Abstract
One of the bottlenecks to bringing new therapies to the clinic has been a lack of vectors for delivering novel therapeutics in a targeted manner. Cell penetrating peptides (CPPs) have received a lot of attention and have been the subject of numerous developments since their identification nearly three decades ago. Known for their transduction abilities, they have generally been considered inert vectors. In this review, we present a schema for their classification, highlight what is known about their mechanism of transduction, and outline the existing literature as well as our own experience, vis a vis the intrinsic anti-inflammatory properties that certain CPPs exhibit. Given the inflammatory responses associated with viral vectors, CPPs represent a viable alternative to such vectors; furthermore, the anti-inflammatory properties of CPPs, mostly through inhibition of the NF-κB pathway, are encouraging. Much more work in relevant animal models, toxicity studies in large animal models, and ultimately human trials are needed before their potential is fully realized.
1. Introduction
Among the most promising forefronts for novel vectors has been the development of protein transduction domains, more commonly referred to as cell penetrating peptides (CPPs). CPPs are small molecules ranging from 5 to 30 amino acids in length, which function to transduce the cell membrane either alone or attached to a variety of cargos [1]. CPPs are unique in their ability to cross the cell membrane without requiring the use of a receptor, a function first identified in 1988 in HIV’s trans-activator of transcription protein (Tat) [2,3]. Notably, the entire protein is not responsible for cell transduction; the identification of a short, 11-amino-acid, primarily cationic sequence within Tat [3], which proved to be responsible for crossing the membrane, has paved the way for the research and development of CPPs as we know them today.
Following the discovery of Tat, other non-cell specific CPPs have been discovered. This includes Antp, also called penetratin, discovered from Drosophila melanogaster [4]. CPPs have traditionally been used mostly for their ability to cross cell membrane barriers and deliver large or hydrophobic cargos, as first demonstrated through the conjugation of proteins such as RNase A, β-galactosidase, and horseradish peroxidase to Tat; in this case, transduction was robustly observed without any cell-type specificity [5]. Such transduction has also been observed in vivo, with protein functionality being preserved after transduction into all tissues including brain tissue [6]. Since then, CPPs, including those exhibiting cell specificity, have been used in a variety of applications including targeted drug delivery [7,8,9], the delivery of RNA interference agents such as siRNA [10,11,12,13,14], enhancing the delivery of viral vectors [15], and radioactive agents [16,17], along with many more applications [18,19,20,21,22].
While CPPs remain an exciting frontier in terms of targeted delivery and enhancing cell transduction, they have generally been considered inert vectors without any intrinsic biological properties. Compared to traditional viral vectors that require immunomodulation to gain long-term expression [23], and AAV (adeno-associated virus) vectors that can lead to immunotoxicity [24] or cytokine release syndrome [25], significant immune responses to CPPs have not been reported. Although studies on both the targeted and non-targeted delivery of potentially therapeutic or diagnostic molecules abound in the literature, the intrinsic biological properties of CPPs have received little attention. Therefore, this review will focus on the existing literature on the intrinsic biological properties of CPPs beyond transduction, which taken together could represent advantages over viral vectors, and potentially provide novel solutions to treat and prevent disease more effectively.
2. Mechanisms and Characteristics of CPPs
2.1. Basic Classification of CPPs
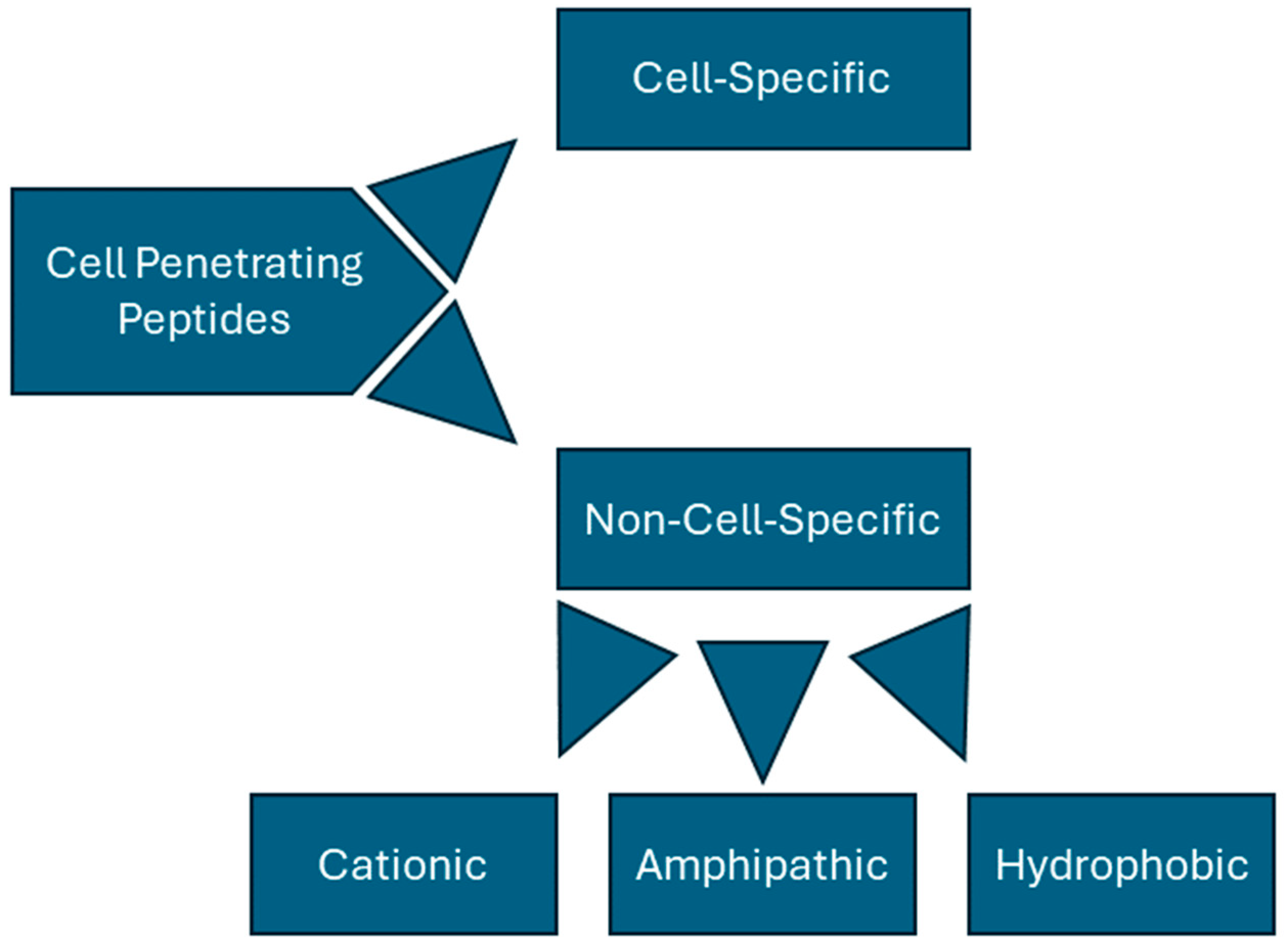
CPPs can be categorized in a variety of ways but are generally divided into non-cell-specific peptides and cell-specific peptides. Non-specific peptides can be further divided based on their structures and associated charges; these peptides are often classified as being cationic, hydrophobic, or amphipathic (Figure 1). Tat (YGRKKRRQRRR) and penetratin (RQIKIWFQNRRMKWKK) are key examples of cationic peptides, rich in both lysine and arginine. Learning from this observation, synthetic, non-naturally occurring peptides have been synthesized by simply using homopolymers of arginine [26] or lysine [27] residues. In fact, Wender et al. found that peptides consisting of only L-arginine or D-arginine were over 20 times better at cell transduction than Tat alone, suggesting that Tat’s cationic properties play a major role in its transduction abilities [26]. Such peptides, however, seem to have a clearly defined length; poly-arginine peptides have been found to be ineffective with less than six residues [28], and poly-lysine polymers show a decrease in transduction with greater than twelve amino acids [29].
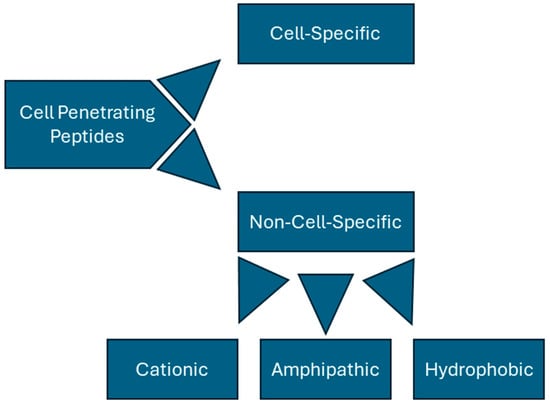
Figure 1.
Classification for CPPs.
Though far less common than the other types of CPPs, hydrophobic CPPs have also been successfully used for membrane transduction. These peptides are often created by the conjugation of hydrophobic signal peptide sequences, which usually function to allow the secretion of proteins from inside the cell membrane. Modifying these sequences can reverse the direction of peptide travel, and instead lead to membrane transduction [1]. Modifications to the structure of these peptides can serve to enhance their stability in solution as well as their transduction ability. One such peptide called TP10, an analog of transportan [30], has been successfully modified to enhance its three-dimensional structure and therefore its transduction abilities [31]. The stabilization of three-dimensional structures, specifically helices, has been shown to enhance the membrane transduction and delivery efficiency [32]. However, hydrophobic CPPs are less stable in solution and present many challenges when it comes to their synthesis and use, such as their tendency to aggregate due to their hydrophobic nature [33].
Amphipathic CPPs, the final type of non-specific cell penetrating peptides, are hybrid peptides created by attaching a hydrophilic sequence to a hydrophobic CPP. For instance, LAH4-L1 is an amphipathic CPP modulated with a nuclear localization signal (NLS) from Simian virus 40; this peptide was able to affect gene expression up to 10-fold compared to the baseline levels of expression [34]. Amphipathic peptides can also be created from a mixture of evenly distributed hydrophobic amino acids and other hydrophilic amino acids [35], allowing them to directly interact with the lipid bilayer for internalization [36].
CPPs can also be classified based on other properties such as the nature of their origin and functional characteristics. Gori et al. [37] have detailed a comprehensive classification system based on multiple properties; however, they admit that a fully rigid system for classifying CPPs is impossible to achieve due to the wide-ranging nature of the peptides. Using their taxonomy, CPPs are classified based on many factors including origin, structure, physical and chemical properties, specificity, and internalization mechanism. These properties tend to overlap; for instance, as discussed above, amphipathic CPPs have both synthetic and natural properties as well as distinct structural classifications (hydrophobic sequence + NLS). CPPs can be naturally occurring, totally synthetic, or a combination of both [4,37,38,39]. Other peptides were engineered to directly interact with various mechanisms of endocytosis [40]. This method of classification can be advantageous, especially in the context of drug delivery, as peptides that exhibit internalization by endocytosis must also exhibit some form of endosomal escape for effective cargo delivery. Furthermore, CPPs that interact with endocytic pathways also have the potential to effect other cellular functions through various signaling pathways. Hence, a classification of CPPs based on protein interactions can also shed light on myriad potential biological effects.
The method of discovery of CPPs can also provide a functional taxonomy for their classification. Traditionally, CPPs have been discovered using a combination of methods as described above, as well as through techniques such as phage display [1,41]. These approaches, while fruitful, are random and rely mostly on trial and error [39]. The continued discovery of CPPs combined with the rise in prevalence of bioinformatics and artificial intelligence (AI) provides another pathway for the discovery and classification of CPPs. For instance, bioinformatic approaches have been used to identify possible CPPs from the proteome of SARS-CoV-2 [42]. Machine learning algorithms such as MLCPP 2.0 [43] and DeepCPPred [44] can effectively predict both CPP sequences and uptake efficiencies. Taken altogether, the classification of CPPs is a continuous subject of study in a massive scientific field, and the readers are referred to several excellent reviews for more information on CPP classification [37,39].
2.2. CPP Transduction
Despite many years of research, the exact mechanism of how CPPs transduce the membrane remains elusive and likely varies between CPP subtypes, the cargoes they are carrying, and concentrations used for delivery. Furthermore, CPP internalization has been observed in both energy dependent and energy independent manners. Which pathway a given peptide uses will depend on both the properties of the cell membrane as well as the properties of the peptide and/or its cargo [45].
Initially, membrane transduction by CPPs was observed to solely occur as an energy independent process. Such transduction with transportan was observed at a range of temperatures from 0 to 37° C and was not prevented by endocytosis inhibition at high concentrations [46]. Tat and penetratin have also been observed to enter the cell by energy independent methods [4,38,39]. In an attempt to explain this phenomenon, Grasso et al. [47] described in detail how membrane mechanics are affected by different CPPs. The binding of various CPPs can rearrange lipid molecules, leading to changes in membrane stiffness and eventual peptide internalization. They also suggest that highly charged CPPs may attract more water to the lipid membrane, further destabilizing it and allowing for peptide entry [39,47]. Indeed, it has been observed that changing the physical shape of a cellular membrane can affect the transduction ability [48]. The changes in the cell membrane that result from CPP association could force the formation of inverted micelles containing the peptide, which then facilitates release into the cytoplasm [49].
Other factors also play a role in direct CPP internalization. Ionization status can affect direct membrane transduction, as described by Grasso et al. [50]. Membrane polarization can also affect peptide internalization in some cases; Wallbrecher et al. observed endocytic uptake of nona-arginine at low concentrations, but direct internalization at high concentrations due to membrane potential dependent sphingomyelinase activation [51]. Concentration and membrane composition are also vital factors in the world of CPP transduction. Walrant et al. [52] described how the membrane bound CPP fraction can be 5-fold to 100-fold higher than the internalized peptide fraction. Changing the concentration of CPPs to increase the bound peptide can cause endocytosis to occur due to increased proteoglycan clustering. Furthermore, the authors note that membrane composition plays a vital role in peptide interactions with the membrane and subsequent internalization; increased peptide–membrane binding due to differences in membrane composition can increase internalization independently of any metabotropic effects the peptide may have on the cell.
Although energy-independent processes for CPP internalization have been widely observed, this process can also occur through endocytic (energy-dependent) pathways, particularly at lower peptide concentrations. Because endocytic pathways create endosomes, CPPs must be able to both induce endocytic uptake and escape the endosome once internalized. Although many endocytic pathways exist, macropinocytosis seems to be the prevailing mechanism for CPP transduction [45]. For instance, Ichimizu et al. [40] described how macropinocytosis facilitates the entry of a peptide called palmitoyl-cyclic-(D-Arg)12/HSA. In this study, this peptide interacted with several key macropinocytosis markers including CXCR4 and pathways such as the PKC and mTOR pathways. Endosomal escape of palmitoyl-cyclic-(D-Arg)12/HSA was also observed to be inhibited by the presence of heparin, suggesting further evidence that electrostatic interactions play a key role in endosomal escape. Interestingly, this finding suggests that the methods CPPs use to transduce cells by changing phospholipid membrane dynamics can also contribute to endosomal escape.
Other endocytic pathways besides macropinocytosis can be responsible for CPP internalization; for instance, clathrin-mediated endocytosis (CME) provides a logical method for CPP transduction. CME occurs after an endocytosis-inducing ligand binds to a receptor [45]. Therefore, it stands to reason that CPPs could possibly act as agonists to a CME-associated receptor, activating endocytosis. This phenomenon has been observed with multiple CPPs including Tat; Richard et al. observed that chlorpromazine, a known inhibitor of CME, inhibited the transduction of Tat in HeLa cells, while filipin III and nystatin, both inhibitors of caveolin-dependent endocytosis, had little to no effect on Tat’s transduction efficiency [53]. CME dependent transduction has also been observed with other CPPs. For instance, siRNA knockdown of syndecan-4, a surface proteoglycan associated with CME, has been shown to prevent the uptake of an eight oligomer of arginine [54]. Other studies have also shown chlorpromazine to be an effective inhibitor of CME-associated CPP transduction in various cell types and with various CPPs [55,56,57,58,59,60]. It does appear, however, that the primary pathway (CME, macropinocytosis, direct transduction, etc.) a peptide uses to transduce the membrane is also dependent on concentration; at lower concentrations, and especially when peptides are attached to large cargo, endocytosis is the driving factor behind peptide internalization. At higher concentrations, peptides appear to transduce the membrane independently of endocytosis [45].
2.3. Cell-Specific CPPs
Although non-cell-specific CPPs have played a vital role in CPP research and present exciting new ways to cross the plasma membrane, they show limited clinical usefulness for targeted cell delivery. Cell-specific CPPs provide several key advantages over non-specific CPPs, namely, requiring lower concentrations and avoiding off-target interactions and hence potential side effects. The identification of such CPPs has largely been accomplished through the use of phage display libraries [1], a technique first identified by Smith [41]. This process has been used successfully many times to identify a variety of cell-specific CPPs including CPPs to target cardiomyocytes [61], dendritic cells [62], B lymphocytes [63], pancreatic islet cells [64], and more, and does not require a priori knowledge of the internalization or binding partners before a cell-specific CPP can be identified.
CPPs with specific biological activity present a new forefront in CPP research. While many studies have been performed to assess the toxicity of such peptides [65,66,67,68,69,70], less research exists on the intrinsic biological and/or therapeutic properties of these peptides. Cell-specific CPPs with inherent therapeutic properties present a new wave of drug development for highly targeted, direct-to-tissue therapies.
3. Biological Effects and Therapeutic Implications
Cell penetrating peptides have been the subject of intense research since the serendipitous discovery of the Tat protein as having intrinsic transduction ability. These studies have focused on identifying new naturally occurring or synthetic CPPs, their mechanism(s) of transduction, and their applications in both diagnostic and therapeutic arenas. To date, CPPs have largely been considered inert, small peptides with robust transduction abilities, but have been assumed to exhibit little intrinsic biological activity beyond transduction. Biological effects, other than transduction, of the CPPs presented here fall into three categories. A handful of studies have looked at the intrinsic anti-inflammatory properties of established CPPs. A second group of peptides are derived from molecular pathways associated with Toll-like receptors (TLRs), or the NF-κB pathways, which were found to also have cell transduction properties. The third, largest group are CPPs not having, or not being studied for, intrinsic anti-inflammatory properties but instead being used to deliver cargo potentially targeting various inflammatory pathways. In these studies, simply the transduction abilities of the CPP were being utilized. Our review details the work in the first two categories, and a summary of the specific biological effects of individual peptides included in this review are detailed in Table 1.

Table 1.
A summary of the specific biological activities of various cell penetrating peptides.
3.1. CPPs with Intrinsic Biological Activity
As stated above, some of the earliest CPPs were cationic and non-cell specific such as Tat, Antennapedia homeodomain-derived peptide, and synthetic homopolymers of arginine (nona-arginine). These three were compared in a head-to-head fashion in vitro in a human cervical cancer and human melanoma cell line. All three inhibited TNF-α mediated signal transduction by inducing the internalization of TNF-α receptors via clathrin mediated endocytosis [71]. The full-length Tat protein had a similar effect, and apoptosis caused by Smac protein (an inhibitor of “inhibitors of apoptosis”, or IAPs) was abrogated by Antennapedia [71]. Another in vitro study investigated the effects of various concentrations of an arginine homopolymer (octa-arginine) with incubations at various lengths of time on a U-937 macrophage cell line. There was an initial burst of super-oxide production at 30 min, but not at later time points, and no increase in the production of pro-inflammatory cytokines like TNF-α, IL-1β, or IL-6 [72]. In another study, antennapedia-derived CPP penetratin decreased the transcriptional activity of NF-κB in TNF-α stimulated L929 fibroblasts and lipopolysaccharide-activated RAW 264.7 macrophages [73]. Moreover, in an in vivo rat model of acute pancreatitis, pre-treatment with 2 mg/Kg of penetratin attenuated the severity of pancreatitis by inhibiting the IκB degradation and nuclear import of NF-κB dimers, inhibiting the expression of several downstream proinflammatory genes [73].
3.2. Anti-Inflammatory Peptides with Intrinsic Transduction Ability
In contrast to the above studies that explored the anti-inflammatory nature and pathways involved of known, well-established CPPs, the following studies aimed to design peptides with cell penetrating properties and the ability to target different inflammatory pathways, or have aimed to identify anti-inflammatory peptides that serendipitously have cell transduction properties. In this regard, the NF-κB signaling pathway, and modulation thereof, has been the subject of numerous studies due to its central role in a number of inflammatory pathologies ranging from, but not limited to, atherosclerosis, diabetes, and rheumatoid arthritis. There are highly conserved DNA-binding domains across all NF-κB members and investigators have searched for peptides that would bind to these domains, thereby inhibiting NK-κB transcriptional activities. One such peptide, anti-inflammatory peptide 6 (AIP6-RLRWR), inhibited the DNA binding and transcriptional activity of the p65 NF-κB subunit in stimulated macrophages in vitro, and inhibited zymosan-induced inflammation in vivo in mice [74]. In other studies, another bifunctional peptide, cSN50.1 (AAVALLPAVLLALLAPCVQRKRQKLMPC), with the molecular wight of 2986 Da, was designed to inhibit the nuclear transport of stress-mediated transcription factors [75]. This peptide mimics the NF-κB/p50 nuclear localizing sequence, with further studies showing that it selectively targets importin α5 [84] and mitigates atherosclerosis, fatty liver, blood glucose, and lipid levels in a mouse model of familial hypercholesterolemia [85]. This peptide has a hydrophobic region (AAVALLPAVLLALLAP) and an arginine/lysine rich nuclear localizing region (CVQRKRQKLMPC); its ability to cross nuclear membranes also translates into a similar ability to cross plasma membranes.
Anti-inflammatory cell penetrating peptides also have applications for the nervous system. Gliomas are intracranial tumors with high mortality and limited options for treatment due to the blood–brain barrier preventing achievement of adequate drug concentrations in tumor tissue. Additionally, NF-κB activation has been correlated with tumor drug resistance. To counter both of these issues, a peptide was specifically designed to have both cell penetrating and NF-κB inhibition capabilities and was conjugated to pegylated liposomes loaded with doxorubicin for delivery to glioma tumors in vivo. This approach decreased the tumor size and significantly prolonged survival in nude mice bearing intracranial gliomas [76]. Finally, a heparin-binding sequence derived from the heparin-binding epidermal growth factor transduced RAW 264.7 macrophages within ten minutes. Pretreatment with this peptide reduced the LPS-induced production of nitric oxide, inducible nitric oxide synthase, and cytokines TNF-α and IL-6, in a dose-dependent manner by blocking the phosphorylation and degradation of IκBα, leading to inhibition of nuclear translocation of the p65 subunit of NF-κB. It also decreased infiltration by polymorphonuclear cells in an in vivo lung inflammation model [77].
Eosinophil mediated inflammation plays a key role in several common allergic lung pathologies including asthma. Eosinophil granules have several proteins including eosinophil cationic protein (ECP), which is thought to play a central role in airway hyper-responsiveness. A 10-amino acid peptide (NYRWRCKNQN) derived from ECP and named CPPecp has a heparan-sulfate binding core. Several glycosaminoglycans present on cell surfaces bind to ligands to mediate myriad immune responses; therefore, it was hypothesized that CPPecp binding to these cell surface receptors will block this interaction and modulate immune responses in various models of lung inflammation. Indeed, this turned out to be true in BEAS-2B, a lung epithelial cell line, where CPPecp decreased ECP mRNA expression, eotaxin secretion, and p-STAT6 activation [78]. In vivo studies also reduced mite-induced airway inflammation by decreasing the neutrophil and eosinophil count in broncho-alveolar lavage fluid, and decreased IL-5, IL-13, IL-17, and eotaxin expression in lung tissue [78]. Further studies identified CPPecp’s anti-inflammatory activity through inhibition of the NLRP3 inflammasome [79].
Acute lung injury (ALI) is a life-threatening, rapidly progressing inflammatory condition driven by TLR4 activation. In an attempt to mitigate the effects of ALI, a decoy CPP designed to inhibit the binding of TLR4 to MyD88 was created and termed TM6 (RQIKIWFQNRRMKWKKENFLRDTWCNFQFY). In a mouse model of lipopolysaccharide (LPS)-induced lung injury, treatment with TM6 alleviated negative histological changes, inhibited myeloperoxidase activity, and lowered the TNF-α, IL-1β, and IL6 levels in lung tissue [80]. Inflammation via TLR4 activation also plays a key pathophysiological role in insulin resistance in type II diabetes mellitus. The death domain of MyD88 has been found to interact with TLR4. Several studies have identified a 10-residue amino acid (M10-RRRLSLFLNV) derived from the death domain of MyD88 to have cell transduction abilities (likely due to its arginine rich residues), which also functioned to inhibit the LPS-induced nuclear translocation of NF-κB, decreased TNF-α and IL6 levels, lowered blood glucose levels, and improved glucose intolerance in db/db mice [81].
CIBG-552 is an anti-tumor peptide with cell penetrating activity developed from the screening of an Ala-library derived from the Limulus anti-LPS factor (LALF) 32–51 region [86]. It has been shown to exert anti-angiogenic and anti-inflammatory effects through inhibition of the NF-κB and HIF-1 pathways via its interaction with COMMD1 [82]. Interestingly, this peptide has been tested in a Phase I clinical trial of 25 patients with advanced, refractory solid tumors [83]. Patients received varying doses of subcutaneous injections of CIBG-522 3x/week for two weeks, in which the maximum tolerated dose was found to be 4.7 mg. Stable disease was reported in five patients, and seven out of ten assessed patients showed a significant change in the ratio of CD4/CD8 cells in response to therapy [83].
3.3. CPPs to Deliver Anti-Inflammatory Cargo
As far as the transduction abilities of CPPs are concerned, numerous studies have utilized these properties to deliver small molecules, or other decoy peptides inhibiting various inflammatory pathways like NF-κB [87,88,89,90,91,92,93,94], the TLR4 pathway [74,95,96,97], and STAT-6 [98,99], to name a few. This list is by no means exhaustive, and we apologize to the authors whose valuable work was inevitably left out due to space limitations.
3.4. Anti-Inflammatory Potential of Cardiac Targeting Peptide, a CPP
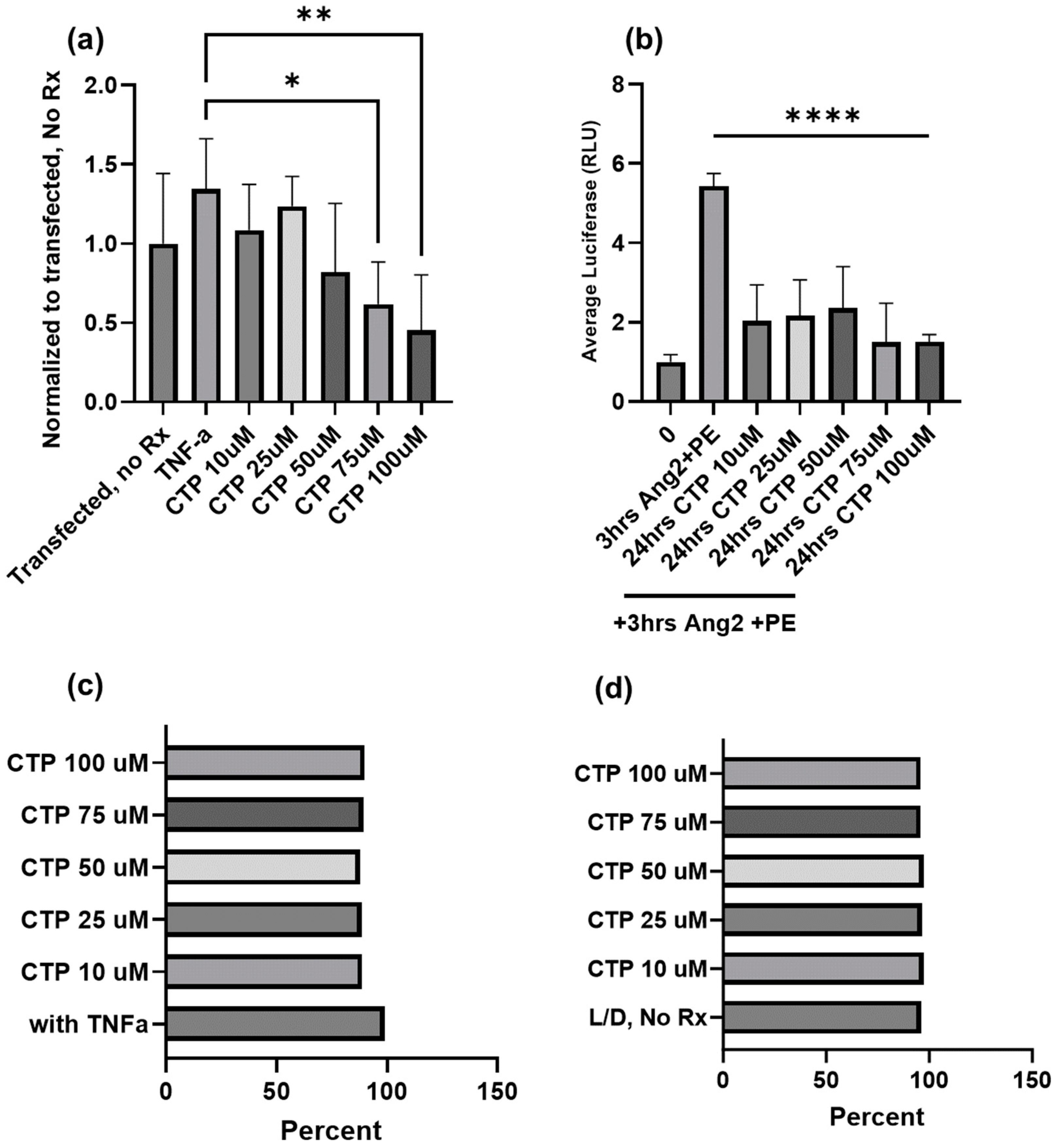
We have had a long-standing interest in cell-type specific CPPs [100], and recently observed our cardiac targeting peptide (CTP: APWHLSSQYSRT) to have intrinsic biological properties beyond transduction. Our work, using a combinatorial in vitro and in vivo phage display methodology [61], identified CTP, which is a cardiomyocyte-specific CPP that transduces heart tissue in as little as 15 min after peripheral intravenous injection in mice [101]. This work has been confirmed by at least three different independent investigators from around the world [102]. Our recent work involved conjugating the N-terminus of CTP to amiodarone, a well-established anti-arrhythmic in routine clinical use via a disulfide bond [103]. The conjugate was injected intraperitoneally into guinea pigs, and after 5 days of daily injections, the animals were euthanized and their hearts placed in a Langendorff perfusion system to measure heart rates, calcium currents, and action potential durations. To our surprise, CTP alone without any cargo displayed several salutary effects on calcium handling and increased heart rates while decreasing the action potential durations [8]. RNA sequencing from these heart extracts revealed that CTP increased alpha-adrenergic receptor expression and decreased beta-adrenergic receptor expression, explaining the changes in heart rate. It also upregulated calcium handling genes (SERCA2a) and surprisingly downregulated several NF-κB pathway genes as well as TNF-α [8]. To further study the physiological effects of CTP, we used a human cardiomyocyte cell line transfected with a reporter plasmid expressing the luciferase gene under an NF-κB promoter. Post-transfection, cells were treated with varying concentrations of CTP and then challenged with TNF-α (20 ng/mL) prior to running a luciferase assay. Cell viability in response to the above manipulations was assessed by FACS using live/dead stain. CTP inhibited TNF-α mediated NF-κB activation in a dose-dependent manner, with significant inhibition seen with CTP at concentrations of 75 µM and above (p < 0.05; Figure 2). Furthermore, challenging cardiomyocytes with angiotensin/phenylephrine resulted in a significant increase in NF-κB activation, an increase prevented by pre-treatment with CTP. Studies looking at the molecular pathways leading to this NF-κB inhibition as well as the expression of downstream cytokines are ongoing.
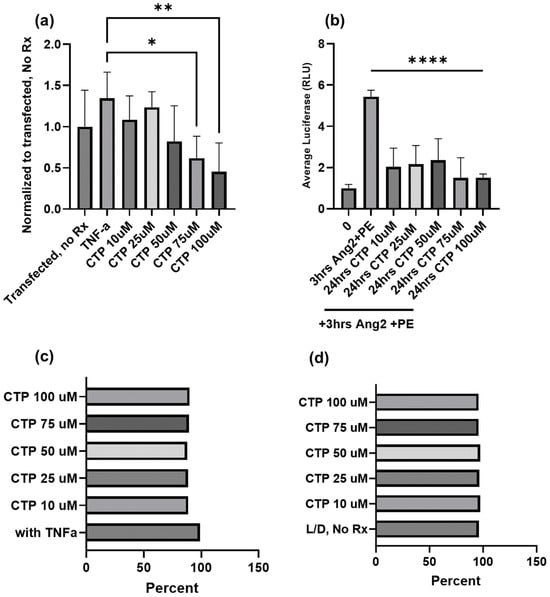
Figure 2.
Inhibition of TNF-α mediated NF-κB activation by CTP in a human cardiomyocyte cell line at the baseline (a) and after angiotensin/phenylephrine challenge (b). No change in cell viability due to either treatments with CTP (c) or treatments with CTP after an angiotensin/phenylephrine challenge (d) as assessed by FACS using live/dead stain. *: p < 0.05, **: p < 0.01, ****: p < 0.0001.
4. Conclusions
The potential of CPPs as novel vectors has been an area of intense study for the last three decades. They have generally been considered inert vectors for the delivery of other diagnostic or therapeutic drugs ranging from small molecules, other peptides of therapeutic potential, to oligonucleotides for gene therapy, as well as most recently RNA-interference therapeutics. Little attention has been paid to the possibility of these CPPs having inherent biological properties beyond transduction. Many of the earliest traditional non-specific CPPs like Tat and penetratin, and more recently our cardiomyocyte-targeting peptide CTP, have shown immense potential as anti-inflammatory agents, which can serve as a stand-alone therapeutic or add to the therapeutic potential of these CPPs. This is of particular significance, as the more traditional viral vectors are limited by pre-existing immunity [23] or the development of rapid immunity to these vectors on first exposure [24]. Beyond developing immunity, these viral vectors also suffer from inciting an acute inflammatory response that can be life-threatening [25] including myocarditis [104] and/or a sepsis-like syndrome [105]. In this review, we make a case for devoting more research to the less obvious, but perhaps equally important biological effects such as transduction that these CPPs exhibit. At a minimum, evolving data indicate that these peptides do not incite an immune response, a highly desirable characteristic of a vector. However, far more longitudinal in vivo studies in large animal models are necessary to confirm these findings.
Author Contributions
Writing—original draft preparation; J.L., J.W., M.Z.; writing-review and editing, M.Z.; data generation, J.W; conceptualization, M.Z.; project administration, J.L.; funding acquisition, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
M.Z. was supported by the National Institutes of Health, grant R01HL153407 awarded to her.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
M.Z. holds a patent on the use of CTP as a cardiac-specific delivery vector along with Paul D. Robbins and the University of Pittsburgh.
References
- Taylor, R.E.; Zahid, M. Cell Penetrating Peptides, Novel Vectors for Gene Therapy. Pharmaceutics 2020, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Fawell, S.; Seery, J.; Daikh, Y.; Moore, C.; Chen, L.L.; Pepinsky, B.; Barsoum, J. Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA 1994, 91, 664–668. [Google Scholar] [CrossRef]
- Schwarze, S.R.; Ho, A.; Vocero-Akbani, A.; Dowdy, S.F. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science 1999, 285, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, B.G.; Hornillos, V.; Luque-Ortega, J.R.; Abengozar, M.A.; Amat-Guerri, F.; Acuna, A.U.; Rivas, L.; Andreu, D. A BODIPY-embedding miltefosine analog linked to cell-penetrating Tat(48-60) peptide favors intracellular delivery and visualization of the antiparasitic drug. Amino Acids 2014, 46, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Weber, B.; Yurko, R.; Islam, K.; Agrawal, V.; Lopuszynski, J.; Yagi, H.; Salama, G. Cardiomyocyte-Targeting Peptide to Deliver Amiodarone. Pharmaceutics 2023, 15, 2107. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Yang, X.Z.; Du, X.; Wang, J.W.; Zhang, R.; Zhao, J.; Wang, F.J.; Dong, Y.; Li, P.F. Enhancing tumor-specific intracellular delivering efficiency of cell-penetrating peptide by fusion with a peptide targeting to EGFR. Amino Acids 2015, 47, 997–1006. [Google Scholar] [CrossRef]
- Vogel, E.; Santos, D.; Huygens, C.; Peeters, P.; Van den Brande, S.; Wynant, N.; Vanden Broeck, J. The Study of Cell-Penetrating Peptides to Deliver dsRNA and siRNA by Feeding in the Desert Locust, Schistocerca gregaria. Insects 2023, 14, 597. [Google Scholar] [CrossRef]
- Furukawa, K.; Tanaka, M.; Oba, M. siRNA delivery using amphipathic cell-penetrating peptides into human hepatoma cells. Bioorg. Med. Chem. 2020, 28, 115402. [Google Scholar] [CrossRef]
- Yan, H.; Duan, X.; Pan, H.; Akk, A.; Sandell, L.J.; Wickline, S.A.; Rai, M.F.; Pham, C.T.N. Development of a peptide-siRNA nanocomplex targeting NF- kappaB for efficient cartilage delivery. Sci. Rep. 2019, 9, 442. [Google Scholar] [CrossRef]
- Falato, L.; Gestin, M.; Langel, U. Cell-Penetrating Peptides Delivering siRNAs: An Overview. Methods Mol. Biol. 2021, 2282, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, P.V.; Gunathunge, B.G.; Wimalasiri, P.N.; Karunaratne, D.N.; Ranatunga, R.J. Trends in the Binding of Cell Penetrating Peptides to siRNA: A Molecular Docking Study. J. Biophys. 2017, 2017, 1059216. [Google Scholar] [CrossRef] [PubMed]
- Vanova, J.; Hejtmankova, A.; Zackova Suchanova, J.; Sauerova, P.; Forstova, J.; Hubalek Kalbacova, M.; Spanielova, H. Influence of cell-penetrating peptides on the activity and stability of virus-based nanoparticles. Int. J. Pharm. 2020, 576, 119008. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, V.; Sharma, V.; Dahlheimer, J.L.; Pica, C.M.; Luker, G.D.; Piwnica-Worms, D. Novel Tat-peptide chelates for direct transduction of technetium-99m and rhenium into human cells for imaging and radiotherapy. Bioconjug. Chem. 2000, 11, 762–771. [Google Scholar] [CrossRef]
- Dong, P.; Cai, H.; Chen, L.; Li, Y.; Yuan, C.; Wu, X.; Shen, G.; Zhou, H.; Zhang, W.; Li, L. Biodistribution and evaluation of (131) I-labeled neuropilin-binding peptide for targeted tumor imaging. Contrast Media Mol. Imaging 2016, 11, 467–474. [Google Scholar] [CrossRef]
- Avula, U.M.; Yoon, H.K.; Lee, C.H.; Kaur, K.; Ramirez, R.J.; Takemoto, Y.; Ennis, S.R.; Morady, F.; Herron, T.; Berenfeld, O.; et al. Cell-selective arrhythmia ablation for photomodulation of heart rhythm. Sci. Transl. Med. 2015, 7, 311ra172. [Google Scholar] [CrossRef]
- Yang, J.; Firdaus, F.; Azuar, A.; Khalil, Z.G.; Marasini, N.; Capon, R.J.; Hussein, W.M.; Toth, I.; Skwarczynski, M. Cell-Penetrating Peptides-Based Liposomal Delivery System Enhanced Immunogenicity of Peptide-Based Vaccine against Group A Streptococcus. Vaccines 2021, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Zhang, W.; Liu, H.; Jiang, J.J.; Wang, W.J.; Jia, Z.Y. Cell-Penetrating Peptide-Modified Graphene Oxide Nanoparticles Loaded with Rictor siRNA for the Treatment of Triple-Negative Breast Cancer. Drug Des. Dev. Ther. 2021, 15, 4961–4972. [Google Scholar] [CrossRef]
- Chu, X.; Wu, B.; Fan, H.; Hou, J.; Hao, J.; Hu, J.; Wang, B.; Liu, G.; Li, C.; Meng, S. PTD-fused p53 as a potential antiviral agent directly suppresses HBV transcription and expression. Antivir. Res. 2016, 127, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.S.; Pierce, G.F.; Arruda, V.R.; Glader, B.; Ragni, M.; Rasko, J.J.; Ozelo, M.C.; Hoots, K.; Blatt, P.; Konkle, B.; et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006, 12, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Duan, D. Lethal immunotoxicity in high-dose systemic AAV therapy. Mol. Ther. 2023, 31, 3123–3126. [Google Scholar] [CrossRef]
- Hinderer, C.; Katz, N.; Buza, E.L.; Dyer, C.; Goode, T.; Bell, P.; Richman, L.K.; Wilson, J.M. Severe Toxicity in Nonhuman Primates and Piglets Following High-Dose Intravenous Administration of an Adeno-Associated Virus Vector Expressing Human SMN. Hum. Gene Ther. 2018, 29, 285–298. [Google Scholar] [CrossRef]
- Wender, P.A.; Mitchell, D.J.; Pattabiraman, K.; Pelkey, E.T.; Steinman, L.; Rothbard, J.B. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13003–13008. [Google Scholar] [CrossRef]
- Mai, J.C.; Shen, H.; Watkins, S.C.; Cheng, T.; Robbins, P.D. Efficiency of protein transduction is cell type-dependent and is enhanced by dextran sulfate. J. Biol. Chem. 2002, 277, 30208–30218. [Google Scholar] [CrossRef]
- Mitchell, D.J.; Kim, D.T.; Steinman, L.; Fathman, C.G.; Rothbard, J.B. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000, 56, 318–325. [Google Scholar] [CrossRef]
- Mi, Z.; Mai, J.; Lu, X.; Robbins, P.D. Characterization of a class of cationic peptides able to facilitate efficient protein transduction in vitro and in vivo. Mol. Ther. 2000, 2, 339–347. [Google Scholar] [CrossRef]
- Soomets, U.; Lindgren, M.; Gallet, X.; Hallbrink, M.; Elmquist, A.; Balaspiri, L.; Zorko, M.; Pooga, M.; Brasseur, R.; Langel, U. Deletion analogues of transportan. Biochim. Biophys. Acta 2000, 1467, 165–176. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Horikoshi, K.; Fujita, M.; Hirano, M.; Miyamoto, M.; Yokoo, H.; Demizu, Y. Development of Hydrophobic Cell-Penetrating Stapled Peptides as Drug Carriers. Int. J. Mol. Sci. 2023, 24, 11768. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Oba, M.; Misawa, T.; Tanaka, M.; Hattori, T.; Naito, M.; Kurihara, M.; Demizu, Y. A Helix-Stabilized Cell-Penetrating Peptide as an Intracellular Delivery Tool. Chembiochem 2016, 17, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.K.; Baumruck, A.C.; Zhdanova, H.; Tietze, A.A. Challenges and Perspectives in Chemical Synthesis of Highly Hydrophobic Peptides. Front. Bioeng. Biotechnol. 2020, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, W.; Qiu, Y.; Cespi, M.; Palmieri, G.F.; Mason, A.J.; Lam, J.K. Incorporation of a Nuclear Localization Signal in pH Responsive LAH4-L1 Peptide Enhances Transfection and Nuclear Uptake of Plasmid DNA. Mol. Pharm. 2016, 13, 3141–3152. [Google Scholar] [CrossRef]
- Sajid, M.I.; Moazzam, M.; Stueber, R.; Park, S.E.; Cho, Y.; Malik, N.U.A.; Tiwari, R.K. Applications of amphipathic and cationic cyclic cell-penetrating peptides: Significant therapeutic delivery tool. Peptides 2021, 141, 170542. [Google Scholar] [CrossRef]
- Alves, I.D.; Goasdoue, N.; Correia, I.; Aubry, S.; Galanth, C.; Sagan, S.; Lavielle, S.; Chassaing, G. Membrane interaction and perturbation mechanisms induced by two cationic cell penetrating peptides with distinct charge distribution. Biochim. Biophys. Acta 2008, 1780, 948–959. [Google Scholar] [CrossRef]
- Gori, A.; Lodigiani, G.; Colombarolli, S.G.; Bergamaschi, G.; Vitali, A. Cell Penetrating Peptides: Classification, Mechanisms, Methods of Study, and Applications. ChemMedChem 2023, 18, e202300236. [Google Scholar] [CrossRef]
- Green, M.; Ishino, M.; Loewenstein, P.M. Mutational analysis of HIV-1 Tat minimal domain peptides: Identification of trans-dominant mutants that suppress HIV-LTR-driven gene expression. Cell 1989, 58, 215–223. [Google Scholar] [CrossRef]
- Zorko, M.; Langel, U. Cell-Penetrating Peptides. Methods Mol. Biol. 2022, 2383, 3–32. [Google Scholar] [CrossRef]
- Ichimizu, S.; Watanabe, H.; Maeda, H.; Hamasaki, K.; Ikegami, K.; Chuang, V.T.G.; Kinoshita, R.; Nishida, K.; Shimizu, T.; Ishima, Y.; et al. Cell-penetrating mechanism of intracellular targeting albumin: Contribution of macropinocytosis induction and endosomal escape. J. Control Release 2019, 304, 156–163. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Kardani, K.; Bolhassani, A. Exploring novel and potent cell penetrating peptides in the proteome of SARS-CoV-2 using bioinformatics approaches. PLoS ONE 2021, 16, e0247396. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, B.; Patra, M.C. MLCPP 2.0: An Updated Cell-penetrating Peptides and Their Uptake Efficiency Predictor. J. Mol. Biol. 2022, 434, 167604. [Google Scholar] [CrossRef]
- Arif, M.; Kabir, M.; Ahmed, S.; Khan, A.; Ge, F.; Khelifi, A.; Yu, D.J. DeepCPPred: A Deep Learning Framework for the Discrimination of Cell-Penetrating Peptides and Their Uptake Efficiencies. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022, 19, 2749–2759. [Google Scholar] [CrossRef]
- Ruseska, I.; Zimmer, A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Pooga, M.; Hallbrink, M.; Zorko, M.; Langel, U. Cell penetration by transportan. FASEB J. 1998, 12, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G.; Muscat, S.; Rebella, M.; Morbiducci, U.; Audenino, A.; Danani, A.; Deriu, M.A. Cell penetrating peptide modulation of membrane biomechanics by Molecular dynamics. J. Biomech. 2018, 73, 137–144. [Google Scholar] [CrossRef]
- Sakamoto, K.; Morishita, T.; Aburai, K.; Ito, D.; Imura, T.; Sakai, K.; Abe, M.; Nakase, I.; Futaki, S.; Sakai, H. Direct entry of cell-penetrating peptide can be controlled by maneuvering the membrane curvature. Sci. Rep. 2021, 11, 31. [Google Scholar] [CrossRef]
- Alves, I.D.; Jiao, C.Y.; Aubry, S.; Aussedat, B.; Burlina, F.; Chassaing, G.; Sagan, S. Cell biology meets biophysics to unveil the different mechanisms of penetratin internalization in cells. Biochim. Biophys. Acta 2010, 1798, 2231–2239. [Google Scholar] [CrossRef]
- Grasso, G.; Mercuri, S.; Danani, A.; Deriu, M.A. Biofunctionalization of Silica Nanoparticles with Cell-Penetrating Peptides: Adsorption Mechanism and Binding Energy Estimation. J. Phys. Chem. B 2019, 123, 10622–10630. [Google Scholar] [CrossRef]
- Wallbrecher, R.; Ackels, T.; Olea, R.A.; Klein, M.J.; Caillon, L.; Schiller, J.; Bovee-Geurts, P.H.; van Kuppevelt, T.H.; Ulrich, A.S.; Spehr, M.; et al. Membrane permeation of arginine-rich cell-penetrating peptides independent of transmembrane potential as a function of lipid composition and membrane fluidity. J. Control Release 2017, 256, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Walrant, A.; Cardon, S.; Burlina, F.; Sagan, S. Membrane Crossing and Membranotropic Activity of Cell-Penetrating Peptides: Dangerous Liaisons? Acc. Chem. Res. 2017, 50, 2968–2975. [Google Scholar] [CrossRef]
- Richard, J.P.; Melikov, K.; Brooks, H.; Prevot, P.; Lebleu, B.; Chernomordik, L.V. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J. Biol. Chem. 2005, 280, 15300–15306. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Takeuchi, T.; Kuwata, K.; Chiba, J.; Hatanaka, Y.; Nakase, I.; Futaki, S. Syndecan-4 Is a Receptor for Clathrin-Mediated Endocytosis of Arginine-Rich Cell-Penetrating Peptides. Bioconjug. Chem. 2016, 27, 1119–1130. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Lu, M.; Xing, H.; Yang, T.; Cai, C.; Zhao, X.; Wei, M.; Yu, J.; Ding, P. Intracellular distribution and internalization pathways of guanidinylated bioresponsive poly(amido amine)s in gene delivery. Asian J. Pharm. Sci. 2018, 13, 360–372. [Google Scholar] [CrossRef]
- Rodrigues, M.; de la Torre, B.G.; Andreu, D.; Santos, N.C. Kinetic uptake profiles of cell penetrating peptides in lymphocytes and monocytes. Biochim. Biophys. Acta 2013, 1830, 4554–4563. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.; Yang, Y.; Zeng, F.; Liu, C. Highly Efficient Delivery of Functional Cargoes by a Novel Cell-Penetrating Peptide Derived from SP140-Like Protein. Bioconjug. Chem. 2016, 27, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.Y.; Raines, R.T. Mechanism of ribonuclease A endocytosis: Analogies to cell-penetrating peptides. Biochemistry 2011, 50, 8374–8382. [Google Scholar] [CrossRef]
- Shen, W.J.; Tian, D.M.; Fu, L.; Jin, B.; Liu, Y.; Xu, Y.S.; Ye, Y.B.; Wang, X.B.; Xu, X.J.; Tang, C.; et al. Elastin-Derived VGVAPG Fragment Decorated Cell-Penetrating Peptide with Improved Gene Delivery Efficacy. Pharmaceutics 2023, 15, 670. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, H.; Cao, W.; Fang, Y.; Chen, Z.; Qi, X.; Luo, D.; Chen, C. Transcytosis mechanisms of cell-penetrating peptides: Cation-independent CC12 and cationic penetratin. J. Pept. Sci. 2022, 28, e3408. [Google Scholar] [CrossRef]
- Zahid, M.; Phillips, B.E.; Albers, S.M.; Giannoukakis, N.; Watkins, S.C.; Robbins, P.D. Identification of a cardiac specific protein transduction domain by in vivo biopanning using a M13 phage peptide display library in mice. PLoS ONE 2010, 5, e12252. [Google Scholar] [CrossRef] [PubMed]
- Chamarthy, S.P.; Jia, L.; Kovacs, J.R.; Anderson, K.R.; Shen, H.; Firestine, S.M.; Meng, W.S. Gene delivery to dendritic cells facilitated by a tumor necrosis factor alpha-competing peptide. Mol. Immunol. 2004, 41, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Adachi, Y.; Komoike, Y.; Kamada, Y.; Koyama, R.; Fukuda, Y.; Kadotani, A.; Asami, T.; Sakamoto, J.I. Novel DOCK2-selective inhibitory peptide that suppresses B-cell line migration. Biochem. Biophys. Res. Commun. 2017, 483, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.K.; Bertera, S.; Bottino, R.; Balamurugan, A.N.; Mai, J.C.; Mi, Z.; Trucco, M.; Robbins, P.D. Protection of islets by in situ peptide-mediated transduction of the Ikappa B kinase inhibitor Nemo-binding domain peptide. J. Biol. Chem. 2003, 278, 9862–9868. [Google Scholar] [CrossRef]
- Sahagun, D.A.; Lopuszynski, J.B.; Feldman, K.S.; Pogodzinski, N.; Zahid, M. Toxicity Studies of Cardiac-Targeting Peptide Reveal a Robust Safety Profile. Pharmaceutics 2024, 16, 73. [Google Scholar] [CrossRef]
- Kilk, K.; Mahlapuu, R.; Soomets, U.; Langel, U. Analysis of in vitro toxicity of five cell-penetrating peptides by metabolic profiling. Toxicology 2009, 265, 87–95. [Google Scholar] [CrossRef]
- Suhorutsenko, J.; Oskolkov, N.; Arukuusk, P.; Kurrikoff, K.; Eriste, E.; Copolovici, D.M.; Langel, U. Cell-penetrating peptides, PepFects, show no evidence of toxicity and immunogenicity in vitro and in vivo. Bioconjug. Chem. 2011, 22, 2255–2262. [Google Scholar] [CrossRef]
- Saar, K.; Lindgren, M.; Hansen, M.; Eiriksdottir, E.; Jiang, Y.; Rosenthal-Aizman, K.; Sassian, M.; Langel, U. Cell-penetrating peptides: A comparative membrane toxicity study. Anal. Biochem. 2005, 345, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Sayers, E.J.; He, L.; Narayan, R.; Williams, T.L.; Mills, E.M.; Allemann, R.K.; Luk, L.Y.P.; Jones, A.T.; Tsai, Y.H. Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Sci. Rep. 2019, 9, 6298. [Google Scholar] [CrossRef]
- Hoffmann, K.; Milech, N.; Juraja, S.M.; Cunningham, P.T.; Stone, S.R.; Francis, R.W.; Anastasas, M.; Hall, C.M.; Heinrich, T.; Bogdawa, H.M.; et al. A platform for discovery of functional cell-penetrating peptides for efficient multi-cargo intracellular delivery. Sci. Rep. 2018, 8, 12538. [Google Scholar] [CrossRef]
- Fotin-Mleczek, M.; Welte, S.; Mader, O.; Duchardt, F.; Fischer, R.; Hufnagel, H.; Scheurich, P.; Brock, R. Cationic cell-penetrating peptides interfere with TNF signalling by induction of TNF receptor internalization. J. Cell Sci. 2005, 118, 3339–3351. [Google Scholar] [CrossRef]
- Kuo, J.H.; Jan, M.S.; Lin, Y.L.; Lin, C. Interactions between octaarginine and U-937 human macrophages: Global gene expression profiling, superoxide anion content, and cytokine production. J. Control Release 2009, 139, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Letoha, T.; Kusz, E.; Papai, G.; Szabolcs, A.; Kaszaki, J.; Varga, I.; Takacs, T.; Penke, B.; Duda, E. In vitro and in vivo nuclear factor-kappaB inhibitory effects of the cell-penetrating penetratin peptide. Mol. Pharmacol. 2006, 69, 2027–2036. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xu, X.; Fan, X.; Zhang, C.; Wei, Q.; Wang, X.; Guo, W.; Xing, W.; Yu, J.; Yan, J.L.; et al. A cell-penetrating peptide suppresses inflammation by inhibiting NF-kappaB signaling. Mol. Ther. 2011, 19, 1849–1857. [Google Scholar] [CrossRef]
- DiGiandomenico, A.; Veach, R.A.; Zienkiewicz, J.; Moore, D.J.; Wylezinski, L.S.; Hutchens, M.A.; Hawiger, J. The “genomic storm” induced by bacterial endotoxin is calmed by a nuclear transport modifier that attenuates localized and systemic inflammation. PLoS ONE 2014, 9, e110183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Hu, Y.; Jiang, K.; Li, Z.; Lin, Y.Z.; Wei, G.; Lu, W. Cell-permeable NF-kappaB inhibitor-conjugated liposomes for treatment of glioma. J. Control Release 2018, 289, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Seo, Y.N.; Park, H.J.; Park, Y.J.; Chung, C.P. The cell-penetrating peptide domain from human heparin-binding epidermal growth factor-like growth factor (HB-EGF) has anti-inflammatory activity in vitro and in vivo. Biochem. Biophys. Res. Commun. 2012, 419, 597–604. [Google Scholar] [CrossRef]
- Fu, L.S.; Wu, Y.R.; Fang, S.L.; Tsai, J.J.; Lin, H.K.; Chen, Y.J.; Chen, T.Y.; Chang, M.D. Cell Penetrating Peptide Derived from Human Eosinophil Cationic Protein Decreases Airway Allergic Inflammation. Sci. Rep. 2017, 7, 12352. [Google Scholar] [CrossRef]
- Yu, S.J.; Liao, E.C.; Sheu, M.L.; Chang, D.T.; Tsai, J.J. Cell-penetrating peptide derived from human eosinophil cationic protein inhibits mite allergen Der p 2 induced inflammasome activation. PLoS ONE 2015, 10, e0121393. [Google Scholar] [CrossRef]
- Hu, X.; Tian, Y.; Qu, S.; Cao, Y.; Li, S.; Zhang, W.; Zhang, Z.; Zhang, N.; Fu, Y. Protective effect of TM6 on LPS-induced acute lung injury in mice. Sci. Rep. 2017, 7, 572. [Google Scholar] [CrossRef]
- Ali, M.; Kumari, T.; Gupta, A.; Akhtar, S.; Verma, R.D.; Ghosh, J.K. Identification of a 10-mer peptide from the death domain of MyD88 which attenuates inflammation and insulin resistance and improves glucose metabolism. Biochem. J. 2024, 481, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Daghero, H.; Fernandez Masso, J.R.; Astrada, S.; Guerra Vallespi, M.; Bollati-Fogolin, M. The Anticancer Peptide CIGB-552 Exerts Anti-Inflammatory and Anti-Angiogenic Effects through COMMD1. Molecules 2020, 26, 152. [Google Scholar] [CrossRef]
- Vallespi, M.G.; Mestre, B.; Marrero, M.A.; Uranga, R.; Rey, D.; Lugiollo, M.; Betancourt, M.; Silva, K.; Corrales, D.; Lamadrid, Y.; et al. A first-in-class, first-in-human, phase I trial of CIGB-552, a synthetic peptide targeting COMMD1 to inhibit the oncogenic activity of NF-kappaB in patients with advanced solid tumors. Int. J. Cancer 2021, 149, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Zienkiewicz, J.; Armitage, A.; Hawiger, J. Targeting nuclear import shuttles, importins/karyopherins alpha by a peptide mimicking the NFkappaB1/p50 nuclear localization sequence. J. Am. Heart Assoc. 2013, 2, e000386. [Google Scholar] [CrossRef]
- Liu, Y.; Major, A.S.; Zienkiewicz, J.; Gabriel, C.L.; Veach, R.A.; Moore, D.J.; Collins, R.D.; Hawiger, J. Nuclear transport modulation reduces hypercholesterolemia, atherosclerosis, and fatty liver. J. Am. Heart Assoc. 2013, 2, e000093. [Google Scholar] [CrossRef]
- Vallespi, M.G.; Fernandez, J.R.; Torrens, I.; Garcia, I.; Garay, H.; Mendoza, O.; Granadillo, M.; Falcon, V.; Acevedo, B.; Ubieta, R.; et al. Identification of a novel antitumor peptide based on the screening of an Ala-library derived from the LALF(32-51) region. J. Pept. Sci. 2010, 16, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S.; May, M.J. Cell penetrating peptide inhibitors of nuclear factor-kappa B. Cell Mol. Life Sci. 2008, 65, 3564–3591. [Google Scholar] [CrossRef]
- Li, N.; Qiao, Y.; Xue, L.; Xu, S.; Zhang, N. Targeted and MMP-2/9 responsive peptides for the treatment of rheumatoid arthritis. Int. J. Pharm. 2019, 569, 118625. [Google Scholar] [CrossRef]
- Kanemaru, Y.; Momiki, Y.; Matsuura, S.; Horikawa, T.; Gohda, J.; Inoue, J.; Okamoto, Y.; Fujita, M.; Otsuka, M. An artificial copper complex incorporating a cell-penetrating peptide inhibits nuclear factor-kappaB (NF-kappaB) activation. Chem. Pharm. Bull. 2011, 59, 1555–1558. [Google Scholar] [CrossRef]
- Lai, J.; Yao, Y.; Zhang, Y.; Liu, Y.; Lu, C.; Meng, C.; Xia, D.; Li, Y.; Cao, K.; Gao, X.; et al. Cell-Penetrating Peptide Conjugated Au Nanoclusters Selectively Suppress Refractory Lymphoma Cells via Targeting Both Canonical and Noncanonical NF-kappaB Signaling Pathways. Bioconjug. Chem. 2023, 34, 228–237. [Google Scholar] [CrossRef]
- Davoudi, Z.; Akbarzadeh, A.; Rahmatiyamchi, M.; Movassaghpour, A.A.; Alipour, M.; Nejati-Koshki, K.; Sadeghi, Z.; Dariushnejad, H.; Zarghami, N. Molecular target therapy of AKT and NF-kB signaling pathways and multidrug resistance by specific cell penetrating inhibitor peptides in HL-60 cells. Asian Pac. J. Cancer Prev. 2014, 15, 4353–4358. [Google Scholar] [CrossRef]
- Hong, S.; Yum, S.; Yoo, H.J.; Kang, S.; Yoon, J.H.; Min, D.; Kim, Y.M.; Jung, Y. Colon-targeted cell-permeable NFkappaB inhibitory peptide is orally active against experimental colitis. Mol. Pharm. 2012, 9, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Urata, M.; Kokabu, S.; Matsubara, T.; Sugiyama, G.; Nakatomi, C.; Takeuchi, H.; Hirata-Tsuchiya, S.; Aoki, K.; Tamura, Y.; Moriyama, Y.; et al. A peptide that blocks the interaction of NF-kappaB p65 subunit with Smad4 enhances BMP2-induced osteogenesis. J. Cell Physiol. 2018, 233, 7356–7366. [Google Scholar] [CrossRef]
- von Bismarck, P.; Winoto-Morbach, S.; Herzberg, M.; Uhlig, U.; Schutze, S.; Lucius, R.; Krause, M.F. IKK NBD peptide inhibits LPS induced pulmonary inflammation and alters sphingolipid metabolism in a murine model. Pulm. Pharmacol. Ther. 2012, 25, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.K.; Patra, M.C.; Shin, H.J.; Gui, X.; Achek, A.; Panneerselvam, S.; Kim, D.J.; Song, S.J.; Hong, R.; Kim, K.S.; et al. A cell-penetrating peptide blocks Toll-like receptor-mediated downstream signaling and ameliorates autoimmune and inflammatory diseases in mice. Exp. Mol. Med. 2019, 51, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, Y.; Song, L.; Kim, D.S.; Wu, H.; Yang, L.; Li, S.; Morgan, K.A.; Adams, D.B.; Wang, H. Cell-Permeable Peptide Blocks TLR4 Signaling and Improves Islet Allograft Survival. Cell Transplant. 2016, 25, 1319–1329. [Google Scholar] [CrossRef]
- Nilsen, K.E.; Zhang, B.; Skjesol, A.; Ryan, L.; Vagle, H.; Boe, M.H.; Orning, P.; Kim, H.; Bakke, S.S.; Elamurugan, K.; et al. Peptide derived from SLAMF1 prevents TLR4-mediated inflammation in vitro and in vivo. Life Sci. Alliance 2023, 6, e202302164. [Google Scholar] [CrossRef]
- McCusker, C.T.; Wang, Y.; Shan, J.; Kinyanjui, M.W.; Villeneuve, A.; Michael, H.; Fixman, E.D. Inhibition of experimental allergic airways disease by local application of a cell-penetrating dominant-negative STAT-6 peptide. J. Immunol. 2007, 179, 2556–2564. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Shan, J.; Fixman, E.; McCusker, C. Effective treatment of experimental ragweed-induced asthma with STAT-6-IP, a topically delivered cell-penetrating peptide. Clin. Exp. Allergy 2011, 41, 1622–1630. [Google Scholar] [CrossRef]
- Zahid, M.; Lu, X.; Mi, Z.; Robbins, P.D. Cationic and tissue-specific protein transduction domains identification, characterization, and therapeutic application. Adv. Genet. 2010, 69, 83–95. [Google Scholar] [CrossRef]
- Zahid, M.; Feldman, K.S.; Garcia-Borrero, G.; Feinstein, T.N.; Pogodzinski, N.; Xu, X.; Yurko, R.; Czachowski, M.; Wu, Y.L.; Mason, N.S.; et al. Cardiac Targeting Peptide, a Novel Cardiac Vector: Studies in Bio-Distribution, Imaging Application, and Mechanism of Transduction. Biomolecules 2018, 8, 147. [Google Scholar] [CrossRef]
- Sahagun, D.; Zahid, M. Cardiac-Targeting Peptide: From Discovery to Applications. Biomolecules 2023, 13, 1690. [Google Scholar] [CrossRef] [PubMed]
- Yurko, R.; Islam, K.; Weber, B.; Salama, G.; Zahid, M. Conjugation of amiodarone to a novel cardiomyocyte cell penetrating peptide for potential targeted delivery to the heart. Front. Chem. 2023, 11, 1220573. [Google Scholar] [CrossRef] [PubMed]
- Silver, E.; Argiro, A.; Hong, K.; Adler, E. Gene therapy vector-related myocarditis. Int. J. Cardiol. 2024, 398, 131617. [Google Scholar] [CrossRef]
- Morales, L.; Gambhir, Y.; Bennett, J.; Stedman, H.H. Broader Implications of Progressive Liver Dysfunction and Lethal Sepsis in Two Boys following Systemic High-Dose AAV. Mol. Ther. 2020, 28, 1753–1755. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).