A DFT Study
Abstract
:1. Introduction
2. Results and Discussion
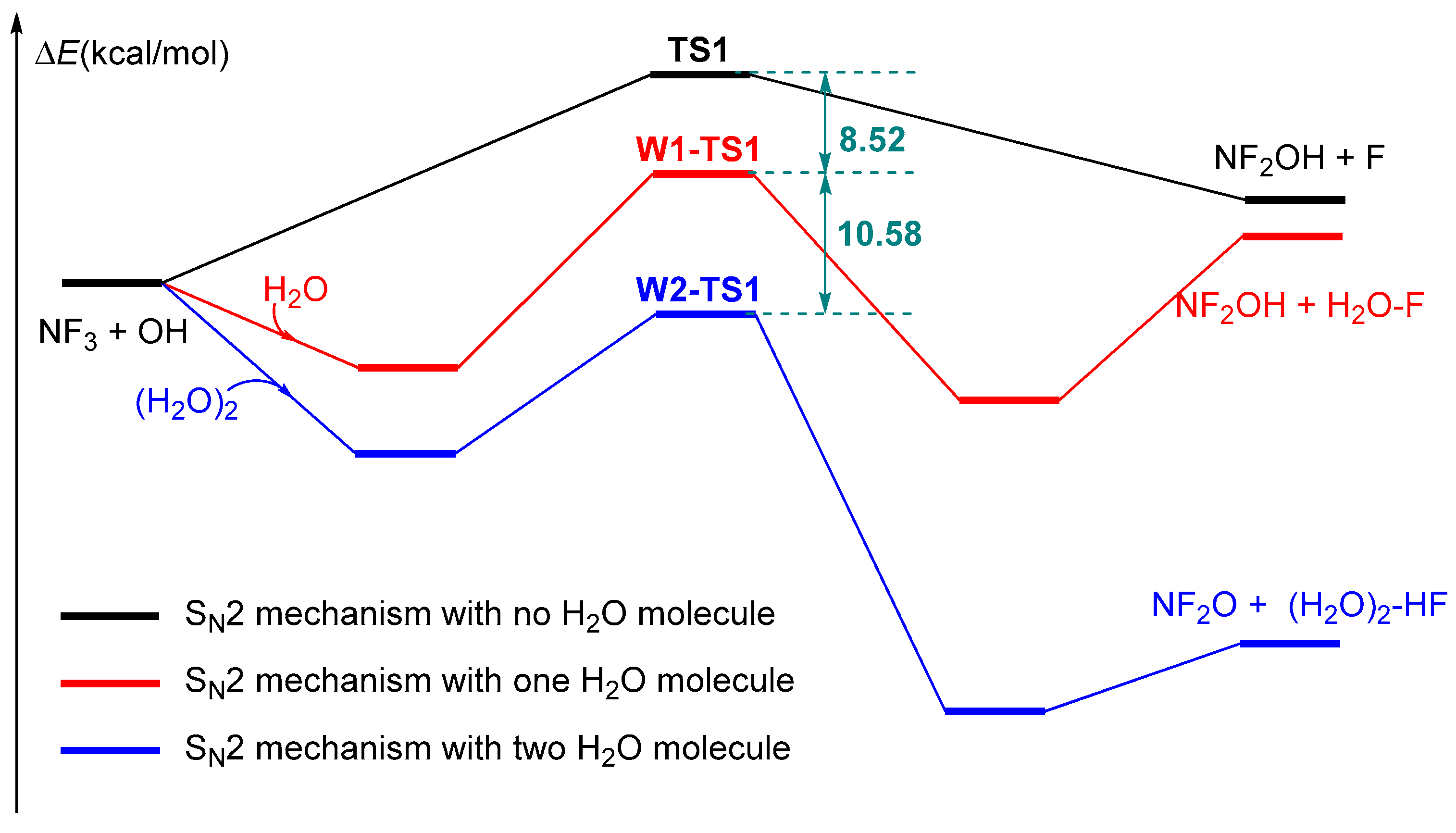
2.1. The Reaction of NF3 + OH
2.2. The Influence of Water Molecules on the Reaction of NF3 + OH
2.3. The Influence of an Additional Two H2O Molecules on the NF3 + OH Reaction
3. Materials and Methods
Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tseng, Y.-H.; Tsui, B.-Y. Microtrenching-free two-step reactive ion etching of 4H-SiC using NF3/HBr/O2 and Cl2/O2. J. Vac. Sci. Technol. A 2014, 32, 031601. [Google Scholar] [CrossRef]
- Tasaka, A.; Takahashi, K.; Tanaka, K.; Shimizu, K.; Mori, K.; Tada, S.; Shimizu, W.; Abe, T.; Inaba, M.; Ogumi, Z. Plasma etching of SiC surface using NF3. J. Vac. Sci. Technol. A 2002, 20, 1254–1260. [Google Scholar] [CrossRef]
- Kastenmeier, B.; Oehrlein, G.; Langan, J.G.; Entley, W.R. Gas utilization in remote plasma cleaning and stripping applications. J. Vac. Sci. Technol. A 2000, 18, 2102–2107. [Google Scholar] [CrossRef]
- Donnelly, V.M. Review Article: Reactions of fluorine atoms with silicon, revisited, again. J. Vac. Sci. Technol. A 2017, 35, 05C202. [Google Scholar] [CrossRef]
- Tasaka, A. Electrochemical synthesis and application of NF3. J. Fluorine Chem. 2007, 128, 296–310. [Google Scholar] [CrossRef]
- Illuzzi, F.; Thewissen, H. Perfluorocompounds emission reduction by the semiconductor industry. J. Integr. Environ. Sci. 2010, 7, 201–210. [Google Scholar] [CrossRef]
- Prather, M.J.; Hsu, J. NF3, the greenhouse gas missing from Kyoto. Geophys. Res. Lett. 2008, 35, L12810. [Google Scholar] [CrossRef]
- Tsai, W.-T. Environmental and health risk analysis of nitrogen trifluoride (NF3), a toxic and potent greenhouse gas. J. Hazard. Mater. 2008, 159, 257–263. [Google Scholar] [CrossRef]
- Molina, L.T.; Wooldridge, P.J.; Molina, M. Atmospheric reactions and ultraviolet and infrared absorptivities of nitrogen trifluoride. Geophys. Res. Lett. 1995, 22, 1873–1876. [Google Scholar] [CrossRef]
- Totterdill, A.; Kovács, T.; Feng, W.; Dhomse, S.; Smith, C.J.; Gómez-Martín, J.C.; Chipperfield, M.P.; Forster, P.M.; Plane, J. Atmospheric lifetimes, infrared absorption spectra, radiative forcings and global warming potentials of NF3 and CF3 CF2 Cl (CFC-115). Atmos. Chem. Phys. 2016, 16, 11451–11463. [Google Scholar] [CrossRef]
- Rogelj, J.; McCollum, D.L.; O’Neill, B.C.; Riahi, K. 2020 emissions levels required to limit warming to below 2 °C. Nat. Clim. Change 2013, 3, 405–412. [Google Scholar] [CrossRef]
- Arnold, T.; Harth, C.M.; Mühle, J.; Manning, A.J.; Salameh, P.K.; Kim, J.; Ivy, D.J.; Steele, L.P.; Petrenko, V.V.; Severinghaus, J.P. Nitrogen trifluoride global emissions estimated from updated atmospheric measurements. Proc. Natl. Acad. Sci. USA 2013, 110, 2029–2034. [Google Scholar] [CrossRef]
- Ji, J.; Xiong, W.; Zhang, X.; Peng, L.; Shi, M.; Wu, Y.; Hu, X. Reversible absorption of NF3 with high solubility in Lewis acidic ionic liquids. Chem. Eng. J. 2022, 440, 135902. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, Y.; Pan, Y.; Li, Y.; Sui, Z.; Xu, X. NF3 decomposition over V2O5, Fe2O3 and Co3O4 coated-Al2O3 reagents: The effect of promoter loadings on reactivity. J. Environ. Chem. Eng. 2020, 8, 103890. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Z.; Li, Y.; Sui, Z.; Liao, W.; Xu, X. NF3 decomposition without water over Cr2O3 coated-Al2O3 reagents. J. Environ. Chem. Eng. 2020, 8, 104166. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Q.; Yin, C.; Pan, Y. NF3 decomposition in the absence of water over some metal oxides coated-Al2O3 reagents. J. Environ. Chem. Eng. 2019, 7, 103192. [Google Scholar] [CrossRef]
- Lai, S.Y.; Pan, W.; Ng, C.F. Catalytic hydrolysis of dichlorodifluoromethane (CFC-12) on unpromoted and sulfate promoted TiO2–ZrO2 mixed oxide catalysts. Appl. Catal. B 2000, 24, 207–217. [Google Scholar] [CrossRef]
- Karmakar, S.; Greene, H.L. Oxidative destruction of chlorofluorocarbons (CFC11 and CFC12) by zeolite catalysts. J. Catal. 1992, 138, 364–376. [Google Scholar] [CrossRef]
- Claudino, D.; Gargano, R.; Carvalho-Silva, V.H.; e Silva, G.M.; Da Cunha, W. Investigation of the abstraction and dissociation mechanism in the nitrogen trifluoride channels: Combined post-Hartree–Fock and Transition State Theory approaches. J. Phys. Chem. A 2016, 120, 5464–5473. [Google Scholar] [CrossRef]
- Ramalho, S.S.; da Cunha, W.F.; Albernaz, A.F.; Neto, P.H.; e Silva, G.M.; Gargano, R. An extensive investigation of reactions involved in the nitrogen trifluoride dissociation. New J. Chem. 2013, 37, 3244–3251. [Google Scholar] [CrossRef]
- Zhao, Z.; Laine, P.L.; Nicovich, J.M.; Wine, P.H. Reactive and nonreactive quenching of O (1D) by the potent greenhouse gases SO2F2, NF3, and SF5CF3. Proc. Natl. Acad. Sci. USA 2010, 107, 6610–6615. [Google Scholar] [CrossRef] [PubMed]
- Baasandorj, M.; Hall, B.; Burkholder, J. Rate coefficients for the reaction of O (1D) with the atmospherically long-lived greenhouse gases NF3, SF5 CF3, CHF3, C2F6, C4 F8, n-C5F12, and n-C6F14. Atmos. Chem. Phys. 2012, 12, 11753–11764. [Google Scholar] [CrossRef]
- Dillon, T.J.; Vereecken, L.; Horowitz, A.; Khamaganov, V.; Crowley, J.N.; Lelieveld, J. Removal of the potent greenhouse gas NF3 by reactions with the atmospheric oxidants O (1D), OH and O3. Phys. Chem. Chem. Phys. 2011, 13, 18600–18608. [Google Scholar] [CrossRef] [PubMed]
- Buszek, R.J.; Francisco, J.S.; Anglada, J.M. Water effects on atmospheric reactions. Int. Rev. Phys. Chem. 2011, 30, 335–369. [Google Scholar] [CrossRef]
- Anglada, J.M.; Gonzalez, J. Different catalytic effects of a single water molecule: The gas-phase reaction of formic acid with hydroxyl radical in water vapor. ChemPhysChem 2009, 10, 3034–3045. [Google Scholar] [CrossRef]
- Gonzalez, J.; Anglada, J.M. Gas phase reaction of nitric acid with hydroxyl radical without and with water. A theoretical investigation. J. Phys. Chem. A 2010, 114, 9151–9162. [Google Scholar] [CrossRef]
- Neeman, E.; González, D.; Blázquez, S.; Ballesteros, B.; Canosa, A.; Antiñolo, M.; Vereecken, L.; Albaladejo, J.; Jiménez, E. The impact of water vapor on the OH reactivity toward CH3CHO at ultra-low temperatures (21.7–135.0 K): Experiments and theory. J. Chem. Phys. 2021, 155, 034306. [Google Scholar] [CrossRef]
- Iuga, C.; Alvarez-Idaboy, J.R.; Reyes, L.; Vivier-Bunge, A. Can a single water molecule really catalyze the acetaldehyde+ OH reaction in tropospheric conditions? J. Phys. Chem. Lett. 2010, 1, 3112–3115. [Google Scholar] [CrossRef]
- Vohringer-Martinez, E.; Hansmann, B.; Hernandez, H.; Francisco, J.; Troe, J.; Abel, B. Water catalysis of a radical-molecule gas-phase reaction. Science 2007, 315, 497–501. [Google Scholar] [CrossRef]
- Bai, F.-Y.; Deng, M.-S.; Chen, M.-Y.; Kong, L.; Ni, S.; Zhao, Z.; Pan, X.-M. Atmospheric oxidation of fluoroalcohols initiated by ˙OH radicals in the presence of water and mineral dusts: Mechanism, kinetics, and risk assessment. Phys. Chem. Chem. Phys. 2021, 23, 13115–13127. [Google Scholar] [CrossRef]
- Gonzalez, J.; Anglada, J.M.; Buszek, R.J.; Francisco, J.S. Impact of water on the OH+ HOCl reaction. J. Am. Chem. Soc. 2011, 133, 3345–3353. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Tan, X.; Ren, D.; Zhang, W. Theoretical study on the water-catalyzed reaction of glyoxal with OH radical. J. Mol. Struct. THEOCHEM 2010, 956, 44–49. [Google Scholar] [CrossRef]
- Iuga, C.; Alvarez-Idaboy, J.R.; Vivier-Bunge, A. Single water-molecule catalysis in the glyoxal+ OH reaction under tropospheric conditions: Fact or fiction? A quantum chemistry and pseudo-second order computational kinetic study. Chem. Phys. Lett. 2010, 501, 11–15. [Google Scholar] [CrossRef]
- Allodi, M.A.; Dunn, M.E.; Livada, J.; Kirschner, K.N.; Shields, G.C. Do hydroxyl radical− water clusters, OH (H2O) n, n= 1− 5, exist in the atmosphere? J. Phys. Chem. A 2006, 110, 13283–13289. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.; Kjaergaard, H.G. Effect of Hydration on the Hydrogen Abstraction Reaction by HO in DMS and its Oxidation Products. J. Phys. Chem. A 2010, 114, 4857–4863. [Google Scholar] [CrossRef]
- Ali, M.A.; Balaganesh, M.; Al-Odail, F.A.; Lin, K. Effect of ammonia and water molecule on OH+ CH3OH reaction under tropospheric condition. Sci. Rep. 2021, 11, 12185. [Google Scholar] [CrossRef]
- Wu, J.; Gao, L.G.; Varga, Z.; Xu, X.; Ren, W.; Truhlar, D.G. Water catalysis of the reaction of methanol with OH radical in the atmosphere is negligible. Angew. Chem. Int. Ed. 2020, 132, 10918–10922. [Google Scholar] [CrossRef]
- Jara-Toro, R.A.; Hernández, F.J.; Taccone, R.A.; Lane, S.I.; Pino, G.A. Water Catalysis of the Reaction between Methanol and OH at 294 K and the Atmospheric Implications. Angew. Chem. Int. Ed. 2017, 56, 2166–2170. [Google Scholar] [CrossRef]
- Schenker, S.; Schneider, C.; Tsogoeva, S.B.; Clark, T. Assessment of popular DFT and semiempirical molecular orbital techniques for calculating relative transition state energies and kinetic product distributions in enantioselective organocatalytic reactions. J. Chem. Theory Comput. 2011, 7, 3586–3595. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Miranda, F.; Occhi, L.; Fontanive, F.; Menziani, M.C.; Pedone, A. Quantum-Chemistry Study of the Hydrolysis Reaction Profile in Borate Networks: A Benchmark. Molecules 2024, 29, 1227. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, H.; Wang, B. Theoretical Study on the Mechanisms and Kinetics of Atmospheric Oxidation of Tetrafluoropropyne and Its Analogues. J. Phys. Chem. A 2024, 128, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tian, Y.; Hou, H.; Wang, B. Mechanistic and kinetic study of the oxidation of trifluoroacetonitrile by hydroxyl and oxygen. Int. J. Quantum Chem. 2023, 123, e27147. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Z.; Zhao, R.; Xu, T. The effect of (H2O) n (n= 1–3) clusters on the reaction of HONO with HCl: A mechanistic and kinetic study. Phys. Chem. Chem. Phys. 2022, 24, 10011–10024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, M.; Liu, Y.; Sun, Y. The influence of a single water molecule on the reaction of BrO+ HONO. J. Mol. Graph. Model. 2022, 116, 108261. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, X.; Wu, X.; Thomsen, D.L.; Bierbaum, V.M.; Xie, J. Single solvent molecules induce dual nucleophiles in gas-phase ion–molecule nucleophilic substitution reactions. J. Phys. Chem. Lett. 2021, 12, 7134–7139. [Google Scholar] [CrossRef]
- Zhang, T.; Zhai, K.; Zhang, Y.; Geng, L.; Geng, Z.; Zhou, M.; Lu, Y.; Shao, X.; Lily, M. Effect of water and ammonia on the HO+ NH3→ NH2+ H2O reaction in troposphere: Competition between single and double hydrogen atom transfer pathways. Comput. Theor. Chem. 2020, 1176, 112747. [Google Scholar] [CrossRef]
- Kumar, A.; Mallick, S.; Kumar, P. Effect of water on the oxidation of CO by a Criegee intermediate. Phys. Chem. Chem. Phys. 2020, 22, 21257–21266. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, M.; Zhang, Y.; Lan, X.; Long, B.; Wang, R.; Yu, X.; Zhao, C.; Wang, W. Atmospheric chemistry of the self-reaction of HO2 radicals: Stepwise mechanism versus one-step process in the presence of (H2O) n (n= 1–3) clusters. Phys. Chem. Chem. Phys. 2019, 21, 24042–24053. [Google Scholar] [CrossRef]
- Chao, W.; Yin, C.; Takahashi, K. Effects of water vapor on the reaction of CH2OO with NH3. Phys. Chem. Chem. Phys. 2019, 21, 22589–22597. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Alipour, M.; Fallahzadeh, P. First principles optimally tuned range-separated density functional theory for prediction of phosphorus–hydrogen spin–spin coupling constants. Phys. Chem. Chem. Phys. 2016, 18, 18431–18440. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Montgomery, J.A., Jr.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J. Chem. Phys. 1999, 110, 2822–2827. [Google Scholar] [CrossRef]
- Montgomery, J.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VII. Use of the minimum population localization method. J. Chem. Phys. 2000, 112, 6532–6542. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
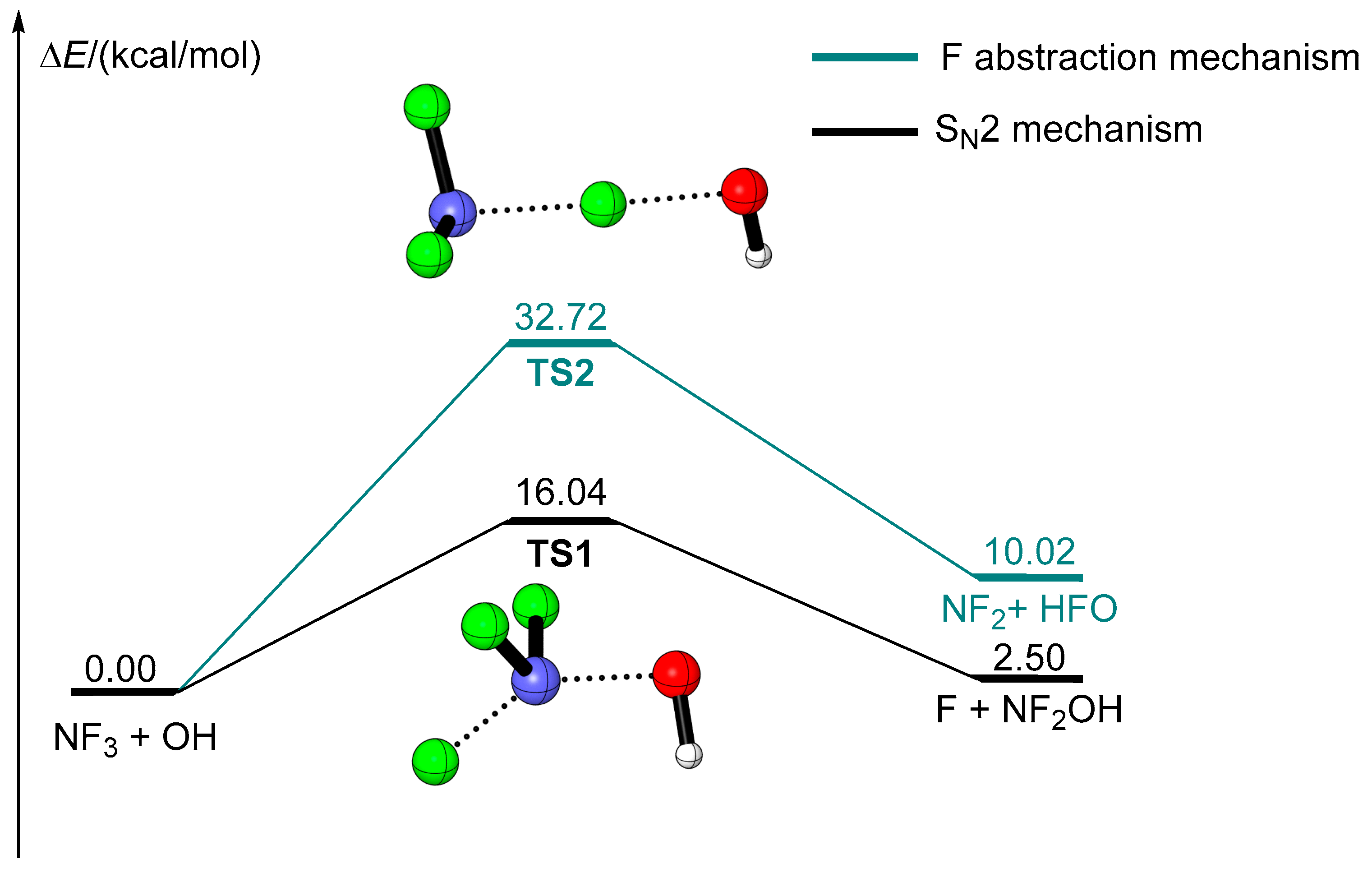
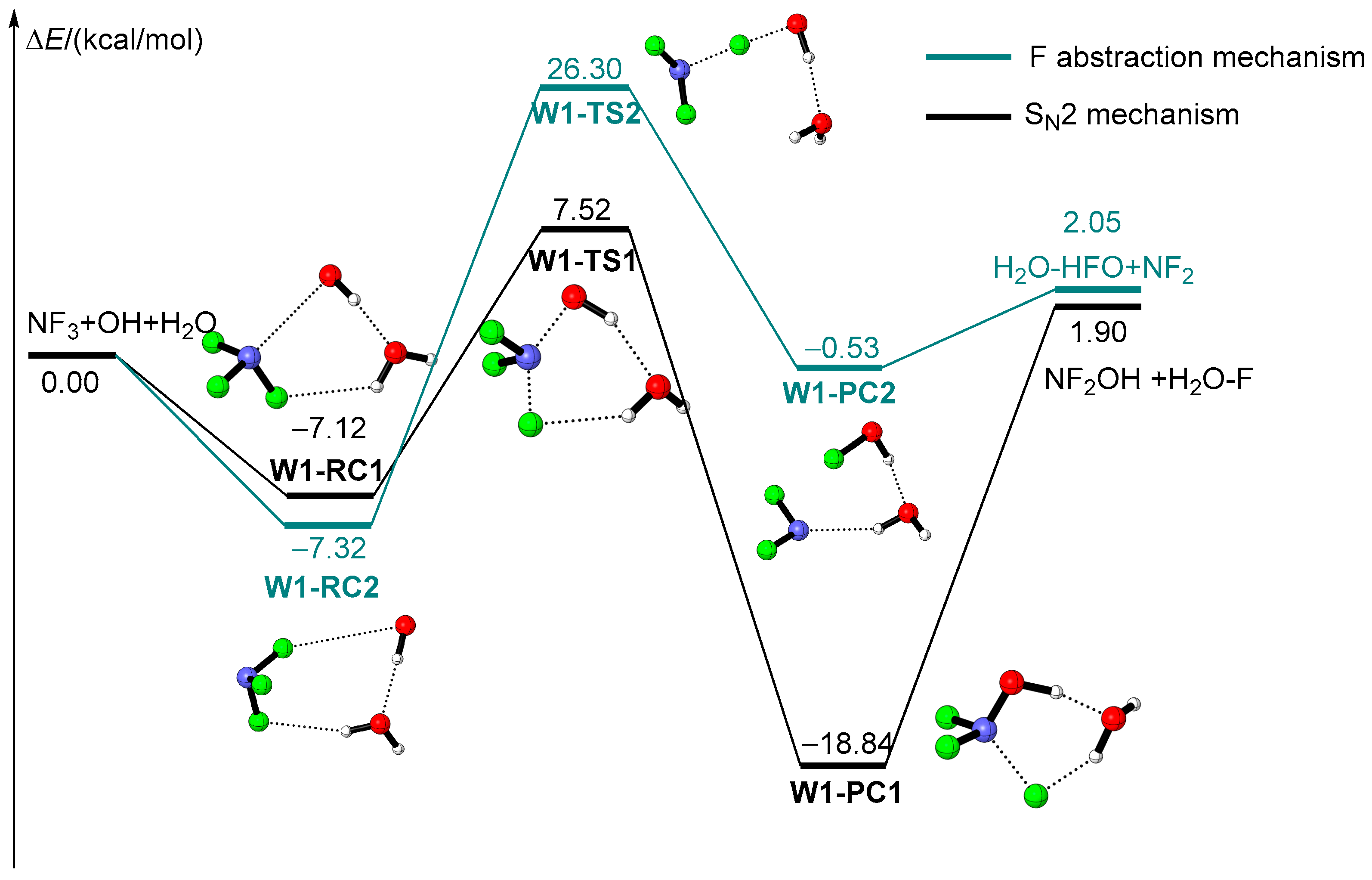
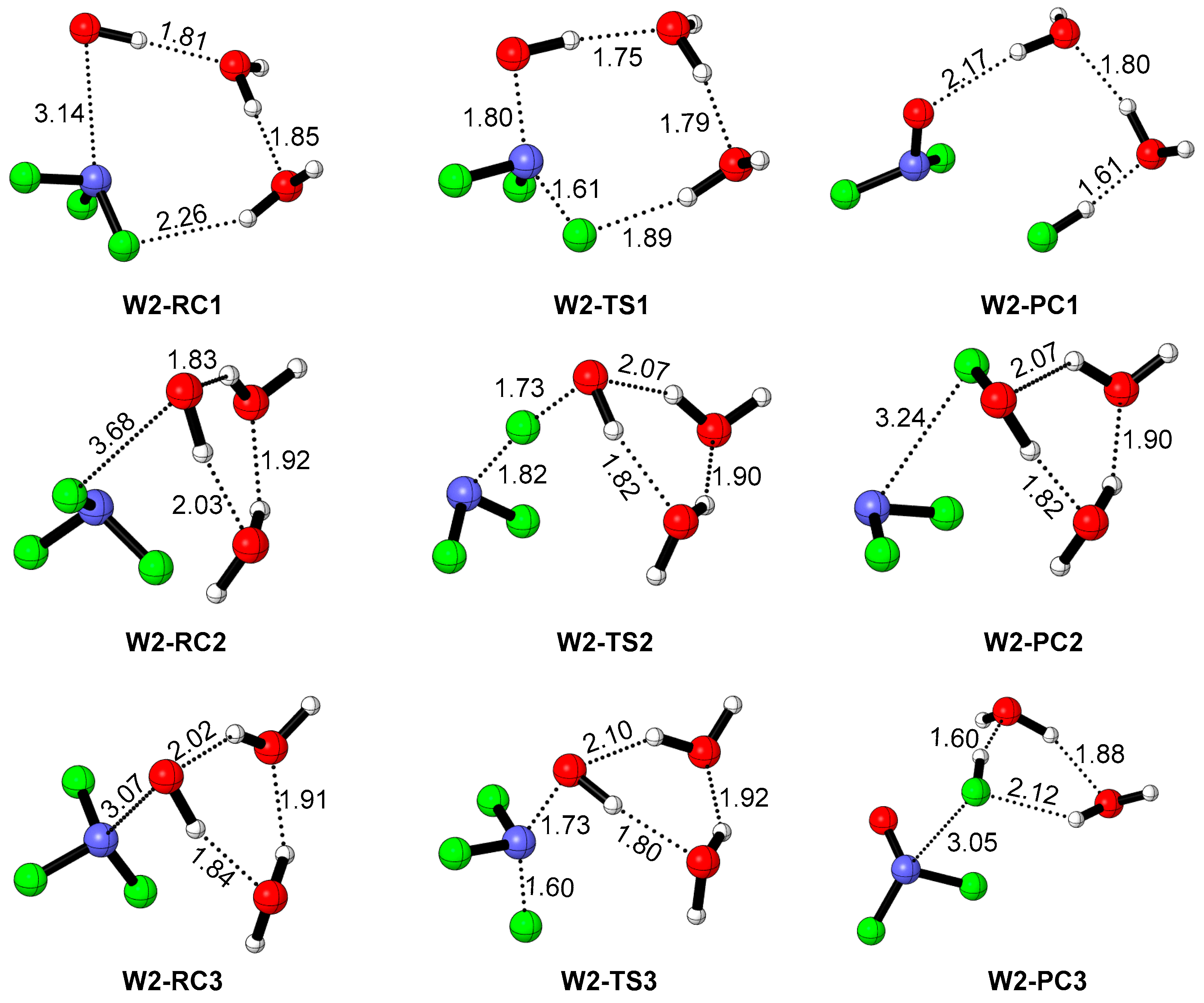
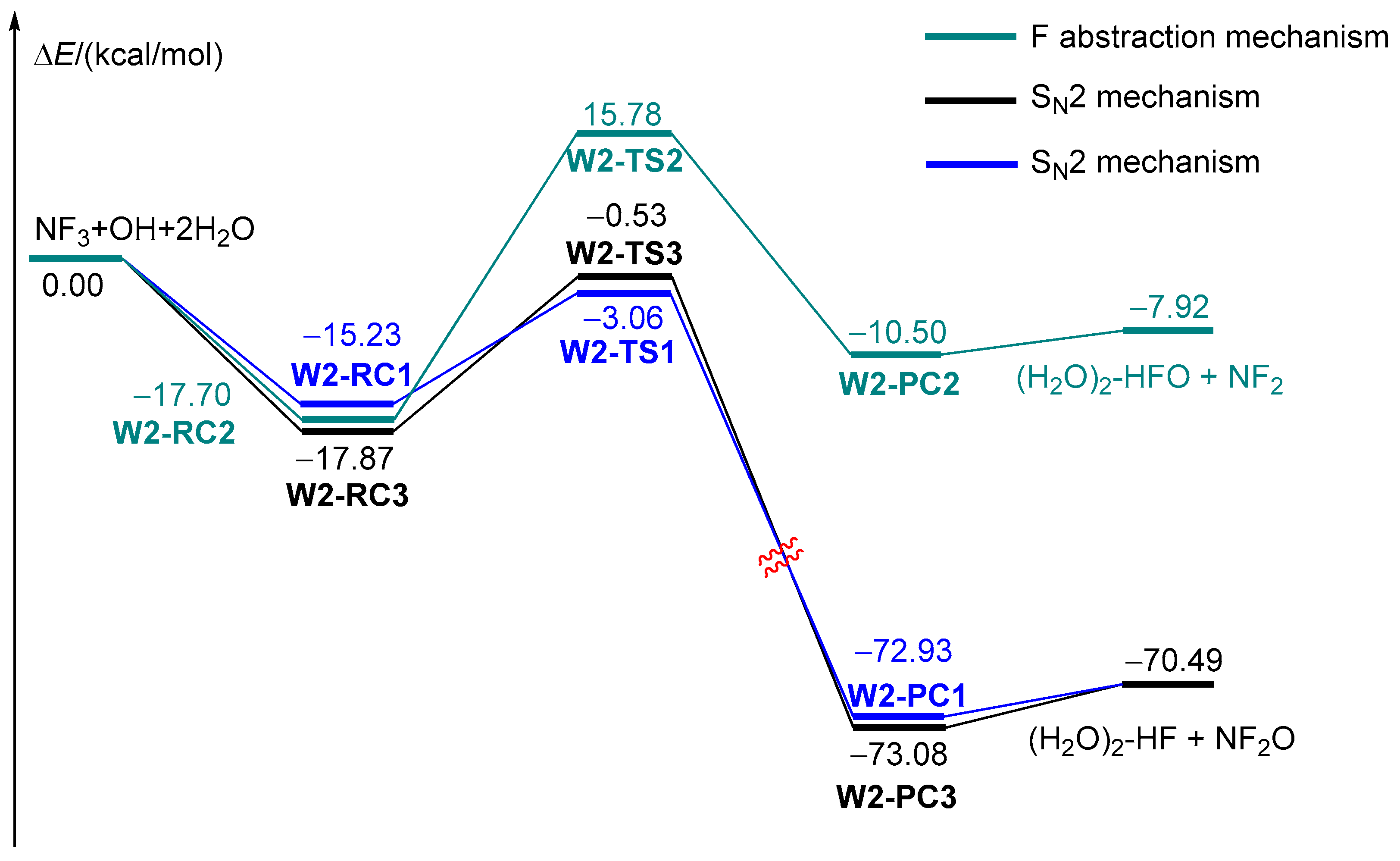
| Species | ZPE (ωB97XD/avtz) | ΔE + ZPE(ωB97XD/avtz) | ΔE (CBS-QB3) |
|---|---|---|---|
| NF3+ OH + nH2O | / | 0 | 0 |
| TS1 | 13.72 | 19.77 | 16.04 |
| TS2 | 12.69 | 32.24 | 32.72 |
| W1-RC1 | 28.24 | −4.40 | −7.12 |
| W1-RC2 | 28.21 | −4.32 | −7.32 |
| W1-TS1 | 29.58 | 13.87 | 7.52 |
| W1-TS2 | 28.00 | 27.75 | 26.30 |
| W1-PC1 | 31.23 | −18.94 | −18.84 |
| W1-PC2 | 29.58 | 2.12 | −0.53 |
| W2-RC1 | 44.22 | −9.60 | −15.23 |
| W2-RC2 | 44.88 | −11.16 | −17.70 |
| W2-RC3 | 44.86 | −11.28 | −17.87 |
| W2-TS1 | 45.73 | 5.99 | −3.06 |
| W2-TS2 | 44.55 | 20.69 | 15.78 |
| W2-TS3 | 45.60 | 8.39 | −0.53 |
| W2-PC1 | 45.98 | −66.14 | −72.93 |
| W2-PC2 | 45.56 | −4.78 | −10.50 |
| W2-PC3 | 46.30 | −66.01 | −73.08 |
| NF2OH + F | 15.06 | 2.79 | 2.50 |
| NF2 + HFO | 12.84 | 8.95 | 10.02 |
| NF2OH + H2O-F | 28.39 | 5.95 | 1.90 |
| NF2 + H2O-HFO | 28.57 | 2.98 | 2.05 |
| NF2O + (H2O)2-HF | 43.85 | −64.63 | −70.49 |
| NF2 + (H2O)2-HFO | 44.99 | −3.90 | −7.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhao, Y.; Lian, X.; Li, D.; Zhang, X.; Chen, J.; Deng, B.; Lan, X.; Shao, Y. Unveiling the Influence of Water Molecules for NF3 Removal by the Reaction of NF3 with OH: A DFT Study. Molecules 2024, 29, 4033. https://doi.org/10.3390/molecules29174033
Liu J, Zhao Y, Lian X, Li D, Zhang X, Chen J, Deng B, Lan X, Shao Y. Unveiling the Influence of Water Molecules for NF3 Removal by the Reaction of NF3 with OH: A DFT Study. Molecules. 2024; 29(17):4033. https://doi.org/10.3390/molecules29174033
Chicago/Turabian StyleLiu, Jiaxin, Yong Zhao, Xueqi Lian, Dongdong Li, Xueling Zhang, Jun Chen, Bin Deng, Xiaobing Lan, and Youxiang Shao. 2024. "Unveiling the Influence of Water Molecules for NF3 Removal by the Reaction of NF3 with OH: A DFT Study" Molecules 29, no. 17: 4033. https://doi.org/10.3390/molecules29174033
APA StyleLiu, J., Zhao, Y., Lian, X., Li, D., Zhang, X., Chen, J., Deng, B., Lan, X., & Shao, Y. (2024). Unveiling the Influence of Water Molecules for NF3 Removal by the Reaction of NF3 with OH: A DFT Study. Molecules, 29(17), 4033. https://doi.org/10.3390/molecules29174033





