Exploiting the Applicability of Polytetrahydrofuran-Modified Polyester for the Fabric Phase Sorptive Extraction of Doxycycline from Human Urine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of FPSE Conditions
2.1.1. Effect of FPSE Membrane Type and Its Surface Area
2.1.2. Effect of Sample pH and Salt Concentration
2.1.3. Effect of the Extraction Time, Stirring Rate, and Sample Using Box-Behnken Design
2.1.4. Effect of the Elution Solvent, Volume and Time
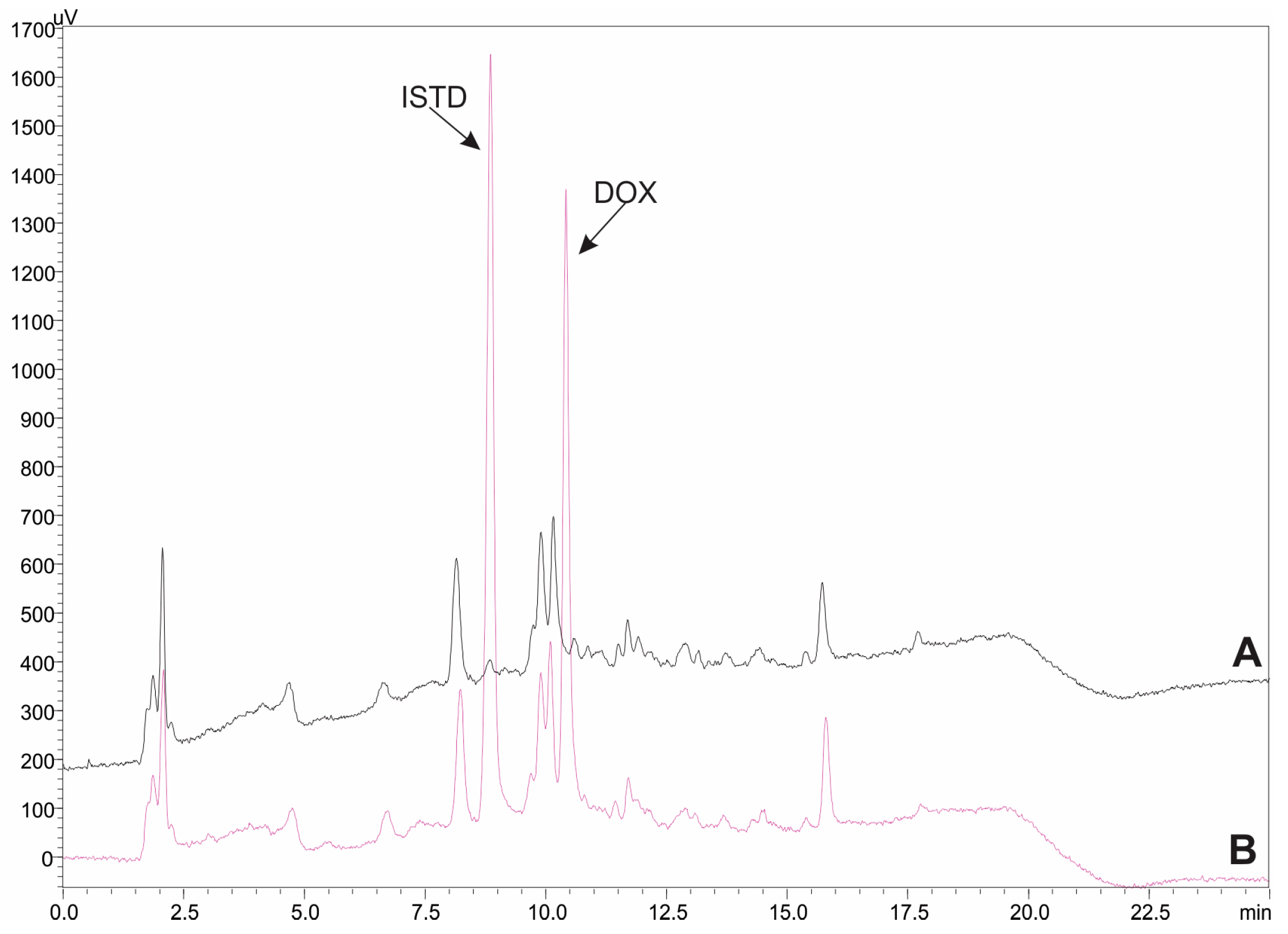
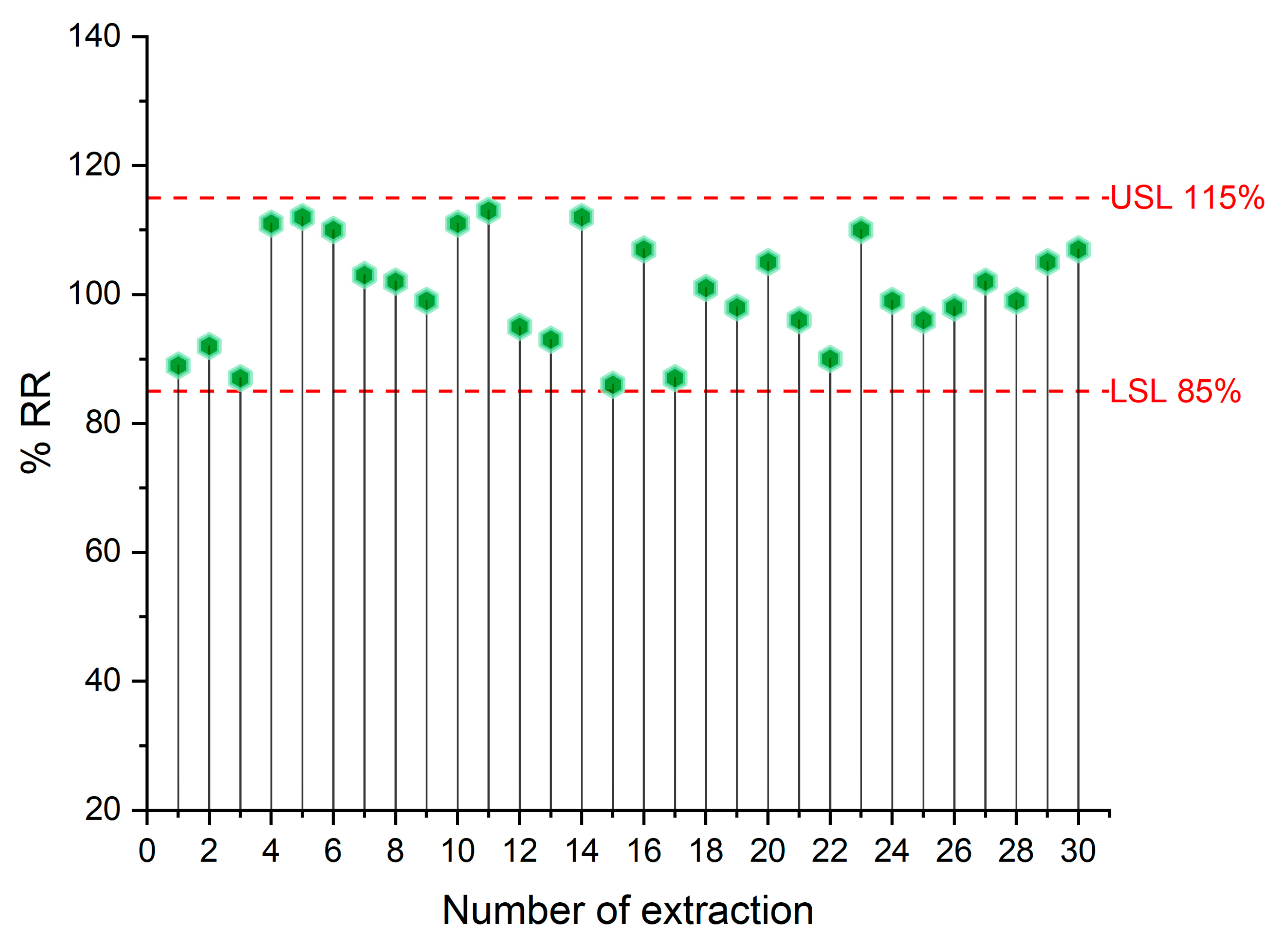
2.2. Method Validation
2.3. Analysis of Real Samples
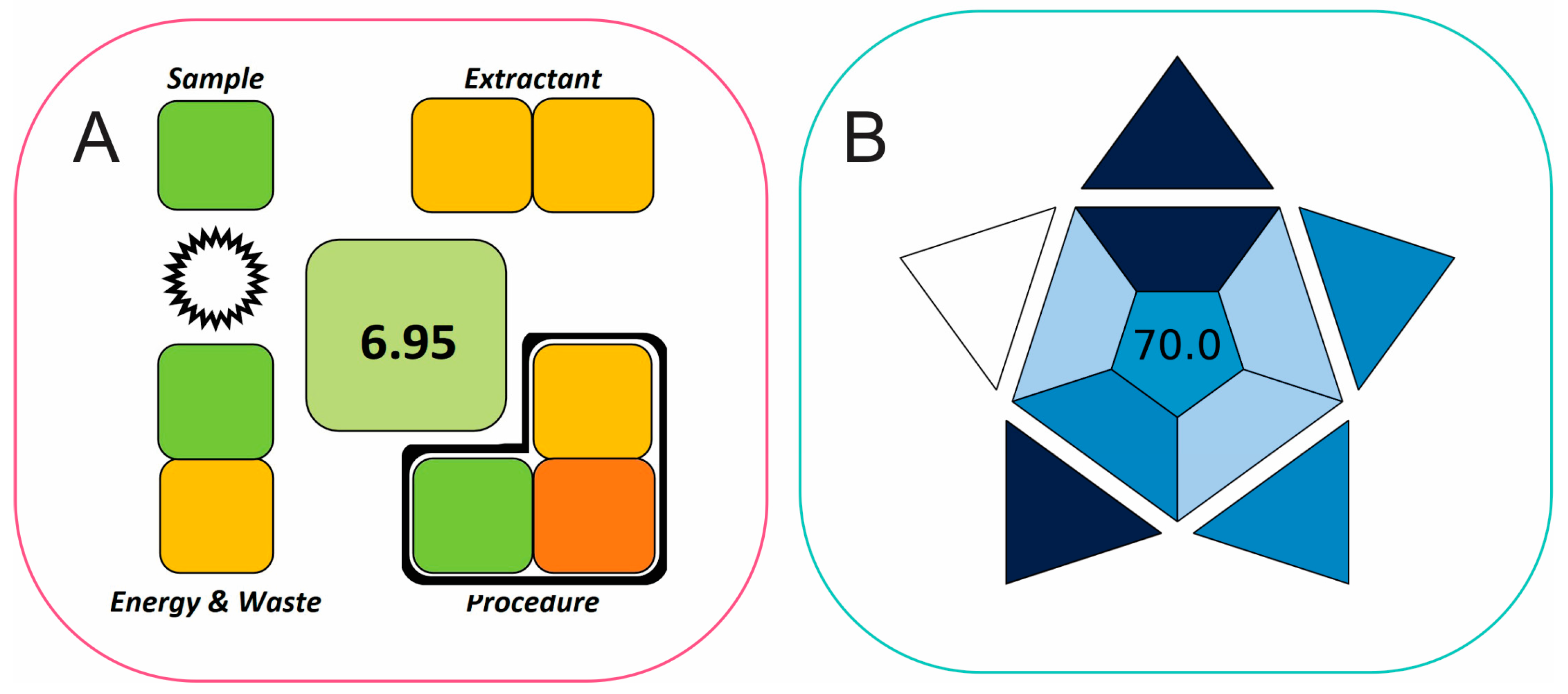
2.4. Evaluation of Method’s Greenness Using SPMS and BAGI Metric Tools
3. Materials and Methods
3.1. Reagents, Solutions, and Materials
3.2. Fabrication of FPSE Membranes
3.3. Instrumentation and HPLC Conditions
3.4. Sample Collection
3.5. FPSE Procedure
- (i)
- Activation: Initially, the FPSE membrane (10 mm × 10 mm) was activated with a 1 mL mixture of MeOH/ACN, 50:50 v/v for 5 min. Then, the device was rinsed with water to remove any solvent traces that might reduce the performance of the extraction process. Then, the device was dried with a lint-free tissue.
- (ii)
- Extraction: 500 μL of sample was transferred to a cylindrical vial (65 × 15 mm) with the FPSE membrane. The extraction of the analyte was performed under constant stirring at 600 rpm for 24 min.
- (iii)
- Elution: After the extraction step, the FPSE membrane was rinsed with water to remove any sample residues left on the surface was dried with a lint-free tissue. Then, it was placed into an Eppendorf tube containing 500 μL ACN followed by vortex for 1 min.
- (iv)
- Cleaning: The FPSE membrane was then immersed in the mixture of MeOH/ACN 50:50 v/v for cleanup and stored until its next use.
3.6. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.; Wang, M.; Zhang, S.; Yangcuo, Z.; He, L.; Xie, L.; Ye, Y.; Xu, G.; Chen, Z.; Cai, Q. Development of a New Synchronous Fluorescence Spectrometry Combined with Al3+ Sensitized for Simultaneous and Rapid Determination of Trace Flumequine, Ciprofloxacin and Doxycycline Hydrochloride Residues in Wastewater. Water Res. 2024, 260, 121941. [Google Scholar] [CrossRef]
- Zermeño-Acosta, M.; Sumano, H.; del Villar, J.L.; Bernad, M.J.; Gutiérrez, L. Pharmacokinetics of Doxycycline Hyclate in Pigs with a New Feed Premix Formulation. J. Vet. Pharmacol. Ther. 2024, 47, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Jantratid, E.; Strauch, S.; Becker, C.; Dressman, J.B.; Amidon, G.L.; Junginger, H.E.; Kopp, S.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; et al. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Doxycycline Hyclate. J. Pharm. Sci. 2010, 99, 1639–1653. [Google Scholar] [CrossRef] [PubMed]
- Ayaslioglu, E.; Erkek, E.; Oba, A.A.; Cebecioǧlu, E. Doxycycline-Induced Staining of Permanent Adult Dentition. Aust. Dent. J. 2005, 50, 273–275. [Google Scholar] [CrossRef]
- Ingle, R.G.; Zeng, S.; Jiang, H.; Fang, W.J. Current Developments of Bioanalytical Sample Preparation Techniques in Pharmaceuticals. J. Pharm. Anal. 2022, 12, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Ocaña-González, J.A.; Fernández-Torres, R.; Bello-López, M.Á.; Ramos-Payán, M. New Developments in Microextraction Techniques in Bioanalysis. A Review. Anal. Chim. Acta 2016, 905, 8–23. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC–Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Alipanahpour Dil, E.; Ghaedi, M.; Asfaram, A.; Tayebi, L.; Mehrabi, F. A Ferrofluidic Hydrophobic Deep Eutectic Solvent for the Extraction of Doxycycline from Urine, Blood Plasma and Milk Samples Prior to Its Determination by High-Performance Liquid Chromatography-Ultraviolet. J. Chromatogr. A 2020, 1613, 460695. [Google Scholar] [CrossRef]
- Sunarić, S.M.; Denić, M.S.; Bojanić, Z.Ž.; Bojanić, V.V. HPLC Method Development for Determination of Doxycycline in Human Seminal Fluid. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 939, 17–22. [Google Scholar] [CrossRef]
- Seelam, R.R.; Terrin, M.; Hassan, H.E. Validated UHPLC–MS/MS Method for Quantification of Doxycycline in Abdominal Aortic Aneurysm Patients. Bioanalysis 2018, 10, 527–539. [Google Scholar] [CrossRef]
- Denić, M.S.; Sunarić, S.M.; Kesić, L.G.; Minić, I.Z.; Obradović, R.R.; Denić, M.S.; Petrović, M.S. RP-HPLC Assay of Doxycycline in Human Saliva and Gingival Crevicular Fluid in Patients with Chronic Periodontal Disease. J. Pharm. Biomed. Anal. 2013, 78–79, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, D.; Krulišová, M.; Gámiz-Gracia, L.; García-Campaña, A.M. Determination of Tetracyclines in Human Urine Samples by Capillary Electrophoresis in Combination with Field Amplified Sample Injection. Electrophoresis 2018, 39, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractor (FPSE). U.S. Patent 14,216,121, 17 March 2014. [Google Scholar]
- Locatelli, M.; Tinari, N.; Grassadonia, A.; Tartaglia, A.; Macerola, D.; Piccolantonio, S.; Sperandio, E.; D’Ovidio, C.; Carradori, S.; Ulusoy, H.I.; et al. FPSE-HPLC-DAD Method for the Quantification of Anticancer Drugs in Human Whole Blood, Plasma, and Urine. J. Chromatogr. B 2018, 1095, 204–213. [Google Scholar] [CrossRef]
- Gazioglu, I.; Evrim Kepekci Tekkeli, S.; Tartaglia, A.; Aslan, C.; Locatelli, M.; Kabir, A. Simultaneous Determination of Febuxostat and Montelukast in Human Plasma Using Fabric Phase Sorptive Extraction and High Performance Liquid Chromatography-Fluorimetric Detection. J. Chromatogr. B 2022, 1188, 123070. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary Green Analytical Procedure Index (ComplexGAPI) and Software. Green Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- Pourmoslemi, S.; Mirfakhraee, S.; Yaripour, S.; Mohammadi, A. Development and Validation of a Stability-Indicating RP-HPLC Method for Rapid Determination of Doxycycline in Pharmaceutical Bulk and Dosage Forms. Pharm. Sci. 2016, 22, 96–104. [Google Scholar] [CrossRef]
- Kogawa, A.C.; Salgado, H.R.N. Quantification of Doxycycline Hyclate in Tablets by HPLC-UV Method. J. Chromatogr. Sci. 2013, 51, 919–925. [Google Scholar] [CrossRef]
- Mamounas, G.; Manousi, N.; Kabir, A.; Furton, K.G.; Mystridis, G.A.; Vizirianakis, I.S.; Zacharis, C.K. Designing an “All-in-One” Microextraction Capsule Device for the Liquid Chromatographic-Fluorescence Determination of Doxorubicin and Its Metabolites in Rat Plasma. J. Chromatogr. A 2022, 1680, 463432. [Google Scholar] [CrossRef]
- Haaland, R.E.; Fountain, J.; Edwards, T.E.; Dinh, C.; Martin, A.; Omoyege, D.; Conway-Washington, C.; Kelley, C.F.; Heneine, W. Pharmacokinetics of Single Dose Doxycycline in the Rectum, Vagina, and Urethra: Implications for Prevention of Bacterial Sexually Transmitted Infections. EBioMedicine 2024, 101, 105037. [Google Scholar] [CrossRef]
- González-Martín, R.; Gutiérrez-Serpa, A.; Pino, V.; Sajid, M. A Tool to Assess Analytical Sample Preparation Procedures: Sample Preparation Metric of Sustainability. J. Chromatogr. A 2023, 1707, 464291. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V.F. Blue Applicability Grade Index (BAGI) and Software. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Brooks, T.; Keevil, C.W. A Simple Artificial Urine for the Growth of Urinary Pathogens. Lett. Appl. Microbiol. 1997, 24, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Mesa, R.; Jurmain, J.; Furton, K.G. Fabric Phase Sorptive Extraction Explained. Separations 2017, 4, 21. [Google Scholar] [CrossRef]
- Food & Drug Administration. Bioanalytical Method Validation Guidance. 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 1 August 2024).


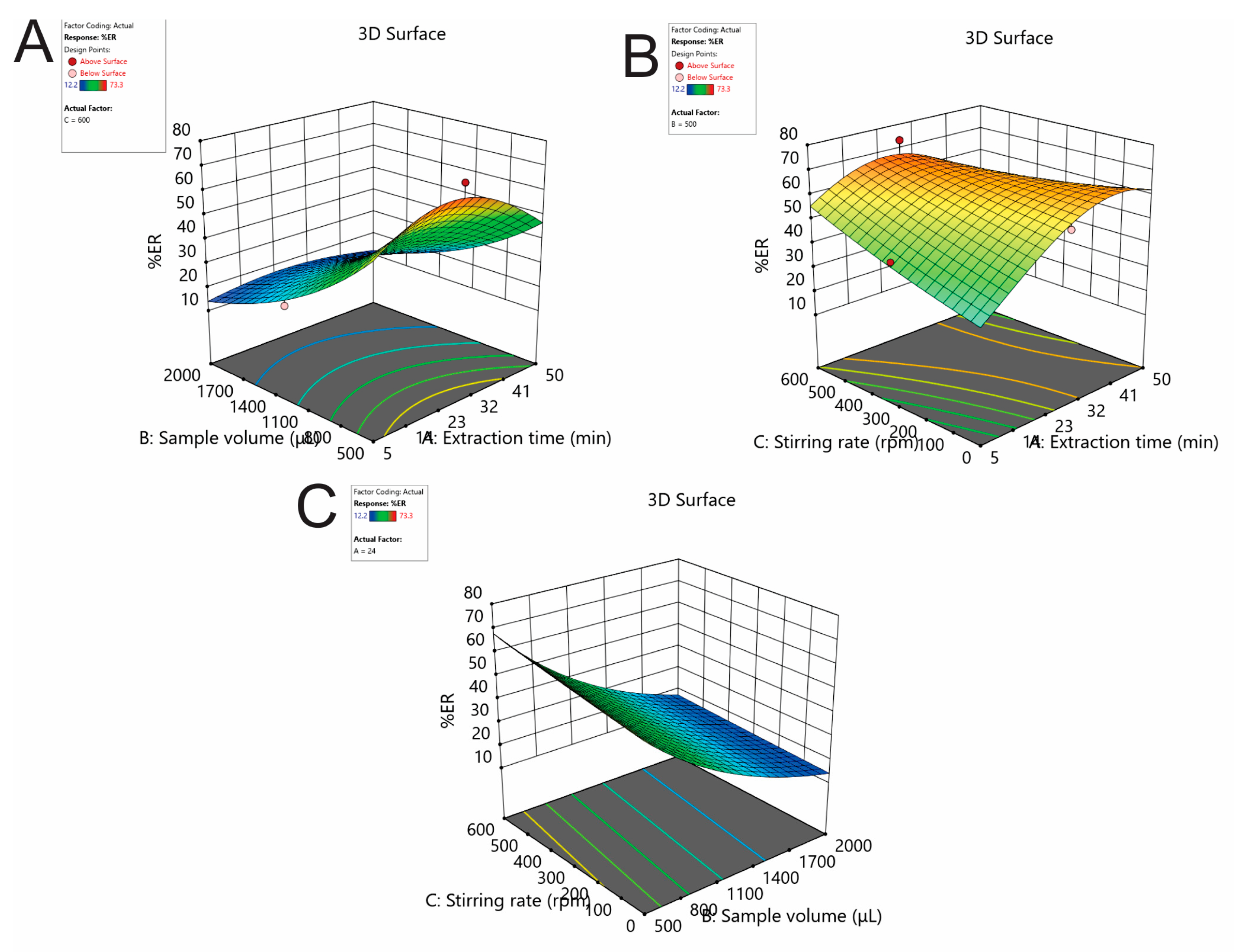


| Run No | Extraction Time (min) (Factor 1) | Sample Volume (μL) (Factor 2) | Stirring Rate (rpm) (Factor 3) | %ER |
|---|---|---|---|---|
| 1 | 27.5 | 500 | 0 | 57 |
| 2 | 50 | 1250 | 600 | 25.7 |
| 3 | 5 | 2000 | 300 | 12.2 |
| 4 | 27.5 | 500 | 600 | 73.3 |
| 5 | 5 | 1250 | 600 | 25.5 |
| 6 | 27.5 | 1250 | 300 | 27.5 |
| 7 | 50 | 500 | 300 | 46.8 |
| 8 | 27.5 | 1250 | 300 | 41.5 |
| 9 | 27.5 | 2000 | 0 | 15.6 |
| 10 | 5 | 1250 | 0 | 14 |
| 11 | 27.5 | 1250 | 300 | 22.5 |
| 12 | 50 | 1250 | 0 | 31.8 |
| 13 | 5 | 500 | 300 | 44.4 |
| 14 | 27.5 | 2000 | 600 | 13.8 |
| 15 | 50 | 2000 | 300 | 13.3 |
| 16 | 27.5 | 1250 | 300 | 35.8 |
| 17 | 27.5 | 1250 | 300 | 33.9 |
| Intra-Day (n = 3) | Inter-Day (n = 3) | |||
|---|---|---|---|---|
| Added Concentration (ng/mL) | Precision (%RSD) | Accuracy RR 1 (%) | Precision (%RSD) | Accuracy RR (%) |
| 100 (LLOQ) | 3.3 | 112.2 | 14.7 | 96.8 |
| 250 (LQC) | 10.3 | 98.5 | 9.9 | 89.6 |
| 750 (MQC) | 7.7 | 100.5 | 3.7 | 91.9 |
| 5000 (HQC) | 8.4 | 99.1 | 4.0 | 95.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzintounas, P.; Ntorkou, M.; Kabir, A.; Zacharis, C.K. Exploiting the Applicability of Polytetrahydrofuran-Modified Polyester for the Fabric Phase Sorptive Extraction of Doxycycline from Human Urine. Molecules 2024, 29, 4076. https://doi.org/10.3390/molecules29174076
Chatzintounas P, Ntorkou M, Kabir A, Zacharis CK. Exploiting the Applicability of Polytetrahydrofuran-Modified Polyester for the Fabric Phase Sorptive Extraction of Doxycycline from Human Urine. Molecules. 2024; 29(17):4076. https://doi.org/10.3390/molecules29174076
Chicago/Turabian StyleChatzintounas, Panagiotis, Marianna Ntorkou, Abuzar Kabir, and Constantinos K. Zacharis. 2024. "Exploiting the Applicability of Polytetrahydrofuran-Modified Polyester for the Fabric Phase Sorptive Extraction of Doxycycline from Human Urine" Molecules 29, no. 17: 4076. https://doi.org/10.3390/molecules29174076







