Agents Targeting the Bacterial Cell Wall as Tools to Combat Gram-Positive Pathogens
Abstract
1. Introduction
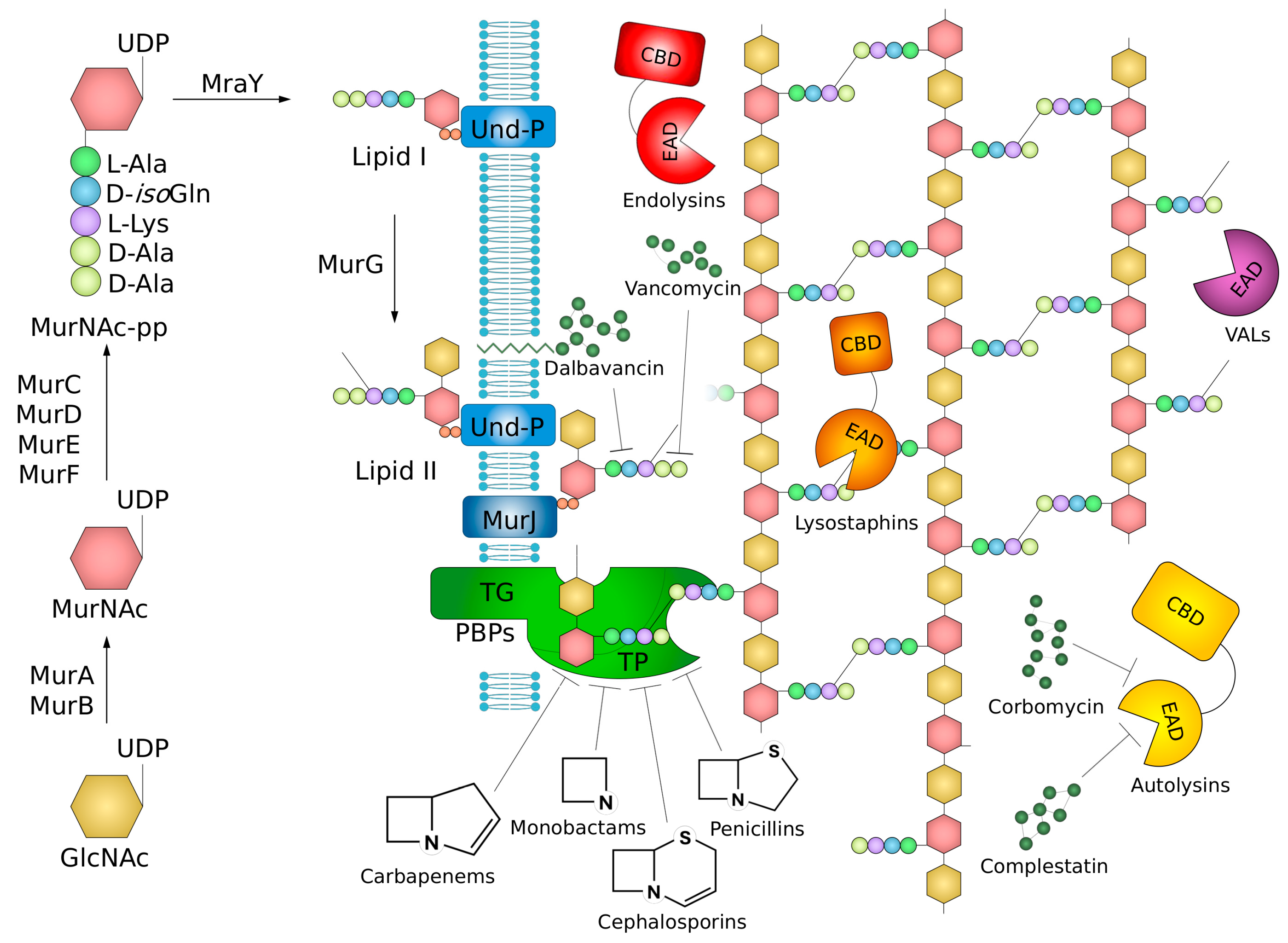
2. Structure, Synthesis, and Remodeling of Peptidoglycan
3. Agents Targeting Peptidoglycan Synthesis
3.1. β-Lactams and Mechanisms of Resistance to Them
3.1.1. Penicillins
3.1.2. Cephalosporins
3.1.3. Carbapenems
3.1.4. Monobactams
3.2. β-Lactamase Based Resistance
3.3. Penicillin-Binding Proteins (PBPs)
3.4. Resistance Mediated by Impermeability or Efflux
3.5. Glycopeptide Antibiotics
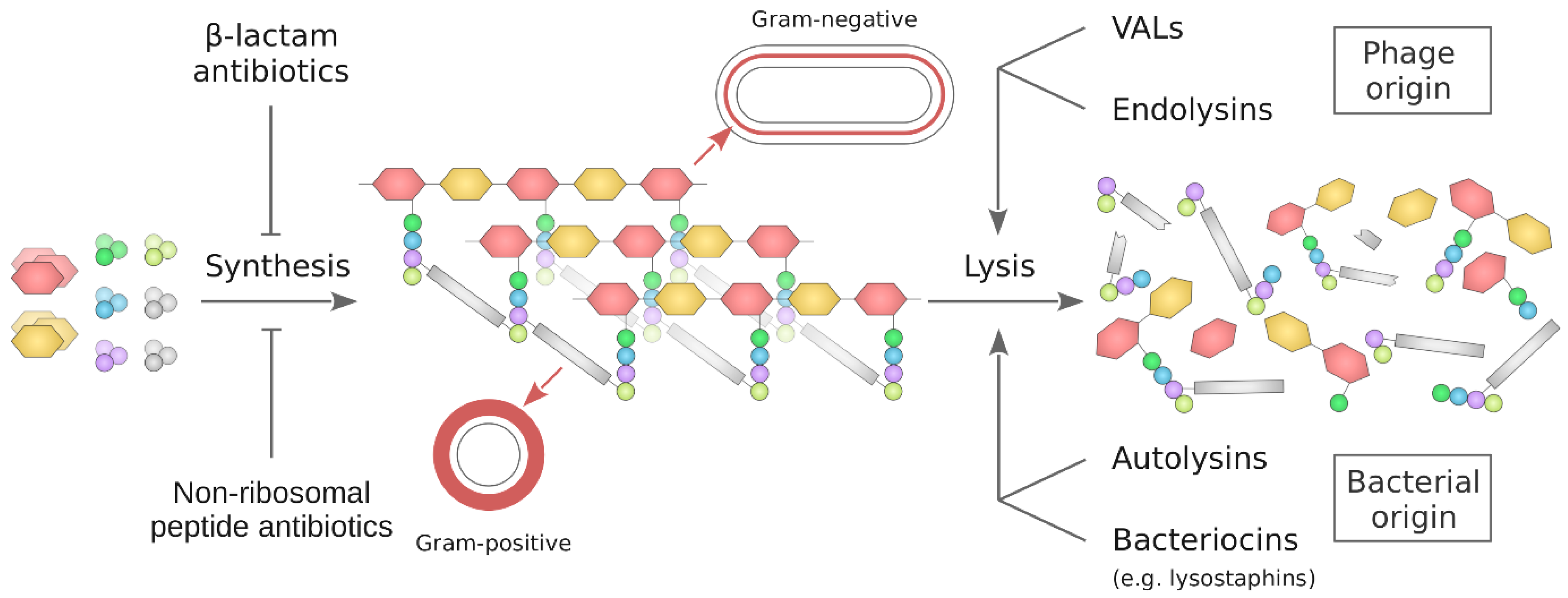
4. Agents Decomposing the Bacterial Cell Wall
4.1. Peptidoglycan Targeting Enzymes of Bacteriophage Origin
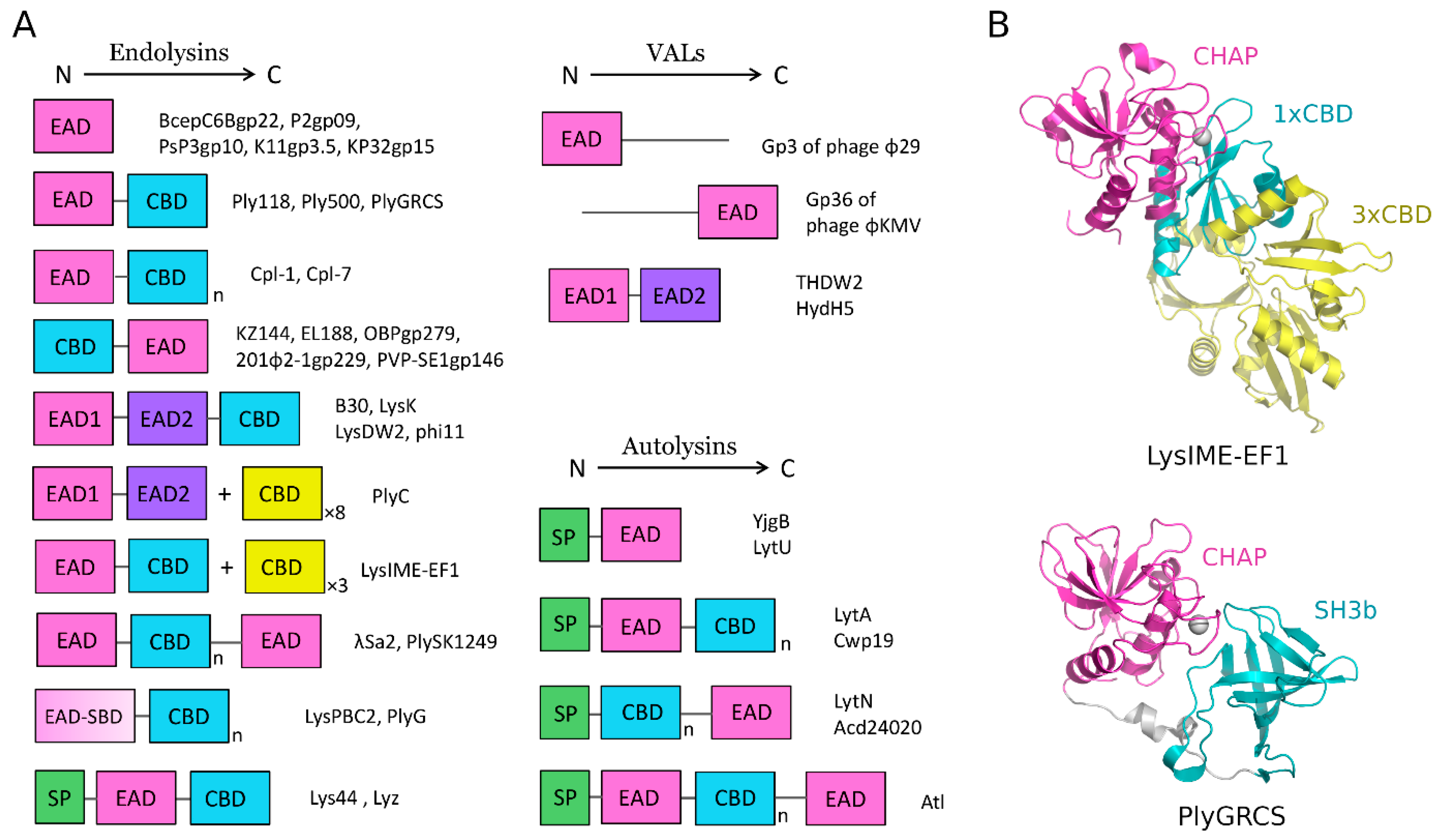
4.1.1. Endolysins
4.1.2. Virion-Associated Lysins
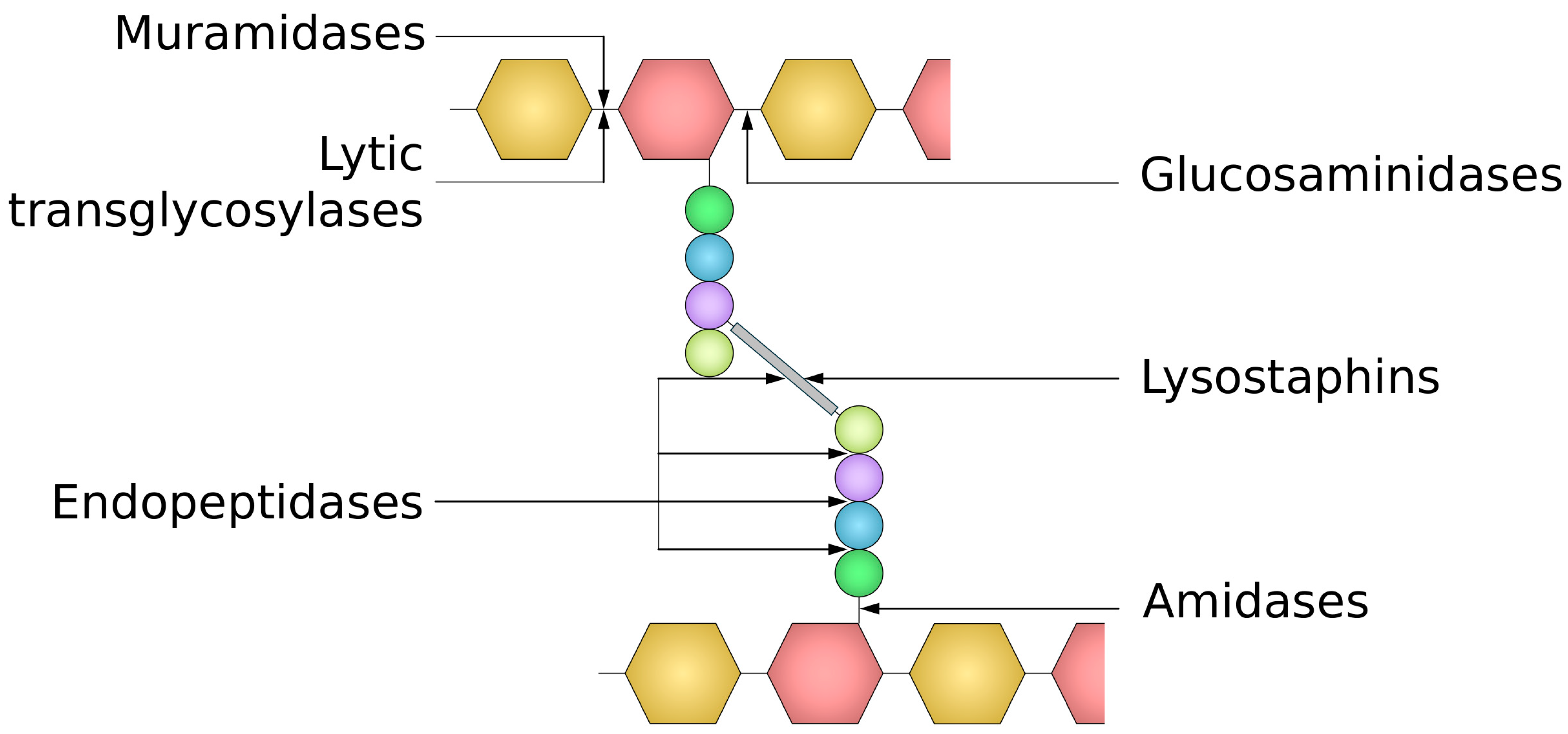
4.2. Peptidoglycan Targeting Enzymes of Bacterial Origin
4.2.1. Autolysins
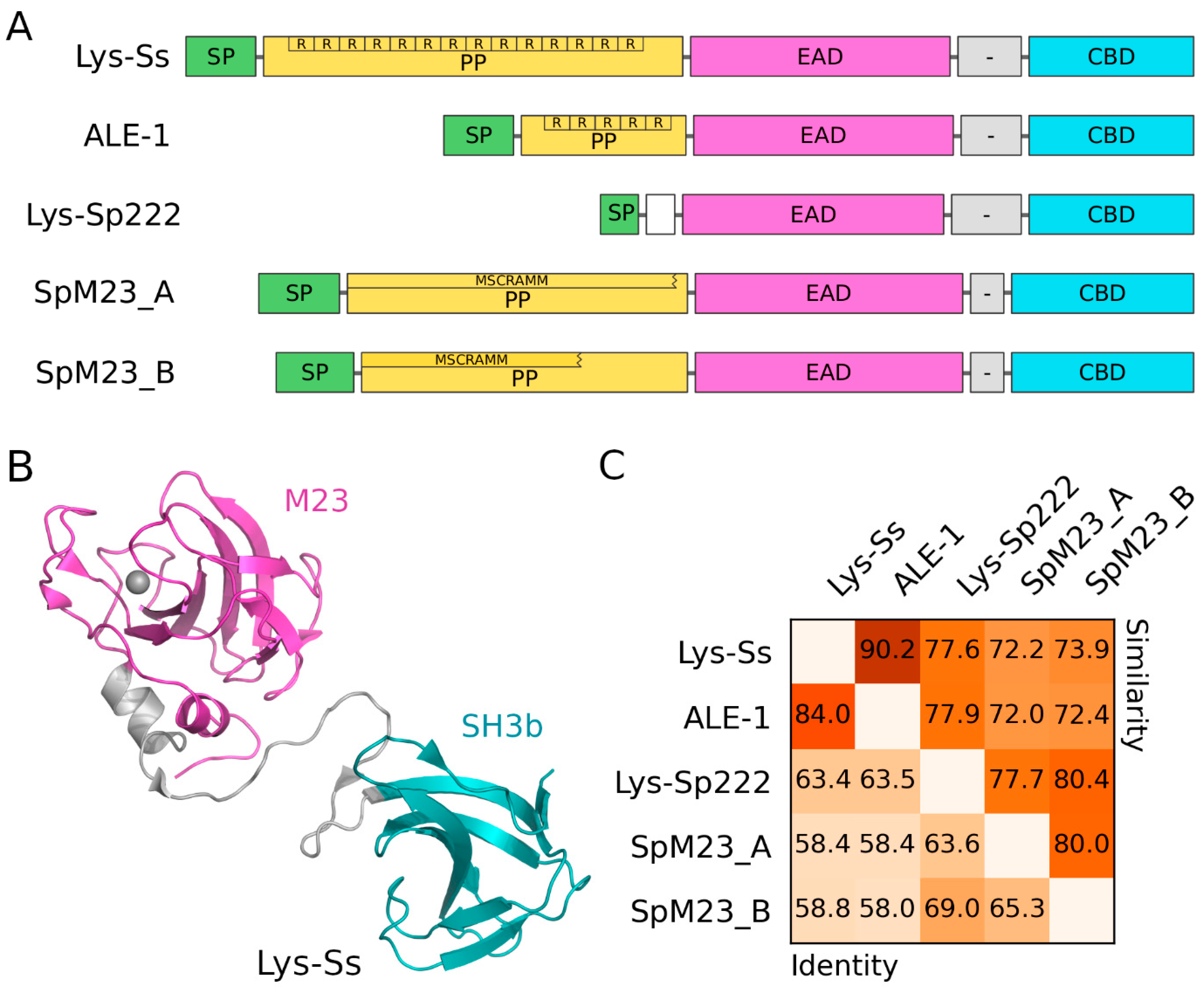
4.2.2. Lysostaphins—Staphylococcus-Genus-Specific Bacteriocins
5. Enzymes Targeting Cell Wall as Potential Antibacterials: Spectrum of Activity and Advantages over Antibiotics
5.1. Resistance to Peptidoglycan Hydrolases
5.2. Lysins as Potential Antibacterials
5.3. Lysostaphins as Potential Antibacterials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- WHO. WHO Bacterial Priority Pathogens List, 2024; WHO: Geneva, Switzerland, 2024; ISBN 978-92-4-009346-1. [Google Scholar]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Garde, S.; Chodisetti, P.K.; Reddy, M. Peptidoglycan: Structure, Synthesis, and Regulation. EcoSal Plus 2021, 9, eESP-0010-2020. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Joris, B.; Charlier, P.; Foster, S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 2008, 32, 259–286. [Google Scholar] [CrossRef]
- Egan, A.J.F.; Errington, J.; Vollmer, W. Regulation of peptidoglycan synthesis and remodelling. Nat. Rev. Microbiol. 2020, 18, 446–460. [Google Scholar] [CrossRef]
- Galinier, A.; Delan-Forino, C.; Foulquier, E.; Lakhal, H.; Pompeo, F. Recent Advances in Peptidoglycan Synthesis and Regulation in Bacteria. Biomolecules 2023, 13, 720. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, M.; Walker, S. Envelope structures of Gram-positive bacteria. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 404, pp. 1–44. [Google Scholar] [CrossRef]
- Rajguru, V.; Chatterjee, S.; Garde, S.; Reddy, M. Crosslink cleaving enzymes: The smart autolysins that remodel the bacterial cell wall. Trends Microbiol. 2024, 32, 494–506. [Google Scholar] [CrossRef]
- Lobanovska, M.; Pilla, G. Penicillin’s Discovery and Antibiotic Resistance: Lessons for the Future? Yale J. Biol. Med. 2017, 90, 135–145. [Google Scholar]
- Duerden, B.I. MRSA: Why have we got it and can we do anything about it? Eye 2012, 26, 218–221. [Google Scholar] [CrossRef][Green Version]
- Bunnell, K.; Duong, A.; Ringsred, T.; Mian, A.; Bhathena, S. Aminopenicillins for treatment of ampicillin-resistant enterococcal urinary tract infections. Am. J. Health Pharm. 2022, 79, 1056–1065. [Google Scholar] [CrossRef]
- Florin, T.A.; Byczkowski, T.; Gerber, J.S.; Ruddy, R.; Kuppermann, N. Diagnostic testing and antibiotic use in young children with community-acquired pneumonia in the United States, 2008–2015. J. Pediatr. Infect. Dis. Soc. 2020, 9, 248–252. [Google Scholar] [CrossRef]
- Hussen, N.H.; Hamid, S.J.; Sabir, M.N.; Hasan, A.H.; Mohammed, S.J.; Shali, A.A.K. Novel Penicillin Derivatives Against Selected Multiple-drug Resistant Bacterial Strains: Design, Synthesis, Structural Analysis, In Silico and In Vitro Studies. Curr. Org. Synth. 2024, 21, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.B.; Veve, M.P.; Wagner, J.L. Cephalosporins: A Focus on Side Chains and β-Lactam Cross-Reactivity. Pharmacy 2019, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kück, U. Cephalosporins as key lead generation beta-lactam antibiotics. Appl. Microbiol. Biotechnol. 2022, 106, 8007–8020. [Google Scholar] [CrossRef]
- Duplessis, C.; Crum-Cianflone, N.F. Ceftaroline: A New Cephalosporin with Activity Against Methicillin-Resistant Staphylococcus aureus (MRSA). Clin. Med. Rev. Ther. 2011, 3, a2466. [Google Scholar] [CrossRef] [PubMed]
- Quiñonez-Flores, A.; Martinez-Guerra, B.A.; Román-Montes, C.M.; Tamez-Torres, K.M.; González-Lara, M.F.; Ponce-de-León, A.; Rajme-López, S. Cephalotin Versus Dicloxacillin for the Treatment of Methicillin-Susceptible Staphylococcus aureus Bacteraemia: A Retrospective Cohort Study. Antibiotics 2024, 13, 176. [Google Scholar] [CrossRef]
- Werth, B.J.; Sakoulas, G.; Rose, W.E.; Pogliano, J.; Tewhey, R.; Rybak, M.J. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 2013, 57, 66–73. [Google Scholar] [CrossRef]
- Jiao, F.; Bao, Y.; Li, M.; Zhang, Y.; Zhang, F.; Wang, P.; Tao, J.; Tong, H.H.Y.; Guo, J. Unraveling the mechanism of ceftaroline-induced allosteric regulation in penicillin-binding protein 2a: Insights for novel antibiotic development against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2023, 67, e0089523. [Google Scholar] [CrossRef]
- Noel, G.J. Clinical profile of ceftobiprole, a novel β-lactam antibiotic. Clin. Microbiol. Infect. 2007, 13, 25–29. [Google Scholar] [CrossRef]
- Méndez, R.; Latorre, A.; González-Jiménez, P. Ceftobiprole medocaril. Rev. Esp. Quimioter. 2022, 35, 25–27. [Google Scholar] [CrossRef]
- Wi, Y.M.; Kwon, K.T.; Jeon, C.H.; Kim, S.H.; Hwang, S.; Bae, S.; Kim, Y.; Chang, H.H.; Kim, S.W.; Cheong, H.S.; et al. Carbapenem Use in the Last Days of Life: A Nationwide Korean Study. Antibiotics 2023, 12, 964. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile β-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.; Fenn, S.J.; Hardie, K.R. JMM Profile: Carbapenems: A broad-spectrum antibiotic. J. Med. Microbiol. 2021, 70, 001462. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; El Ouweini, A.; Chahine, E.B.; Karaoui, L.R. Imipenem/cilastatin/relebactam: A new carbapenem β-lactamase inhibitor combination. Am. J. Health Pharm. 2021, 78, 674–683. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.N.; Lodise, T.P. New Perspectives on Antimicrobial Agents: Imipenem-Relebactam. Antimicrob. Agents Chemother. 2022, 66, e0025622. [Google Scholar] [CrossRef]
- Bassetti, M.; Magnè, F.; Giacobbe, D.R.; Bini, L.; Vena, A. New antibiotics for Gram-negative pneumonia. Eur. Respir. Rev. 2022, 31, 220119. [Google Scholar] [CrossRef]
- Thu, Z.M.; Sun, J.; Ji, J.; He, L.; Ji, J.; Iqbal, Z.; Myo, K.K.; Gao, Y.; Zhai, L.; Mu, Y.; et al. Synthesis and antibacterial evaluation of new monobactams. Bioorg. Med. Chem. Lett. 2021, 39, 127878. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.M.; Davies, C. Penicillin-binding protein 3 is essential for growth of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61, e01651-16. [Google Scholar] [CrossRef]
- Freischem, S.; Grimm, I.; López-Pérez, A.; Willbold, D.; Klenke, B.; Vuong, C.; Dingley, A.J.; Weiergräber, O.H. Interaction mode of the novel monobactam AIC499 targeting penicillin binding protein 3 of Gram-negative bacteria. Biomolecules 2021, 11, 1057. [Google Scholar] [CrossRef]
- Blais, J.; Lopez, S.; Li, C.; Ruzin, A.; Ranjitkar, S.; Dean, C.R.; Leeds, J.A.; Casarez, A.; Simmons, R.L.; Reck, F. In vitro activity of LYS228, a novel monobactam antibiotic, against multidrug-resistant enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e00552-18. [Google Scholar] [CrossRef]
- Sun, Y.; Liao, X.; Huang, Z.; Xie, Y.; Liu, Y.; Ma, C.; Jiang, B.; Zhang, L.; Yan, Y.; Li, G.; et al. Therapeutic effect and mechanisms of the novel monosulfactam 0073. Antimicrob. Agents Chemother. 2020, 64, e00529-20. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Z.; Lu, X.; Ma, X.; Wang, X.; Zhang, R.; Hu, X.; Wang, Y.; Pang, J.; Fan, T.; et al. Evolution and development of potent monobactam sulfonate candidate IMBZ18g as a dual inhibitor against MDR Gram-negative bacteria producing ESBLs. Acta Pharm. Sin. B 2023, 13, 3067–3079. [Google Scholar] [CrossRef] [PubMed]
- Schalk, I.J. A Trojan-Horse Strategy Including a Bacterial Suicide Action for the Efficient Use of a Specific Gram-Positive Antibiotic on Gram-Negative Bacteria. J. Med. Chem. 2018, 61, 3842–3844. [Google Scholar] [CrossRef] [PubMed]
- Meini, M.R.; Llarrull, L.I.; Vila, A.J. Evolution of metallo-β-lactamases: Trends revealed by natural diversity and in vitro evolution. Antibiotics 2014, 3, 285–316. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Philippon, A.; Slama, P.; Dény, P.; Labia, R. A structure-based classification of class A β-Lactamases, a broadly diverse family of enzymes. Clin. Microbiol. Rev. 2016, 29, 29–57. [Google Scholar] [CrossRef]
- Eiamphungporn, W.; Schaduangrat, N.; Malik, A.A.; Nantasenamat, C. Tackling the antibiotic resistance caused by class a β-lactamases through the use of β-lactamase inhibitory protein. Int. J. Mol. Sci. 2018, 19, 2222. [Google Scholar] [CrossRef]
- Yagi, T.; Wachino, J.I.; Kurokawa, H.; Suzuki, S.; Yamane, K.; Doi, Y.; Shibata, N.; Kato, H.; Shibayama, K.; Arakawa, Y. Practical methods using boronic acid compounds for identification of class C β-lactamase-producing Klebsiella pneumoniae and Escherichia coli. J. Clin. Microbiol. 2005, 43, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Philippon, A.; Arlet, G.; Labia, R.; Iorga, B.I. Class C β-Lactamases: Molecular Characteristics. Clin. Microbiol. Rev. 2022, 35, e0015021. [Google Scholar] [CrossRef] [PubMed]
- Kaitany, K.C.J.; Klinger, N.V.; June, C.M.; Ramey, M.E.; Bonomo, R.A.; Powers, R.A.; Leonard, D.A. Structures of the class D carbapenemases OXA-23 and OXA-146: Mechanistic basis of activity against carbapenems, extended-spectrum cephalosporins, and aztreonam. Antimicrob. Agents Chemother. 2013, 57, 4848–4855. [Google Scholar] [CrossRef]
- Antunes, N.T.; Lamoureaux, T.L.; Toth, M.; Stewart, N.K.; Frase, H.; Vakulenko, S.B. Class D β-lactamases: Are they all carbapenemases? Antimicrob. Agents Chemother. 2014, 58, 2119–2125. [Google Scholar] [CrossRef]
- Kim, Y.; Maltseva, N.; Wilamowski, M.; Tesar, C.; Endres, M.; Joachimiak, A. Structural and biochemical analysis of the metallo-β-lactamase L1 from emerging pathogen Stenotrophomonas maltophilia revealed the subtle but distinct di-metal scaffold for catalytic activity. Protein Sci. 2020, 29, 723–743. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.E.; Livermore, D.M.; Hooper, D.C.; Hope, W.W. Metallo-β-lactamases: Structure, function, epidemiology, treatment options, and the development pipeline. Antimicrob. Agents Chemother. 2020, 64, e00397-20. [Google Scholar] [CrossRef] [PubMed]
- López-Agudelo, V.A.; Gómez-Ríos, D.; Ramirez-Malule, H. Clavulanic acid production by streptomyces clavuligerus: Insights from systems biology, strain engineering, and downstream processing. Antibiotics 2021, 10, 84. [Google Scholar] [CrossRef]
- Mauri, C.; Maraolo, A.E.; Di Bella, S.; Luzzaro, F.; Principe, L. The revival of aztreonam in combination with avibactam against metallo-β-lactamase-producing Gram-negatives: A systematic review of in vitro studies and clinical cases. Antibiotics 2021, 10, 1012. [Google Scholar] [CrossRef]
- Lahiri, S.D.; Johnstone, M.R.; Ross, P.L.; McLaughlin, R.E.; Olivier, N.B.; Alm, R.A. Avibactam and class C β -lactamases: Mechanism of inhibition, conservation of the binding pocket, and implications for resistance. Antimicrob. Agents Chemother. 2014, 58, 5704–5713. [Google Scholar] [CrossRef]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef]
- Fishovitz, J.; Hermoso, J.A.; Chang, M.; Mobashery, S. Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. IUBMB Life 2014, 66, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Otero, L.H.; Rojas-Altuve, A.; Llarrull, L.I.; Carrasco-López, C.; Kumarasiri, M.; Lastochkin, E.; Fishovitz, J.; Dawley, M.; Hesek, D.; Lee, M.; et al. How allosteric control of Staphylococcus aureus penicillin binding protein 2a enables methicillin resistance and physiological function. Proc. Natl. Acad. Sci. USA 2013, 110, 16808–16813. [Google Scholar] [CrossRef]
- Hunashal, Y.; Kumar, G.S.; Choy, M.S.; D’Andréa, É.D.; Da Silva Santiago, A.; Schoenle, M.V.; Desbonnet, C.; Arthur, M.; Rice, L.B.; Page, R.; et al. Molecular basis of β-lactam antibiotic resistance of ESKAPE bacterium E. faecium Penicillin Binding Protein PBP5. Nat. Commun. 2023, 14, 4268. [Google Scholar] [CrossRef]
- Bartsch, A.; Ives, C.M.; Kattner, C.; Pein, F.; Diehn, M.; Tanabe, M.; Munk, A.; Zachariae, U.; Steinem, C.; Llabrés, S. An antibiotic-resistance conferring mutation in a neisserial porin: Structure, ion flux, and ampicillin binding. Biochim. Biophys. Acta—Biomembr. 2021, 1863, 183601. [Google Scholar] [CrossRef]
- Abavisani, M.; Kodori, M.; Akrami, F.; Radfar, A.; Hashemi, A. Relationships between Efflux Pumps and Emergence of Heteroresistance: A Comprehensive Study on the Current Findings. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3916980. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Huang, J.; Xu, Z. Antibiotic influx and efflux in Pseudomonas aeruginosa: Regulation and therapeutic implications. Microb. Biotechnol. 2024, 17, e14487. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Hansford, K.A.; Blaskovich, M.A.T.; Halai, R.; Cooper, M.A. Glycopeptide antibiotics: Back to the future. J. Antibiot. 2014, 67, 631–644. [Google Scholar] [CrossRef]
- Sarkar, P.; Yarlagadda, V.; Ghosh, C.; Haldar, J. A review on cell wall synthesis inhibitors with an emphasis on glycopeptide antibiotics. Medchemcomm 2017, 8, 516–533. [Google Scholar] [CrossRef]
- Binda, E.; Marinelli, F.; Marcone, G.L. Old and new glycopeptide antibiotics: Action and resistance. Antibiotics 2014, 3, 572–594. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Rigol, S. A brief history of antibiotics and select advances in their synthesis. J. Antibiot. 2018, 71, 153–184. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Hansford, K.A.; Butler, M.S.; Jia, Z.; Mark, A.E.; Cooper, M.A. Developments in Glycopeptide Antibiotics. ACS Infect. Dis. 2018, 4, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, M.; Geng, J.; Wang, M. Teicoplanin combined with conventional vancomycin therapy for the treatment of pulmonary methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis infections. World J. Clin. Cases 2021, 9, 10549–10556. [Google Scholar] [CrossRef] [PubMed]
- Jovetic, S.; Zhu, Y.; Marcone, G.L.; Marinelli, F.; Tramper, J. β-Lactam and glycopeptide antibiotics: First and last line of defense? Trends Biotechnol. 2010, 28, 596–604. [Google Scholar] [CrossRef]
- Leadbetter, M.R.; Adams, S.M.; Bazzini, B.; Fatheree, P.R.; Karr, D.E.; Krause, K.M.; Lam, B.M.T.; Linsell, M.S.; Nodwell, M.B.; Pace, J.L.; et al. Hydrophobic vancomycin derivatives with improved ADME properties: Discovery of telavancin (TD-6424). J. Antibiot. 2004, 57, 326–336. [Google Scholar] [CrossRef]
- Culp, E.J.; Waglechner, N.; Wang, W.; Fiebig-Comyn, A.A.; Hsu, Y.P.; Koteva, K.; Sychantha, D.; Coombes, B.K.; Van Nieuwenhze, M.S.; Brun, Y.V.; et al. Evolution-guided discovery of antibiotics that inhibit peptidoglycan remodelling. Nature 2020, 578, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage lytic enzymes: Novel anti-infectives. Trends Microbiol. 2005, 13, 491–496. [Google Scholar] [CrossRef]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, L.; Blasco, L.; Lopez, M.; Bou, G.; García-Contreras, R.; Wood, T.; Tomas, M. Toxin-antitoxin systems in clinical pathogens. Toxins 2016, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- São-José, C. Engineering of phage-derived lytic enzymes: Improving their potential as antimicrobials. Antibiotics 2018, 7, 29. [Google Scholar] [CrossRef]
- Wang, I.N.; Deaton, J.; Young, R. Sizing the holin lesion with an endolysin-β-galactosidase fusion. J. Bacteriol. 2003, 185, 779–787. [Google Scholar] [CrossRef]
- Wang, I.N.; Smith, D.L.; Young, R. Holins: The protein clocks of bacteriophage infections. Annu. Rev. Microbiol. 2000, 54, 799–825. [Google Scholar] [CrossRef]
- Vukov, N.; Moll, I.; Bläsi, U.; Scherer, S.; Loessner, M.J. Functional regulation of the Listeria monocytogenes bacteriophage A118 holin by an intragenic inhibitor lacking the first transmembrane domain. Mol. Microbiol. 2003, 48, 173–186. [Google Scholar] [CrossRef]
- Young, R. Phage lysis: Do we have the hole story yet? Curr. Opin. Microbiol. 2013, 16, 790–797. [Google Scholar] [CrossRef]
- Borysowski, J.; Weber-Dąbrowska, B.; Górski, A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp. Biol. Med. 2006, 231, 366–377. [Google Scholar] [CrossRef]
- Payne, K.M.; Hatfull, G.F. Mycobacteriophage endolysins: Diverse and modular enzymes with multiple catalytic activities. PLoS ONE 2012, 7, e34052. [Google Scholar] [CrossRef] [PubMed]
- Hermoso, J.A.; García, J.L.; García, P. Taking aim on bacterial pathogens: From phage therapy to enzybiotics. Curr. Opin. Microbiol. 2007, 10, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Fernández, L.; Rodríguez, A.; García, P. Are phage lytic proteins the secret weapon to kill Staphylococcus aureus? MBio 2018, 9, e01923-17. [Google Scholar] [CrossRef]
- Love, M.J.; Abeysekera, G.S.; Muscroft-Taylor, A.C.; Billington, C.; Dobson, R.C.J. On the catalytic mechanism of bacteriophage endolysins: Opportunities for engineering. Biochim. Biophys. Acta—Proteins Proteom. 2020, 1868, 140302. [Google Scholar] [CrossRef] [PubMed]
- Loessner, M.J. Bacteriophage endolysins—Current state of research and applications. Curr. Opin. Microbiol. 2005, 8, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Sass, P.; Bierbaum, G. Lytic activity of recombinant bacteriophage φ11 and φ12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl. Environ. Microbiol. 2007, 73, 347–352. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Coffey, A.; Meaney, W.; Fitzgerald, G.F.; Ross, R.P. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 2005, 187, 7161–7164. [Google Scholar] [CrossRef]
- Rashel, M.; Uchiyama, J.; Ujihara, T.; Uehara, Y.; Kuramoto, S.; Sugihara, S.; Yagyu, K.I.; Muraoka, A.; Sugai, M.; Hiramatsu, K.; et al. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage φMR11. J. Infect. Dis. 2007, 196, 1237–1247. [Google Scholar] [CrossRef]
- Obeso, J.M.; Martínez, B.; Rodríguez, A.; García, P. Lytic activity of the recombinant staphylococcal bacteriophage ΦH5 endolysin active against Staphylococcus aureus in milk. Int. J. Food Microbiol. 2008, 128, 212–218. [Google Scholar] [CrossRef]
- Schmelcher, M.; Loessner, M.J. Bacteriophage endolysins: Applications for food safety. Curr. Opin. Biotechnol. 2016, 37, 76–87. [Google Scholar] [CrossRef]
- Love, M.J.; Bhandari, D.; Dobson, R.C.J.; Billington, C. Potential for bacteriophage endolysins to supplement or replace antibiotics in food production and clinical care. Antibiotics 2018, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Krishnappa, G.; Mandal, M.; Ganesan, S.; Babu, S.; Padavattan, S.; Haradara Bahubali, V.K.; Padmanabhan, B. Structural and biochemical insights into the bacteriophage PlyGRCS endolysin targeting methicillin-resistant Staphylococcus aureus (MRSA) and serendipitous discovery of its interaction with a cold shock protein C (CspC). Protein Sci. 2023, 32, e4737. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Melo, L.D.R.; Santos, S.B.; Nóbrega, F.L.; Ferreira, E.C.; Cerca, N.; Azeredo, J.; Kluskens, L.D. Molecular Aspects and Comparative Genomics of Bacteriophage Endolysins. J. Virol. 2013, 87, 4558–4570. [Google Scholar] [CrossRef]
- Broendum, S.S.; Buckle, A.M.; McGowan, S. Catalytic diversity and cell wall binding repeats in the phage-encoded endolysins. Mol. Microbiol. 2018, 110, 879–896. [Google Scholar] [CrossRef]
- Korndörfer, I.P.; Danzer, J.; Schmelcher, M.; Zimmer, M.; Skerra, A.; Loessner, M.J. The Crystal Structure of the Bacteriophage PSA Endolysin Reveals a Unique Fold Responsible for Specific Recognition of Listeria Cell Walls. J. Mol. Biol. 2006, 364, 678–689. [Google Scholar] [CrossRef]
- Wong, K.Y.; Megat Mazhar Khair, M.H.; Song, A.A.L.; Masarudin, M.J.; Chong, C.M.; In, L.L.A.; Teo, M.Y.M. Endolysins against Streptococci as an antibiotic alternative. Front. Microbiol. 2022, 13, 935145. [Google Scholar] [CrossRef] [PubMed]
- Haddad Kashani, H.; Schmelcher, M.; Sabzalipoor, H.; Seyed Hosseini, E.; Moniri, R. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: Current status of research and novel delivery strategies. Clin. Microbiol. Rev. 2018, 31, e00071-17. [Google Scholar] [CrossRef]
- Donovan, D.M.; Foster-Frey, J.; Dong, S.; Rousseau, G.M.; Moineau, S.; Pritchard, D.G. The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain. Appl. Environ. Microbiol. 2006, 72, 5108–5112. [Google Scholar] [CrossRef]
- Donovan, D.M.; Lardeo, M.; Foster-Frey, J. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol. Lett. 2006, 265, 133–139. [Google Scholar] [CrossRef]
- Becker, S.C.; Dong, S.; Baker, J.R.; Foster-Frey, J.; Pritchard, D.G.; Donovan, D.M. LysK CHAP endopeptidase domain is required for lysis of live staphylococcal cells. FEMS Microbiol. Lett. 2009, 294, 52–60. [Google Scholar] [CrossRef]
- Pritchard, D.G.; Dong, S.; Kirk, M.C.; Cartee, R.T.; Baker, J.R. LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl. Environ. Microbiol. 2007, 73, 7150–7154. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F.; Daraspe, J.; Giddey, M.; Moreillon, P.; Resch, G. In vitro characterization of PlySK1249, a novel phage lysin, and assessment of its antibacterial activity in a mouse model of Streptococcus agalactiae bacteremia. Antimicrob. Agents Chemother. 2013, 57, 6276–6283. [Google Scholar] [CrossRef]
- Kong, M.; Na, H.; Ha, N.C.; Ryu, S. LysPBC2, a novel endolysin harboring a Bacillus cereus spore binding domain. Appl. Environ. Microbiol. 2018, 85, e02462-18. [Google Scholar] [CrossRef] [PubMed]
- Alreja, A.B.; Linden, S.B.; Lee, H.R.; Chao, K.L.; Herzberg, O.; Nelson, D.C. Understanding the Molecular Basis for Homodimer Formation of the Pneumococcal Endolysin Cpl-1. ACS Infect. Dis. 2023, 9, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Silva-Martin, N.; Molina, R.; Angulo, I.; Mancheño, J.M.; García, P.; Hermoso, J.A. Crystallization and preliminary crystallographic analysis of the catalytic module of endolysin from Cp-7, a phage infecting Streptococcus pneumoniae. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhen, X.; Zhou, H.; Zhao, F.; Fan, C.; Perčulija, V.; Tong, Y.; Mi, Z.; Ouyang, S. Structural and functional insights into a novel two-component endolysin encoded by a single gene in Enterococcus faecalis phage. PLoS Pathog. 2020, 16, e1008394. [Google Scholar] [CrossRef]
- Nelson, D.; Schuch, R.; Chahales, P.; Zhu, S.; Fischetti, V.A. PlyC: A multimeric bacteriophage lysin. Proc. Natl. Acad. Sci. USA 2006, 103, 10765–10770. [Google Scholar] [CrossRef]
- McGowan, S.; Buckle, A.M.; Mitchell, M.S.; Hoopes, J.T.; Gallagher, D.T.; Heselpoth, R.D.; Shen, Y.; Reboul, C.F.; Law, R.H.P.; Fischetti, V.A.; et al. X-ray crystal structure of the streptococcal specific phage lysin PlyC. Proc. Natl. Acad. Sci. USA 2012, 109, 12752–12757. [Google Scholar] [CrossRef]
- Wladyka, B.; Bonar, E. Application of Staphylococci in the Food Industry and Biotechnology; Savini, V., Ed.; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128135488. [Google Scholar]
- Schmelcher, M.; Powell, A.M.; Becker, S.C.; Camp, M.J.; Donovan, D.M. Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl. Environ. Microbiol. 2012, 78, 2297–2305. [Google Scholar] [CrossRef]
- Walmagh, M.; Boczkowska, B.; Grymonprez, B.; Briers, Y.; Drulis-Kawa, Z.; Lavigne, R. Characterization of five novel endolysins from Gram-negative infecting bacteriophages. Appl. Microbiol. Biotechnol. 2013, 97, 4369–4375. [Google Scholar] [CrossRef]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B.; Delattre, A.-S.; Lavigne, R. Learning from Bacteriophages—Advantages and Limitations of Phage and Phage-Encoded Protein Applications. Curr. Protein Pept. Sci. 2013, 13, 699–722. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, J.; Low, L.Y.; Kamal, N.; Saile, E.; Forsberg, L.S.; Gutierrez-Sanchez, G.; Hoffmaster, A.R.; Liddington, R.; Quinn, C.P.; Carlson, R.W.; et al. The secondary cell wall polysaccharide of Bacillus anthracis provides the specific binding ligand for the C-terminal cell wall-binding domain of two phage endolysins, PlyL and PlyG. Glycobiology 2013, 23, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Novel method to control pathogenic bacteria on human mucous membranes. Ann. N. Y. Acad. Sci. 2003, 987, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Pastagia, M.; Schuch, R.; Fischetti, V.A.; Huang, D.B. Lysins: The arrival of pathogen-directed anti-infectives. J. Med. Microbiol. 2013, 62, 1506–1516. [Google Scholar] [CrossRef]
- Loessner, M.J.; Kramer, K.; Ebel, F.; Scherer, S. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 2002, 44, 335–349. [Google Scholar] [CrossRef]
- Yang, H.; Xue, J.; Li, J.; Hu, G.; Li, H.; Lu, S.; Fu, Z. Green fluorescent protein-fused bacteriophage cellular wall-binding domain as broad-spectrum signal probe for fluorimetry of methicillin-resistant Staphylococcus aureus strains. Anal. Chim. Acta 2022, 1207, 339799. [Google Scholar] [CrossRef]
- Jado, I.; López, R.; García, E.; Fenoll, A.; Casal, J.; García, P.; Pallares, R.; de la Campa, A.G.; Bouza, E.; Baquero, F.; et al. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Chemother. 2003, 52, 967–973. [Google Scholar] [CrossRef]
- Linden, S.B.; Alreja, A.B.; Nelson, D.C. Application of bacteriophage-derived endolysins to combat streptococcal disease: Current State and perspectives. Curr. Opin. Biotechnol. 2021, 68, 213–220. [Google Scholar] [CrossRef]
- Horgan, M.; O’Flynn, G.; Garry, J.; Cooney, J.; Coffey, A.; Fitzgerald, G.F.; Paul Ross, R.; McAuliffe, O. Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl. Environ. Microbiol. 2009, 75, 872–874. [Google Scholar] [CrossRef]
- Alaksandr, Ž.; Sergey, G.; Maksim, P.; Sergey, K.; Niyaz, S.; Uladzimir, P.; Mikhail, S. Efficient matrix-assisted refolding of the recombinant anti-staphylococcal truncated endolysin LysKCA and its structural and enzymatic description. Protein Expr. Purif. 2020, 174, 105683. [Google Scholar] [CrossRef]
- Van Tassell, M.L.; Angela Daum, M.; Kim, J.S.; Miller, M.J. Creative lysins: Listeria and the engineering of antimicrobial enzymes. Curr. Opin. Biotechnol. 2016, 37, 88–96. [Google Scholar] [CrossRef]
- Kong, M.; Ryu, S. Bacteriophage PBC1 and its endolysin as an antimicrobial agent against Bacillus cereus. Appl. Environ. Microbiol. 2015, 81, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Donovan, D.M. Bacteriophage and Peptidoglycan Degrading Enzymes with Antimicrobial Applications. Recent Pat. Biotechnol. 2007, 1, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Low, L.Y.; Yang, C.; Perego, M.; Osterman, A.; Liddington, R. Role of net charge on catalytic domain and influence of cell wall binding domain on bactericidal activity, specificity, and host range of phage lysins. J. Biol. Chem. 2011, 286, 34391–34403. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, A.; Mixon, M.; Collins, C.H.; Dordick, J.S. Opportunities for broadening the application of cell wall lytic enzymes. Appl. Microbiol. Biotechnol. 2020, 104, 9019–9040. [Google Scholar] [CrossRef]
- Latka, A.; Maciejewska, B.; Majkowska-Skrobek, G.; Briers, Y.; Drulis-Kawa, Z. Bacteriophage-encoded virion-associated enzymes to overcome the carbohydrate barriers during the infection process. Appl. Microbiol. Biotechnol. 2017, 101, 3103–3119. [Google Scholar] [CrossRef]
- Wu, X.; Kwon, S.J.; Kim, J.; Kane, R.S.; Dordick, J.S. Biocatalytic nanocomposites for combating bacterial pathogens. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 87–113. [Google Scholar] [CrossRef]
- Roach, D.R.; Donovan, D.M. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 2015, 5, e1062590. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Martínez, B.; Donovan, D.M.; Rodríguez, A.; García, P. Bacteriophage virion-associated peptidoglycan hydrolases: Potential new enzybiotics. Crit. Rev. Microbiol. 2013, 39, 427–434. [Google Scholar] [CrossRef]
- Keary, R.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Genome analysis of the staphylococcal temperate phage DW2 and functional studies on the endolysin and tail hydrolase. Bacteriophage 2014, 4, e28451. [Google Scholar] [CrossRef]
- Sekiya, H.; Tamai, E.; Kawasaki, J.; Murakami, K.; Kamitori, S. Structural and biochemical characterizations of the novel autolysin Acd24020 from Clostridioides difficile and its full-function catalytic domain as a lytic enzyme. Mol. Microbiol. 2021, 115, 684–698. [Google Scholar] [CrossRef]
- Chapot-Chartier, M.P. Chapter 13 Bacterial Autolysins. In Prokaryotic Cell Wall Compounds, Structure and Biochemistry; Konig, H., Claus, H., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 383–406. ISBN 978-3-642-05061-9. [Google Scholar]
- Raulinaitis, V.; Tossavainen, H.; Aitio, O.; Juuti, J.T.; Hiramatsu, K.; Kontinen, V.; Permi, P. Identification and structural characterization of LytU, a unique peptidoglycan endopeptidase from the lysostaphin family. Sci. Rep. 2017, 7, 6020. [Google Scholar] [CrossRef] [PubMed]
- Odintsov, S.G.; Sabala, I.; Marcyjaniak, M.; Bochtler, M. Latent LytM at 1.3 Å resolution. J. Mol. Biol. 2004, 335, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Wydau-Dematteis, S.; El Meouche, I.; Courtin, P.; Hamiot, A.; Lai-Kuen, R.; Saubaméa, B.; Fenaille, F.; Butel, M.J.; Pons, J.L.; Dupuy, B.; et al. Cwp19 is a novel lytic transglycosylase involved in stationary-phase autolysis resulting in toxin release in Clostridium difficile. MBio 2018, 9, e00648-18. [Google Scholar] [CrossRef]
- Eckert, C.; Lecerf, M.; Dubost, L.; Arthur, M.; Mesnage, S. Functional analysis of AtlA, the major N-acetylglucosaminidase of Enterococcus faecalis. J. Bacteriol. 2006, 188, 8513–8519. [Google Scholar] [CrossRef]
- Götz, F.; Heilmann, C.; Stehle, T. Functional and structural analysis of the major amidase (Atl) in Staphylococcus. Int. J. Med. Microbiol. 2014, 304, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Redko, Y.; Courtin, P.; Mézange, C.; Huard, C.; Chapot-Chartier, M.P. Lactococcus lactis gene YjgB encodes a γ-D-glutaminyl-L-lysyl- endopeptidase which hydrolyzes peptidoglycan. Appl. Environ. Microbiol. 2007, 73, 5825–5831. [Google Scholar] [CrossRef]
- Frankel, M.B.; Hendrickx, A.P.A.; Missiakas, D.M.; Schneewind, O. LytN, a murein hydrolase in the cross-wall compartment of Staphylococcus aureus, is involved in proper bacterial growth and envelope assembly. J. Biol. Chem. 2011, 286, 32593–32605. [Google Scholar] [CrossRef]
- Rodríguez-Cerrato, V.; García, P.; Huelves, L.; García, E.; Del Prado, G.; Gracia, M.; Ponte, C.; López, R.; Soriano, F. Pneumococcal LytA autolysin, a potent therapeutic agent in experimental peritonitis-sepsis caused by highly β-lactam-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2007, 51, 3371–3373. [Google Scholar] [CrossRef]
- Bonnet, J.; Durmort, C.; Jacq, M.; Mortier-Barrière, I.; Campo, N.; VanNieuwenhze, M.S.; Brun, Y.V.; Arthaud, C.; Gallet, B.; Moriscot, C.; et al. Peptidoglycan O-acetylation is functionally related to cell wall biosynthesis and cell division in Streptococcus pneumoniae. Mol. Microbiol. 2017, 106, 832–846. [Google Scholar] [CrossRef]
- Leonard, A.C.; Goncheva, M.I.; Gilbert, S.E.; Shareefdeen, H.; Petrie, L.E.; Thompson, L.K.; Khursigara, C.M.; Heinrichs, D.E.; Cox, G. Autolysin-mediated peptidoglycan hydrolysis is required for the surface display of Staphylococcus aureus cell wall-anchored proteins. Proc. Natl. Acad. Sci. USA 2023, 120, e2301414120. [Google Scholar] [CrossRef] [PubMed]
- Atilano, M.L.; Pereira, P.M.; Vaz, F.; Catalão, M.J.; Reed, P.; Grilo, I.R.; Sobral, R.G.; Ligoxygakis, P.; Pinho, M.G.; Filipe, S.R. Bacterial autolysins trim cell surface peptidoglycan to prevent detection by the drosophila innate immune system. eLife 2014, 3, e02277. [Google Scholar] [CrossRef] [PubMed]
- Osipovitch, D.C.; Therrien, S.; Griswold, K.E. Discovery of novel S. aureus autolysins and molecular engineering to enhance bacteriolytic activity. Appl. Microbiol. Biotechnol. 2015, 99, 6315–6326. [Google Scholar] [CrossRef] [PubMed]
- Osipovitch, D.C.; Griswold, K.E. Fusion with a cell wall binding domain renders autolysin LytM a potent anti-Staphylococcus aureus agent. FEMS Microbiol. Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Verma, D.; Griswold, K.E.; Bailey-Kellogg, C. Building blocks and blueprints for bacterial autolysins. PLoS Comput. Biol. 2021, 17, e1008889. [Google Scholar] [CrossRef]
- Recsei, P.A.; Gruss, A.D.; Novick, R.P. Cloning, sequence, and expression of the lysostaphin gene from Staphylococcus simulans. Proc. Natl. Acad. Sci. USA 1987, 84, 1127–1131. [Google Scholar] [CrossRef]
- Wysocka, A.; Jagielska, E.; Łężniak, Ł.; Sabała, I. Two New M23 Peptidoglycan Hydrolases With Distinct Net Charge. Front. Microbiol. 2021, 12, 719689. [Google Scholar] [CrossRef]
- Sugai, M.; Fujiwara, T.; Akiyama, T.; Ohara, M.; Komatsuzawa, H.; Inoue, S.; Suginaka, H. Purification and molecular characterization of glycylglycine endopeptidase produced by Staphylococcus capitis EPK1. J. Bacteriol. 1997, 179, 1193–1202. [Google Scholar] [CrossRef]
- Thumm, G.; Götz, F. Studies on prolysostaphin processing and characterization of the lysostaphin immunity factor (Lif) of Stphylococcus simulans biovar staphylolyticus. Mol. Microbiol. 1997, 23, 1251–1265. [Google Scholar] [CrossRef]
- Fujiwara, T.; Aoki, S.; Komatsuzawa, H.; Nishida, T.; Ohara, M.; Suginaka, H.; Sugai, M. Mutation analysis of the histidine residues in the glycylglycine endopeptidase ALE-1. J. Bacteriol. 2005, 187, 480–487. [Google Scholar] [CrossRef][Green Version]
- Rawlings, N.D.; Morton, F.R.; Kok, C.Y.; Kong, J.; Barrett, A.J. MEROPS: The peptidase database. Nucleic Acids Res. 2008, 36, D320–D325. [Google Scholar] [CrossRef]
- Hirakawa, H.; Akita, H.; Fujiwara, T.; Sugai, M.; Kuhara, S. Structural insight into the binding mode between the targeting domain of ALE-1 (92AA) and pentaglycine of peptidoglycan. Protein Eng. Des. Sel. 2009, 22, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Sabala, I.; Jagielska, E.; Bardelang, P.T.; Czapinska, H.; Dahms, S.O.; Sharpe, J.A.; James, R.; Than, M.E.; Thomas, N.R.; Bochtler, M. Crystal structure of the antimicrobial peptidase lysostaphin from Staphylococcus simulans. FEBS J. 2014, 281, 4112–4122. [Google Scholar] [CrossRef]
- Lu, J.Z.; Fujiwara, T.; Komatsuzawa, H.; Sugai, M.; Sakon, J. Cell wall-targeting domain of glycylglycine endopeptidase distinguishes among peptidoglycan cross-bridges. J. Biol. Chem. 2006, 281, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.A.; Schuhardt, V.T. Lysostaphin: A New Bacteriolytic Agent for the Staphylococcus. Proc. Natl. Acad. Sci. USA 1964, 51, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Sloan, G.L.; Robinson, J.M.; Kloos, W.E. Identification of Staphylococcus staphylolyticus NRRL B-2628 as a biovar of Staphylococcus simulans. Int. J. Syst. Bacteriol. 1982, 32, 170–174. [Google Scholar] [CrossRef]
- Heath, L.S.; Heath, H.E.; Sloan, G.L. Plasmid-encoded lysostaphin endopeptidase gene of Staphylococcus simulans biovar staphylolyticus. FEMS Microbiol. Lett. 1987, 44, 129–133. [Google Scholar] [CrossRef]
- Browder, H.P.; Zygmunt, W.A.; Young, J.R.; Tavormina, P.A. Lysostaphin: Enzymatic mode of action. Biochem. Biophys. Res. Commun. 1965, 19, 383–389. [Google Scholar] [CrossRef]
- Iversen, O.-J.; Grov, A. Studies on Lysostaphin: Separation and Characterization of Three Enzymes. Eur. J. Biochem. 1973, 38, 293–300. [Google Scholar] [CrossRef]
- Baba, T.; Schneewind, O. Target cell specificity of a bacteriocin molecule: A C-terminal signal directs lysostaphin to the cell wall of Staphylococcus aureus. EMBO J. 1996, 15, 4789–4797. [Google Scholar] [CrossRef]
- Gründling, A.; Schneewind, O. Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus. J. Bacteriol. 2006, 188, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Mitkowski, P.; Jagielska, E.; Nowak, E.; Bujnicki, J.M.; Stefaniak, F.; Niedziałek, D.; Bochtler, M.; Sabała, I. Structural bases of peptidoglycan recognition by lysostaphin SH3b domain. Sci. Rep. 2019, 9, 5965. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Delgado, L.S.; Walters-Morgan, H.; Salamaga, B.; Robertson, A.J.; Hounslow, A.M.; Jagielska, E.; Sabała, I.; Williamson, M.P.; Lovering, A.L.; Mesnage, S. Two-site recognition of Staphylococcus aureus peptidoglycan by lysostaphin SH3b. Nat. Chem. Biol. 2020, 16, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Bonar, E.; Bukowski, M.; Chlebicka, K.; Madry, A.; Bereznicka, A.; Kosecka-Strojek, M.; Dubin, G.; Miedzobrodzki, J.; Mak, P.; Wladyka, B. Human skin microbiota-friendly lysostaphin. Int. J. Biol. Macromol. 2021, 183, 852–860. [Google Scholar] [CrossRef]
- Kamiryo, T.; Matsuhashi, M. The biosynthesis of the cross-linking peptides in the cell wall peptidoglycan of Staphylococcus aureus. J. Biol. Chem. 1972, 247, 6306–6311. [Google Scholar] [CrossRef]
- Rohrer, S.; Ehlert, K.; Tschierske, M.; Labischinski, H.; Berger-Bachi, B. The essential Staphylococcus aureus gene fmhB is involved in the first step of peptidoglycan pentaglycine interpeptide formation. Proc. Natl. Acad. Sci. USA 1999, 96, 9351–9356. [Google Scholar] [CrossRef]
- Kopp, U.; Roos, M.; Wecke, J.; Labischinski, H. Staphylococcal peptidoglycan interpeptide bridge biosynthesis: A novel antistaphylococcal target? Microb. Drug Resist. 1996, 2, 29–41. [Google Scholar] [CrossRef]
- Ehlert, K.; Schroder, W.; Labischinski, H. Specificities of FemA and FemB for different glycine residues: FemB cannot substitute for FemA in staphylococcal peptidoglycan pentaglycine side chain formation. J. Bacteriol. 1997, 179, 7573–7576. [Google Scholar] [CrossRef]
- Strandén, A.M.; Ehlert, K.; Labischinski, H.; Berger-BÄCHI, B. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J. Bacteriol. 1997, 179, 9–16. [Google Scholar] [CrossRef]
- Robinson, J.M.; Hardman, J.K.; Sloan, G.L. Relationship between lysostaphin endopeptidase production and cell wall composition in Staphylococcus staphylolyticus. J. Bacteriol. 1979, 137, 1158–1164. [Google Scholar] [CrossRef]
- DeHart, H.P.; Heath, H.E.; Heath, L.S.; LeBlanc, P.A.; Sloan, G.L. The lysostaphin endopeptidase resistance gene (epr) specifies modification of peptidoglycan cross bridges in Staphylococcus simulans and Staphylococcus aureus. Appl. Environ. Microbiol. 1995, 61, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Tschierske, M.; Ehlert, K.; Strandén, A.M.; Berger-Bächi, B. Lif, the lysostaphin immunity factor, complements FemB in staphylococcal peptidoglycan interpeptide bridge formation. FEMS Microbiol. Lett. 1997, 153, 261–264. [Google Scholar] [CrossRef]
- Ehlert, K.; Tschierske, M.; Mori, C.; Schröder, W.; Berger-Bächi, B. Site-specific serine incorporation by Lif and Epr into positions 3 and 5 of the staphylococcal peptidoglycan interpeptide bridge. J. Bacteriol. 2000, 182, 2635–2638. [Google Scholar] [CrossRef] [PubMed]
- Sugai, M.; Fujiwara, T.; Ohta, K.; Komatsuzawa, H.; Ohara, M.; Suginaka, H. epr, which encodes glycylglycine endopeptidase resistance, is homologous to femAB and affects serine content of peptidoglycan cross bridges in Staphylococcus capitis and Staphylococcus aureus. J. Bacteriol. 1997, 179, 4311–4318. [Google Scholar] [CrossRef]
- Ajuebor, J.; McAuliffe, O.; O’Mahony, J.; Ross, R.P.; Hill, C.; Coffey, A. Bacteriophage endolysins and their applications. Sci. Prog. 2016, 99, 183–199. [Google Scholar] [CrossRef]
- Loeffler, J.M.; Nelson, D.; Fischetti, V.A. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 2001, 294, 2170–2172. [Google Scholar] [CrossRef] [PubMed]
- Schuch, R.; Nelson, D.; Fischetti, V.A. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 2002, 418, 884–889. [Google Scholar] [CrossRef]
- Pastagia, M.; Euler, C.; Chahales, P.; Fuentes-Duculan, J.; Krueger, J.G.; Fischetti, V.A. A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrob. Agents Chemother. 2011, 55, 738–744. [Google Scholar] [CrossRef]
- Becker, S.C.; Roach, D.R.; Chauhan, V.S.; Shen, Y.; Foster-Frey, J.; Powell, A.M.; Bauchan, G.; Lease, R.A.; Mohammadi, H.; Harty, W.J.; et al. Triple-acting Lytic Enzyme Treatment of Drug-Resistant and Intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 25063. [Google Scholar] [CrossRef]
- Singh, P.K.; Donovan, D.M.; Kumar, A. Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob. Agents Chemother. 2014, 58, 4621–4629. [Google Scholar] [CrossRef]
- Grishin, A.V.; Karyagina, A.S.; Vasina, D.V.; Vasina, I.V.; Gushchin, V.A.; Lunin, V.G. Resistance to peptidoglycan-degrading enzymes. Crit. Rev. Microbiol. 2020, 46, 703–726. [Google Scholar] [CrossRef] [PubMed]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Kusuma, C.; Jadanova, A.; Chanturiya, T.; Kokai-Kun, J.F. Lysostaphin-resistant variants of Staphylococcus aureus demonstrate reduced fitness in vitro and in vivo. Antimicrob. Agents Chemother. 2007, 51, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Stogios, P.J.; Savchenko, A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef]
- Yang, H.; Yu, J.; Wei, H. Engineered bacteriophage lysins as novel anti-infectives. Front. Microbiol. 2014, 5, 542. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Donovan, D.M.; García, P. Enhanced staphylolytic activity of the Staphylococcus aureus bacteriophage vB_SauS-phiiPla88 HydH5 Virion-associated peptidoglycan hydrolase: Fusions, deletions, and synergy with LysH5. Appl. Environ. Microbiol. 2012, 78, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.; Ross, P.; Mcauliffe, O.; O’Mahony, J.; Coffey, A. Recombinant bacteriophage lysins as antibacterials. Bioeng. Bugs 2010, 1, 9–16. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Gutiérrez, D.; Donovan, D.M.; Martínez, B.; Rodríguez, A.; García, P. Phage lytic proteins: Biotechnological applications beyond clinical antimicrobials. Crit. Rev. Biotechnol. 2016, 36, 542–552. [Google Scholar] [CrossRef]
- São-José, C.; Costa, A.R.; Melo, L.D.R. Editorial: Bacteriophages and Their Lytic Enzymes as Alternative Antibacterial Therapies in the Age of Antibiotic Resistance. Front. Microbiol. 2022, 13, 884176. [Google Scholar] [CrossRef]
- van der Ploeg, J.R. Characterization of Streptococcus gordonii prophage PH15: Complete genome sequence and functional analysis of phage-encoded integrase and endolysin. Microbiology 2008, 154, 2970–2978. [Google Scholar] [CrossRef]
- Muharram, M.M.; Abulhamd, A.T.; Aldawsari, M.F.; Alqarni, M.H.; Labrou, N.E. Development of staphylococcus enzybiotics: The ph28 gene of Staphylococcus epidermidis phage ph15 is a two-domain endolysin. Antibiotics 2020, 9, 148. [Google Scholar] [CrossRef]
- Yoong, P.; Schuch, R.; Nelson, D.; Fischetti, V.A. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J. Bacteriol. 2004, 186, 4808–4812. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.M.; Rowley, D.T.; Young, C.; Franks, A.; Hyman, P.; Donovan, D.M. The endolysin from the Enterococcus faecalis bacteriophage VD13 and conditions stimulating its lytic activity. FEMS Microbiol. Lett. 2016, 363, fnw216. [Google Scholar] [CrossRef]
- Zimmer, M.; Vukov, N.; Scherer, S.; Loessner, M.J. The murein hydrolase of the bacteriophage φ3626 dual lysis system is active against all tested Clostridium perfringens strains. Appl. Environ. Microbiol. 2002, 68, 5311–5317. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.J.; Narbad, A.; Gasson, M.J. Molecular characterization of a Clostridium difficile bacteriophage and its cloned biologically active endolysin. J. Bacteriol. 2008, 190, 6734–6740. [Google Scholar] [CrossRef]
- Mayer, M.J.; Payne, J.; Gasson, M.J.; Narbad, A. Genomic sequence and characterization of the virulent bacteriophage φCTP1 from Clostridium tyrobutyricum and heterologous expression of its endolysin. Appl. Environ. Microbiol. 2010, 76, 5415–5422. [Google Scholar] [CrossRef] [PubMed]
- Griego, A.; Antinori, B.; Spitaleri, A.; Muzzolini, I.; Muzzioli, S. Endolysin B as a new archetype in M. tuberculosis treatment. bioRxiv 2023. [Google Scholar] [CrossRef]
- Gaeng, S.; Scherer, S.; Neve, H.; Loessner, M.J. Gene cloning and expression and secretion of Listeria monocytogenes bacteriophage-lyric enzymes in Lactococcus lactis. Appl. Environ. Microbiol. 2000, 66, 2951–2958. [Google Scholar] [CrossRef]
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int. J. Antimicrob. Agents 2020, 55, 105844. [Google Scholar] [CrossRef]
- Rahman, M.U.; Wang, W.; Sun, Q.; Shah, J.A.; Li, C.; Sun, Y.; Li, Y.; Zhang, B.; Chen, W.; Wang, S. Endolysin, a promising solution against antimicrobial resistance. Antibiotics 2021, 10, 1277. [Google Scholar] [CrossRef]
- Lai, M.J.; Lin, N.T.; Hu, A.; Soo, P.C.; Chen, L.K.; Chen, L.H.; Chang, K.C. Antibacterial activity of Acinetobacter baumannii phage ΦaB2 endolysin (LysAB2) against both Gram-positive and Gram-negative bacteria. Appl. Microbiol. Biotechnol. 2011, 90, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, S.M.; Guezenec, S.; Piot, M.; Foster, S.; Lortal, S. Mur-LH, the Broad-Spectrum Endolysin of Lactobacillus helveticus Temperate Bacteriophage φ-0303. Appl. Environ. Microbiol. 2004, 70, 96–103. [Google Scholar] [CrossRef]
- Wang, J.; Liang, S.; Lu, X.; Xu, Q.; Zhu, Y.; Yu, S.; Zhang, W.; Liu, S.; Xie, F. Bacteriophage endolysin Ply113 as a potent antibacterial agent against polymicrobial biofilms formed by enterococci and Staphylococcus aureus. Front. Microbiol. 2023, 14, 1304932. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jin, J.S.; Choi, Y.J.; Kim, J. LysSAP26, a New Recombinant Phage Endolysin with a Broad Spectrum Antibacterial Activity. Viruses 2020, 12, 1340. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef]
- Schmelcher, M.; Loessner, M.J. Bacteriophage endolysins—Extending their application to tissues and the bloodstream. Curr. Opin. Biotechnol. 2021, 68, 51–59. [Google Scholar] [CrossRef]
- De Maesschalck, V.; Gutiérrez, D.; Paeshuyse, J.; Lavigne, R.; Briers, Y. Advanced engineering of third-generation lysins and formulation strategies for clinical applications. Crit. Rev. Microbiol. 2020, 46, 548–564. [Google Scholar] [CrossRef]
- Schaffner, W.; Melly, M.A.; Hash, J.H.; Koenig, M.G. Lysostaphin: An enzymatic approach to staphylococcal disease. I. In vitro studies. Yale J. Biol. Med. 1967, 39, 215–229. [Google Scholar] [PubMed]
- King, B.F.; Biel, M.L.; Wilkinson, B.J. Facile penetration of the Staphylococcus aureus capsule by lysostaphin. Infect. Immun. 1980, 29, 892–896. [Google Scholar] [CrossRef]
- Bastos, M.D.C.D.F.; Coutinho, B.G.; Coelho, M.L.V. Lysostaphin: A staphylococcal bacteriolysin with potential clinical applications. Pharmaceuticals 2010, 3, 1139–1161. [Google Scholar] [CrossRef]
- Jayakumar, J.; Kumar, V.A.; Biswas, L.; Biswas, R. Therapeutic applications of lysostaphin against Staphylococcus aureus. J. Appl. Microbiol. 2021, 131, 1072–1082. [Google Scholar] [CrossRef]
- Harrison, E.F.; Zygmunt, W.A. Lysostaphin in experimental renal infections. J. Bacteriol. 1967, 93, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, W.; Melly, M.A.; Koenig, M.G. Lysostaphin: An enzymatic approach to staphylococcal disease. II. In vivo studies. Yale J. Biol. Med. 1967, 39, 230–244. [Google Scholar]
- Dixon, R.E.; Goodman, J.S.; Koenig, M.G. Lysostaphin: An enzymatic approach to staphylococcal disease. 3. Combined lysostaphin-methicillin therapy of established staphylococcal abscesses in mice. Yale J. Biol. Med. 1968, 41, 62–68. [Google Scholar]
- Climo, M.W.; Patron, R.L.; Goldstein, B.P.; Archer, G.L. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob. Agents Chemother. 1998, 42, 1355–1360. [Google Scholar] [CrossRef]
- Dajcs, J.J.; Thibodeaux, B.A.; Girgis, D.O.; Shaffer, M.D.; Delvisco, S.M.; O’Callaghan, R.J. Immunity to lysostaphin and its therapeutic value for ocular MRSA infections in the rabbit. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3712–3716. [Google Scholar]
- Placencia, F.X.; Kong, L.; Weisman, L.E. Treatment of methicillin-resistant Staphylococcus aureus in neonatal mice: Lysostaphin versus vancomycin. Pediatr. Res. 2009, 65, 420–424. [Google Scholar] [CrossRef]
- Walsh, S.; Shah, A.; Mond, J. Improved pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol. Antimicrob. Agents Chemother. 2003, 47, 554–558. [Google Scholar] [CrossRef]
- Shah, A.; Mond, J.; Walsh, S. Lysostaphin-coated catheters eradicate Staphylococccus aureus challenge and block surface colonization. Antimicrob. Agents Chemother. 2004, 48, 2704–2707. [Google Scholar] [CrossRef]
- Zhao, H.; Brooks, S.A.; Eszterhas, S.; Heim, S.; Li, L.; Xiong, Y.Q.; Fang, Y.; Kirsch, J.R.; Verma, D.; Bailey-Kellogg, C.; et al. Globally deimmunized lysostaphin evades human immune surveillance and enables highly efficacious repeat dosing. Sci. Adv. 2020, 6, eabb9011. [Google Scholar] [CrossRef]
- Mądry, A.; Jendroszek, A.; Dubin, G.; Wladyka, B. Production of Lysostaphin by Nonproprietary Method Utilizing a Promoter from Toxin–Antitoxin System. Mol. Biotechnol. 2019, 61, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Verma, D.; Li, W.; Choi, Y.; Ndong, C.; Fiering, S.N.; Bailey-Kellogg, C.; Griswold, K.E. Depletion of T cell epitopes in lysostaphin mitigates anti-drug antibody response and enhances antibacterial efficacy in vivo. Chem. Biol. 2015, 22, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.E.; Plaut, K.; Bramley, A.J.; Williamson, C.M.; Lax, A.J.; Moore, K.; Wells, K.D.; Wall, R.J. Lysostaphin expression in mammary glands confers protection against staphylococcal infection in transgenic mice. Nat. Biotechnol. 2001, 19, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.R.; White, A. The reacquisition of staphylococci by treated carriers: A demonstration of bacterial interference. J. Lab. Clin. Med. 1968, 71, 791–797. [Google Scholar] [PubMed]
- Quickel, K.E.; Selden, R.; Caldwell, J.R.; Nora, N.F.; Schaffner, W. Efficacy and safety of topical lysostaphin treatment of persistent nasal carriage of Staphylococcus aureus. Appl. Microbiol. 1971, 22, 446–450. [Google Scholar] [CrossRef]
- Stark, F.R.; Thornsvard, C.; Flannery, E.P.; Artenstein, M.S. Systemic Lysostaphin in Man—Apparent Antimicrobial Activity in a Neutropenic Patient. N. Engl. J. Med. 1974, 291, 239–240. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhydzetski, A.; Głowacka-Grzyb, Z.; Bukowski, M.; Żądło, T.; Bonar, E.; Władyka, B. Agents Targeting the Bacterial Cell Wall as Tools to Combat Gram-Positive Pathogens. Molecules 2024, 29, 4065. https://doi.org/10.3390/molecules29174065
Zhydzetski A, Głowacka-Grzyb Z, Bukowski M, Żądło T, Bonar E, Władyka B. Agents Targeting the Bacterial Cell Wall as Tools to Combat Gram-Positive Pathogens. Molecules. 2024; 29(17):4065. https://doi.org/10.3390/molecules29174065
Chicago/Turabian StyleZhydzetski, Aliaksandr, Zuzanna Głowacka-Grzyb, Michal Bukowski, Tomasz Żądło, Emilia Bonar, and Benedykt Władyka. 2024. "Agents Targeting the Bacterial Cell Wall as Tools to Combat Gram-Positive Pathogens" Molecules 29, no. 17: 4065. https://doi.org/10.3390/molecules29174065
APA StyleZhydzetski, A., Głowacka-Grzyb, Z., Bukowski, M., Żądło, T., Bonar, E., & Władyka, B. (2024). Agents Targeting the Bacterial Cell Wall as Tools to Combat Gram-Positive Pathogens. Molecules, 29(17), 4065. https://doi.org/10.3390/molecules29174065






