The Composition Optimization of Curcumin-Loaded Double Oil–Water–Oil Emulsions and Their Stability Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results of Design Experiment and ANOVA Statistical Analysis
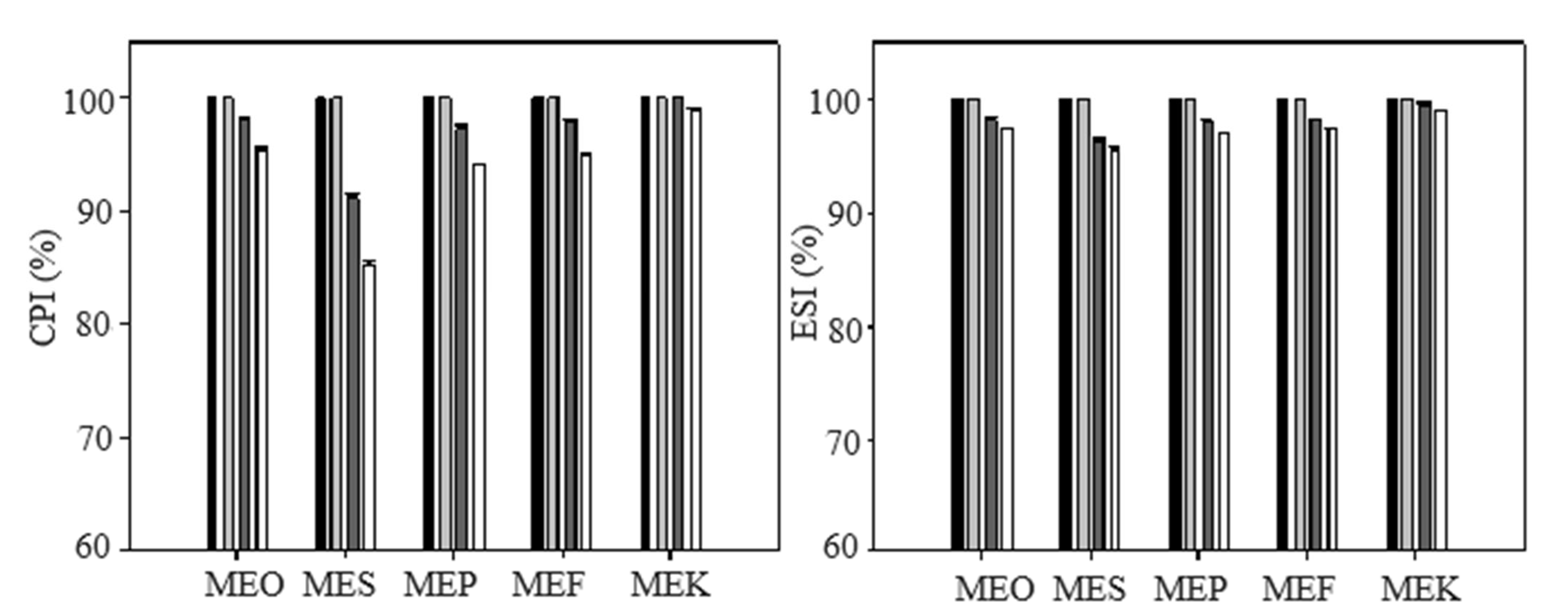
2.2. Determination of Emulsion Stability by the Creaming Index (CPI)
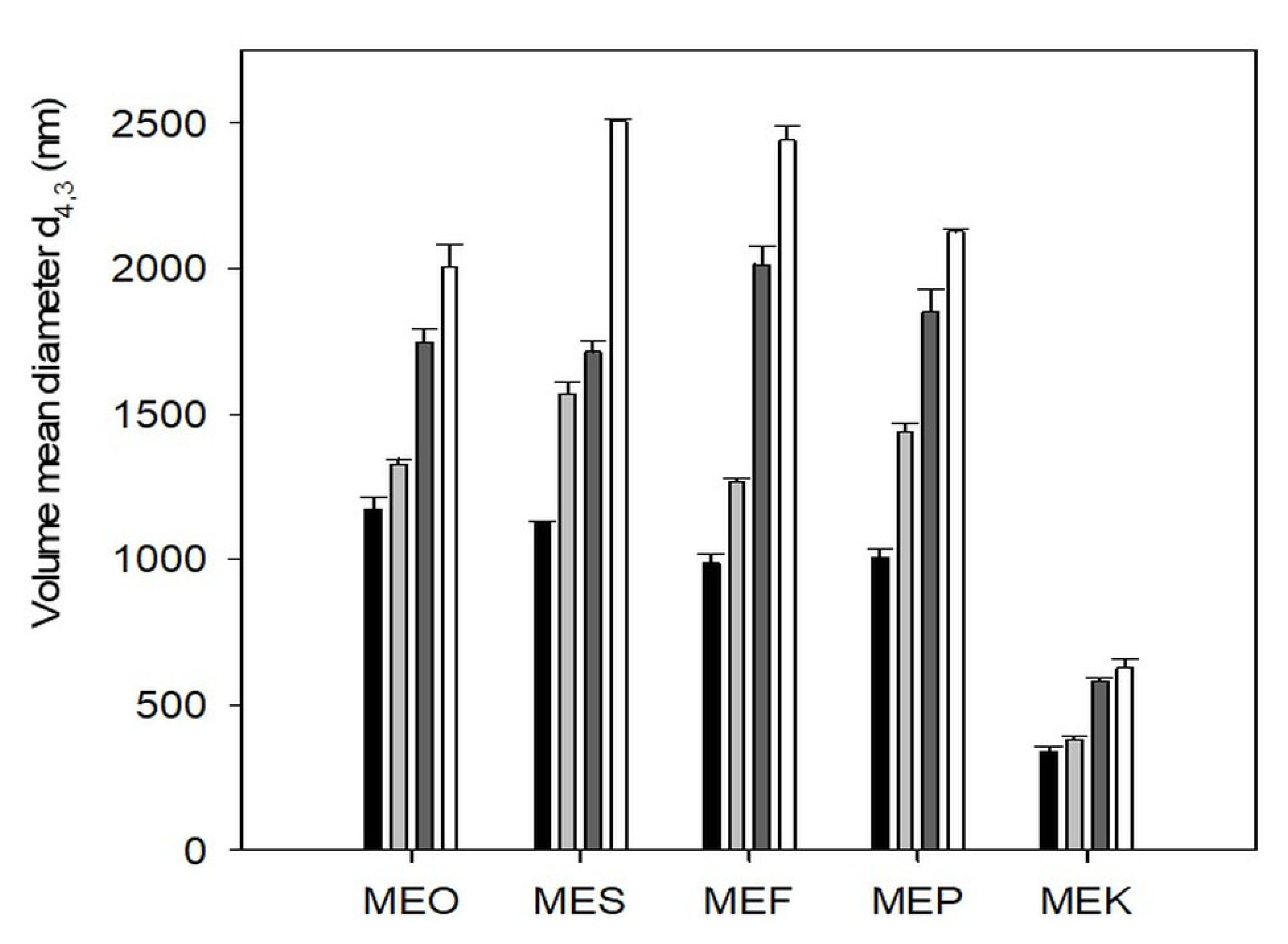
2.3. Particle Size
2.4. Results of Encapsulation Efficiency
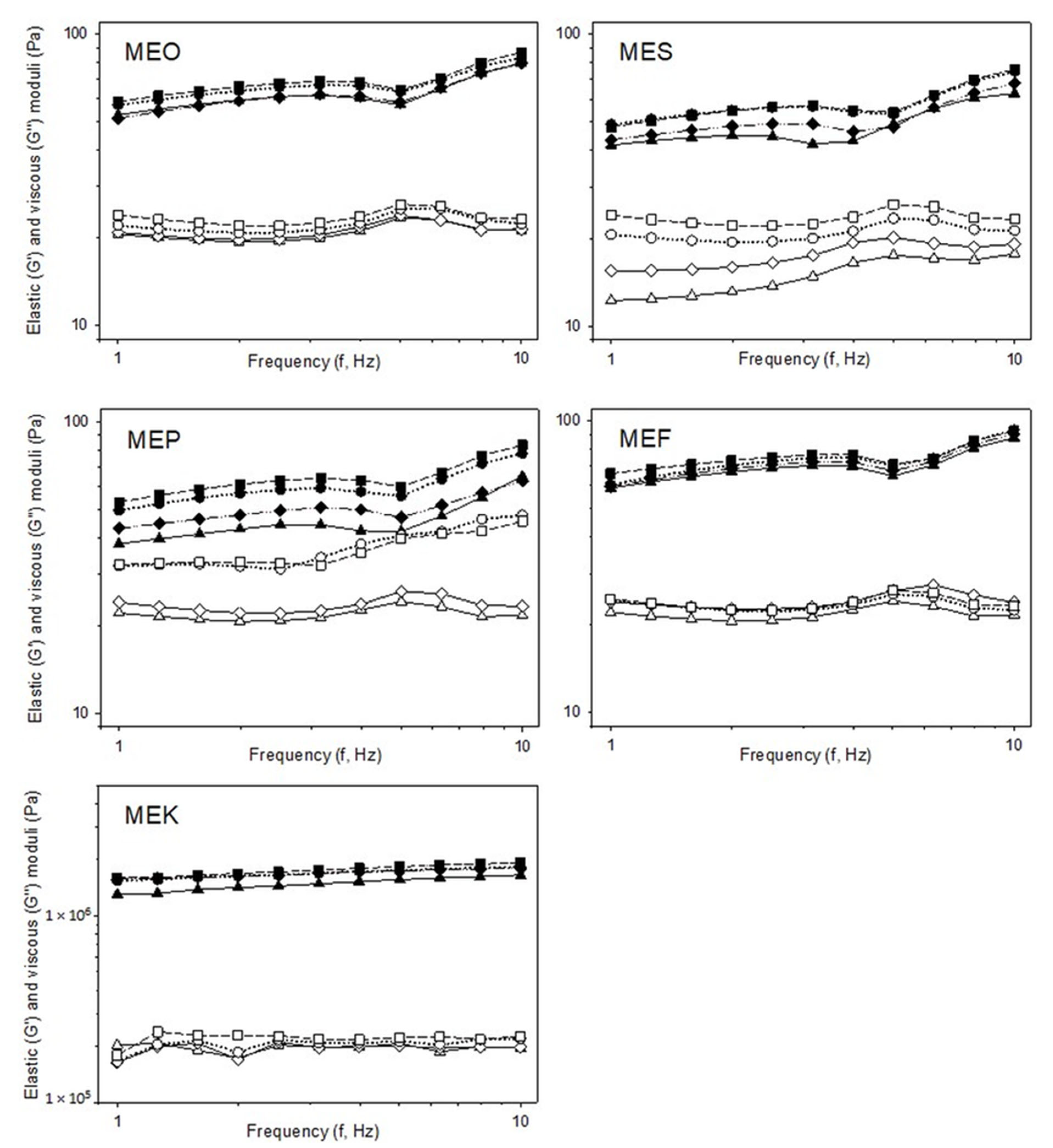
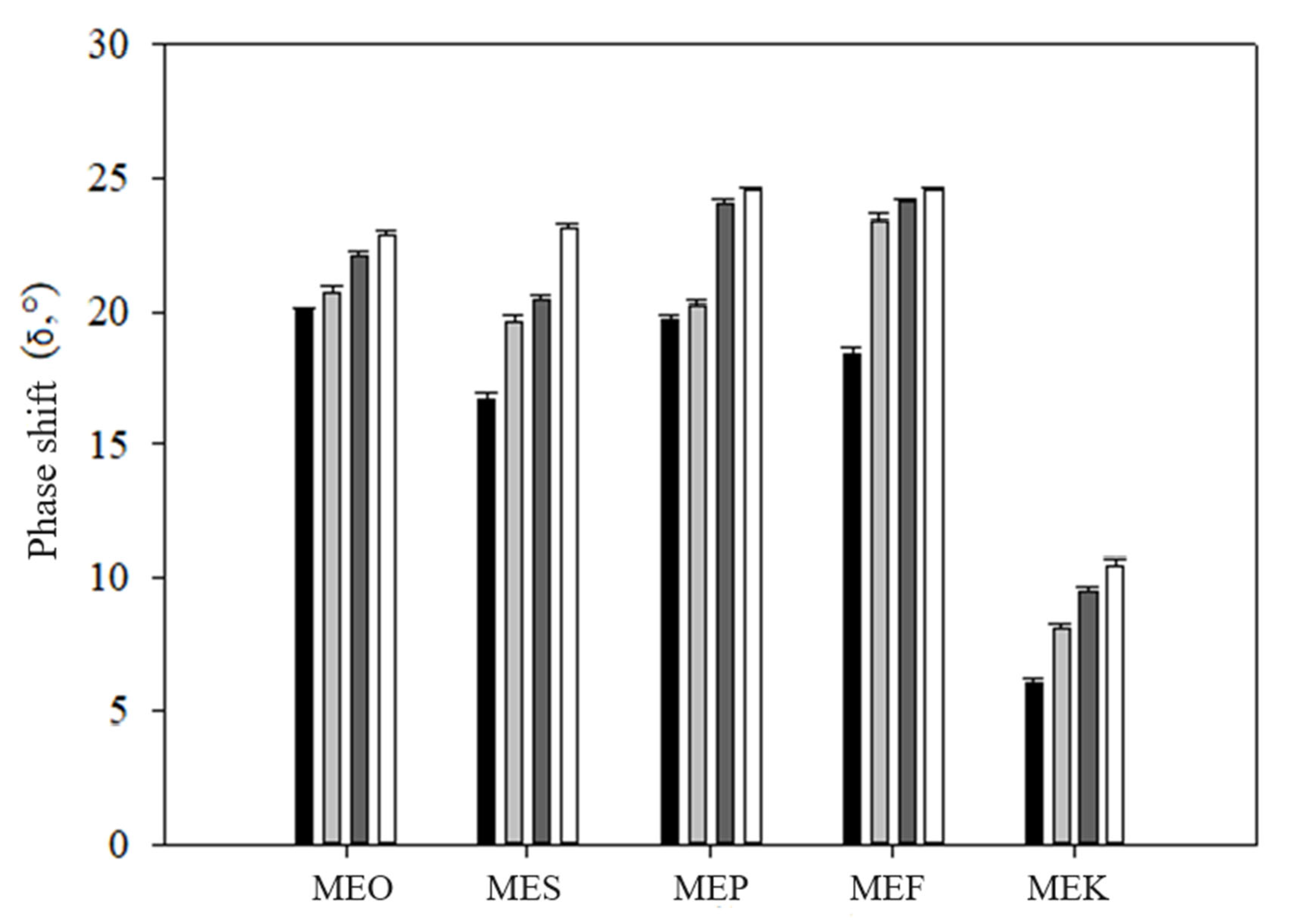
2.5. Rheology
2.6. Differential Scanning Calorimetry
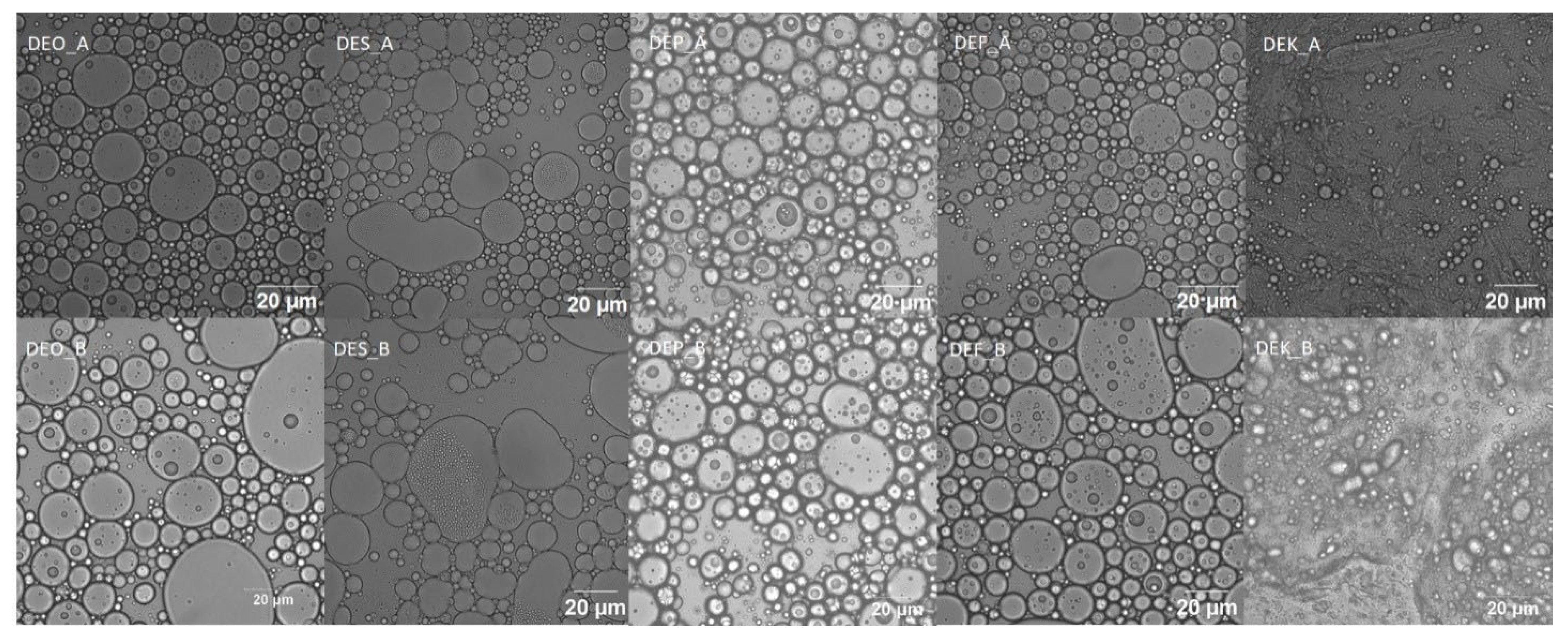
2.7. Results of the Confocal Laser Microscopy
3. Materials and Methods
3.1. Materials
Design Experiment and ANOVA Statistical Analysis
3.2. Preparation of Model Samples
3.2.1. Preparation of the Inner Emulsion Phase (O/W)
3.2.2. Preparation of the Multiple Emulsion (O/W/O)
3.3. Determination of Emulsion Stability by the Creaming Index (CI)
3.4. Determination of the Emulsion Stability Index (ESI)
3.5. Particle Size Measurement
3.6. Encapsulation Efficiency
3.7. Dynamic Oscillatory Rheology
3.8. Determination of Emulsion Stability by Differential Scanning Calorimetry (DSC)
3.9. Confocal Laser Microscopy
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Campelo, M.S.; Aragão, C.B.; de Melo, D.G.; Barbosa, M.L.F.; de Oliveira, R.N.M.P.S.; Muniz, C.R.; Ribeiro, W.L.C.; Ribeiro, M.E.N.P. Curcumin-Loaded Emulsion Improves Its In Vitro Anthelmintic Activity against Benzimidazole-Resistant Haemonchus contortus. Food Biosci. 2024, 59, 104052. [Google Scholar] [CrossRef]
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverrí, J. Renoprotective Effect of the Antioxidant Curcumin: Recent Findings. Redox Biol. 2013, 1, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Keramat, M.; Golmakani, M. Antioxidant Potency and Inhibitory Mechanism of Curcumin and Its Derivatives in Oleogel and Emulgel Produced by Linseed Oil. Food Chem. 2024, 445, 136814. [Google Scholar] [CrossRef]
- Kaur, R. Turmeric: A Golden Herb with Health-Promoting Components. Just Agric. 2024, 4, 5. [Google Scholar]
- Bradford, P.G. Curcumin and Obesity. Biofactors 2013, 39, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Akram, M.; Shahab-Uddin, A.A.; Usmanghani, K.; Hannan, A.; Mohiuddin, E.; Asif, M. Curcuma Longa and Curcumin: A Review Article. Rom. J. Biol. Plant Biol. 2010, 55, 65–70. [Google Scholar]
- Kim, K.; Kim, J.-J.; Jung, Y.; Noh, J.-Y.; Syed, A.S.; Kim, C.Y.; Lee, M.-Y.; Lim, K.-M.; Bae, O.-N.; Chung, J.-H. Cyclocurcumin, an antivasoconstrictive constituent of Curcuma longa (Turmeric). J. Nat. Prod. 2017, 80, 196–200. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Zhang, L.; Wang, M.; Li, D.; Liu, N.; Liu, D.; Zhao, M.; Yao, X. Construction, Characterization, and Bioactivity Evaluation of Curcumin Nanocrystals with Extremely High Solubility and Dispersion Prepared by Ultrasound-Assisted Method. Ultrason. Sonochem. 2024, 104, 106835. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Cao, W.; Yan, Y.; Guo, F.; Li, J.; Li, W. Stability and Gastrointestinal Behavior of Curcumin-Loaded Emulsion Stabilized by Multi-Conformation Soy Proteins: Influence of Oil Volume Fraction. Food Chem. 2024, 440, 138215. [Google Scholar] [CrossRef]
- Bako, H.K.; Ibeogu, H.I.; Bassey, A.P.; Yar, M.S.; Zhou, T.; Li, C. Optimization and Characterization of Double Emulsion Derived from Rice Starch, Rice Protein Isolates, and Rice Bran Oil. Int. J. Biol. Macromol. 2024, 258, 128966. [Google Scholar] [CrossRef]
- Alouk, I.; Xu, D.; Cao, Y. Encapsulation of Natural Pigments by Double Emulsion: A Review. Food Biosci. 2023, 56, 103411. [Google Scholar] [CrossRef]
- Leister, N.; Karbstein, H.P. Evaluating the Stability of Double Emulsions—A Review of the Measurement Techniques for the Systematic Investigation of Instability Mechanisms. Colloids Interfaces 2020, 4, 8. [Google Scholar] [CrossRef]
- Zhao, J.; Bhandari, B.; Gaiani, C.; Prakash, S. Fermentation of Almond-Based Gel Incorporated with Double Emulsion (W1/O/W2): A Study on Gel Properties and Effectiveness of Double Emulsion as a Fat Replacer. Food Struct. 2023, 36, 100322. [Google Scholar] [CrossRef]
- Zhi, Z.; Li, H.; Geurs, I.; Lewille, B.; Liu, R.; Van der Meeren, P.; Dewettinck, K.; van Bockstaele, F. Dual Stabilization of O/W/O Double Emulsions by Proteins: An Interfacial Perspective. Food Hydrocoll. 2024, 148, 109488. [Google Scholar] [CrossRef]
- Heidari, F.; Jafari, S.M.; Ziaiifar, A.M.; Malekjani, N. Stability and Release Mechanisms of Double Emulsions Loaded with Bioactive Compounds: A Critical Review. Adv. Colloid Interface Sci. 2022, 299, 102567. [Google Scholar] [CrossRef]
- Eslami, P.; Forootan, K.; Davarpanah, L.; Vahabzadeh, F. Incorporation of Lactobacillus Casei into the Inner Phase of the Water-in-Oil-in-Water (W1/O/W2) Emulsion Prepared with Β-Cyclodextrin and Bacterial Survival in a Model Gastric Environment. Appl. Food Biotechnol. 2020, 7, 171–182. [Google Scholar] [CrossRef]
- Klojdová, I.; Stathopoulos, C. The Potential Application of Pickering Multiple Emulsions in Food. Foods 2022, 11, 1558. [Google Scholar] [CrossRef]
- Gu, Y.; Li, H.; Liu, L.; Li, J.; Zhang, B.; Ma, H. Constructing CNTs-Based Composite Membranes for Oil/Water Emulsion Separation via Radiation-Induced “Grafting to” Strategy. Carbon 2021, 178, 678–687. [Google Scholar] [CrossRef]
- Elaine, E.; Bhandari, B.; Tan, C.P.; Nyam, K.L. Recent Advances in the Formation, Stability, and Emerging Food Application of Water-in-Oil-in-Water Double Emulsion Carriers. Food Bioprocess Technol. 2024, 1–21. [Google Scholar] [CrossRef]
- Bhattacharya, S. Central Composite Design for Response Surface Methodology and Its Application in PharmacyIn Response Surface Methodology in Engineering Science; IntechOpen: London, UK, 2021; ISBN 978-1-83968-918-5. [Google Scholar]
- Izadiyan, P.; Hemmateenejad, B. Multi-Response Optimization of Factors Affecting Ultrasonic-Assisted Extraction from Iranian Basil Using Central Composite Design. Food Chem. 2016, 190, 864–870. [Google Scholar] [CrossRef]
- Rajmohan, T.; Palanikumar, K. Application of the Central Composite Design in Optimization of Machining Parameters in Drilling Hybrid Metal Matrix Composites. Measurement 2013, 46, 1470–1481. [Google Scholar] [CrossRef]
- Gautam, S.; Lapcik, L.; Lapcikova, B.; Repka, D.; Szyk-Warszyńska, L. Physicochemical Characterisation of Polysaccharide Films with Embedded Bioactive Substances. Foods 2023, 12, 4454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, W.; Wang, J.; Zhang, R.; Zhang, J. Comparison of oil-in-water emulsions prepared by ultrasound, high-pressure homogenization and high-speed homogenization. Ultrason. Sonochem. 2022, 82, 105885. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, I.; Chang, J.; Chou, T. The impact of the surfactant type on physicochemical properties, encapsulation, and in vitro biocompatibility of coconut oil nanoemulsions. J. Taiwan Inst. Chem. Eng. 2022, 137, 104217. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef]
- Beegum, P.P.S.; Ramesh, S.V.; Pandiselvam, R.; Neema, M.; Daliyamol, M.R.; Manikantan, H.; Hebbar, K.B. Perspectives on the cardioprotective, neuroprotective and anti-obesity functions of coconut (Cocos nucifera L.). Food Biosci. 2024, 58, 103756. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Joyner, H.S. Explaining food texture through rheology. Curr. Opin. Food Sci. 2018, 21, 7–14. [Google Scholar] [CrossRef]
- Pal, R. Rheology of double emulsions. J. Colloid Interface Sci. 2007, 307, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Georges, P.C.; Hvidt, S. Basic rheology for biologists. Methods Cell Biol. 2007, 83, 3–27. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Nikzade, V.; Tehrani, M.M.; Saadatmand-Tarzjan, M. Optimization of low-cholesterol–low-fat mayonnaise formulation: Effect of using soy milk and some stabilizer by a mixture design approach. Food Hydrocoll. 2012, 28, 344–352. [Google Scholar] [CrossRef]
- Prieto, C.; Lagaron, J.M. Nanodroplets of docosahexaenoic acid-enriched algae oil encapsulated within microparticles of hydrocolloids by emulsion electrospraying assisted by pressurized gas. Nanomaterials 2020, 10, 270. [Google Scholar] [CrossRef]
- Kůrová, V.; Salek, R.N.; Vašina, M.; Vinklárková, K.; Zálešáková, L.; Gál, R.; Adámek, R.; Buňka, F. The effect of homogenization and addition of polysaccharides on the viscoelastic properties of processed cheese sauce. J. Dairy Sci. 2022, 105, 6563–6577. [Google Scholar] [CrossRef]
- Dalmazzone, C.; Noïk, C.; Clausse, D. Application of DSC for emulsified system characterization. Oil Gas Sci. Technol. 2009, 64, 543–555. [Google Scholar] [CrossRef]
- Tylewicz, U.; Aganovic, K.; Vannini, M.; Toepfl, S.; Bortolotti, V.; Dalla Rosa, M.; Oey, I.; Heinz, V. Effect of pulsed electric field treatment on water distribution of freeze-dried apple tissue evaluated with DSC and TD-NMR techniques. Innov. Food Sci. Emerg. Technol. 2016, 37, 352–358. [Google Scholar] [CrossRef]
- Granato, D.; de Araújo Calado, V.M.; Jarvis, B. Observations on the use of statistical methods in food science and technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]


| Ingredients | Composition 1 |
|---|---|
| Curcumin (g) | 1 |
| Nigella seed oil (mL) | 10 |
| Distilled water (mL) | 90 |
| Tween 20 (g) | 0.5 |
| κ-carrageenan (g) | 1 |
| Processing Parameters | |
| Homogenization speed (RPM) 2 | 25,000 |
| Homogenization time (min) | 5 |
| Temperature (°C) | 25 |
| Oil Type (Producer) | Content of Fatty Acids (g/100 mL) | ||
|---|---|---|---|
| Saturated Fatty Acid | Monounsaturated Fatty Acids | Polyunsaturated Fatty Acids | |
| Olive (100% Bio extra virgin oil, Headquartes esporao, Espana) | 13.1 | 71.8 | 6.3 |
| Sunflower (Bio virgin oil, Country life, Slovakia) | 6.5 | 85.8 a | |
| Flaxseed (100% Bio virgin oil, Greenspol, Hungary) | 10.0 | 20.0 | 70.0 |
| Pumpkin (Bio 100% virgin oil, Helth lins, The Netherlands) | 17.0 | 25.0 | 50.0 |
| Coconut (Fair trade, Bio virgin oil, Purity Vision, Czechia Republic) | 95.0 | 5.0 a | |
| Ingredients | MEO | MES | MEF | MEP | MEK |
|---|---|---|---|---|---|
| Inner phase (mL) | 40 | 40 | 40 | 40 | 40 |
| SPAN 80 (g) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Oil (mL) | 60 | 60 | 60 | 60 | 60 |
| Oil type | olive | sunflower | flax | pumpkin | coconut |
| Processing parameters | |||||
| Homogenization speed (RPM) 1 | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 |
| Homogenization time (min) | 5 | 5 | 5 | 5 | 5 |
| Temperature (°C) | 25 | 25 | 25 | 25 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opustilová, K.; Lapčíková, B.; Kocourková, K.; Lapčík, L. The Composition Optimization of Curcumin-Loaded Double Oil–Water–Oil Emulsions and Their Stability Evaluation. Molecules 2024, 29, 4035. https://doi.org/10.3390/molecules29174035
Opustilová K, Lapčíková B, Kocourková K, Lapčík L. The Composition Optimization of Curcumin-Loaded Double Oil–Water–Oil Emulsions and Their Stability Evaluation. Molecules. 2024; 29(17):4035. https://doi.org/10.3390/molecules29174035
Chicago/Turabian StyleOpustilová, Kristýna, Barbora Lapčíková, Karolina Kocourková, and Lubomír Lapčík. 2024. "The Composition Optimization of Curcumin-Loaded Double Oil–Water–Oil Emulsions and Their Stability Evaluation" Molecules 29, no. 17: 4035. https://doi.org/10.3390/molecules29174035






