Study on the Hydrophobic Modification Mechanism of Stearic Acid on the Surface of Coal Gasification Fly Ash
Abstract
:1. Introduction
2. Results and Discussion
2.1. FA Characteristics
2.2. FA Modification Characteristics
2.2.1. Activation Index and Oil Absorption Value
2.2.2. Thermogravimetric Curve
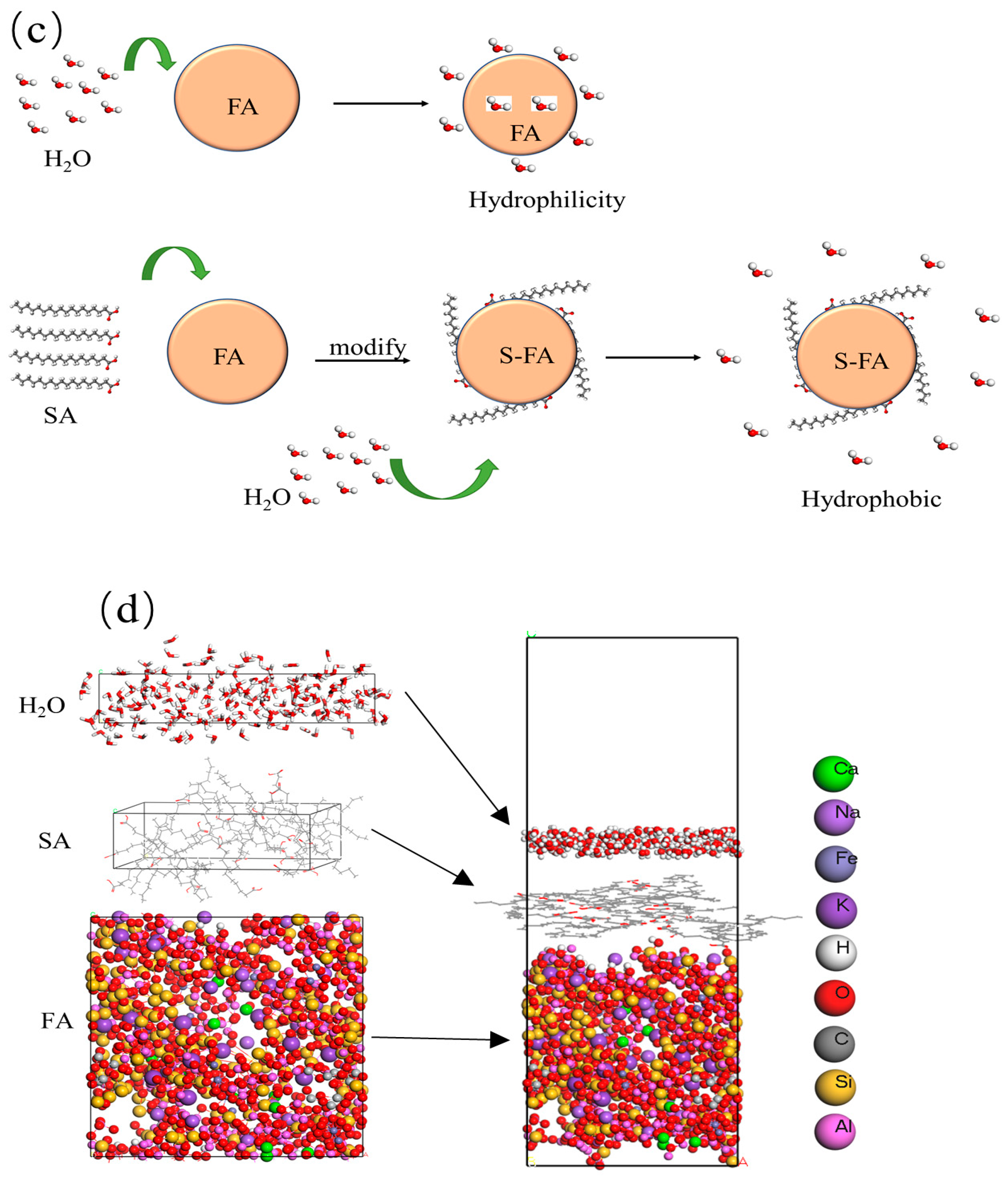
2.3. Research on the Microscopic Mechanism
2.3.1. Model Construction
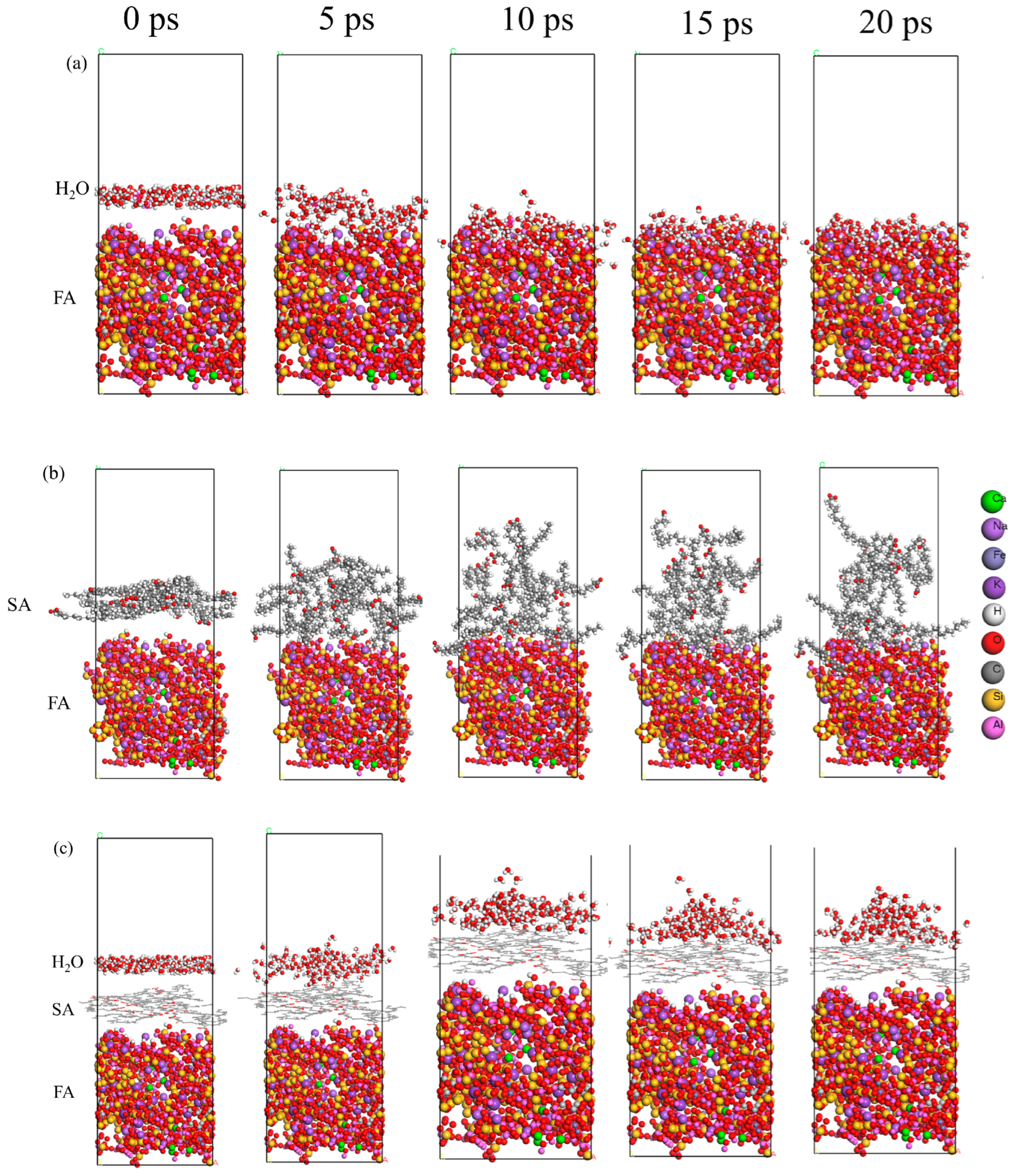
2.3.2. Molecular Dynamics Relaxation
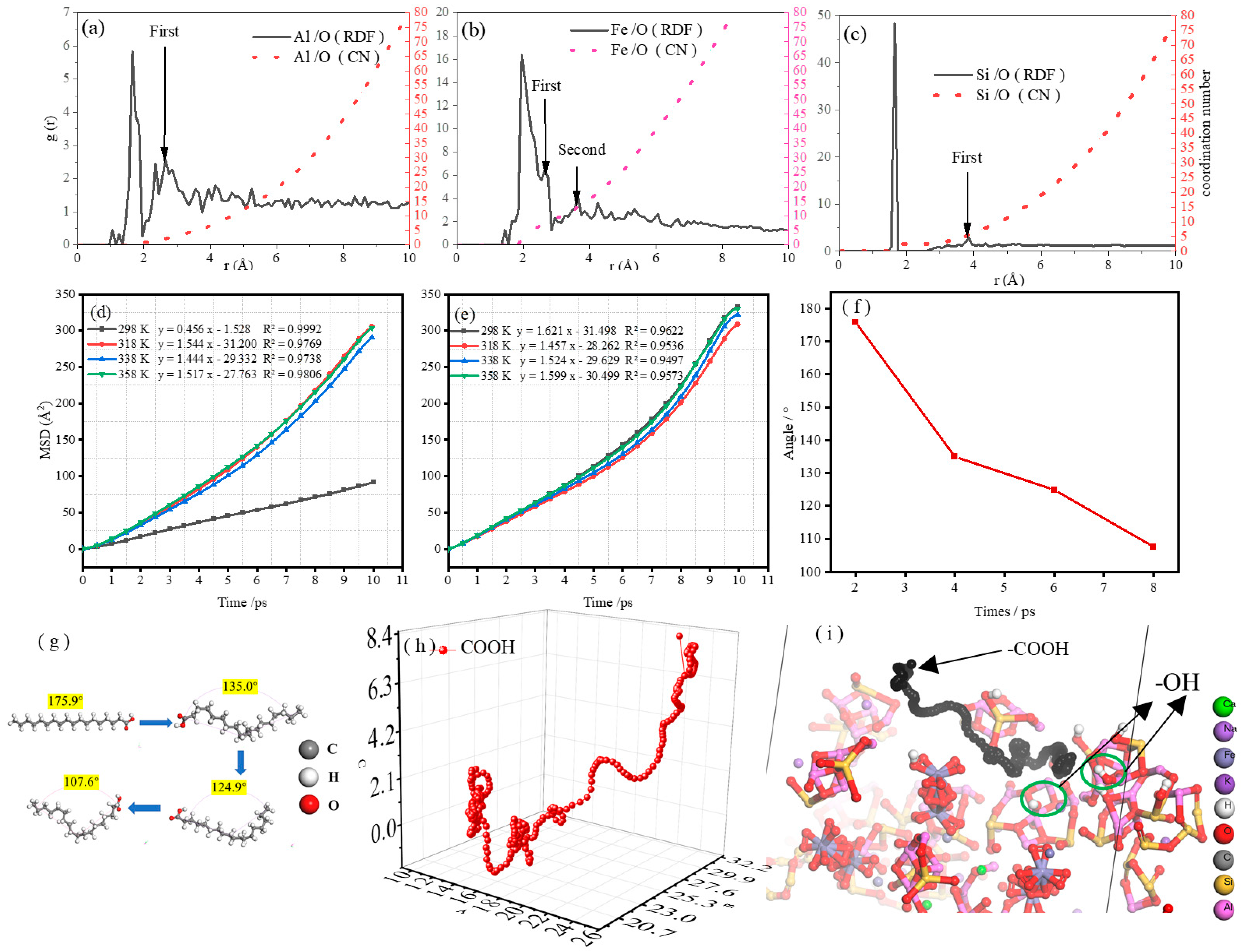
2.3.3. Radial Distribution Function and Coordination Number
2.3.4. Mean Square Displacement
2.3.5. SA Bond Angle and Carboxyl Trajectory
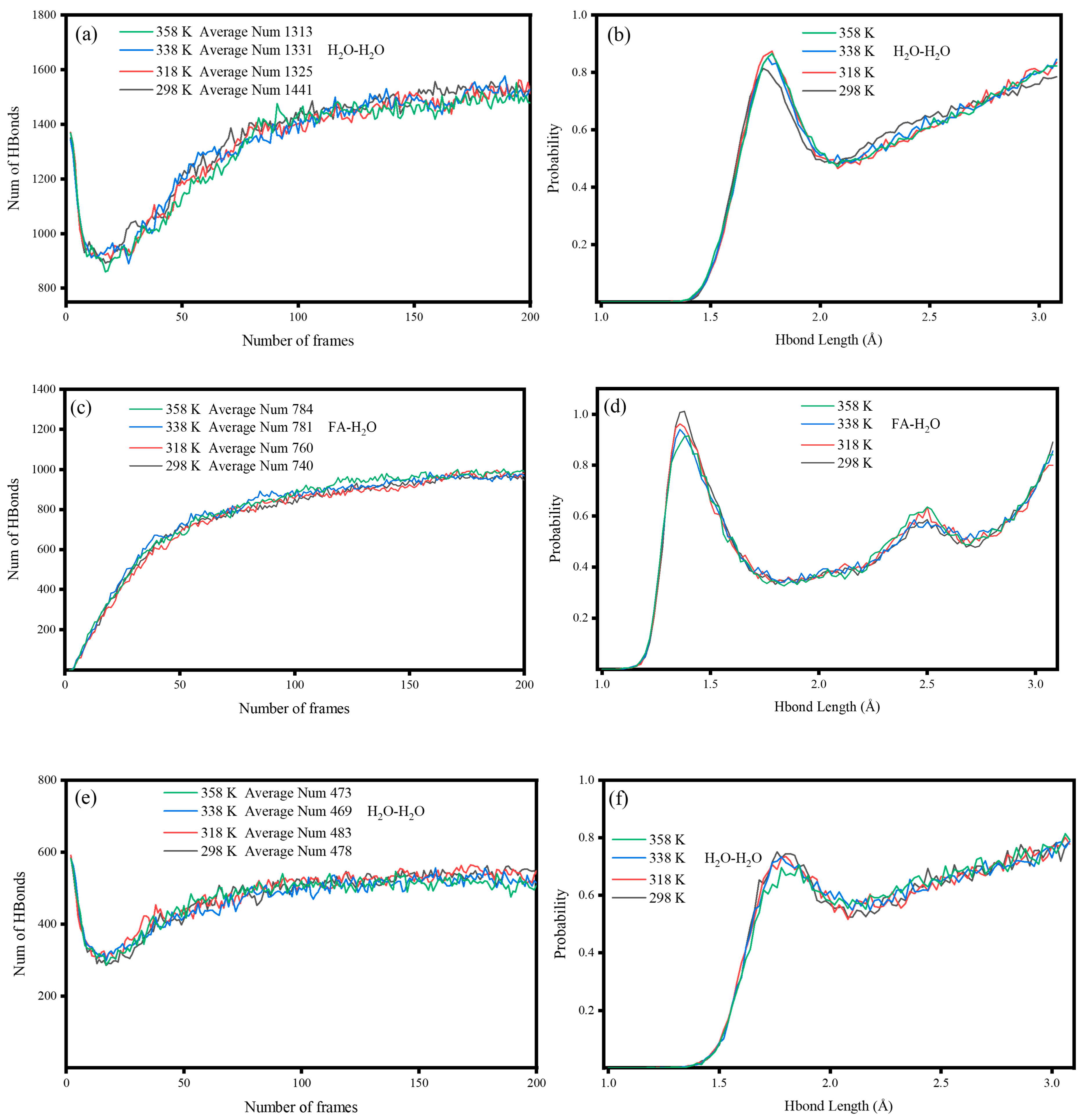
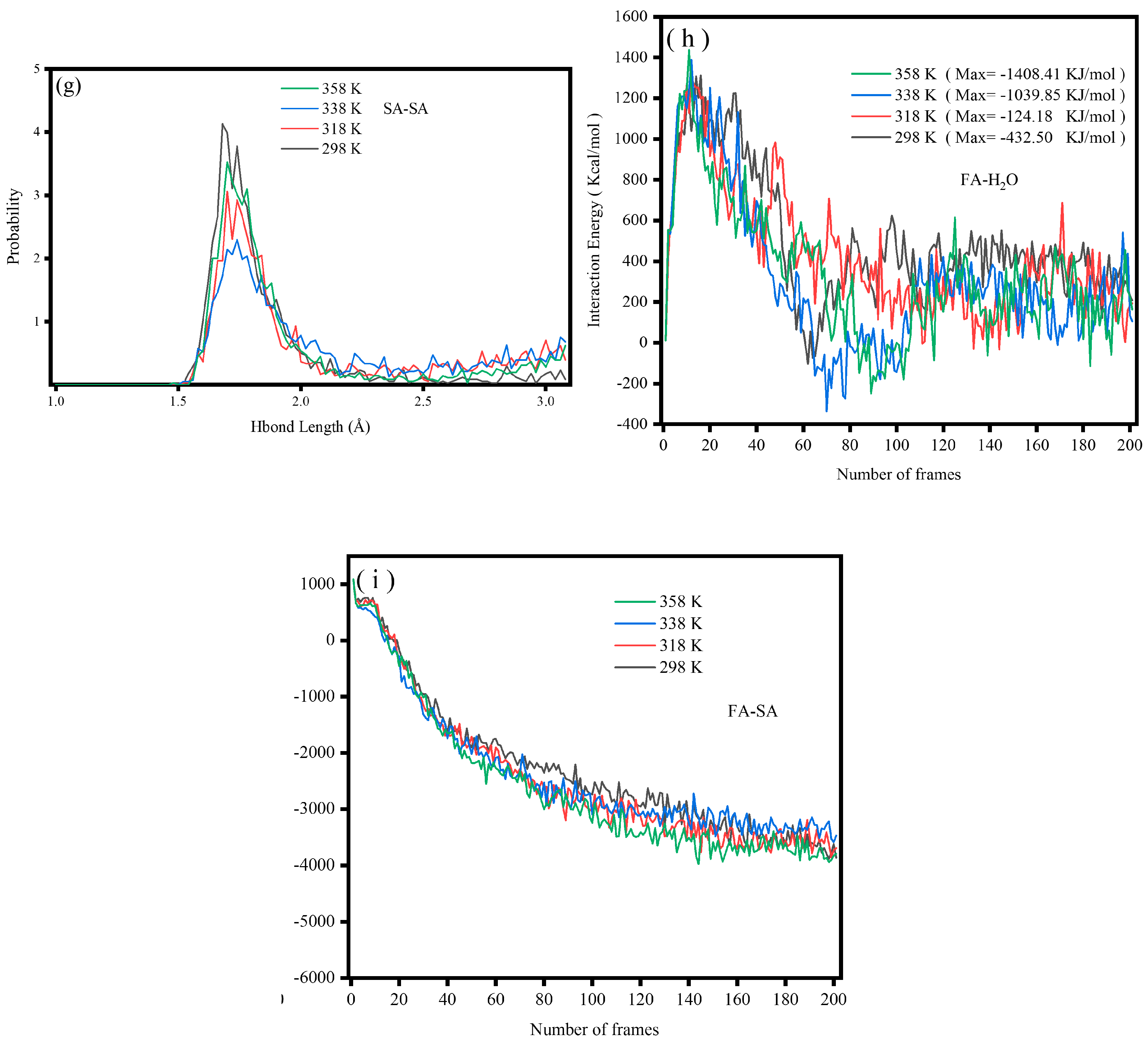
2.3.6. Hydrogen Bonds
2.3.7. Interaction Energy
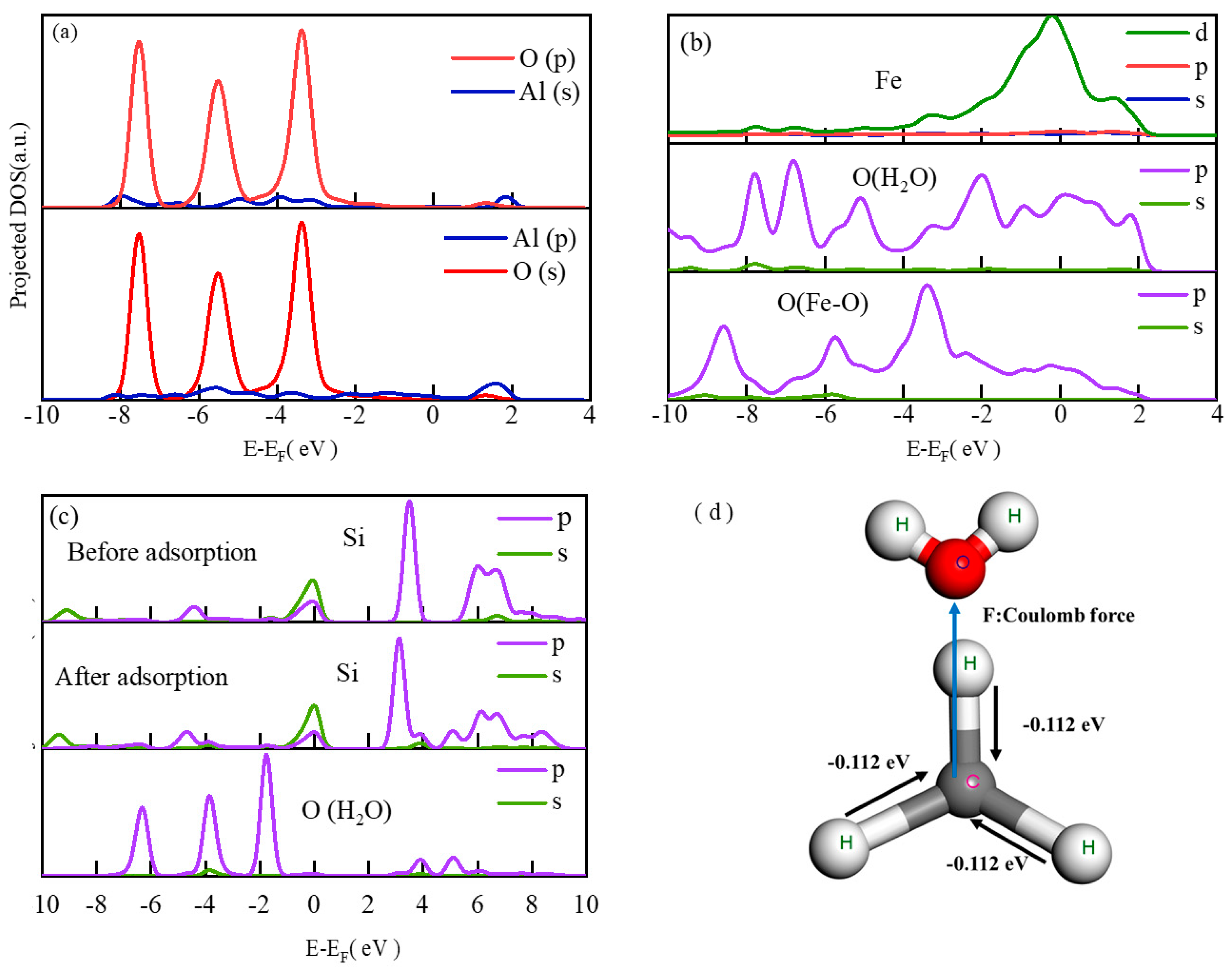
2.3.8. Density of States
2.3.9. Mulliken Charge Distribution Analysis
3. Materials and Methods
3.1. Materials
3.2. Modification Methods
3.3. Oil Absorption Value
3.4. Activation Index
3.5. Characterization Method
3.6. Coverage and Coverage Test
3.7. Model Building
3.7.1. Construction of the Initial FA Model
3.7.2. Calculation Method
4. Conclusions
- (1)
- FA was changed from a hydrophilic structure to a hydrophobic structure through the surface modification of SA. When the activation index increased from 0 to 0.98, the contact angle increased from 23.4° to 127.2°, and the oil absorption decreased from 0.564 g/g to 0.510 g/g.
- (2)
- The adsorption effect of FA on SA was mainly pore adsorption, and the pore-blocking effect was preferentially completed to start the surface coating outside the pores. In addition, the pore-blocking effect and the adhesion between particles caused by SA modification reduced the BET-specific surface area from 13.973 m2/g to 3.218 m2/g, which weakened the adsorption performance of FA.
- (3)
- In the temperature range of 298–358 K, the surface wettability of FA became more hydrophilic with increasing temperature, while the surface wettability of S-FA was not affected in this temperature range. When there was an excess of SA, a multilayer unstable interface formed on the surface of FA, which was less stable in water than the monolayer interface formed by SA, thus reducing the activation index. In addition, when the temperature was greater than 210 °C, the SA layer began to decompose and volatilize, and the SA coating rate on the FA surface was indirectly adjusted by adjusting the reaction time and temperature to dynamically regulate the surface hydrophobicity.
- (4)
- Al, Fe, and Si served as the main active adsorption sites of FA on H2O. The top adsorption of the Al atom on water molecules was strong chemical adsorption, with new chemical bonds between Al and O as well as Fe and O. The adsorption of Si atoms on water molecules was physical adsorption. In addition, the adsorbed water molecules on the surface of FA formed hydrogen bonds with lengths of 1.5–2.5 Å, which resulted in the aggregation of FA in water.
- (5)
- In the temperature range of 298–358 K, heating increased the interaction energy between FA and SA, and the combination of FA and SA became more stable with increasing temperature. However, according to the intramolecular hydrogen bond length of SA, 338 K was considered as the best temperature for SA grafting on the FA surface.
- (6)
- The long alkyl chain in the hydrophobic layer of SA mainly relied on the central carbon atom in the -CH3′ structure to obtain electrons from the H atoms in different directions in the space, increasing the Coulomb repulsion with the O atoms in the water molecule to achieve the hydrophobic effect. In addition, the carboxyl group on the long chain of SA tended to attack the hydroxyl group on the surface of FA, but the interchain interactions of SA molecules led to steric hindrance and closed-loop effects. When there was an excess of SA, the carboxyl group (-COOH) in SA was hindered from attacking the hydroxyl group (-OH) on the surface of FA, resulting in the instability of the formed hydrophobic layer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, D.; Rout, P.K. A Review of Coal Fly Ash Utilization to Save the Environment. Water Air Soil Pollut. 2023, 234, 128. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Samiotis, G.; Lefteri, L.; Amanatidou, E. Cr(VI) Leached from Lignite Fly Ash—Assessment of Groundwater Contamination Risk. Water Air Soil Pollut. 2020, 231, 373. [Google Scholar] [CrossRef]
- Chatterjee, A.; Hu, X.; Lam, F.L.-Y. Modified coal fly ash waste as an efficient heterogeneous catalyst for dehydration of xylose to furfural in biphasic medium. Fuel 2019, 239, 726–736. [Google Scholar] [CrossRef]
- Torralvo, F.A.; Fernández-Pereira, C.; Campanario, M.C. Recovery of Germanium from Aqueous Solutions by Ion-Exchange Extraction of Its Catechol Complex. Ind. Eng. Chem. Res. 2010, 49, 4817–4823. [Google Scholar] [CrossRef]
- Qin, S.; Sun, Y.; Li, Y.; Wang, J.; Zhao, C.; Gao, K. Coal deposits as promising alternative sources for gallium. Earth Sci. Rev. 2015, 150, 95–101. [Google Scholar] [CrossRef]
- He, P.; Qin, H.; Zhang, Y.; Zhao, X.; Chen, N.; Wu, J. Influence of mercury retention on mercury adsorption of fly ash. Energy 2020, 204, 117927. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X.; Li, F. Methylene blue adsorption properties of mechanochemistry modified coal fly ash. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 2133–2141. [Google Scholar] [CrossRef]
- Qi, L.; Luo, J.; Wang, W.; Zhao, W. Adsorption of SO2 from sintering flue gas by alkali modified fly ash in electrostatic precipitator. Sep. Sci. Technol. 2023, 58, 1237–1251. [Google Scholar] [CrossRef]
- Garbalińska, H.; Bochenek, M.; Stasiak, M. Experimental and Modeling Investigations on the Water Sorption Behaviors of Autoclaved Aerated Concrete. Materials 2021, 14, 6235. [Google Scholar] [CrossRef]
- Backiel-Brzozowska, B.; Nikitsin, V.I.; Alsabry, A.; Nikitsin, S.K.; Rutkowska, W. Peculiarities of Calculating the Thermal Conductivity of Moist Autoclaved Aerated Concrete. Energies 2022, 15, 5831. [Google Scholar] [CrossRef]
- Zhiliang, C.; Minghui, T.; Shengyong, L.; Buekens, A.; Jiamin, D.; Qili, Q.; Jianhua, Y. Mechanochemical degradation of PCDD/Fs in fly ash within different milling systems. Chemosphere 2019, 223, 188–195. [Google Scholar] [CrossRef]
- Luo, T.; Wang, Q.; Fang, Z. Effect of graphite on the self-sensing properties of cement and alkali-activated fly ash/slag based composite cementitious materials. J. Build. Eng. 2023, 77, 107493. [Google Scholar] [CrossRef]
- Rong, X.; Zhang, X.; Zhang, J.; Xu, W.; Zhang, Z. Study on mechanical and thermal properties of alkali-excited fly ash aerogel foam concrete. Constr. Build. Mater. 2023, 408, 133770. [Google Scholar] [CrossRef]
- Vu, D.-H.; Bui, H.-B.; Kalantar, B.; Bui, X.-N.; Nguyen, D.-A.; Le, Q.-T.; Do, N.-H.; Nguyen, H. Composition and Morphology Characteristics of Magnetic Fractions of Coal Fly Ash Wastes Processed in High-Temperature Exposure in Thermal Power Plants. Appl. Sci. 2019, 9, 1964. [Google Scholar] [CrossRef]
- Qu, B.; Martin, A.; Pastor, J.; Palomo, A.; Jiménez, A.F. Effects of elevated temperatures on properties of hybrid alkaline-belite cement with high level of fly ash. J. Mater. Res. Technol. 2022, 21, 2455–2470. [Google Scholar] [CrossRef]
- Mushtaq, S.; Aslam, Z.; Ali, R.; Aslam, U.; Naseem, S.; Ashraf, M.; Bello, M.M. Surfactant modified waste ash for the removal of chloro and nitro group substituted benzene from wastewater. Water Sci. Technol. 2022, 86, 1969–1980. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Lam, E.S.-S.; Jang, J.G. Effects of modified binders on flowability of grout and properties of preplaced aggregate concrete. J. Build. Eng. 2023, 68, 106235. [Google Scholar] [CrossRef]
- Zhigang, P.; Jian, Z.; Qian, F.; Changjun, Z.; Yong, Z.; Bojian, Z.; Jinhua, H. Synthesis and retarder mechanism study of a novel amphoteric composite high temperature-resistant retarder for oil well cement. RSC Adv. 2018, 8, 14812–14822. [Google Scholar] [CrossRef]
- Fu, J.-X.; Wang, K.; Wang, J. Internal pore evolution and early hydration characterization of fly ash cement backfill. J. Build. Eng. 2023, 72, 106716. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Yi, P.; Li, Y.; Luo, Y.; Chen, Q.; Xing, F. The early hydration and rheological characteristics of cement paste containing co-combustion fly ash. J. Build. Eng. 2023, 78, 107736. [Google Scholar] [CrossRef]
- Qu, L.; Song, W.; Wang, Q.; Xu, S.; Hou, C. Effects of hydrophobic modified fly ash on resistance of chloride corrosion and water penetration of cement mortar in the early hydration stage. J. Build. Eng. 2022, 64, 105573. [Google Scholar] [CrossRef]
- Koga, G.Y.; Albert, B.; Nogueira, R.P. On the hydration of Belite-Ye’elimite-Ferrite (BYF) cement pastes: Effect of the water-to-cement ratio and presence of fly ash. Cem. Concr. Res. 2020, 137, 106215. [Google Scholar] [CrossRef]
- Ihsanullah; Asmaly, H.A.; Saleh, T.A.; Laoui, T.; Gupta, V.K.; Atieh, M.A. Enhanced adsorption of phenols from liquids by aluminum oxide/carbon nanotubes: Comprehensive study from synthesis to surface properties. J. Mol. Liq. 2015, 206, 176–182. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, Y.; Zhang, J.; Chen, L.; Meng, X.; Xiao, F.-S. Adsorptive and catalytic properties in the removal of volatile organic compounds over zeolite-based materials. Chin. J. Catal. 2016, 37, 800–809. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wei, Y.; Zeng, J.; Shi, W.; Wang, Y. Preparation and adsorption performance of palm fiber-based nanoporous carbon materials with high specific surface area. J. Porous Mater. 2016, 23, 1059–1064. [Google Scholar] [CrossRef]
- Li, P.T. Bending and Internal Tension of an Intramolecular Kissing Complex. Biophys. J. 2009, 96, 575a. [Google Scholar] [CrossRef]
- Ha, J.; Jeon, M.; Park, J.; Kim, J.; Moon, H.R. Effect of steric hindrance on the interfacial connection of MOF-on-MOF architectures. Nanoscale Adv. 2023, 5, 2111–2117. [Google Scholar] [CrossRef]
- Zheng, W.; Tang, C.; Xie, J.; Gui, Y. Micro-scale effects of nano-SiO2 modification with silane coupling agents on the cellulose/nano-SiO2 interface. Nanotechnology 2019, 30, 445701. [Google Scholar] [CrossRef]
- Mousavizadeh, S.M.; He, Z.; Wang, X.; Shen, P.; Wang, W.; Gilchrist, M.D.; Zhang, N. Surface modification of WE43 magnesium alloy via alkali/stearic acid treatment for biodegradable drug-eluting stent applications. Mater. Chem. Phys. 2023, 308, 128249. [Google Scholar] [CrossRef]
- Daikh, S.; Ouis, D.; Benyoucef, A.; Mouffok, B. Equilibrium, kinetic and thermodynamic studies for evaluation of adsorption capacity of a new potential hybrid adsorbent based on polyaniline and chitosan for Acetaminophen. Chem. Phys. Lett. 2022, 798, 139565. [Google Scholar] [CrossRef]
- Shaik, S.; Danovich, D.; Hiberty, P.C. On the nature of the chemical bond in valence bond theory. J. Chem. Phys. 2022, 157, 090901. [Google Scholar] [CrossRef]
- Itas, Y.S.; Razali, R.; Alamri, S.; Osman, H.; Khandaker, M.U. CO2 capture and storage by metal and non-metal decorated silicon carbide nanotubes: A DFT study. Phys. Scr. 2024, 99, 015920. [Google Scholar] [CrossRef]
- Woerner, P.; Nair, A.G.; Taira, K.; Oates, W.S. Sparsification of long range force networks for molecular dynamics simulations. PLoS ONE 2019, 14, e0213262. [Google Scholar] [CrossRef] [PubMed]
- Arabani, M.; Tahami, S.A.; Hamedi, G.H. Performance evaluation of dry process crumb rubber-modified asphalt mixtures with nanomaterial. Road Mater. Pavement Des. 2017, 19, 1241–1258. [Google Scholar] [CrossRef]
- Akasaka, M.; Hashimoto, H.; Makino, K.; Hino, R. 57Fe Moessbauer and X-ray Rietveld studies of ferrian prehnite from Kouragahana, Shimane Peninsula, Japan. J. Miner. Pet. Sci. 2003, 98, 31–40. [Google Scholar] [CrossRef]
- Albash, T.; Lidar, D.A. Demonstration of a Scaling Advantage for a Quantum Annealer over Simulated Annealing. Phys. Rev. X 2018, 8, 031016. [Google Scholar] [CrossRef]
- Yuan, Q.; Yang, J.; Sui, Y.; Zhao, Y.-P. Dynamics of Dissolutive Wetting: A Molecular Dynamics Study. Langmuir 2017, 33, 6464–6470. [Google Scholar] [CrossRef]
- Yeston, J. A checkup on density functional theory. Science 2017, 356, 1041–1042. [Google Scholar] [CrossRef]



| Density (g/cm3) | 1.0 | 1.2 | 1.4 |
|---|---|---|---|
| Rwp (%) | 6.55 | 6.24 | 6.95 |
| Rp (%) | 3.43 | 3.66 | 4.08 |
| Atom | Before Interaction | After Interaction |
|---|---|---|
| H(-CH3) | 0.053 | 0.165 |
| C(-CH3) | −0.159 | −0.488 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, L.; Wang, W. Study on the Hydrophobic Modification Mechanism of Stearic Acid on the Surface of Coal Gasification Fly Ash. Molecules 2024, 29, 4071. https://doi.org/10.3390/molecules29174071
Yang J, Li L, Wang W. Study on the Hydrophobic Modification Mechanism of Stearic Acid on the Surface of Coal Gasification Fly Ash. Molecules. 2024; 29(17):4071. https://doi.org/10.3390/molecules29174071
Chicago/Turabian StyleYang, Jian, Longjiang Li, and Wenyuan Wang. 2024. "Study on the Hydrophobic Modification Mechanism of Stearic Acid on the Surface of Coal Gasification Fly Ash" Molecules 29, no. 17: 4071. https://doi.org/10.3390/molecules29174071





