The Cytoprotective Effect of C60 Derivatives in the Self-Microemulsifying Drug Delivery System against Triptolide-Induced Cytotoxicity In Vitro
Abstract
:1. Introduction
2. Results
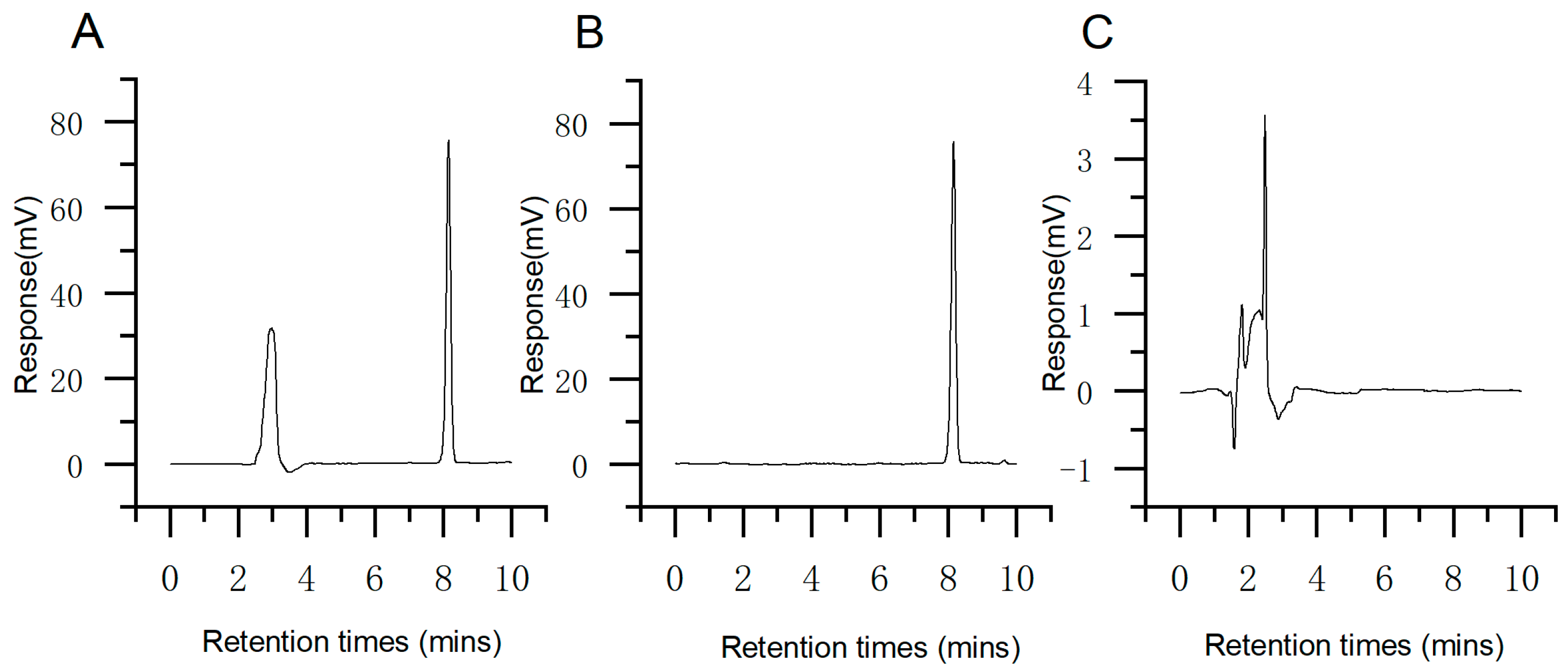
2.1. HPLC Analysis of TP
2.2. Preparation and Optimization of the TP-Loaded C60-SMEDDS
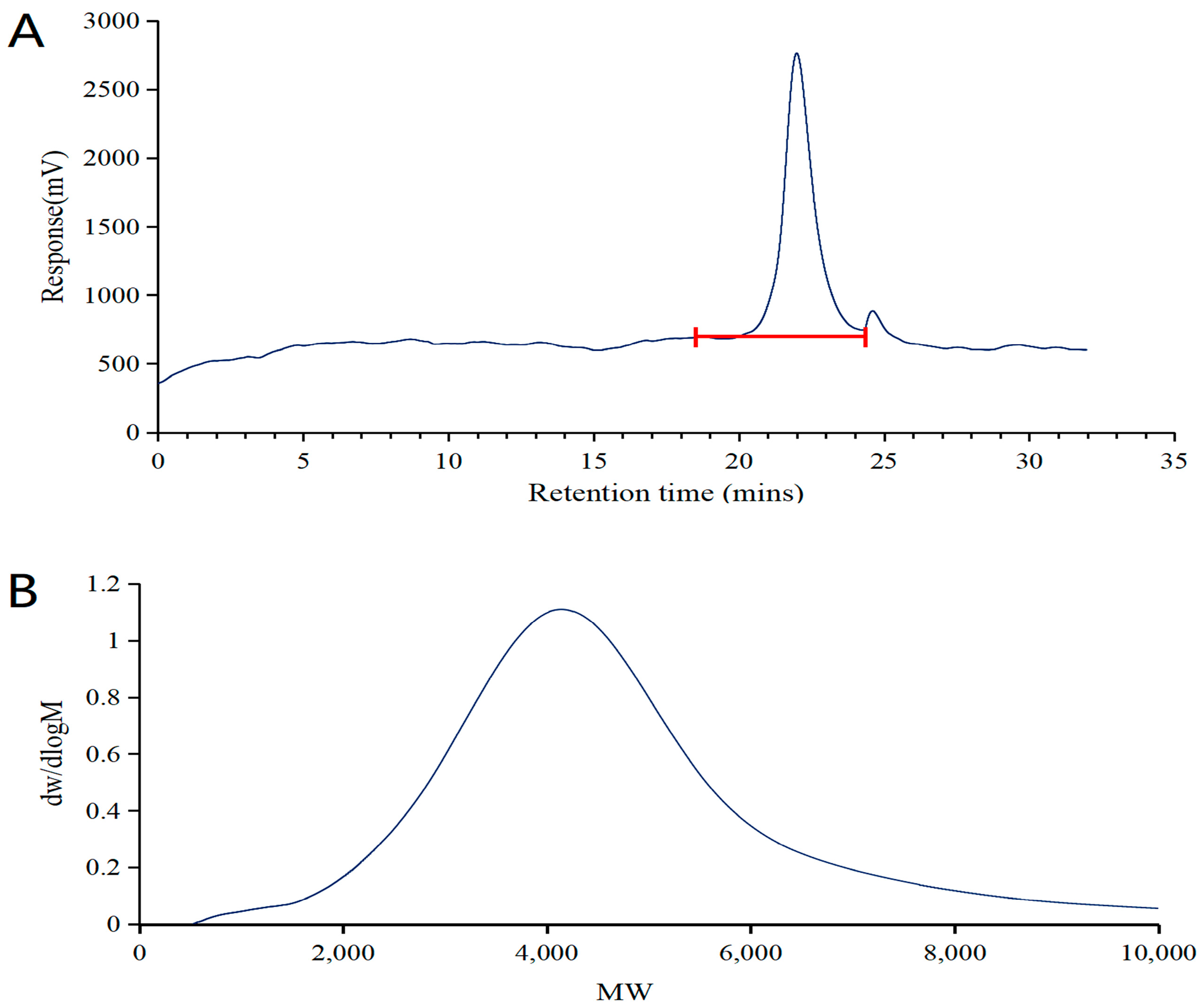
2.2.1. GPC Analysis of DSPE-PEG-C60
2.2.2. Pseudo-Ternary Phase Diagram
2.2.3. Preparation of the TP-Loaded SMEDDS
2.3. Characterization of Preparations
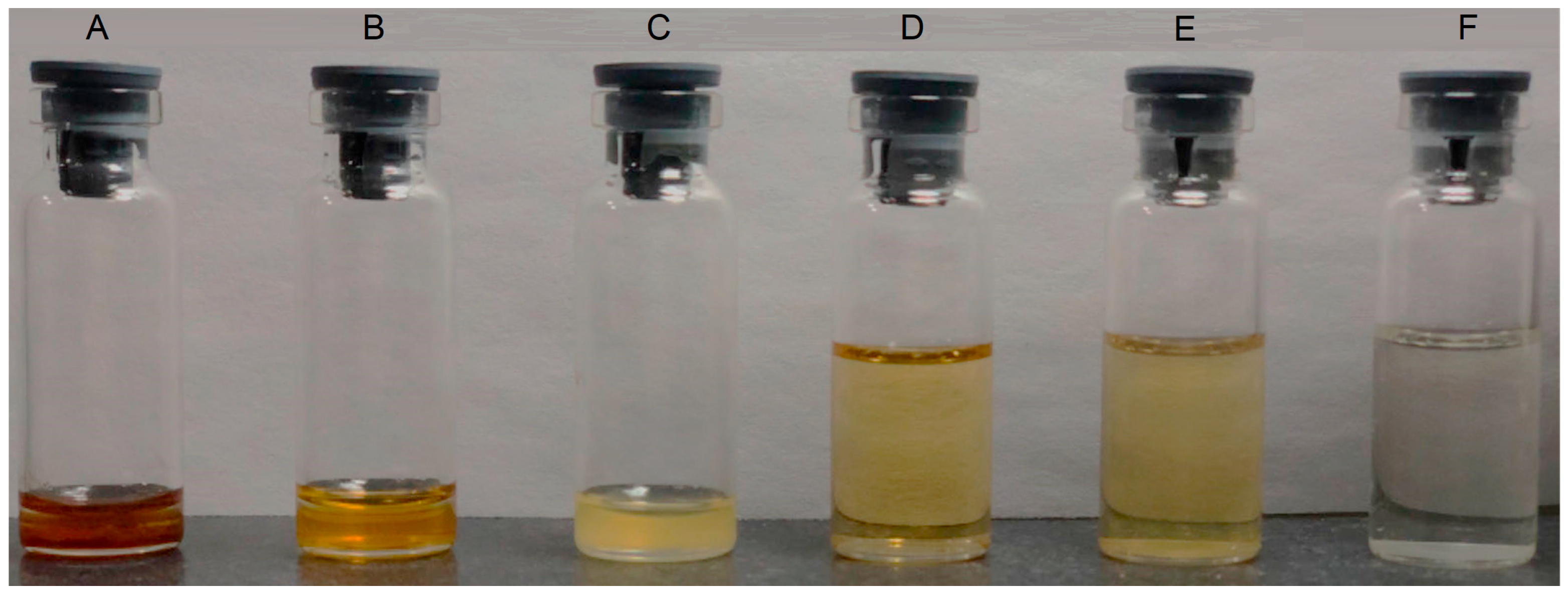
2.3.1. Dispersibility Test
2.3.2. Analysis of Droplet Size and Zeta Potential
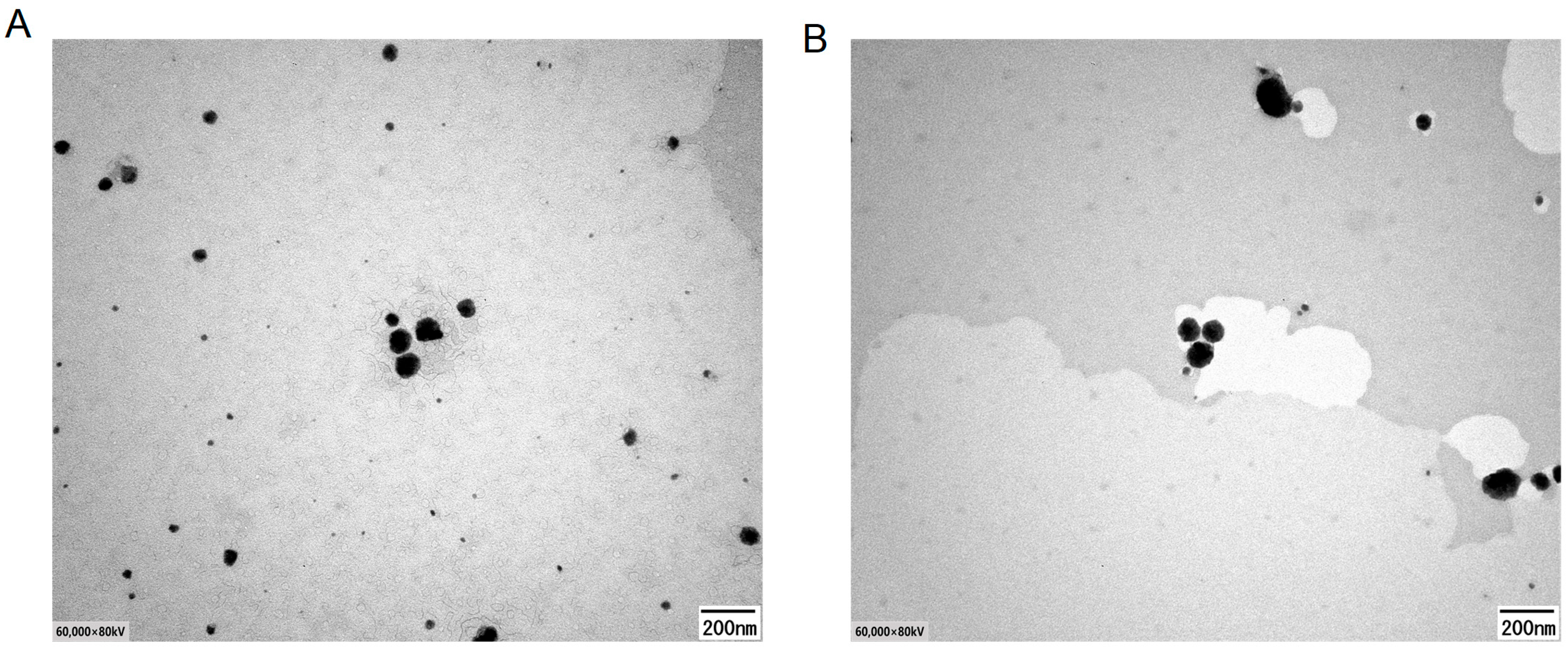
2.3.3. Morphological Analysis
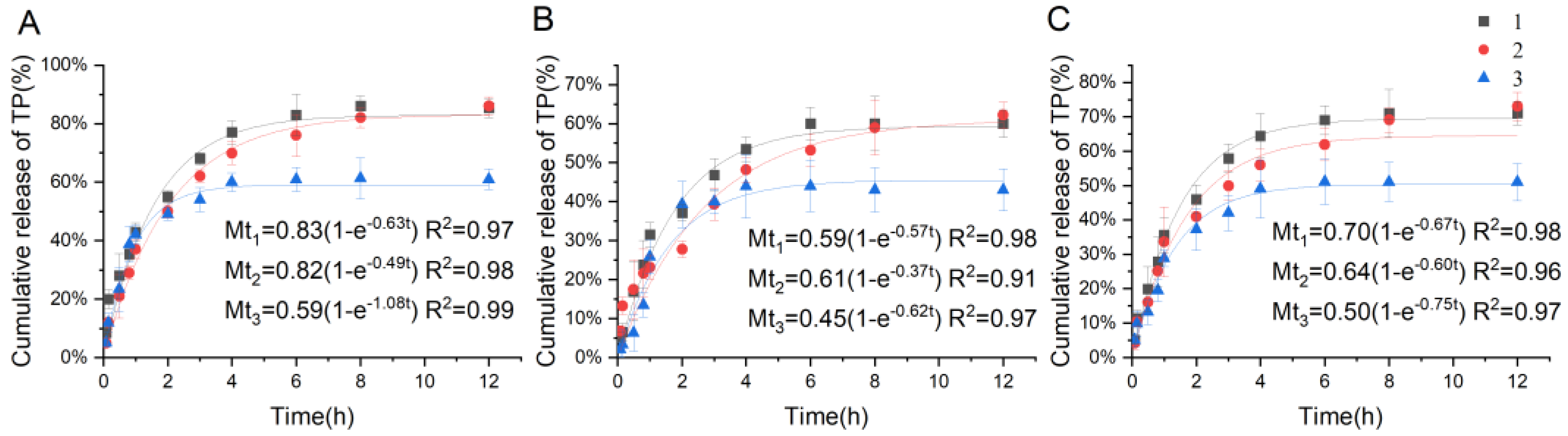
2.3.4. Drug Release Assay
2.4. In Vitro Cytotoxicity Examination
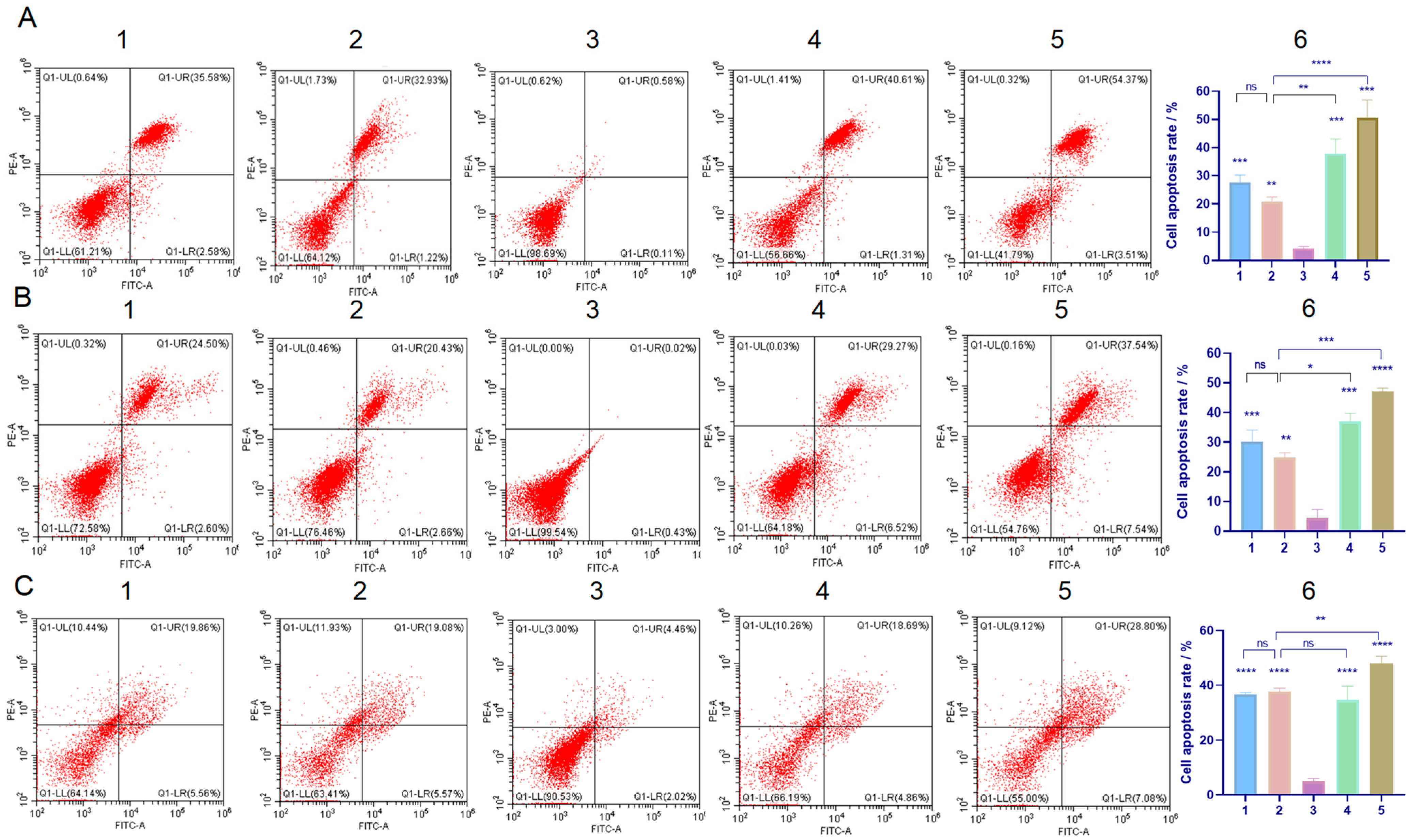
2.5. Detection of Cell Apoptosis
2.6. Evaluation of Intracellular Levels of ROS
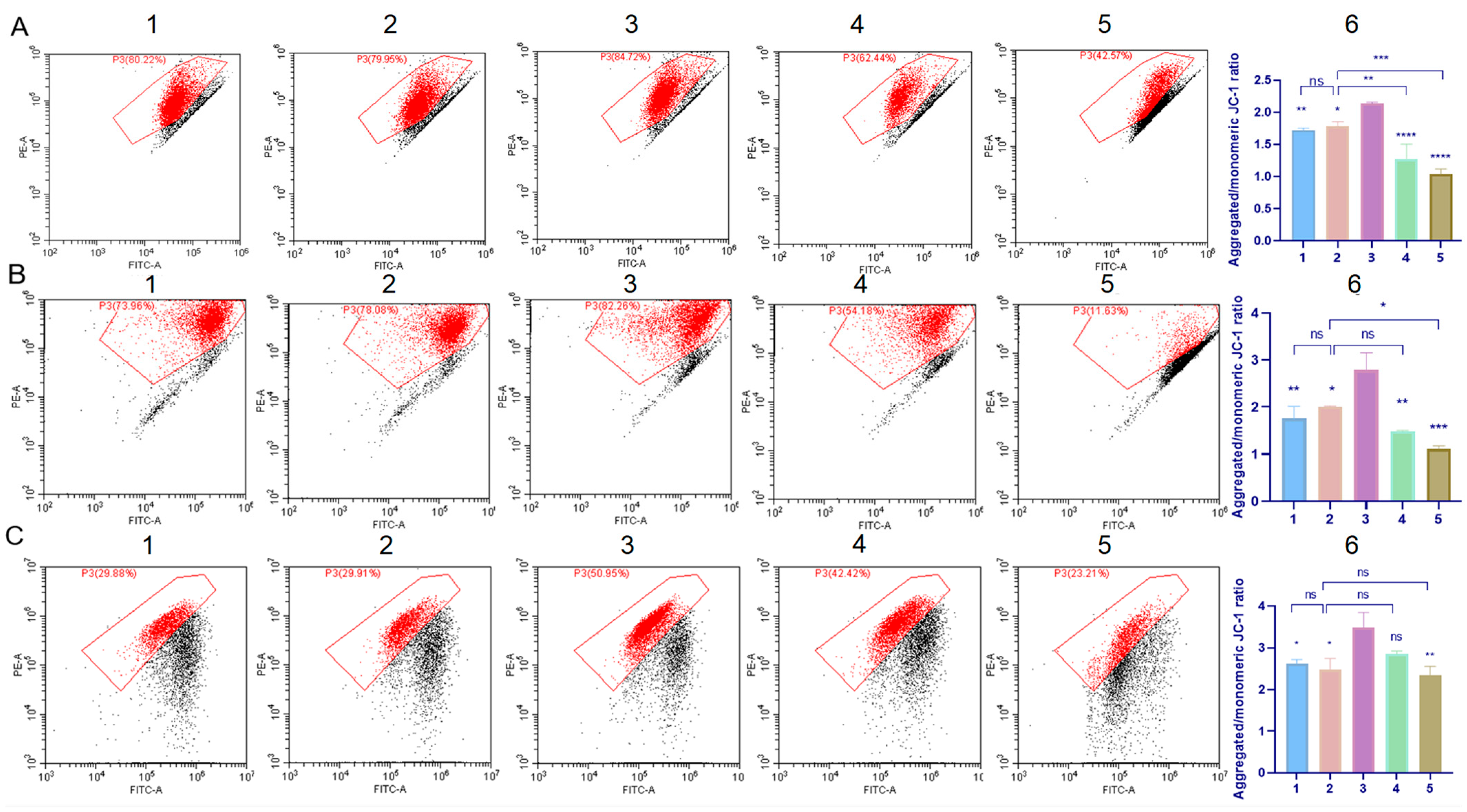
2.7. Cell Mitochondrial Function Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Determination of TP Content
4.3. Preparation and Optimization of the TP-Loaded C60-SMEDDS
4.3.1. Synthesis of DSPE-PEG-C60
4.3.2. Characterization of DSPE-PEG-C60
4.3.3. Construction of Pseudo-Ternary Phase Diagram
4.3.4. Preparation of the TP-Loaded SMEDDS
4.4. Characterization of Preparations
4.4.1. Dispersibility Test
4.4.2. Droplet Size and Zeta Potential
4.4.3. Morphology
4.4.4. Drug Release Study
4.5. Cell Culture
4.6. CCK-8 Assay
4.7. Apoptosis Detection
4.8. ROS Production Detection
4.9. Mitochondria Membrane Potential Determination
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TP | Triptolide |
| SMEDDS | Self-microemulsifying drug delivery system |
| O/W | Oil-in-water |
| MCT | Medium-chain triglycerides |
| EL | Cremophor EL |
| ROS | Reactive Oxygen Species |
| LD50 | Median lethal dose |
| EPR | Effect enhanced permeability and retention effect |
| DSPE-PEG | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy-[poly(ethylene glycol)]; PEG MW = 2000 |
| C60(OH)22 | Fullerenol |
| DSPE-PEG-C60 | Fullerenol-Grafted 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy-[poly(ethylene glycol)] |
| CCK-8 | Cell Counting Kit-8 |
| FITC | 3′,6′-dihydroxy-5-isothiocyanato-3H-spiro[isobenzofuran-1,9′-xanthen]-3-one |
| PI | Propidium iodide |
| PE | Phycoerythrin |
| HPLC | High-Performance Liquid Chromatography |
| TEM | Transmission Electron Microscopy |
| DLS | Dynamic light scattering |
| DCFDA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| IC50 | Half-maximal inhibitory concentration |
| HCC | Human hepatocellular carcinoma |
References
- Hou, Z.-Y.; Tong, X.-P.; Peng, Y.-B.; Zhang, B.-K.; Yan, M. Broad targeting of triptolide to resistance and sensitization for cancer therapy. Biomed. Pharmacother. 2018, 104, 771–780. [Google Scholar] [CrossRef]
- Hong, O.-Y.; Jang, H.-Y.; Park, K.-H.; Jeong, Y.-J.; Kim, J.-S.; Chae, H.S. Triptolide inhibits matrix metalloproteinase-9 expression and invasion of breast cancer cells through the inhibition of NF-κB and AP-1 signaling pathways. Oncol. Lett. 2021, 22, 562. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, P.; Xiao, Z.; Zhou, J.; Xue, X.; Jiang, N.; Peng, C.; Wu, L.; Tian, H.; Popper, H.; et al. Triptolide inhibits epithelial-mesenchymal transition phenotype through the p70S6k/GSK3/β-catenin signaling pathway in taxol-resistant human lung adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Guo, Q.; Shen, J.; Zheng, K.; Lu, C.; Zhang, G.; Lu, A.; He, X. The effect of triptolide in rheumatoid arthritis: From basic research towards clinical translation. Int. J. Mol. Sci. 2018, 19, 376. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.-J.; Ding, T.-Y.; Dong, F.-L.; Gao, Y.-J.; Jiang, B.-C. Intravenous administration of triptonide attenuates CFA-induced pain hypersensitivity by inhibiting DRG AKT signaling pathway in mice. J. Pain Res. 2020, 13, 3195–3206. [Google Scholar] [CrossRef]
- Yang, J.; Tang, X.; Ke, X.; Dai, Y.; Shi, J. Triptolide suppresses NF-κB-mediated inflammatory responses and activates expression of Nrf2-mediated antioxidant genes to alleviate caerulein-induced acute pancreatitis. Int. J. Mol. Sci. 2022, 23, 1252. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Takahashi, A.; Mukai, T.; Hori, A.; Narita, M.; Tojo, A.; Yang, T.; Nagamura-Inoue, T. The immunomodulatory effect of triptolide on mesenchymal stromal cells. Front. Immunol. 2021, 12, 686356. [Google Scholar] [CrossRef]
- Tang, Y.H.; Liu, L.; Liu, J.; Jiang, M.; Yu, L. Regulation of JAK/STAT signaling pathway by triptolide on inflammatory immunity in mice with systemic lupus erythematosus. J. Chin. Med. Mater. 2021, 8, 1986–1990. [Google Scholar]
- Zheng, N.; Wei, A.; Wu, T.; Long, L.; Yang, H.; Li, H.; Wang, L. Triptolide and atorvastatin synergistically promote hepatotoxicity in cultured hepatocytes and female Sprague-Dawley rats by inhibiting pregnane X receptor-mediated transcriptional activation of CYP3A4. Toxicol. Lett. 2021, 342, 85–94. [Google Scholar] [CrossRef]
- Fu, Q.; Jiang, Z.Z.; Zhang, L.Y. Impairment of triptolide on liver mitochondria in isolated liver mitochondria and HL7702 cell line. Chin. J. Integr. Med. 2013, 19, 683–688. [Google Scholar] [CrossRef]
- Hasnat, M.; Yuan, Z.; Naveed, M.; Khan, A.; Raza, F.; Xu, D.; Ullah, A.; Sun, L.; Zhang, L.; Jiang, Z. Drp1-associated mitochondrial dysfunction and mitochondrial autophagy: A novel mechanism in triptolide-induced hepatotoxicity. Cell Biol. Toxicol. 2019, 35, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Jiang, L.; Zhu, M.; Li, Y.; Luo, M.; Jiang, P.; Tong, S.; Zhang, H.; Yan, J. The genus Tripterygium: A phytochemistry and pharmacological review. Fitoterapia 2019, 137, 104190. [Google Scholar] [CrossRef]
- Injac, R.; Boskovic, M.; Perse, M.; Koprivec-Furlan, E.; Cerar, A.; Djordjevic, A.; Strukelj, B. Acute doxorubicin nephrotoxicity in rats with malignant neoplasm can be successfully treated with fullerenol C60(OH)24 via suppression of oxidative stress. Pharmacol. Rep. 2008, 60, 742–749. [Google Scholar]
- Injac, R.; Perse, M.; Cerne, M.; Potocnik, N.; Radic, N.; Govedarica, B.; Djordjevic, A.; Cerar, A.; Strukelj, B. Protective effects of fullerenol C60(OH)24 against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats with colorectal cancer. Biomaterials 2009, 30, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, D.; Seke, M.; Borovic, M.L.; Jovic, D.; Borisev, I.; Srdjenovic, B.; Rakocevic, Z.; Pavlovic, V.; Djordjevic, A. Hepatoprotective effect of fullerenol/doxorubicin nanocomposite in acute treatment of healthy rats. Exp. Mol. Pathol. 2018, 104, 199–211. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, R.; Guo, M.; Shao, L.; Liu, Y.; Zhao, Y.; Zhang, S.; Wu, Y.; Chen, C. Nucleosome-inspired nanocarrier obtains encapsulation efficiency enhancement and side effects reduction in chemotherapy by using fullerenol assembled with doxorubicin. Biomaterials 2018, 167, 205–215. [Google Scholar] [CrossRef]
- Ding, M.; Li, M.; Zhang, E.-M.; Yang, H.-L. FULLEROL alleviates myocardial ischemia-reperfusion injury by reducing inflammation and oxidative stress in cardiomyocytes via activating the Nrf2/HO-1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9665–9674. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ding, Z.; Hu, Y.; Zhang, T.; Shi, S.; Yu, G.; Qi, X. Preparation and Evaluation of the Cytoprotective Activity of Micelles with DSPE-PEG-C60 as a Carrier Against Doxorubicin-Induced Cytotoxicity. Front. Pharmacol. 2022, 13, 952800. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Xu, B.; Zhang, H.; Wang, Z.; Sun, L.; Tang, M.; Ding, M.; Zhang, T.; Shi, S. Norcantharidin-Encapsulated C60-Modified Nanomicelles: A Potential Approach to Mitigate Cytotoxicity in Renal Cells and Simultaneously Enhance Anti-Tumor Activity in Hepatocellular Carcinoma Cells. Molecules 2023, 28, 7609. [Google Scholar] [CrossRef]
- Zhu, Y.; Ye, J.; Zhang, Q. Self-emulsifying Drug Delivery System Improve Oral Bioavailability: Role of Excipients and Physico-chemical Characterization. Pharm. Nanotechnol. 2020, 8, 290–301. [Google Scholar] [CrossRef]
- Salawi, A. Self-emulsifying drug delivery systems: A novel approach to deliver drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef]
- Kadian, R.; Nanda, A. A Comprehensive Insight on Recent Advancements in Self-emulsifying Drug Delivery Systems. Curr. Drug Deliv. 2023, 20, 1095–1114. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.U.; Farid, A.; Shah, S.U.; Dar, M.J.; Rehman, A.U.; Ahmed, N.; Rashid, S.A.; Shaukat, I.; Shah, M.; Albadrani, G.M.; et al. Self-Emulsifying Drug Delivery Systems (SEDDS): Measuring Energy Dynamics to Determine Thermodynamic and Kinetic Stability. Pharmaceuticals 2022, 15, 1064. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Fukumori, Y.; Okonogi, S.; Ichikawa, H. Preparation of lipid nanoemulsions incorporating curcumin for cancer therapy. J. Nanosci. Nanotechnol. 2012, 41, 270383. [Google Scholar] [CrossRef]
- Xia, F.; Fan, W.; Jiang, S.; Ma, Y.; Lu, Y.; Qi, J.; Ahmad, E.; Dong, X.; Zhao, W.; Wu, W. Size-dependent translocation of nanoemulsions via oral delivery. ACS Appl. Mater. Interfaces 2017, 9, 21660–21672. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.-P.; Lu, Y.; Dong, X.-C.; Zhao, W.-L.; Wu, W. In vivo fate study of drug nanocarriers: The applications of environment-responsive fluorescent dyes. Acta Pharm. Sin. 2019, 54, 1965–1975. [Google Scholar]



| MCT (%) | EL (%) | PEG-400 (%) | Self-Emulsifying Time | Appearance | Size (nm) | |
|---|---|---|---|---|---|---|
| 1 | 10 | 60 | 30 | <30 s | Clear | 19.14 ± 0.07 |
| 2 | 25 | 50 | 25 | <30 s | Milky light | 50.06 ± 0.19 |
| 3 | 40 | 40 | 20 | <30 s | Milky light | 91.22 ± 1.59 |
| 4 | 55 | 30 | 15 | <30 s | Milky light | 29.85 ± 0.33 |
| 5 | 70 | 20 | 10 | >30 s | Clear | 176 ± 1.25 |
| 6 | 85 | 10 | 5 | >30 s | Cloudy | 441.8 ± 7.0 |
| DSPE-PEG-C60:TP = 10:1 (W/W) | DSPE-PEG-C60:TP = 15:1 (W/W) | DSPE-PEG:TP = 10:1 (W/W) | DSPE-PEG:TP = 15:1 (W/W) | Regular SMEDDS/TP | |
|---|---|---|---|---|---|
| PS (nm) | 50 ± 0.19 | 50 ± 0.28 | 69 ± 0.59 | 68 ± 0.12 | 187 ± 0.18 |
| PDI | 0.21 ± 0.049 | 0.20 ± 0.008 | 0.29 ± 0.010 | 0.29 ± 0.015 | 0.19 ± 0.015 |
| ZP (mv) | −1.60 | −2.81 | +1.52 | −0.16 | +1.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Wang, Z.; Zhang, H.; Xu, X.; Tang, M.; Wang, G.; Ding, Z.; Yu, R.; Ding, M.; Zhang, T.; et al. The Cytoprotective Effect of C60 Derivatives in the Self-Microemulsifying Drug Delivery System against Triptolide-Induced Cytotoxicity In Vitro. Molecules 2024, 29, 4073. https://doi.org/10.3390/molecules29174073
Xu B, Wang Z, Zhang H, Xu X, Tang M, Wang G, Ding Z, Yu R, Ding M, Zhang T, et al. The Cytoprotective Effect of C60 Derivatives in the Self-Microemulsifying Drug Delivery System against Triptolide-Induced Cytotoxicity In Vitro. Molecules. 2024; 29(17):4073. https://doi.org/10.3390/molecules29174073
Chicago/Turabian StyleXu, Beihua, Zhenyu Wang, Huimin Zhang, Xiao Xu, Mengjie Tang, Gang Wang, Zhongpeng Ding, Ruihao Yu, Meihong Ding, Ting Zhang, and et al. 2024. "The Cytoprotective Effect of C60 Derivatives in the Self-Microemulsifying Drug Delivery System against Triptolide-Induced Cytotoxicity In Vitro" Molecules 29, no. 17: 4073. https://doi.org/10.3390/molecules29174073
APA StyleXu, B., Wang, Z., Zhang, H., Xu, X., Tang, M., Wang, G., Ding, Z., Yu, R., Ding, M., Zhang, T., & Shi, S. (2024). The Cytoprotective Effect of C60 Derivatives in the Self-Microemulsifying Drug Delivery System against Triptolide-Induced Cytotoxicity In Vitro. Molecules, 29(17), 4073. https://doi.org/10.3390/molecules29174073





