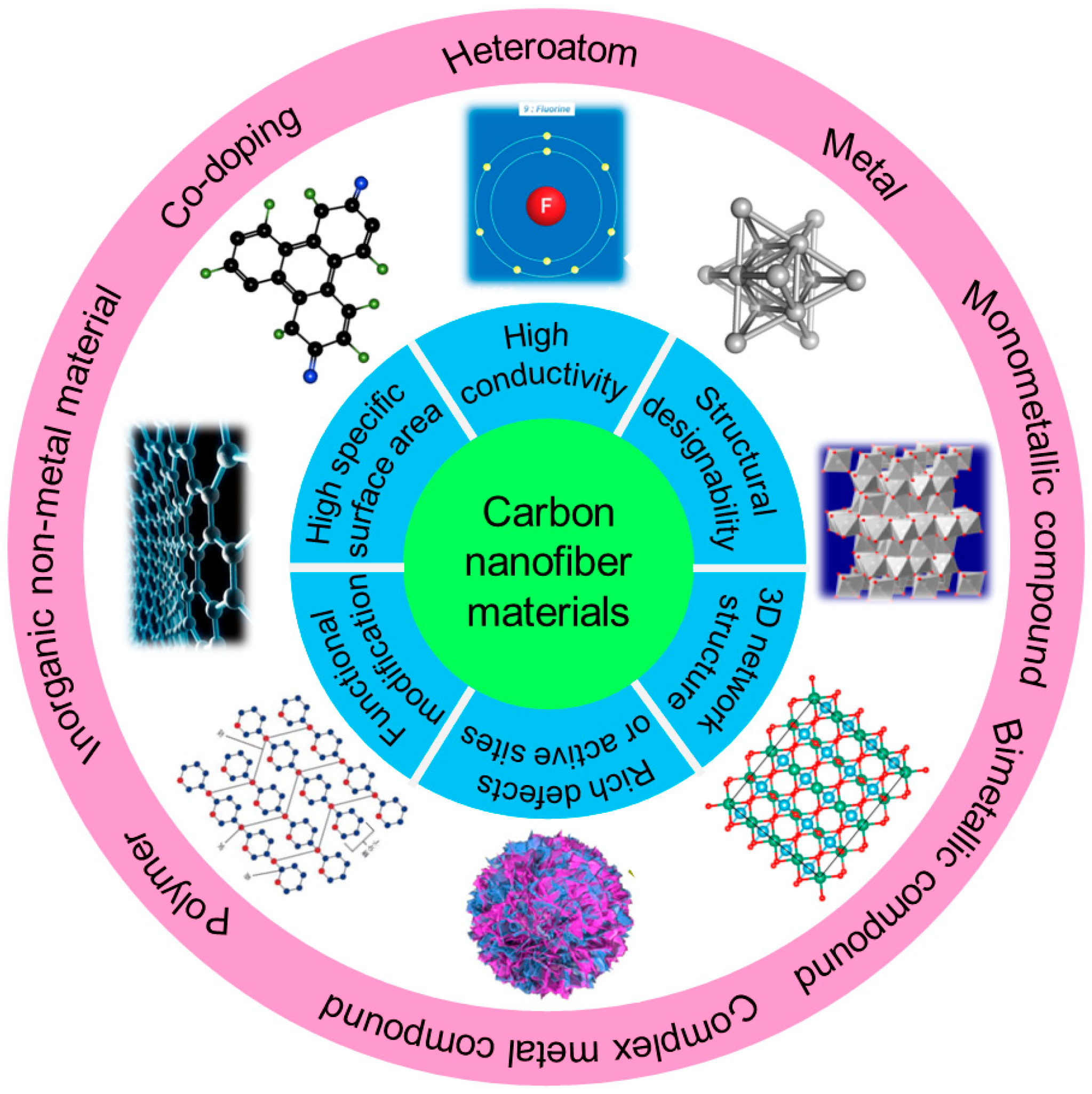
A Review of Carbon Nanofiber Materials for Dendrite-Free Lithium-Metal Anodes
Abstract
:1. Introduction
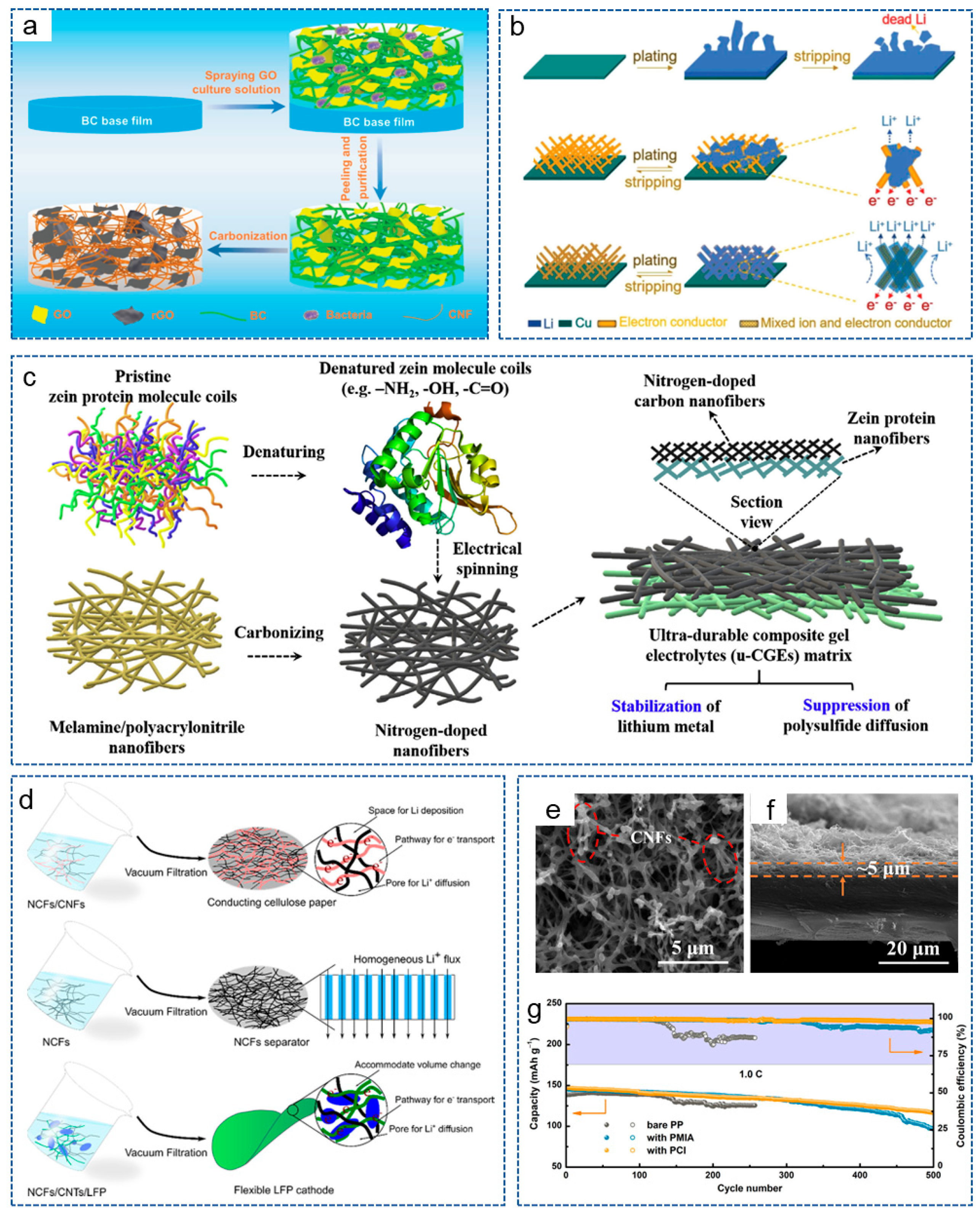
2. Preparation Technology for CNFs
2.1. CVD
2.2. Template Method
2.3. Electrospinning
3. CNFs for Dendrite-Free LMA
3.1. Neat CNFs
3.2. CNF-Based Composites
3.2.1. Heteroatom/CNF Composites
3.2.2. Metal/CNFs Composites
3.2.3. Metal Compound/CNFs Composites
- Monometallic Compound/CNF Composites
- Metal Oxide/CNFs Composites
- Metal Fluoride/CNF Composites
- Metal Sulfide/CNFs Composites
- Other Metal Compound/CNFs Composites
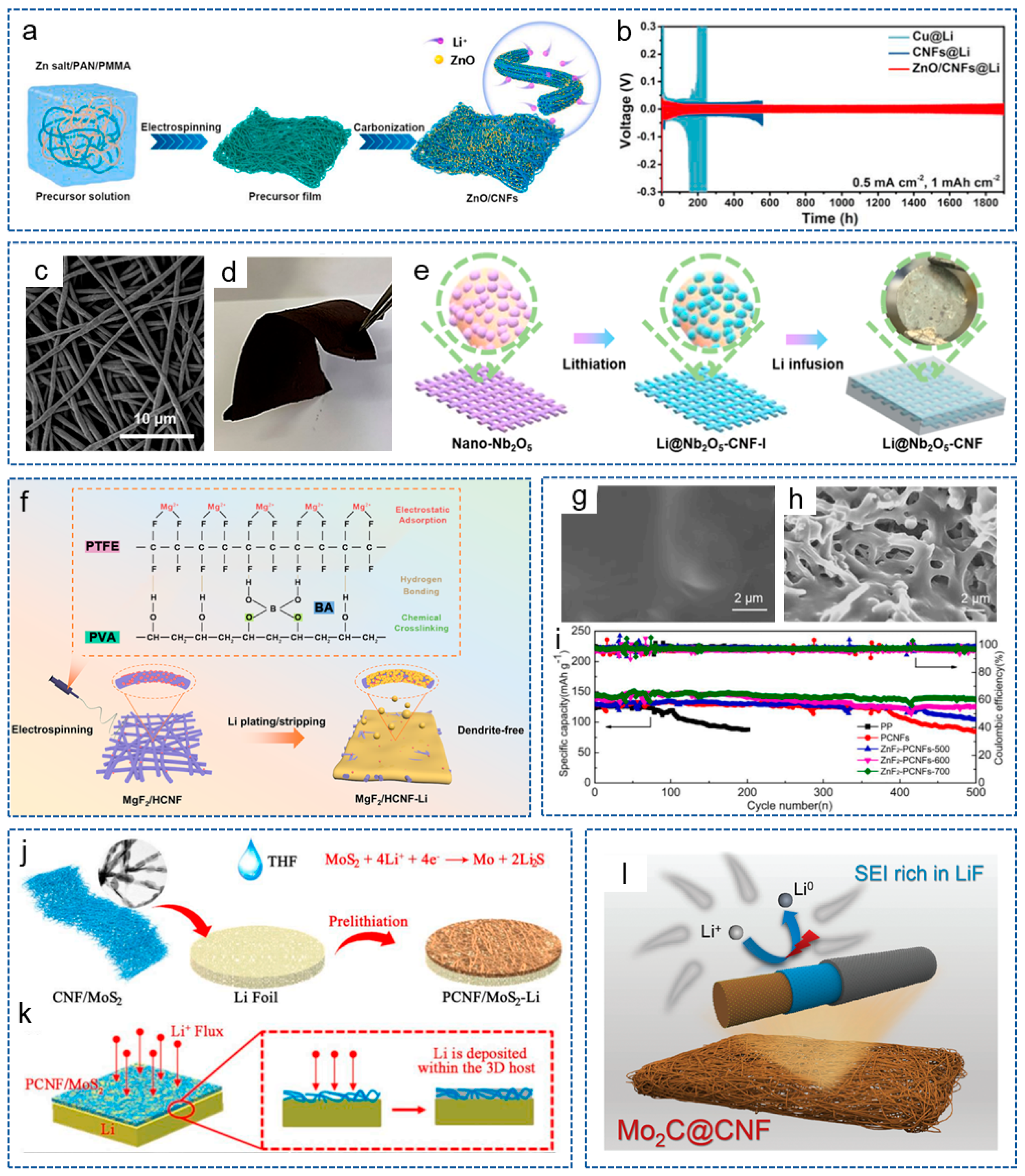
- Bimetallic Compound/CNFs Composites
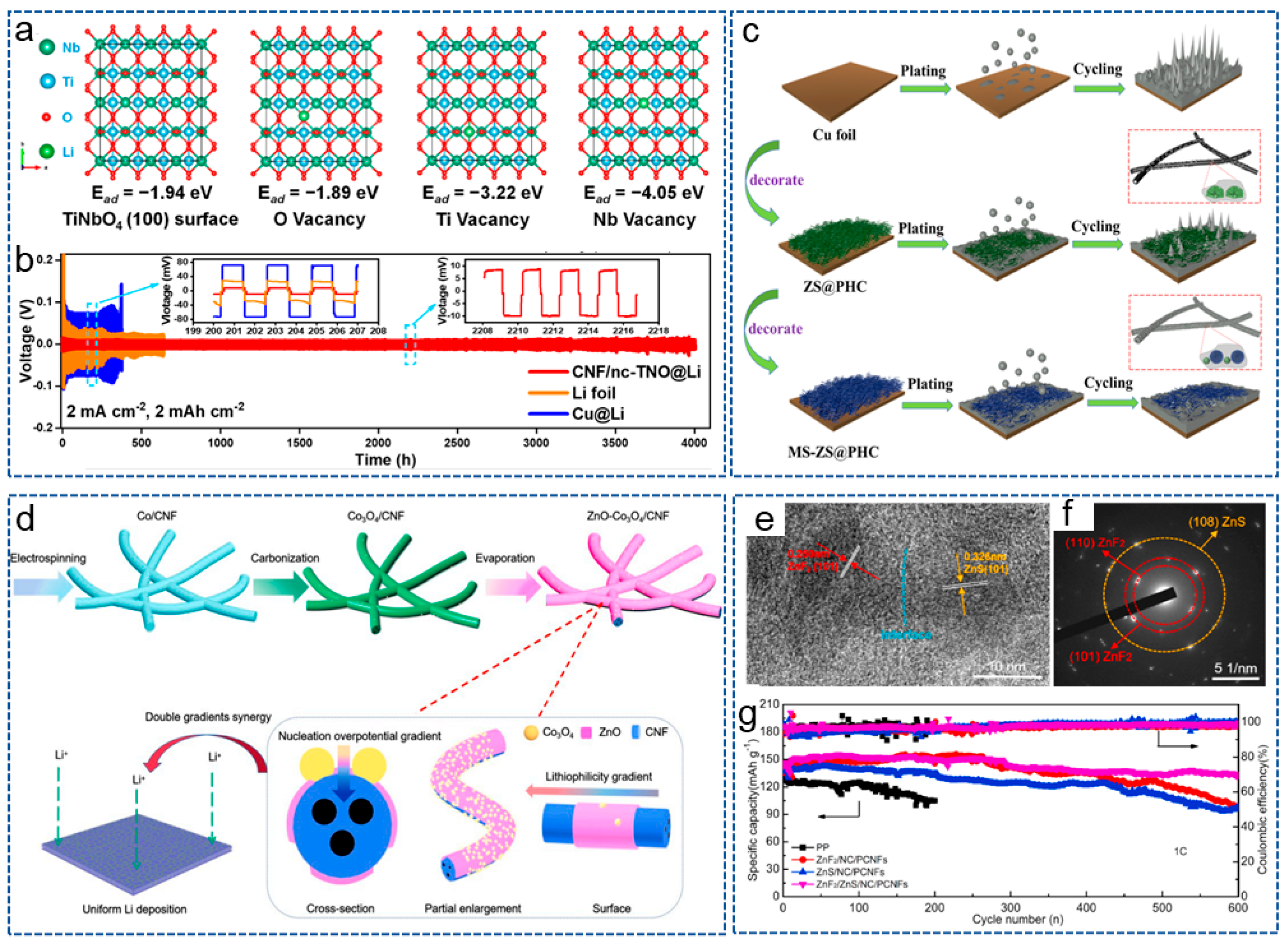
- Complex Metal Compound/CNFs Composites
3.2.4. Inorganic Non-Metal Material/CNFs Composites
3.2.5. Polymer/CNFs Composites
3.2.6. Co-Doping/CNFs Composites
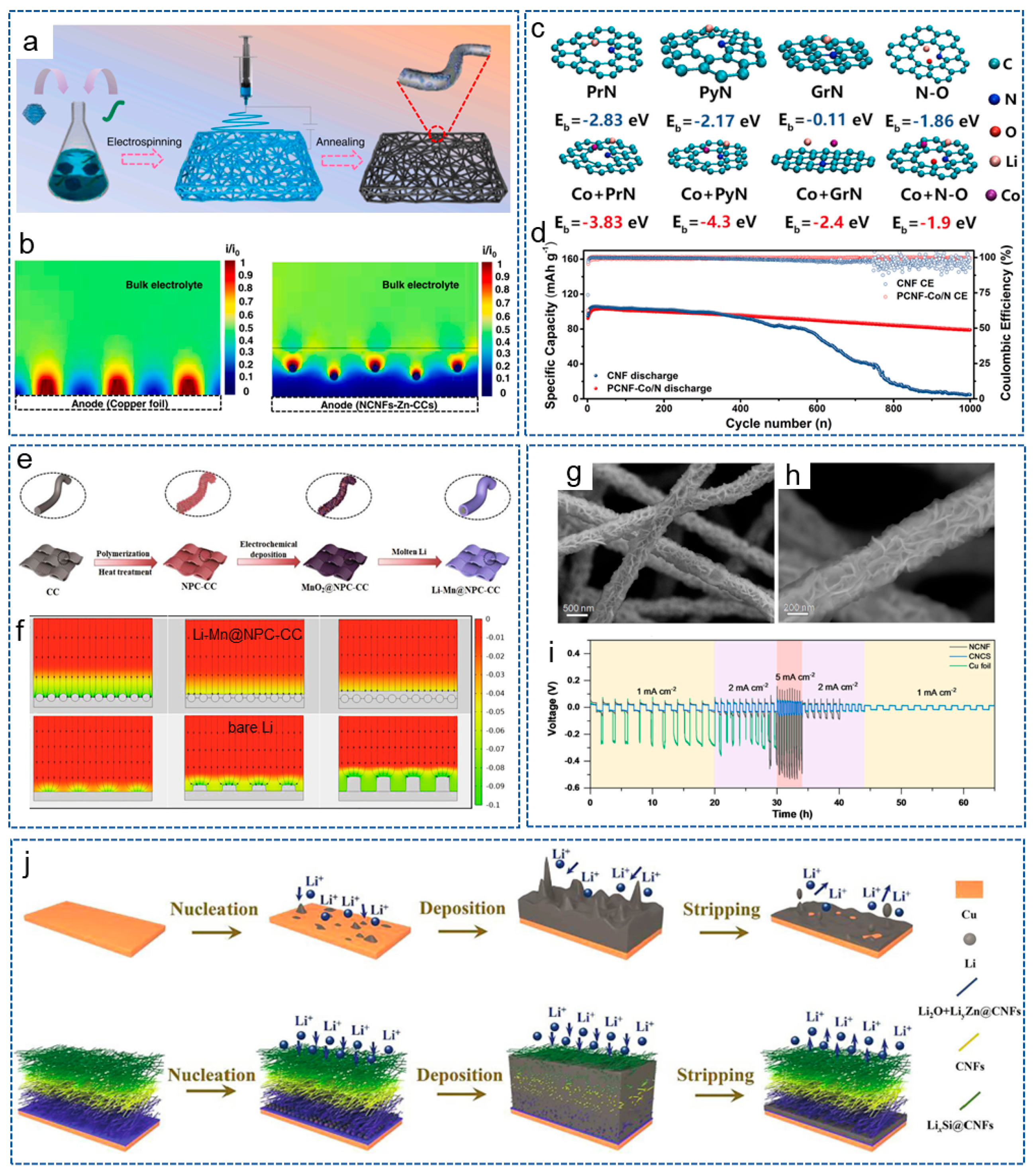
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lee, S.W.; Lin, D.; Shi, F.; Wang, S.; Sendek, A.D.; Cui, Y. Enhancing Ionic Conductivity in Composite Polymer Electrolytes with Well-Aligned Ceramic Nanowires. Nat. Energy 2017, 2, 17035. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Xin, L.; Liu, Y.; Yang, F.; Stach, E.A.; Xie, J. Making Li-Metal Electrodes Rechargeable by Controlling the Dendrite Growth Direction. Nat. Energy 2017, 2, 17083. [Google Scholar] [CrossRef]
- Xu, Z.L.; Liu, X.; Luo, Y.; Zhou, L.; Kim, J.K. Nanosilicon Anodes for High Performance Rechargeable Batteries. Prog. Mater. Sci. 2017, 90, 1–44. [Google Scholar] [CrossRef]
- Grande, L.; Paillard, E.; Hassoun, J.; Park, J.-B.; Lee, Y.-J.; Sun, Y.-K.; Passerini, S.; Scrosati, B. ChemInform Abstract: The Lithium/Air Battery: Still an Emerging System or a Practical Reality? Adv. Mater. 2015, 27, 784–800. [Google Scholar] [CrossRef]
- Gao, X.; Li, G.; Xu, Y.; Hong, Z.; Liang, C.; Lin, Z. TiO2 Microboxes with Controlled Internal Porosity for High-Performance Lithium Storage. Angew. Chem. Int. Ed. 2015, 54, 14331–14335. [Google Scholar] [CrossRef]
- Zhang, W.; Sayavong, P.; Xiao, X.; Oyakhire, S.T.; Shuchi, S.B.; Vilá, R.A.; Boyle, D.T.; Kim, S.C.; Kim, M.S.; Holmes, S.E.; et al. Recovery of Isolated Lithium through Discharged State Calendar Ageing. Nature 2024, 626, 306–312. [Google Scholar] [CrossRef]
- Lu, Y.; Tikekar, M.; Mohanty, R.; Hendrickson, K.; Ma, L.; Archer, L.A. Stable Cycling of Lithium Metal Batteries Using High Transference Number Electrolytes. Adv. Energy Mater. 2015, 5, 1402073. [Google Scholar] [CrossRef]
- Wang, R.; Luo, C.; Wang, T.; Zhou, G.; Deng, Y.; He, Y.; Zhang, Q.; Kang, F.; Lv, W.; Yang, Q.H. Bidirectional Catalysts for Liquid–Solid Redox Conversion in Lithium–Sulfur Batteries. Adv. Mater. 2020, 32, 2000315. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li-S Batteries with High Energy Storage. Nat. Mater. 2012, 11, 172. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Zou, J.; Gao, P.; Niu, X.; Li, H.; Chen, L. Li-Free Cathode Materials for High Energy Density Lithium Batteries. Joule 2019, 3, 2086–2102. [Google Scholar] [CrossRef]
- Cheng, X.B.; Hou, T.Z.; Zhang, R.; Peng, H.J.; Zhao, C.Z.; Huang, J.Q.; Zhang, Q. Dendrite-Free Lithium Deposition Induced by Uniformly Distributed Lithium Ions for Efficient Lithium Metal Batteries. Adv. Mater. 2016, 28, 2888–2895. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Sun, W.; Sun, K. Dendrite-Free Lithium Metal Anodes in High Performance Lithium-Sulfur Batteries with Bifunctional Carbon Nanofiber Interlayers. Electrochim. Acta 2017, 252, 127–137. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, J.; Yu, J.; Ding, B. Advances in Nanofibrous Materials for Stable Lithium-Metal Anodes. ACS Nano 2022, 16, 17891–17910. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Huang, S.; Yan, D.; Zhang, J.; Fang, D.; Lim, Y.V.; Wang, Y.; Li, T.C.; Li, Y.; Guo, L.; et al. Tuning Lithiophilicity and Stability of 3D Conductive Scaffold via Covalent Ag-S Bond for High-Performance Lithium Metal Anode. Energy Environ. Mater. 2023, 6, 2–9. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, X.; Zhou, Y.; Liu, S.; Fang, G.; Liang, S. Towards Establishing Uniform Metrics for Evaluating the Safety of Lithium Metal Batteries. Adv. Powder Mater. 2023, 2, 100139. [Google Scholar] [CrossRef]
- Ning, Z.; Li, G.; Melvin, D.L.R.; Chen, Y.; Bu, J.; Spencer-Jolly, D.; Liu, J.; Hu, B.; Gao, X.; Perera, J.; et al. Dendrite Initiation and Propagation in Lithium Metal Solid-State Batteries. Nature 2023, 618, 287–293. [Google Scholar] [CrossRef]
- Sleight, A.W. Historical Development of Secondary Lithium Batteries. Mater. Res. Bull. 1995, 30, 1589. [Google Scholar]
- Pokharel, J.; Cresce, A.; Pant, B.; Yang, M.Y.; Gurung, A.; He, W.; Baniya, A.; Lamsal, B.S.; Yang, Z.; Gent, S.; et al. Manipulating the Diffusion Energy Barrier at the Lithium Metal Electrolyte Interface for Dendrite-Free Long-Life Batteries. Nat. Commun. 2024, 15, 3085. [Google Scholar] [CrossRef]
- Deng, N.; Liu, Y.; Li, Q.; Yan, J.; Lei, W.; Wang, G.; Wang, L.; Liang, Y.; Kang, W.; Cheng, B. Functional Mechanism Analysis and Customized Structure Design of Interlayers for High Performance Li-S Battery. Energy Storage Mater. 2019, 23, 314–349. [Google Scholar] [CrossRef]
- Fan, L.; Zhuang, H.L.; Gao, L.; Lu, Y.; Archer, L.A. Regulating Li Deposition at Artificial Solid Electrolyte Interphases. J. Mater. Chem. A 2017, 5, 3483–3492. [Google Scholar] [CrossRef]
- Liu, W.; Lin, D.; Pei, A.; Cui, Y. Stabilizing Lithium Metal Anodes by Uniform Li-Ion Flux Distribution in Nanochannel Confinement. J. Am. Chem. Soc. 2016, 138, 15443–15450. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.C.; Park, K.; Jang, J.H.; Goodenough, J.B. Cellulose-Based Porous Membrane for Suppressing Li Dendrite Formation in Lithium-Sulfur Battery. ACS Energy Lett. 2016, 1, 633–637. [Google Scholar] [CrossRef]
- Deng, Y.; Gao, J.; Wang, M.; Luo, C.; Zhou, C.; Wu, M. Homogenizing the Li-Ion Flux by Multi-Element Alloying Modified for 3D Dendrite-Free Lithium Anode. Energy Storage Mater. 2022, 48, 114–122. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, X.; Ye, Y.; Lee, H.K.; Zhang, P.; Cui, A.; Xiao, X.; Yang, Y.; Cui, Y. Lithiophilic Hydrogen-Substituted Graphdiyne Aerogels with Ionically Conductive Channels for High-Performance Lithium Metal Batteries. Nano Lett. 2024, 24, 3044–3050. [Google Scholar] [CrossRef]
- Wei, L.; Deng, N.; Ju, J.; Kang, J.; Wang, X.; Ding, L.; Kang, W.; Cheng, B. A Review on Nanofiber Materials for Lithium-Metal Batteries to Suppress the Dendritic Lithium Growth. Chem. Eng. J. 2022, 433, 134392. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the Lithium Metal Anode for High-Energy Batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Yuan, Y.; Pu, S.D.; Pérez-Osorio, M.A.; Li, Z.; Zhang, S.; Yang, S.; Liu, B.; Gong, C.; Menon, A.S.; Piper, L.F.J.; et al. Diagnosing the Electrostatic Shielding Mechanism for Dendrite Suppression in Aqueous Zinc Batteries. Adv. Mater. 2024, 36, 2307708. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, J.; Xu, W.; Russell, S.M.; Chen, X.; Nasybulin, E.; Bhattacharya, P.; Engelhard, M.H.; Mei, D.; Cao, R.; et al. Dendrite-Free Lithium Deposition with Self-Aligned Nanorod Structure. Nano Lett. 2014, 14, 6889–6896. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.; Shao, Y.; Engelhard, M.H.; Nie, Z.; Xiao, J.; et al. Dendrite-Free Lithium Deposition via Self-Healing Electrostatic Shield Mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef]
- Su, D.S.; Schlögl, R. Nanostructured Carbon and Carbon Nanocomposites for Electrochemical Energy Storage Applications. ChemSusChem 2010, 3, 136–168. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, T.; Xiao, H.; Liang, X.; Zhang, F.; Deng, J.; Gao, Q. 3D Free-Standing Carbon Nanofibers Modified by Lithiophilic Metals Enabling Dendrite-Free Anodes for Li Metal Batteries. Energy Environ. Mater. 2023, 6, e12470. [Google Scholar] [CrossRef]
- Wu, J.; Ihsan-Ul-Haq, M.; Ciucci, F.; Huang, B.; Kim, J.K. Rationally Designed Nanostructured Metal Chalcogenides for Advanced Sodium-Ion Batteries. Energy Storage Mater. 2021, 34, 582–628. [Google Scholar] [CrossRef]
- Thavasi, V.; Singh, G.; Ramakrishna, S. Electrospun Nanofibers in Energy and Environmental Applications. Energy Environ. Sci. 2008, 1, 205–222. [Google Scholar] [CrossRef]
- Luo, C.J.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus Fibre Production Methods: From Specifics to Technological Convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiao, Y.; Liao, M.; Wang, B.; Peng, H. Carbon Nanomaterials for Flexible Lithium Ion Batteries. Carbon 2017, 124, 79–88. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, F.; Tarascon, J.M.; Kim, J.K. Recent Advances in Electrospun Carbon Nanofibers and Their Application in Electrochemical Energy Storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Chen, L.F.; Feng, Y.; Liang, H.W.; Wu, Z.Y.; Yu, S.H. Macroscopic-Scale Three-Dimensional Carbon Nanofiber Architectures for Electrochemical Energy Storage Devices. Adv. Energy Mater. 2017, 7, 1700826. [Google Scholar] [CrossRef]
- Weng, W.; Kurihara, R.; Wang, J.; Shiratori, S. Electrospun Carbon Nanofiber-Based Composites for Lithium-Ion Batteries: Structure Optimization towards High Performance. Compos. Commun. 2019, 15, 135–148. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Y.; Su, W.; Fu, W.; Wang, C.H. Carbon Deposition Behavior on Biochar during Chemical Vapor Deposition Process. Chem. Eng. J. 2024, 485, 149726. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Huang, Z.; Oh, S.; Yu, Y.; Mai, Y.W.; Kim, J.K. Urchin-like Li4Ti5O12-Carbon Nanofiber Composites for High Rate Performance Anodes in Li-Ion Batteries. J. Mater. Chem. 2012, 22, 12133–12140. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow Carbon Nanofiber-Encapsulated Sulfur Cathodes for High Specific Capacity Rechargeable Lithium Batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, M.; Wei, X.; Sun, J.; Xu, D. Recent Advances in Morphology, Aperture Control, Functional Control and Electrochemical Sensors Applications of Carbon Nanofibers. Anal. Biochem. 2022, 656, 114882. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, S.; Chen, S. Recent Advances in Electrospinning Nanofiber Materials for Aqueous Zinc Ion Batteries. Chem. Sci. 2023, 14, 13346–13366. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Xu, M.; Ren, S.; Li, W.; Wang, C.; Gao, M.; Lu, X. Modulating the Electronic Structure of Ni(OH)2 by Coupling with Low-Content Pt for Boosting the Urea Oxidation Reaction Enables Significantly Promoted Energy-Saving Hydrogen Production. Energy Environ. Sci. 2024, 17, 1984–1996. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre Diameter Fibres of Polymer, Produced by Electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, H.; Wu, J.; Chu, X.; He, Y.B.; Han, C.; Miao, C.; Wang, S.; Li, B.; Kang, F. Fe3O4 Nanoparticles Encapsulated in Electrospun Porous Carbon Fibers with a Compact Shell as High-Performance Anode for Lithium Ion Batteries. Carbon 2016, 87, 347–356. [Google Scholar] [CrossRef]
- Xu, Z.L.; Zhang, B.; Kim, J.K. Electrospun Carbon Nanofiber Anodes Containing Monodispersed Si Nanoparticles and Graphene Oxide with Exceptional High Rate Capacities. Nano Energy 2014, 6, 27–35. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, W.J. Hierarchically Mesoporous Flower-like Cobalt Oxide/Carbon Nanofiber Composites with Shell-Core Structure as Anodes for Lithium Ion Batteries. Carbon 2015, 89, 197–207. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Wu, Q.; Zhang, Q.; Chen, H.; Jing, H.; Wang, J.; Mi, S.B.; Rogach, A.L.; Niu, C. Encapsulating Silica/Antimony into Porous Electrospun Carbon Nanofibers with Robust Structure Stability for High-Efficiency Lithium Storage. ACS Nano 2018, 12, 3406–3416. [Google Scholar] [CrossRef]
- Liu, M.; Deng, N.; Ju, J.; Fan, L.; Wang, L.; Li, Z.; Zhao, H.; Yang, G.; Kang, W.; Yan, J.; et al. A Review: Electrospun Nanofiber Materials for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1905467. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Adair, K.R.; Sun, X.; Yu, Y. Carbon Nanofiber-Based Nanostructures for Lithium-Ion and Sodium-Ion Batteries. J. Mater. Chem. A 2017, 5, 13882–13906. [Google Scholar] [CrossRef]
- Li, L.; Peng, S.; Lee, J.K.Y.; Ji, D.; Srinivasan, M.; Ramakrishna, S. Electrospun Hollow Nanofibers for Advanced Secondary Batteries. Nano Energy 2017, 39, 111–139. [Google Scholar] [CrossRef]
- Qanati, M.V.; Rasooli, A.; Rezvani, M. Main Structural and Mechanical Properties of Electrospun PAN-Based Carbon Nanofibers as a Function of Carbonization Maximum Temperature. Polym. Bull. 2022, 79, 331–355. [Google Scholar] [CrossRef]
- Agostini, M.; Hwang, J.Y.; Kim, H.M.; Bruni, P.; Brutti, S.; Croce, F.; Matic, A.; Sun, Y.K. Minimizing the Electrolyte Volume in Li–S Batteries: A Step Forward to High Gravimetric Energy Density. Adv. Energy Mater. 2018, 8, 1801560. [Google Scholar] [CrossRef]
- Jin, M.; Sun, G.; Wang, Y.; Yuan, J.; Zhao, H.; Wang, G.; Zhou, J.; Xie, E.; Pan, X. Boosting Charge Transport and Catalytic Performance in MoS2 by Zn2+ Intercalation Engineering for Lithium-Sulfur Batteries. ACS Nano 2024, 18, 2017–2029. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Silva, S.R.P.; Ding, B.; Zhang, P.; Shao, G. Lithium–Sulfur Batteries Meet Electrospinning: Recent Advances and the Key Parameters for High Gravimetric and Volume Energy Density. Adv. Sci. 2022, 9, 2103879. [Google Scholar] [CrossRef]
- Wei, L.; Deng, N.; Zhao, H.; Ju, J.; Wang, G.; Wang, X.; Kang, J.; Kang, W.; Cheng, B. ZnF2/ZnS Heterostructures@NC Doped Porous Carbon Nanofibers as Interlayers for Stable Lithium Metal Anodes. Compos. Part B Eng. 2022, 230, 109531. [Google Scholar] [CrossRef]
- Liu, M.; Deng, N.; Ju, J.; Wang, L.; Wang, G.; Ma, Y.; Kang, W.; Yan, J. Silver Nanoparticle-Doped 3D Porous Carbon Nanofibers as Separator Coating for Stable Lithium Metal Anodes. ACS Appl. Mater. Interfaces 2019, 11, 17843–17852. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, M.; Wang, T.; Wu, H.; Zhang, Y. A 3D Hierarchical Host with Gradient-Distributed Dielectric Properties toward Dendrite-Free Lithium Metal Anode. Angew. Chem. Int. Ed. 2024, 63, e202403399. [Google Scholar] [CrossRef]
- Gu, Y.; Cui, R.C.; Wang, G.Y.; Yang, C.C.; Jiang, Q. Sb/N-Doped Carbon Nanofiber as a Sodium-Ion Battery Anode. Energy Technol. 2022, 10, 2200746. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Z.; Wang, C.C.; Yang, C.C.; Jiang, Q. MOF-Derived Fe7S8 Nanoparticles/N-Doped Carbon Nanofibers as an Ultra-Stable Anode for Sodium-Ion Batteries. Small 2021, 17, 2102349. [Google Scholar] [CrossRef]
- He, B.; Yin, K.; Gong, W.; Xiong, Y.; Zhang, Q.; Yang, J.; Wang, Z.; Wang, Z.; Chen, M.; Man, P.; et al. NaTi2(PO4)3 Hollow Nanoparticles Encapsulated in Carbon Nanofibers as Novel Anodes for Flexible Aqueous Rechargeable Sodium-Ion Batteries. Nano Energy 2021, 82, 105764. [Google Scholar] [CrossRef]
- Gao, T.; Le, T.H.; Yang, Y.; Yu, Z.; Huang, Z.; Kang, F. Effects of Electrospun Carbon Nanofibers Interlayers on High-Performance Lithium-Sulfur Batteries. Materials 2017, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Qpufoujbm, D.; Wt, P.G.; Izesphfo, T.; Up, D.; Uif, T.; Pg, H.; Boe, E.; Uif, S.; Boe, Q.; Hsbqifof, S.; et al. Intrinsic Lithiophilic Carbon Host Derived from Bacterial Cellulosenanofiber for Dendrite-Free and Long-Life Lithium Metal Anode. Nano Res. 2024, 17, 4203–4210. [Google Scholar]
- Rajendran, S.; Sekar, A.; Li, J. Deciphering the Effect of Nucleation/Growth Kinetics on the Morphology of Li Plating/Stripping on Vertically Aligned Carbon Nanofibers—A Low-Tortuosity 3-D Nanostructured Carbon Host. Chem. Eng. J. 2024, 484, 149515. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.-R.; Chen, X.; Cheng, X.-B.; Zhang, X.-Q.; Yan, C.; Zhang, Q. Lithiophilic Sites in Doped Graphene Guide Uniform Lithium Nucleation for Dendrite-Free Lithium Metal Anodes. Angew. Chem. 2017, 129, 7872–7876. [Google Scholar] [CrossRef]
- Guo, F.; Wang, Y.; Kang, T.; Liu, C.; Shen, Y.; Lu, W.; Wu, X.; Chen, L. A Li-Dual Carbon Composite as Stable Anode Material for Li Batteries. Energy Storage Mater. 2018, 15, 116–123. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Q. Three-Dimensional Matrix for Lithium Metal Anode for next-Generation Rechargeable Batteries: Structure Design and Interface Engineering. J. Energy Chem. 2019, 33, 167–168. [Google Scholar] [CrossRef]
- Chen, Y.; Elangovan, A.; Zeng, D.; Zhang, Y.; Ke, H.; Li, J.; Sun, Y.; Cheng, H. Vertically Aligned Carbon Nanofibers on Cu Foil as a 3D Current Collector for Reversible Li Plating/Stripping toward High-Performance Li–S Batteries. Adv. Funct. Mater. 2020, 30, 1906444. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, D.; Tang, Z.; Xie, C.; Yang, J.; Tang, J.; Zhou, X.; Tang, Y.; Wang, H. Scalable Slurry-Coating Induced Integrated 3D Lithiophilic Architecture for Stable Lithium Metal Anodes. J. Power Sources 2021, 485, 229334. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zong, W.; Wang, J.; Xu, Z.; Mo, L.; Lai, F.; Xu, Z.L.; Miao, Y.E.; Liu, T. Multi-Scale Uniform Li Regulation Triggered by Tunable Electric Field Distribution on Oxygen-Functionalized Porous Framework for Flexible Li-S Full Batteries. Energy Storage Mater. 2021, 42, 68–77. [Google Scholar] [CrossRef]
- Yang, C.; Yao, Y.; He, S.; Xie, H.; Hitz, E.; Hu, L. Ultrafine Silver Nanoparticles for Seeded Lithium Deposition toward Stable Lithium Metal Anode. Adv. Mater. 2017, 29, 1702714. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Yang, H.; Jia, P.; Zhao, C.; Wang, L.; Liu, T. Steady Cycling of Lithium Metal Anode Enabled by Alloying Sn-Modified Carbon Nanofibers. J. Mater. Chem. A 2023, 11, 15237–15245. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, W.; Chen, X.; Liu, X. Bifunctional 3D Hierarchical Hairy Foam toward Ultrastable Lithium/Sulfur Electrochemistry. Adv. Funct. Mater. 2020, 30, 2004650. [Google Scholar] [CrossRef]
- Huang, T.; Sun, Y.; Wu, J.; Jin, J.; Wei, C.; Shi, Z.; Wang, M.; Cai, J.; An, X.T.; Wang, P.; et al. A Dual-Functional Fibrous Skeleton Implanted with Single-Atomic Co-NxDispersions for Longevous Li-S Full Batteries. ACS Nano 2021, 15, 14105–14115. [Google Scholar] [CrossRef]
- Kuna, J.J.; Voïtchovsky, K.; Singh, C.; Jiang, H.; Mwenifumbo, S.; Ghorai, P.K.; Stevens, M.M.; Glotzer, S.C.; Stellacci, F. The Effect of Nanometre-Scale Structure on Interfacial Energy. Nat. Mater. 2009, 8, 837–842. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Li, C.; Li, X.; Wu, Y.; Chen, X.; Wu, J.; Li, X.; Chen, Y. Electrospun Carbon Nanofibers for Lithium Metal Anodes: Progress and Perspectives. Chin. Chem. Lett. 2022, 33, 141–152. [Google Scholar] [CrossRef]
- Lai, Y.; Yang, T.; Yang, Y.; Li, W.; Xie, Y.; Cheng, S.; Qiao, L.; Wang, X. A Lithiophilic and Conductive Interlayer for Dendrite-Free Lithium Metal Anodes. Chem. Eng. J. 2023, 462, 142223. [Google Scholar] [CrossRef]
- Wang, Z.F.; Wang, H.Y.; Liu, X.L.; Chen, Y.X.; Zhao, Y.; Zhang, Y.G.; Han, Q.Q.; Qin, C.L.; Bakenov, Z.; Wang, Y.C.; et al. Single Zn Atoms Anchored on Hollow Carbon Nanofiber Network for Dendrite-Free Lithium Metal Anode of Flexible Li–S Full Cell. Rare Met. 2023, 42, 3705–3717. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Z.; Lee, H.; Xiong, F.; Hsu, P.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective Deposition and Stable Encapsulation of Lithium through Heterogeneous Seeded Growth. Nat. Energy 2016, 3, 16010. [Google Scholar] [CrossRef]
- Liu, T.; Hu, J.; Li, C.; Wang, Y. Unusual Conformal Li Plating on Alloyable Nanofiber Frameworks to Enable Dendrite Suppression of Li Metal Anode. ACS Appl. Energy Mater. 2019, 2, 4379–4388. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, J.; Lv, X.; Li, C.; Liu, X.; Yang, S.; Liu, Y. Nitrogen-Doped Porous Carbon Nanofiber Decorated with FeNi Alloy for Dendrite-Free High-Performance Lithium Metal Anode. J. Alloys Compd. 2022, 925, 166691. [Google Scholar] [CrossRef]
- Cheng, X.; Ban, J.; Wang, Q.; Xu, H.; Shao, G.; Hu, J.; Cao, G. “Mechanical–Electrochemical” Coupling Structure and the Application as a Three-Dimensional Current Collector for Lithium Metal Anode. Appl. Surf. Sci. 2021, 563, 150247. [Google Scholar] [CrossRef]
- Zhao, C.; Yao, X.; Yang, H.; Jiao, X.; Wang, L. Hierarchical Porous Carbon Nanofibers with Lithiophilic Metal Oxide Crystalline Grains for Long-Life Li Metal Anodes. Compos. Commun. 2021, 26, 100789. [Google Scholar] [CrossRef]
- Shao, S.; Liu, H.; Wang, C.; Li, D.; Zhao, X.; Fan, L.Z. Copper Oxide Decorated Carbon Nanofiber Skeleton for Dendrite-Free Lithium-Metal Anodes. Batter. Supercaps 2023, 6, 6–13. [Google Scholar] [CrossRef]
- Yi, S.; Hong, D.; Su, Z.; Tian, L.; Zhang, W.; Chen, M.; Hu, M.; Niu, B.; Zhang, Y.; Long, D. In Situ Formed Lithiophilic LixNbyO in a Carbon Nanofiber Network for Dendrite-Free Li-Metal Anodes. ACS Appl. Mater. Interfaces 2021, 13, 56498–56509. [Google Scholar] [CrossRef]
- Chen, A.L.; Gao, M.; Mo, L.; Wang, J.; Xu, Z.; Miao, Y.E.; Liu, T. Homogeneous Electric Field and Li+ Flux Regulation in Three-Dimensional Nanofibrous Composite Framework for Ultra-Long-Life Lithium Metal Anode. J. Colloid Interface Sci. 2022, 614, 138–146. [Google Scholar] [CrossRef]
- Qu, Q.; Guo, J.; Wang, H.; Zhang, K.; Li, J. Carbon Nanofibers Implanted Porous Catalytic Metal Oxide Design as Efficient Bifunctional Electrode Host Material for Lithium-Sulfur Battery. Electrochim. Acta 2024, 473, 143454. [Google Scholar] [CrossRef]
- Xie, J.; Liao, L.; Gong, Y.; Li, Y.; Shi, F.; Pei, A.; Sun, J.; Zhang, R.; Kong, B.; Subbaraman, R.; et al. Stitching H-BN by Atomic Layer Deposition of LiF as a Stable Interface for Lithium Metal Anode. Sci. Adv. 2017, 3, 3170. [Google Scholar] [CrossRef]
- Shang, Y.; Chu, T.; Shi, B.; Fu, K. Scalable Synthesis of LiF-Rich 3D Architected Li Metal Anode via Direct Lithium-Fluoropolymer Pyrolysis to Enable Fast Li Cycling. Energy Environ. Mater. 2021, 4, 213–221. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, X.; Liu, Y.; Li, S.; Guan, W.; Du, Z.; Ai, W. Stabilizing Lithium-Metal Host Anodes by Covalently Binding MgF2 Nanodots to Honeycomb Carbon Nanofibers. ACS Appl. Mater. Interfaces 2024, 16, 4530–4539. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, H.; Naveed, A.; Nuli, Y.; Yang, J.; Cao, Y.; Yang, H.; Wang, J. AlF3-Modified Carbon Nanofibers as a Multifunctional 3D Interlayer for Stable Lithium Metal Anodes. Chem. Commun. 2018, 54, 8347–8350. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Deng, N.; Ju, J.; Zhao, H.; Wang, G.; Xiang, H.; Kang, W.; Cheng, B. ZnF2 Doped Porous Carbon Nanofibers as Separator Coating for Stable Lithium-Metal Batteries. Chem. Eng. J. 2021, 424, 130346. [Google Scholar] [CrossRef]
- Deng, N.; Peng, Z.; Tian, X.; Li, Y.; Yan, J.; Liu, Y.; Kang, W. Yttrium Trifluoride Doped Polyacrylonitrile Based Carbon Nanofibers as Separator Coating Layer for High Performance Lithium-Metal Batteries. J. Colloid Interface Sci. 2023, 634, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Deng, N.; Wang, X.; Zhao, H.; Yan, J.; Yang, Q.; Kang, W.; Cheng, B. Flexible Ordered MnS@CNC/Carbon Nanofibers Membrane Based on Microfluidic Spinning Technique as Interlayer for Stable Lithium-Metal Battery. J. Membr. Sci. 2021, 637, 119615. [Google Scholar] [CrossRef]
- Yu, L.; Su, Q.; Li, B.; Huang, L.; Du, G.; Ding, S.; Zhao, W.; Zhang, M.; Xu, B. Pre-Lithiated Edge-Enriched MoS2 Nanoplates Embedded into Carbon Nanofibers as Protective Layers to Stabilize Li Metal Anodes. Chem. Eng. J. 2022, 429, 132479. [Google Scholar] [CrossRef]
- Fu, C.; Xu, W.; Tao, C.; Wang, L.; Liu, T. Lithiophilic Nickel Phosphide Modifying Carbon Nanofibers for a Highly Reversible Lithium-Metal Anode. ACS Appl. Energy Mater. 2022, 5, 4733–4742. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Li, P.; Ayranci, C.; Li, G. High Mass Loading of Edge-Exposed Cu3P Nanocrystal in 3D Freestanding Matrix Regulating Lithiophilic Sites for High-Performance Lithium Metal Anode. ACS Appl. Mater. Interfaces 2023, 15, 29352–29362. [Google Scholar] [CrossRef]
- Huang, A.; Wu, Y.; Huang, H.; Li, C.; Sun, Y.; Li, L.; Peng, S. Lithiophilic Mo2C Clusters-Embedded Carbon Nanofibers for High Energy Density Lithium Metal Batteries. Adv. Funct. Mater. 2023, 33, 2303111. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Shao, Y.; Zhang, L.; Li, Y.; Wu, Y.; Hao, X. Transition-Metal Oxynitride: A Facile Strategy for Improving Electrochemical Capacitor Storage. Adv. Mater. 2019, 31, 1806088. [Google Scholar] [CrossRef]
- Yao, Y.; Feng, Q.; Zhu, S.; Li, J.; Yao, Y.; Wang, Y.; Wang, Q.; Gu, M.; Wang, H.; Li, H.; et al. Chromium Oxynitride Electrocatalysts for Electrochemical Synthesis of Ammonia under Ambient Conditions. Small Methods 2019, 3, 1800324. [Google Scholar] [CrossRef]
- Xiao, J.; Xiao, N.; Liu, C.; Li, H.; Pan, X.; Zhang, X.; Bai, J.; Guo, Z.; Ma, X.; Qiu, J. In Situ Growing Chromium Oxynitride Nanoparticles on Carbon Nanofibers to Stabilize Lithium Deposition for Lithium Metal Anodes. Small 2020, 16, 2003827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Ding, Z.; Zhang, L.; Deng, L.; Yao, L.; Yang, H.Y. Cationic Defect-Modulated Li-Ion Migration in High-Voltage Li-Metal Batteries. ACS Nano 2023, 17, 25519–25531. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Liang, Q.; Chen, M.; Yang, C.; Yu, Z.; Cheng, J.; Kang, F.; Yang, Y. A Triple-Gradient Host for Long Cycling Lithium Metal Anodes at Ultrahigh Current Density. Small 2020, 16, e2001992. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Song, X.; Li, X.; Yan, C.; Zou, J.; Wu, H.; Zhang, Q.; Zeng, X. Facile Synthesis of Hierarchical MoS2/ZnS@Porous Hollow Carbon Nanofibers for a Stable Li Metal Anode. J. Colloid Interface Sci. 2022, 622, 347–356. [Google Scholar] [CrossRef]
- Yang, X.; Cao, S.; He, Z.; Xu, C.; Wang, H.; Liu, G. Lithiophilic Metal Oxide-Based Double Gradient Anode Host Design for Lithium-Sulfur Batteries. Electrochim. Acta 2023, 469, 143259. [Google Scholar] [CrossRef]
- Hong, X.; Kim, J.; Shi, S.; Zhang, Y.; Jin, C.; Sun, Y.; Tongay, S.; Wu, J.; Zhang, Y.; Wang, F. Ultrafast Charge Transfer in Atomically Thin MoS2/WS2 Heterostructures. Nat. Nanotechnol. 2014, 9, 682–686. [Google Scholar] [CrossRef]
- Fang, Y.; Lv, Y.; Gong, F.; Elzatahry, A.A.; Zheng, G. Synthesis of 2D-Mesoporous-Carbon/MoS2 Heterostructures with Well-Defined Interfaces for High-Performance Lithium-Ion Batteries. Adv. Mater. 2016, 28, 9385–9390. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, X. Heterostructured Materials. Prog. Mater. Sci. 2023, 131, 101019. [Google Scholar] [CrossRef]
- Song, X.; Zeng, X.; Zou, J.; Zhao, F.; Wu, H. Carbon Nanotubes Loaded with Carbon Nanofibers as Scaffold for Li Metal Battery Anodes. J. Alloys Compd. 2021, 854, 157122. [Google Scholar] [CrossRef]
- Shi, H.; Qin, J.; Huang, K.; Lu, P.; Zhang, C.; Dong, Y.; Ye, M.; Liu, Z.; Wu, Z. A Two-Dimensional Mesoporous Polypyrrole–Graphene Oxide Heterostructure as a Dual-Functional Ion Redistributor for Dendrite-Free Lithium Metal Anodes. Angew. Chem. Int. Ed. 2020, 132, 12245–12251. [Google Scholar] [CrossRef]
- Hu, Z.; Su, H.; Zhou, M.; Liu, J.; Wan, Y.; Hu, J.; Xu, Y. Lithiophilic Carbon Nanofiber/Graphene Nanosheet Composite Scaffold Prepared by a Scalable and Controllable Biofabrication Method for Ultrastable Dendrite-Free Lithium-Metal Anodes. Small 2022, 18, 2104735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, S.; Li, G.; Zhang, C.; Liu, X.; Luo, J. Incorporating Ionic Paths into 3D Conducting Scaffolds for High Volumetric and Areal Capacity,High Rate Lithium-Metal Anodes. Adv. Mater. 2018, 30, 1801328. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Huang, L.; Guo, Y.; Lan, J.-L.; Yu, Y.; Fu, X.; Zhong, W.H.; Yang, X. An Ultra-Durable Gel Electrolyte Stabilizing Ion Deposition and Trapping Polysulfides for Lithium-Sulfur Batteries. Energy Storage Mater. 2020, 27, 25–34. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, R.; Sun, R.; Edström, K.; Strømme, M.; Nyholm, L. Nanocellulose Structured Paper-Based Lithium Metal Batteries. ACS Appl. Energy Mater. 2018, 1, 4341–4350. [Google Scholar] [CrossRef]
- Liu, H.; Peng, D.; Xu, T.; Cai, K.; Sun, K.; Wang, Z. Porous Conductive Interlayer for Dendrite-Free Lithium Metal Battery. J. Energy Chem. 2021, 53, 412–418. [Google Scholar] [CrossRef]
- Cao, D.; Xing, Y.; Tantratian, K.; Wang, X.; Ma, Y.; Mukhopadhyay, A.; Cheng, Z.; Zhang, Q.; Jiao, Y.; Chen, L.; et al. 3D Printed High-Performance Lithium Metal Microbatteries Enabled by Nanocellulose. Adv. Mater. 2019, 31, 1807313. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zheng, Z.J.; Feng, Y.Q.; Ye, H.; Cao, F.F.; Guo, Z.P. Topological Design of Ultrastrong MXene Paper Hosted Li Enables Ultrathin and Fully Flexible Lithium Metal Batteries. Nano Energy 2020, 74, 104817. [Google Scholar] [CrossRef]
- Zeng, W.; Yang, C.; Zhu, H.; Wang, G.; Li, J.; Ye, J.; Zhang, W.; Zhang, G.; Duan, H. Three-Dimensional MOF-Derived Host with Surface-Preferred and Spatial-Selective Effect for Dendrite-Free Lithium Metal Battery. J. Alloys Compd. 2023, 938, 168542. [Google Scholar] [CrossRef]
- Liu, X.; Qian, X.; Tang, W.; Luo, H.; Zhao, Y.; Tan, R.; Qiao, M.; Gao, X.; Hua, Y.; Wang, H.; et al. Designer Uniform Li Plating/Stripping through Lithium–Cobalt Alloying Hierarchical Scaffolds for Scalable High-Performance Lithium-Metal Anodes. J. Energy Chem. 2020, 52, 385–392. [Google Scholar] [CrossRef]
- Zhao, C.; Xiong, S.; Li, H.; Li, Z.; Qi, C.; Yang, H.; Wang, L.; Zhao, Y.; Liu, T. A Dendrite-Free Composite Li Metal Anode Enabled by Lithiophilic Co, N Codoped Porous Carbon Nanofibers. J. Power Sources 2021, 483, 229188. [Google Scholar] [CrossRef]
- Song, X.; Wang, H.; Wu, H.; Zou, J.; Zhao, F.; Wu, H.; Zeng, X. Free-Standing Hollow Carbon Nanofibers Scaffold with Spherical Nanocavities and Lithiophilic N/ZnO Heteroatoms as Stable Dendrite-Free Lithium Metal Anode. Appl. Surf. Sci. 2021, 565, 150589. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Ma, Y.; Chi, Z.; Yin, H.; Liu, J.; Huang, J.; Guo, Z.; Wang, L. MnO2 Nanosheet Modified N, P Co-Doping Carbon Nanofibers on Carbon Cloth as Lithiophilic Host to Construct High-Performance Anodes for Li Metal Batteries. J. Energy Chem. 2022, 69, 270–281. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhang, H.; Li, G. Fast Capture and Stabilization of Li-Ions via Physicochemical Dual Effects for an Ultra-Stable Self-Supporting Li Metal Anode. Carbon Energy 2023, 5, 348. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, H.; Liu, B.; Xie, Q.; Chen, Q.; Lin, L.; Lin, J.; Qu, B.; Wang, L.; Peng, D.L. Homogeneous Bottom-Growth of Lithium Metal Anode Enabled by Double-Gradient Lithiophilic Skeleton. J. Energy Chem. 2021, 57, 392–400. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, L.; Li, W.; Yan, F.; Wang, H.; Zhao, Z.; Li, J.; Giri Prakash Kottapalli, A.; Pei, Y. Nitrogen-Doped Porous Carbon Nanofibers Embedded with Cu/Cu3P Heterostructures as Multifunctional Current Collectors for Stabilizing Lithium Anodes in Lithium-Sulfur Batteries. Chem. Eng. J. 2023, 472, 145089. [Google Scholar] [CrossRef]


| Preparation Technology | Diameter | Length | Electrical Conductivity | Cost |
|---|---|---|---|---|
| CVD | 50–200 nm | 50–100 µm | 103–104 S cm−1 | >500 USD/kg |
| Templated method | 50–200 nm | 1–60 µm | 1–10 S cm−1 | N/A |
| Electrospinning | 10 nm–10 μm | 103–104 µm | 1–600 S cm−1 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Ji, D.; Zhao, F.; Tian, X.; Guo, Y.; Yan, J. A Review of Carbon Nanofiber Materials for Dendrite-Free Lithium-Metal Anodes. Molecules 2024, 29, 4096. https://doi.org/10.3390/molecules29174096
Wei L, Ji D, Zhao F, Tian X, Guo Y, Yan J. A Review of Carbon Nanofiber Materials for Dendrite-Free Lithium-Metal Anodes. Molecules. 2024; 29(17):4096. https://doi.org/10.3390/molecules29174096
Chicago/Turabian StyleWei, Liying, Dawei Ji, Fulai Zhao, Xuwang Tian, Yongshi Guo, and Jianhua Yan. 2024. "A Review of Carbon Nanofiber Materials for Dendrite-Free Lithium-Metal Anodes" Molecules 29, no. 17: 4096. https://doi.org/10.3390/molecules29174096
APA StyleWei, L., Ji, D., Zhao, F., Tian, X., Guo, Y., & Yan, J. (2024). A Review of Carbon Nanofiber Materials for Dendrite-Free Lithium-Metal Anodes. Molecules, 29(17), 4096. https://doi.org/10.3390/molecules29174096







