Heteroleptic Complexes of Ruthenium Nitrosyl with Pyridine and Bypiridine—Synthesis and Photoisomerization
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure
2.1.1. [RuNO(Py)(Bpy)ClOH]PF6
2.1.2. [RuNO(Py)(Bpy)ClF]PF6
2.2. Photogeneration of Linkage Isomers in Solid State
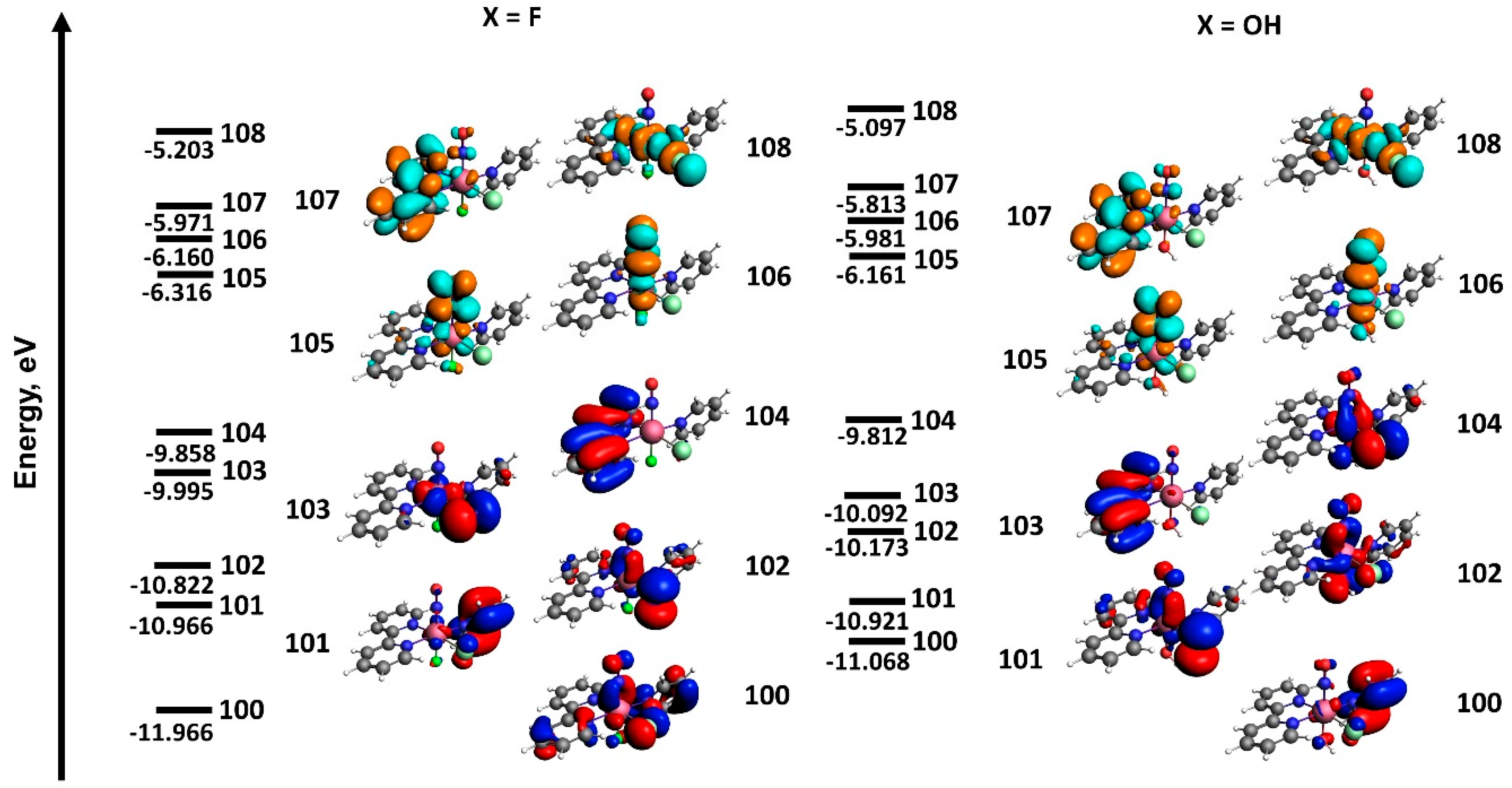
2.3. Molecular Orbitals and Electronic Transitions
3. Materials and Methods
3.1. Synthesis of [RuNO(Py)(Bpy)ClOH]PF6 (1)
3.2. Synthesis of [RuNO(Py)(Bpy)ClF]PF6 (2)
3.3. IR-Spectroscopy
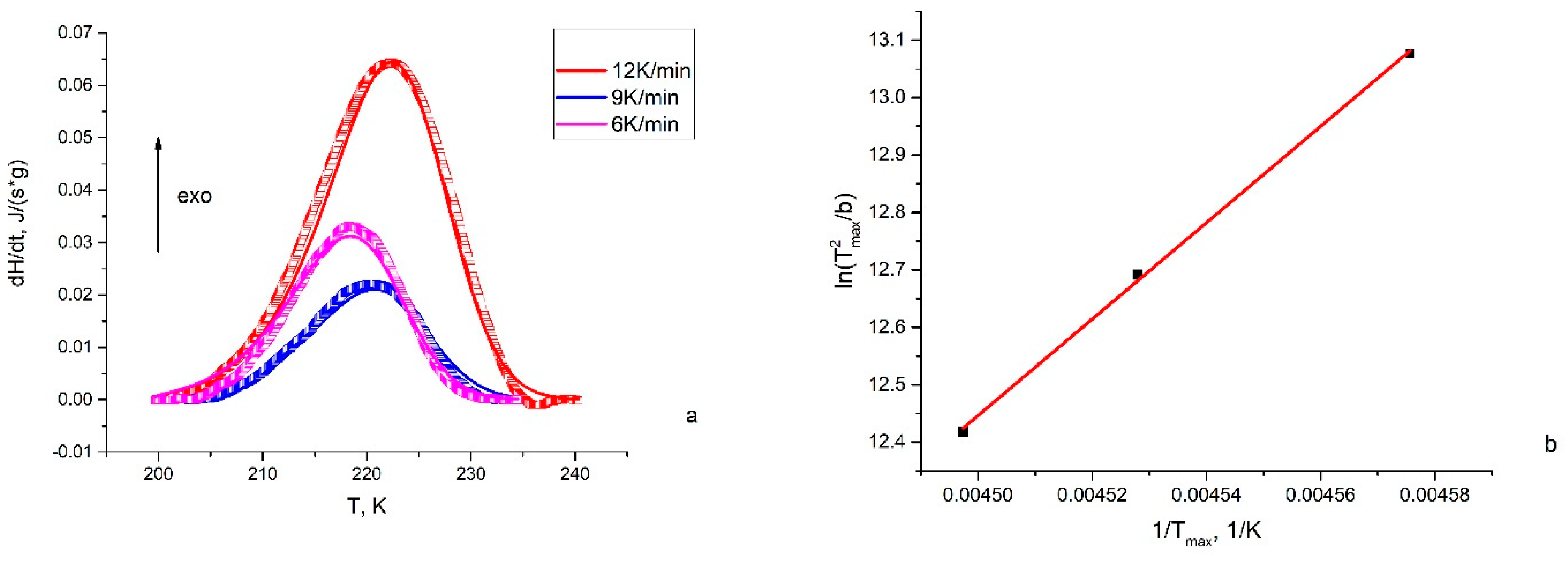
3.4. Differential Scanning Calorimetry (DSC)
3.5. Single-Crystal X-ray Diffraction
3.6. Density Functional Theory (DFT) Calculations
3.7. The Hirshfeld Surfaces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balzani, V.; Ceroni, P.; Credi, A.; Venturi, M. Ruthenium Tris(Bipyridine) Complexes: Interchange between Photons and Electrons in Molecular-Scale Devices and Machines. Coord. Chem. Rev. 2021, 433, 213758. [Google Scholar] [CrossRef]
- Shum, J.; Leung, P.K.-K.; Lo, K.K.-W. Luminescent Ruthenium(II) Polypyridine Complexes for a Wide Variety of Biomolecular and Cellular Applications. Inorg. Chem. 2019, 58, 2231–2247. [Google Scholar] [CrossRef]
- Dixon, I.M.; Lebon, E.; Sutra, P.; Igau, A. Luminescent Ruthenium–Polypyridine Complexes & Phosphorus Ligands: Anything but a Simple Story. Chem. Soc. Rev. 2009, 38, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.J.; Godwin, J.B. Preparation of Ruthenium Nitrosyl Complexes Containing 2,2’-Bipyridine and 1,10-Phenanthroline. Inorg. Chem. 1971, 10, 471–474. [Google Scholar] [CrossRef]
- Hirano, T.; Ueda, K.; Mukaida, M.; Nagao, H.; Oi, T. Reactions of [RuCl2(NO)(Terpy)]+ (Terpy = 2,2′: 6′,2″-Terpyridine) with Mono Anions Such as NO2−, Br− and N3−, and Structural Studies on Terpyridineruthenium Having a Nitrosyl Ligand. J. Chem. Soc. Dalt. Trans. 2001, 16, 2341–2345. [Google Scholar] [CrossRef]
- Togano, T.; Kuroda, H.; Nagao, N.; Maekawa, Y.; Nishimura, H.; Howell, F.S.; Mukaida, M. Synthesis, Properties and Molecular Structure of Trans-Hydroxobis(2, 2′-Bipyridine)Nitrosylruthenium(2+): Influence of Axial Ligand on Characteristics of Nitrosyl Moiety in Trans-[Ru(NO)XL4]N+ (X=OH, Cl; L=py, 1/2(Bpy)) Type Complexes. Inorg. Chim. Acta 1992, 196, 57–63. [Google Scholar] [CrossRef]
- Stepanenko, I.; Zalibera, M.; Schaniel, D.; Telser, J.; Arion, V.B. Ruthenium-Nitrosyl Complexes as NO-Releasing Molecules, Potential Anticancer Drugs, and Photoswitches Based on Linkage Isomerism. Dalt. Trans. 2022, 51, 5367–5393. [Google Scholar] [CrossRef]
- da Silva, R.S.; de Lima, R.G.; de Paula Machado, S. Chapter Six—Design, Reactivity, and Biological Activity of Ruthenium Nitrosyl Complexes. In Advances in Inorganic Chemistry; van Eldik, R., Olabe, J.A., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 67, pp. 265–294. [Google Scholar] [CrossRef]
- Awasabisah, D.; Richter-Addo, G.B. Chapter One—NOx Linkage Isomerization in Metal Complexes. In Advances in Inorganic Chemistry; van Eldik, R., Olabe, J.A., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 67, pp. 1–86. [Google Scholar] [CrossRef]
- Coppens, P.; Novozhilova, I.; Kovalevsky, A. Photoinduced Linkage Isomers of Transition-Metal Nitrosyl Compounds and Related Complexes. Chem. Rev. 2002, 102, 861–884. [Google Scholar] [CrossRef]
- Enriquez-Cabrera, A.; Sasaki, I.; Bukhanko, V.; Tassé, M.; Mallet-Ladeira, S.; Lacroix, P.G.; Barba-Barba, R.M.; Ramos-Ortiz, G.; Farfán, N.; Voitenko, Z.; et al. Replacing Two Chlorido Ligands by a Bipyridine Ligand in Ruthenium Nitrosyl Complexes with NO-Release Capabilities: A Comparative Study. Eur. J. Inorg. Chem. 2017, 2017, 1446–1456. [Google Scholar] [CrossRef]
- Amabilino, S.; Tasse, M.; Lacroix, P.G.; Mallet-Ladeira, S.; Pimienta, V.; Akl, J.; Sasaki, I.; Malfant, I. Photorelease of Nitric Oxide (NO) on Ruthenium Nitrosyl Complexes with Phenyl Substituted Terpyridines. New J. Chem. 2017, 41, 7371–7383. [Google Scholar] [CrossRef]
- Sasaki, I.; Amabilino, S.; Mallet-Ladeira, S.; Tassé, M.; Sournia-Saquet, A.; Lacroix, P.G.; Malfant, I. Further Studies on the Photoreactivities of Ruthenium–Nitrosyl Complexes with Terpyridyl Ligands. New J. Chem. 2019, 43, 11241–11250. [Google Scholar] [CrossRef]
- Labra-Vázquez, P.; Bocé, M.; Tassé, M.; Mallet-Ladeira, S.; Lacroix, P.G.; Farfán, N.; Malfant, I. Chemical and Photochemical Behavior of Ruthenium Nitrosyl Complexes with Terpyridine Ligands in Aqueous Media. Dalt. Trans. 2020, 49, 3138–3154. [Google Scholar] [CrossRef]
- Fry, N.L.; Heilman, B.J.; Mascharak, P.K. Dye-Tethered Ruthenium Nitrosyls Containing Planar Dicarboxamide Tetradentate N4 Ligands: Effects of In-Plane Ligand Twist on NO Photolability. Inorg. Chem. 2011, 50, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.J.; Mascharak, P.K. Photoactive Ruthenium Nitrosyls: Effects of Light and Potential Application as NO Donors. Coord. Chem. Rev. 2008, 252, 2093–2114. [Google Scholar] [CrossRef] [PubMed]
- Fry, N.L.; Mascharak, P.K. Photoactive Ruthenium Nitrosyls as NO Donors: How To Sensitize Them toward Visible Light. Acc. Chem. Res. 2011, 44, 289–298. [Google Scholar] [CrossRef]
- Fry, N.L.; Mascharak, P.K. Photolability of NO in Designed Metal Nitrosyls with Carboxamido-N Donors: A Theoretical Attempt to Unravel the Mechanism. Dalt. Trans. 2012, 41, 4726–4735. [Google Scholar] [CrossRef] [PubMed]
- Cormary, B.; Ladeira, S.; Jacob, K.; Lacroix, P.G.; Woike, T.; Schaniel, D.; Malfant, I. Structural Influence on the Photochromic Response of a Series of Ruthenium Mononitrosyl Complexes. Inorg. Chem. 2012, 51, 7492–7501. [Google Scholar] [CrossRef]
- Ferlay, S.; Schmalle, H.W.; Francese, G.; Stoeckli-Evans, H.; Imlau, M.; Schaniel, D.; Woike, T. Light-Induced Metastable States in Oxalatenitrosylruthenium(II) and Terpyridinenitrosylruthenium(II) Complexes. Inorg. Chem. 2004, 43, 3500–3506. [Google Scholar] [CrossRef]
- Giglmeier, H.; Kerscher, T.; Klüfers, P.; Schaniel, D.; Woike, T. Nitric-Oxide Photorelease and Photoinduced Linkage Isomerism on Solid [Ru(NO)(Terpy)(L)]BPh4 (L = Glycolate Dianion). Dalt. Trans. 2009, 42, 9113–9116. [Google Scholar] [CrossRef]
- Kovalevsky, A.Y.; King, G.; Bagley, K.A.; Coppens, P. Photoinduced Oxygen Transfer and Double-Linkage Isomerism in Acis-(NO)(NO2) Transition-Metal Complex by Photocrystallography, FT-IR Spectroscopy and DFT Calculations. Chem.—Eur. J. 2005, 11, 7254–7264. [Google Scholar] [CrossRef]
- Mikhailov, A.A.; Wenger, E.; Kostin, G.A.; Schaniel, D. Room-Temperature Photogeneration of Nitrosyl Linkage Isomers in Ruthenium Nitrosyl Complexes. Chem.—Eur. J. 2019, 25, 7569–7574. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, A.A.; Komarov, V.Y.; Sukhikh, A.S.; Pishchur, D.P.; Schaniel, D.; Kostin, G.A. The Impact of Counterion on the Metastable State Properties of Nitrosyl Ruthenium Complexes. New J. Chem. 2020, 44, 18014–18024. [Google Scholar] [CrossRef]
- Mikhailov, A.A.; Sukhikh, T.S.; Kuratieva, N.V.; Pishchur, D.P.; Kostin, G.A. Remarkable Thermal Stability of Light-Induced Ru-ON Linkage Isomers in Mixed Salts of a Ruthenium Amine Complex with Atrans-ON-Ru-F Coordinate. Dalt. Trans. 2021, 50, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Enemark, J.H.; Feltham, R.D. Principles of Structure, Bonding, and Reactivity for Metal Nitrosyl Complexes. Coord. Chem. Rev. 1974, 13, 339–406. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The Nature of .Pi.-.Pi. Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the Term “Pi-Stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef]
- Vorobyev, V.; Alferova, N.I.; Emelyanov, V.A. Infrared Detection with Temperature Sweep for Stability Determination of Ru-ON Metastable States. Inorg. Chem. 2019, 58, 1007–1011. [Google Scholar] [CrossRef]
- Mikhailov, A.A.; Brovko, A.O.; Kuratieva, N.V.; Eltsov, I.V.; Schaniel, D.; Kostin, G.A. The influence of hydroxide to fluoride substitution in the trans-position to NO on the photochemistry of ruthenium nitrosyl complexes in the solid state and in solution. New. J. Chem. 2023, 47, 15506–15513. [Google Scholar] [CrossRef]
- Mikhailov, A.A.; Kostin, G.A.; Schaniel, D. The Influence of the Trans-Ligand to NO on the Thermal Stability of the Photoinduced Side-Bond Coordinated Linkage Isomer. New J. Chem. 2022, 46, 12641–12650. [Google Scholar] [CrossRef]
- Cormary, B.; Malfant, I.; Buron-Le Cointe, M.; Toupet, L.; Delley, B.; Schaniel, D.; Mockus, N.; Woike, T.; Fejfarová, K.; Petříček, V.; et al. [Ru(Py)4Cl(NO)](PF6)2·0.5H2O: A Model System for Structural Determination and Ab Initio Calculations of Photo-Induced Linkage NO Isomers. Acta Crystallogr. Sect. B 2009, 65, 612–623. [Google Scholar] [CrossRef]
- Taylor, S.M.; Fryer, P.J. A Numerical Study of the Use of the Kissinger Analysis of DSC Thermograms to Obtain Reaction Kinetic Parameters. Thermochim. Acta 1992, 209, 111–125. [Google Scholar] [CrossRef]
- Wellen, R.M.R.; Canedo, E.L. On the Kissinger Equation and the Estimate of Activation Energies for Non-Isothermal Cold Crystallization of PET. Polym. Test. 2014, 40, 33–38. [Google Scholar] [CrossRef]
- Morioka, Y.; Ishikawa, A.; Tomizawa, H.; Miki, E.-I. Light-Induced Metastable States in Nitrosyl-Rutheniuni Complexes Containing Ethylenediamine and Oxalate Ion Ligands. J. Chem. Soc. Dalt. Trans. 2000, 54, 781–786. [Google Scholar] [CrossRef]
- Schaniel, D.; Woike, T. Necessary Conditions for the Photogeneration of Nitrosyl Linkage Isomers. Phys. Chem. Chem. Phys. 2009, 11, 4391–4395. [Google Scholar] [CrossRef]
- Schaniel, D.; Cormary, B.; Malfant, I.; Valade, L.; Woike, T.; Delley, B.; Krämer, K.W.; Güdel, H.-U. Photogeneration of Two Metastable NO Linkage Isomers with High Populations of up to 76% in Trans-[RuCl(Py)4(NO)][PF6]2·½H2O. Phys. Chem. Chem. Phys. 2007, 9, 3717–3724. [Google Scholar] [CrossRef]
- Hasil, A.; Konieczny, K.A.; Mikhailov, A.; Pillet, S.; Schaniel, D. CW or Pulsed Laser—That Is the Question: Comparative Steady-State Photocrystallographic Analysis of Metal Nitrosyl Linkage Isomers. ChemPhotoChem 2024, 8, e202300149. [Google Scholar] [CrossRef]
- Makhinya, A.N.; Il’In, M.A.; Kabin, E.V.; Baidina, I.A.; Gallyamov, M.R.; Alferova, N.I. Nitrosoruthenium Hydroxo and Aqua Complexes of the Trans-Dipyridine Series: Synthesis, Structures, and Characterization. Russ. J. Coord. Chem. Khimiya 2014, 40, 297–303. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-Type Basis Sets for the Elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, A.; Asanova, T.; Asanov, I.; Pis, I.; Magnano, E.; Kostin, G.; Schaniel, D. N, O and F K-edges XAS and DFT combination for the exploration of linkage isomers of coordinated nitric oxide in a ruthenium nitrosyl complex. J. Electr. Spectr. Rel. Phen. 2023, 266, 147342. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
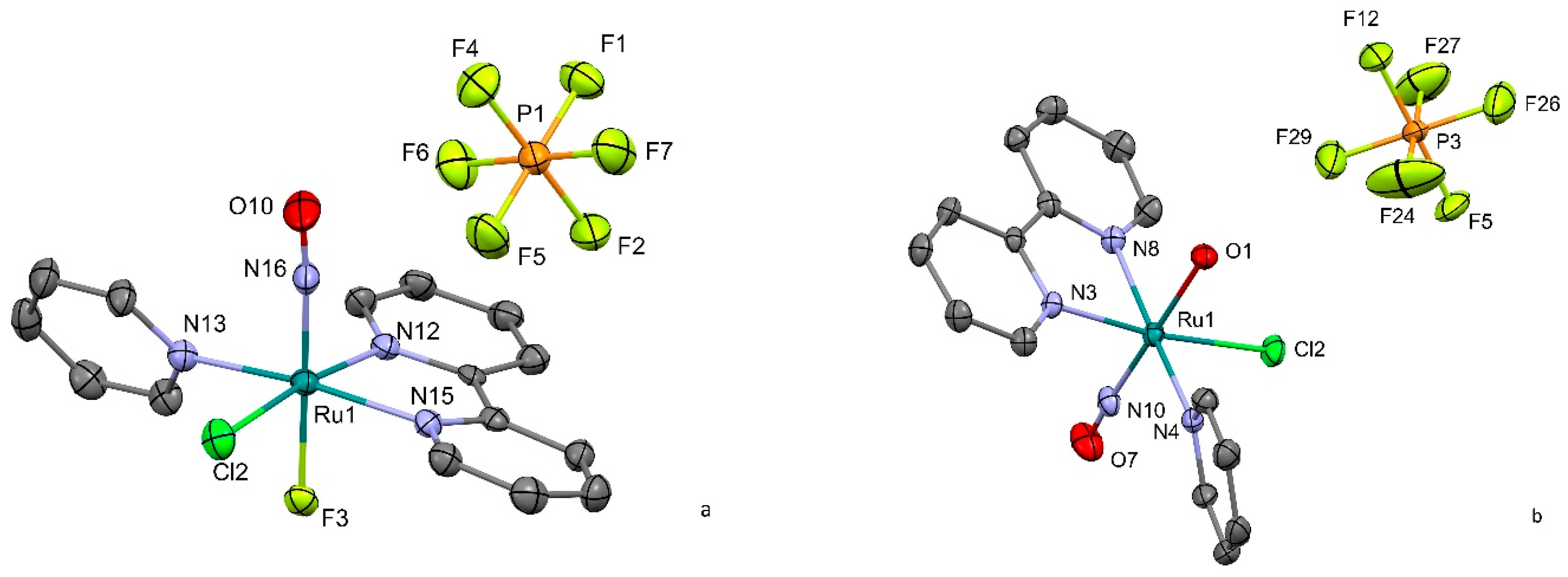
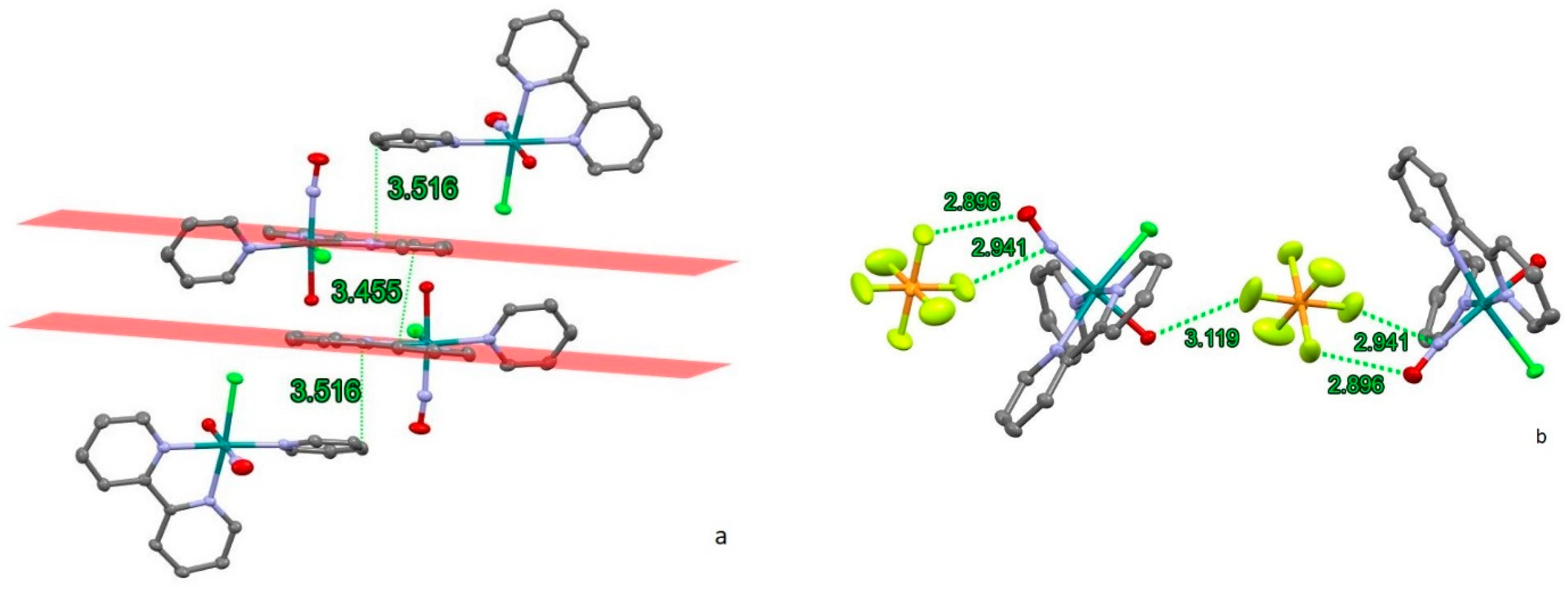
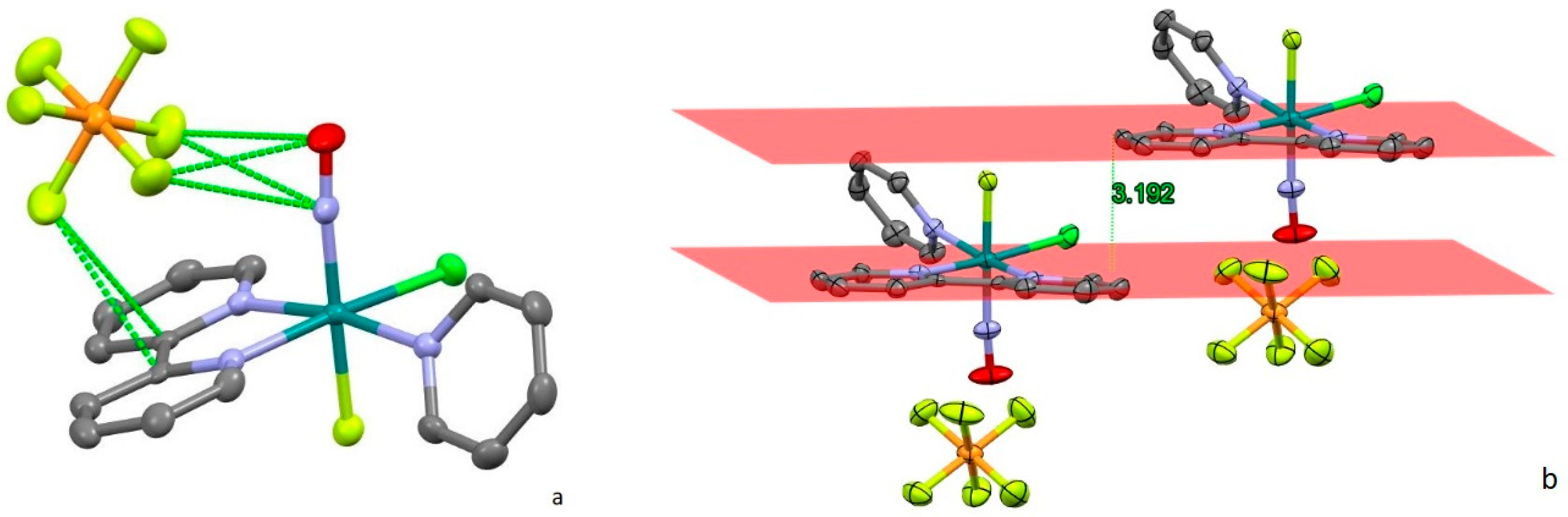



| Parameter | [RuNO(Py)(Bpy)ClOH]PF6 (1) | [RuNO(Py)(Bpy)ClF]PF6 (2) |
|---|---|---|
| d(Ru–Cl), Å | 2.361(1) | 2.371(1) |
| d(Ru–NPy), Å | 2.108(2) | 2.103(4) |
| d(Ru–Nbpy), Å | 2.078(2)–2.082(2) | 2.063(4)–2.070(4) |
| d(Ru–NO), Å | 1.758(2) | 1.727(4) |
| d(Ru–X), Å (X = F, OH) | 1.936(2) | 1.948(3) |
| d(N–O), Å | 1.153(3) | 1.154(5) |
| ∠Ru–N–O, ° | 170.6(2) | 174.4(4) |
| ∠X-Ru-Cl | 88.51(2) | 88.97(3) |
| ∠X-Ru–NPy | 88.12(2) | 87.03(2) |
| ∠X-Ru–Nbpy | 85.17–85.54 | 84.92–85.44 |
| ∠Bpy-Py, ° | 56.07 | 53.26 |
| [RuNO(Py)(Bpy)ClF]PF6 | [RuNO(Py)(Bpy)ClOH]PF6 | ||||
|---|---|---|---|---|---|
| Wavelength, nm | Transition occ.-virt. | Contribution, % | Wavelength, nm | Transition occ.-virt. | Contribution, % |
| 428.4 | 103–105 | 92 | 430.4 | 104–105 102–105 102–106 | 51 19 7 |
| 422.7 | 103–106 | 94 | 424.3 | 102–105 104–105 | 52 33 |
| 395.5 | 102–105 102–106 104–105 | 39 18 11 | 417.2 | 104–106 102–106 | 71 21 |
| 367.9 | 104–105 103–108 102–106 | 57 20 10 | 373.2 | 101–105 102–106 103–105 | 34 22 11 |
| 363.0 | 103–108 104–105 | 61 19 | 370.6 | 104–108 101–106 102–108 | 40 14 12 |
| 358.6 | 104–106 104–105 | 80 7 | 362.7 | 101–106 104–108 103–106 | 37 21 7 |
| 352.6 | 102–106 102–105 100–106 | 41 24 10 | 350.8 | 103–105 | 80 |
| 344.1 | 100–105 100–106 102–105 | 20 19 9 | 340.0 | 103–106 101–106 | 84 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brovko, A.O.; Kuratieva, N.V.; Pishchur, D.P.; Kostin, G.A. Heteroleptic Complexes of Ruthenium Nitrosyl with Pyridine and Bypiridine—Synthesis and Photoisomerization. Molecules 2024, 29, 4039. https://doi.org/10.3390/molecules29174039
Brovko AO, Kuratieva NV, Pishchur DP, Kostin GA. Heteroleptic Complexes of Ruthenium Nitrosyl with Pyridine and Bypiridine—Synthesis and Photoisomerization. Molecules. 2024; 29(17):4039. https://doi.org/10.3390/molecules29174039
Chicago/Turabian StyleBrovko, Anastasiya O., Natalya V. Kuratieva, Denis P. Pishchur, and Gennadiy A. Kostin. 2024. "Heteroleptic Complexes of Ruthenium Nitrosyl with Pyridine and Bypiridine—Synthesis and Photoisomerization" Molecules 29, no. 17: 4039. https://doi.org/10.3390/molecules29174039
APA StyleBrovko, A. O., Kuratieva, N. V., Pishchur, D. P., & Kostin, G. A. (2024). Heteroleptic Complexes of Ruthenium Nitrosyl with Pyridine and Bypiridine—Synthesis and Photoisomerization. Molecules, 29(17), 4039. https://doi.org/10.3390/molecules29174039







