Insights into the Synergistic Effect and Inhibition Mechanism of Composite Conditioner on Sulfur-Containing Gases during Sewage Sludge Pyrolysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sulfur Distribution in Products
2.1.1. Sulfur Balance
2.1.2. Release of Sulfur-Containing Gases
2.2. Effect of Conditioners on H2S Release
2.2.1. Effect of Single Conditioner
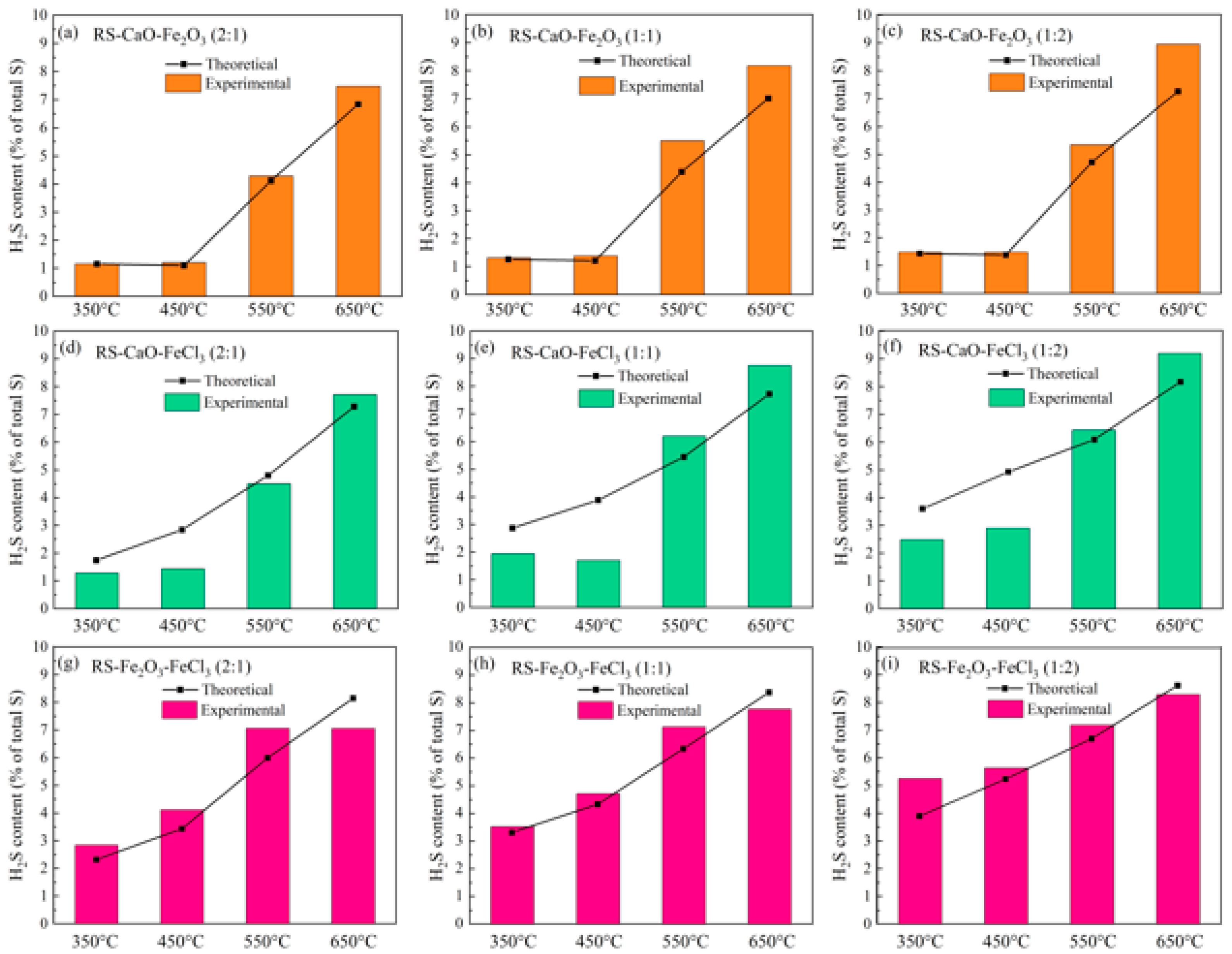
2.2.2. Synergistic Effect of Composite Conditioner
2.3. Effect of Conditioners on Sulfur Species in Char
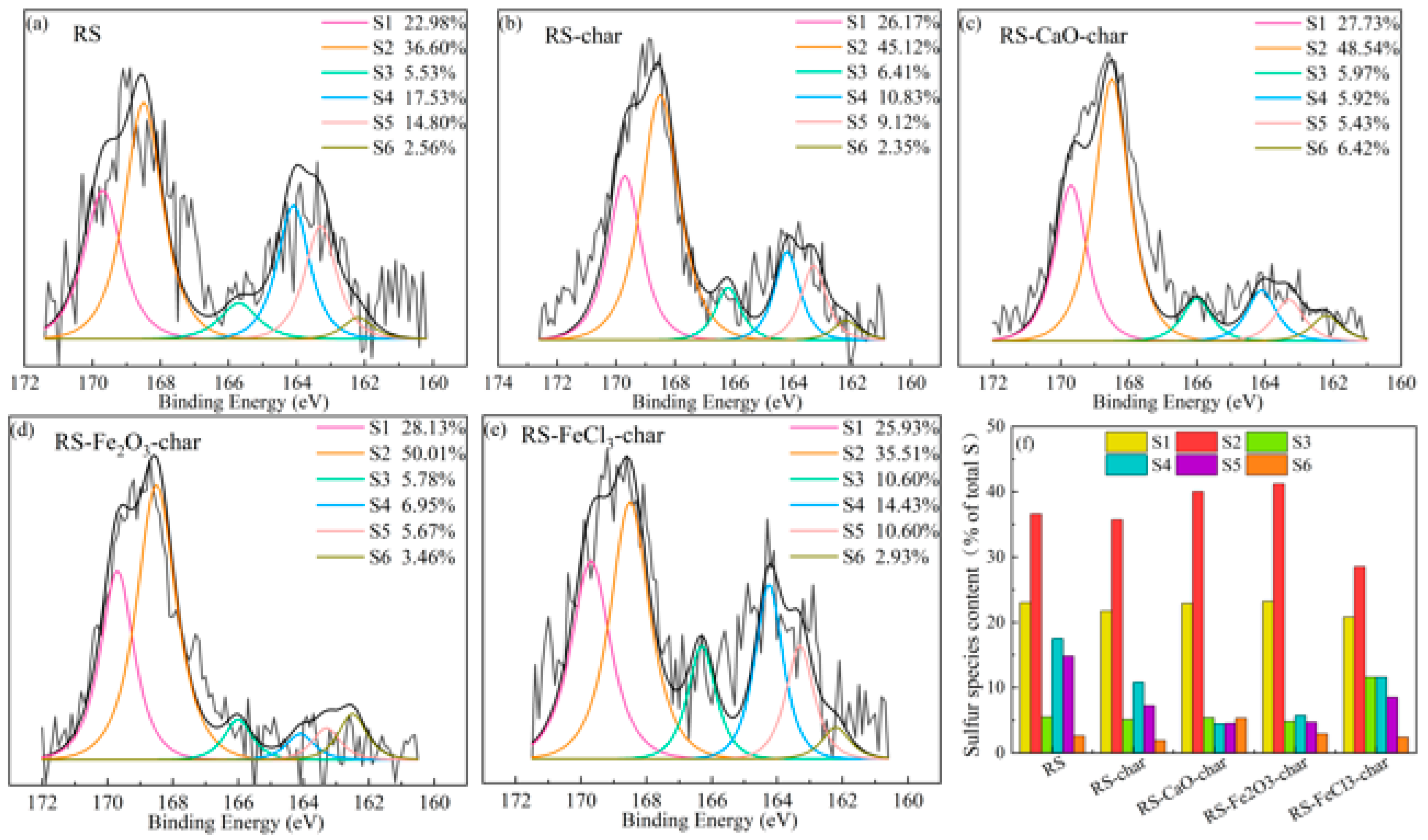
2.3.1. Effect of Single Conditioner on Sulfur Species
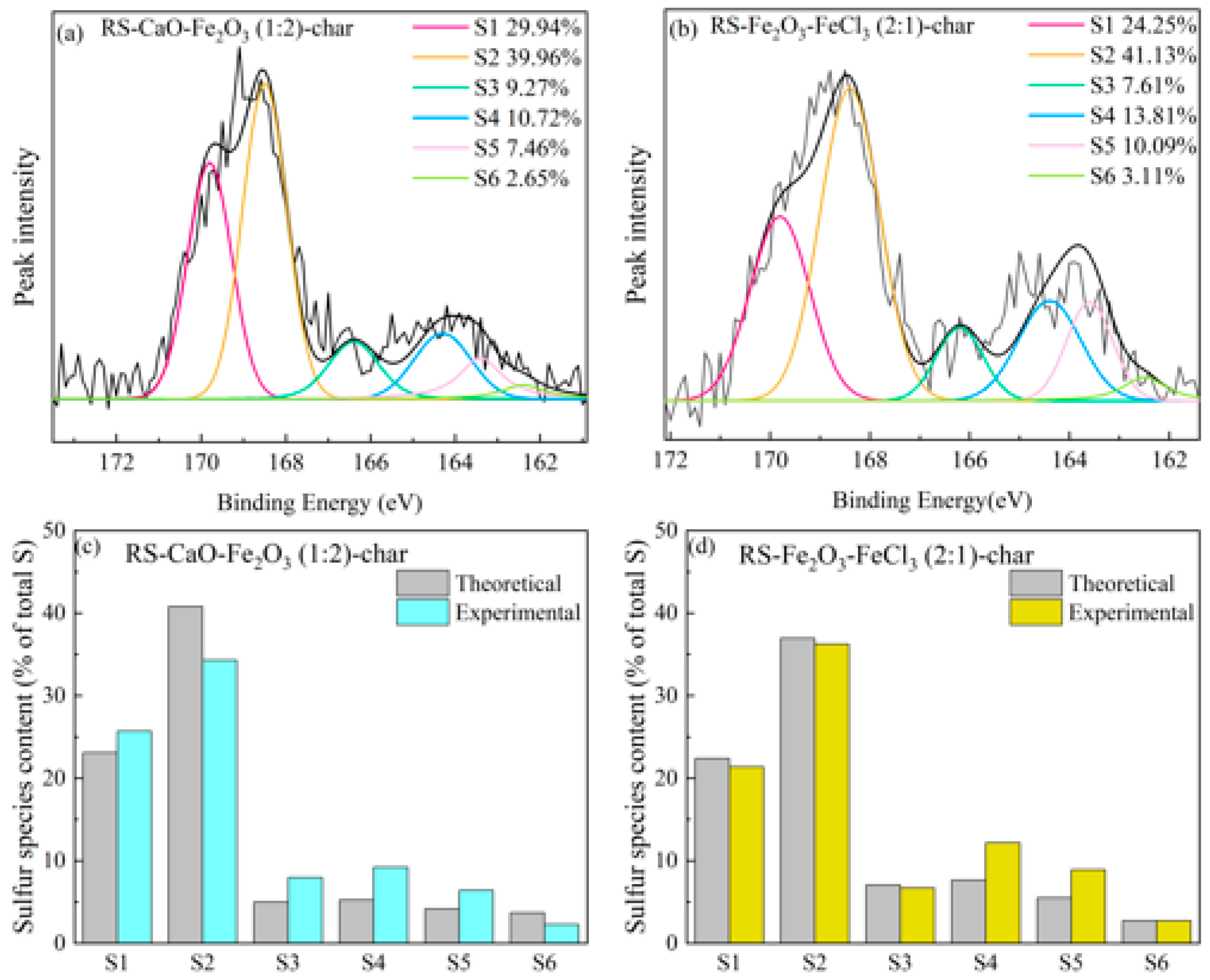
2.3.2. Synergistic Effect of Composite Conditioners on Sulfur Species
2.3.3. Sulfur-Containing Mineral Components
3. Materials and Methods
3.1. Sample Preparation
3.2. Pyrolysis Experiment
3.3. Analytic Methods
3.4. Calculation Method
4. Conclusions
- (1)
- The release of H2S was suppressed by CaO, Fe2O3, and FeCl3 by 35.8%, 23.2%, and 9.1% at 650 °C, respectively. CaO mitigated gas-S release by promoting the transformation of organic sulfur into more stable oxidized forms, and by capturing gas-S. Fe2O3 demonstrated enhanced oxidizing capabilities, while FeCl3 inhibited the decomposition of organic sulfur and captured gas-S at high temperatures.
- (2)
- Utilizing a composite of CaO and FeCl3 within the temperature range from 350 to 450 °C, along with a composite of Fe2O3 and FeCl3 at 650 °C, can synergistically suppress the H2S release. Conversely, between 350 °C and 550 °C, the combination of Fe2O3 with FeCl3, and between approximately 550 and 650 °C, the composite of Ca-based and Fe-based conditioners, demonstrate a synergistic enhancement effect on the release of H2S.
- (3)
- In composite conditioning, the strong alkalinity of CaO neutralizes HCl, an acidic gas generated by the decomposition of FeCl3. This neutralization prevents HCl from interacting with inorganic or organic sulfides in the sludge, thus synergistically inhibiting H2S release. At 450 °C, this synergistic effect inhibits H2S release by 56.3%. Nonetheless, the formation of FeCa2O4 above 550 °C, reduces the sulfur-fixing capacity of the composite conditioners, and at 650 °C, it synergistically promotes the release of H2S by 26.3%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, H.; Gao, B.Q.; Ren, J.; Li, A.; Yang, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Chu, Z.W.; Li, Y.J.; Zhang, C.X.; Fang, Y.; Zhao, J.L. A review on resource utilization of oil sludge based on pyrolysis and gasification. J. Environ. Chem. Eng. 2023, 11, 109692. [Google Scholar] [CrossRef]
- Lin, B.C.; Alhadj Mallah, M.M.; Huang, Q.X.; Ali, M.; Chi, Y. Effects of temperature and potassium compounds on the transformation behavior of sulfur during pyrolysis of oily sludge. Energy Fuels 2017, 31, 7004–7014. [Google Scholar] [CrossRef]
- Liu, M.S.; Yuan, C.W.; Ru, S.Q.; Li, J.; Lei, Z.F.; Zhang, Z.Y.; Shimizu, K.; Yuan, T.; Li, F.T. Combined organic reagents for co-conditioning of sewage sludge: High performance in deep dewatering and significant contribution to the floc property. J. Water Process Eng. 2022, 48, 102855. [Google Scholar] [CrossRef]
- Li, J.T.; Sun, B.Y.; Lin, F.W.; Tian, W.Y.; Yan, B.B.; Song, Y.J.; Chen, G.Y.; He, C. Transformation and regulation of nitrogen and sulfur during pyrolysis of oily sludge with N/S model compounds. Fuel 2022, 324, 124651. [Google Scholar] [CrossRef]
- Ogugua, P.C.; Su, H.H.; Wang, E. Synergistic blending of biomass, sewage sludge, and coal for enhanced bioenergy production: Exploring residue combinations and optimizing thermal conversion parameters. J. Environ. Manag. 2024, 352, 120035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.G.; Hu, J.J.; Lee, D.J.; Chang, Y.J.; Lee, Y.J. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Zhen, Y.H.; Liu, L.L.; Ning, X.Y.; Wang, C.Z.; Li, K.; Zhao, L.; Lu, Q. Comprehensive competitiveness assessment of four typical municipal sludge treatment routes in China based on environmental and techno-economic analysis. Sci. Total Environ. 2023, 895, 165123. [Google Scholar] [CrossRef]
- Kwon, E.E.; Lee, T.; Ok, Y.S.; Tsang, D.C.; Park, C.; Lee, J. Effects of calcium carbonate on pyrolysis of sewage sludge. Energy 2018, 153, 726–731. [Google Scholar] [CrossRef]
- Cao, C.Y.; Cheng, Y.T.; Hu, H.Y.; Wang, H.; Liu, S.; Hu, M.; Li, X.; Yao, H. Products distribution and sulfur fixation during the pyrolysis of CaO conditioned textile dyeing sludge: Effects of pyrolysis temperature and heating rate. Waste Manag. 2022, 153, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Huang, L.M.; Zou, H.H.; Xie, W.M.; Evrendilek, D.E.; Luo, G.Q.; Ninomiya, Y. Do FeCl3 and FeCl3/CaO conditioners change pyrolysis and incineration performances, emissions, and elemental fates of textile dyeing sludge? J. Hazard. Mater. 2021, 413, 125334. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Xing, H.X.; Hu, H.Y.; Li, A.J.; Yao, H. Product distribution and sulfur behavior in sewage sludge pyrolysis: Synergistic effect of Fenton peroxidation and CaO conditioning. Fuel 2015, 159, 68–75. [Google Scholar] [CrossRef]
- Cheng, S.; Qiao, Y.; Huang, J.C.; Cao, L.W.; Yang, H.Z.; Liu, H.; Yu, Y.; Xu, M.H. Effect of alkali addition on sulfur transformation during low temperature pyrolysis of sewage sludge. Proc. Combust. Inst. 2017, 36, 2253–2261. [Google Scholar] [CrossRef]
- Wang, M.Q.; Pan, X.W.; Xia, Y.P.; Zhu, A.L.; Wu, Y.; Fu, C.; Zhang, P.Y.; Zhao, J.G.; Li, J.R.; Fu, J.R. Effect of dewatering conditioners on pollutants with nitrogen, sulfur, and chlorine releasing characteristics during sewage sludge pyrolysis. Fuel 2022, 307, 121834. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Hu, H.Y.; Xiao, R.X.; Li, A.J.; Qiao, Y.; Yao, H.; Naruse, I. Dual role of conditioner CaO in product distributions and sulfur transformation during sewage sludge pyrolysis. Fuel 2014, 134, 514–520. [Google Scholar] [CrossRef]
- Lu, M.X.; Xue, Y.J.; Zhao, H.; Zhang, X.S.; Wang, T. Effect of electromagnetic induction drying on the drying–incineration process of dyeing sludge: Focus on migration and conversion of sulfur. Waste Manag. 2023, 171, 522–531. [Google Scholar] [CrossRef]
- Liu, S.; Wei, M.M.; Qiao, Y.; Yang, Z.L.; Gui, B.; Yu, Y.; Xu, M.H. Release of organic sulfur as sulfur-containing gases during low temperature pyrolysis of sewage sludge. Proc. Combust. Inst. 2015, 35, 2767–2775. [Google Scholar] [CrossRef]
- Liu, G.C.; Liao, Y.F.; Wu, Y.T.; Ma, X.Q. Synthesis gas production from microalgae gasification in the presence of Fe2O3 oxygen carrier and CaO additive. Appl. Energy 2018, 212, 955–965. [Google Scholar] [CrossRef]
- Ying, C.D.; Wang, Q.H.; Yang, Y.J.; Huang, Z.M.; Zhu, Y.; Cen, J.M. Experimental investigation on sulfur transformation during coal steam gasification with Ca-based absorbent. Fuel 2023, 343, 127908. [Google Scholar] [CrossRef]
- Hu, Y.J.; Xia, Z.P.; Wang, X.; Xing, B.; Chen, Q.; Yu, F.; Zhou, N. Enhanced co-pyrolysis of textile and leather waste with Ca/Fe-enriched sludge ash: Catalytic effect on thermal behavior, volatile composition, and biochar formation. Fuel 2024, 373, 132272. [Google Scholar] [CrossRef]
- Folgueras, M.B.; DíAz, R.M.; Xiberta, J. Sulphur retention during co-combustion of coal and sewage sludge. Fuel 2004, 83, 1315–1322. [Google Scholar] [CrossRef]
- Leng, L.J.; Yuan, X.Z.; Huang, H.J.; Peng, X.; Chen, H.M.; Wang, H.; Wang, L.L.; Chen, X.H.; Zeng, G.M. Distribution behavior and risk assessment of metals in bio-oils produced by liquefaction/pyrolysis of sewage sludge. Environ. Sci. Pollut. Res. 2015, 22, 18945–18955. [Google Scholar] [CrossRef] [PubMed]
- GB/T 28731-2012; Proximate Analysis of Solid Biofuels. Standardization Administration of the People’s Republic of China: Beijing, China, 2012.
- GB/T31391-2015; UItimate Analysis of Coal. Standardization Administration of the People’s Republic of China: Beijing, China, 2015.
- Leng, E.W.; Costa, M.; Gong, X.; Zheng, A.Q.; Liu, S.J.; Xu, M.H. Effects of KCl and CaCl2 on the evolution of anhydro sugars in reaction intermediates during cellulose fast pyrolysis. Fuel 2019, 251, 307–315. [Google Scholar] [CrossRef]
- Cheng, S.; Tian, H.; Huang, J.C.; Wei, Y.Y.; Yang, T.; Qiao, Y. Effect of NaCl/MgCl2 on generation of NOx precursors during aspartic acid pyrolysis: A experimental and theoretical study. Fuel 2023, 354, 129335. [Google Scholar] [CrossRef]
- Yao, D.D.; Hu, Q.; Wang, D.Q.; Yang, H.P.; Wu, C.F. Hydrogen production from biomass gasification using biochar as a catalyst/support. Bioresour. Technol. 2016, 216, 159–164. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.Q.; Yang, H.P.; Li, K.X.; Chen, X.; Chen, H.P. Investigation on biomass nitrogen-enriched pyrolysis: Influence of temperature. Bioresour. Technol. 2018, 249, 247–253. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, J.C.; Luo, R.; Tian, H.; Yin, Y.S.; Qiao, Y. Experimental and theoretical study on the transformation of typical organic sulfur during hydrothermal carbonization of sludge. Fuel 2023, 332, 126064. [Google Scholar] [CrossRef]




| Char-S (%) | Gas-S (%) | Tas-S (%) | |
|---|---|---|---|
| RS-700 | 79.1 | 15.1 | - |
| RS-CaO | 90.6 | 7.0 | - |
| RS-Fe2O3 | 89.8 | 6.9 | - |
| RS-FeCl3 | 85.5 | 9.9 | - |
| Samples | Proximate Analysis (wt.%) a | Ultimate Analysis (wt.%) a | ||||||
|---|---|---|---|---|---|---|---|---|
| Volatile Matter | Ash | Fixed Carbon | C | H | N | S | O b | |
| RS | 31.2 | 64.7 | 4.1 | 15.88 | 2.64 | 2.52 | 1.11 | 13.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Chen, L.; Wang, S.; Yao, K.; Tian, H. Insights into the Synergistic Effect and Inhibition Mechanism of Composite Conditioner on Sulfur-Containing Gases during Sewage Sludge Pyrolysis. Molecules 2024, 29, 4110. https://doi.org/10.3390/molecules29174110
Cheng S, Chen L, Wang S, Yao K, Tian H. Insights into the Synergistic Effect and Inhibition Mechanism of Composite Conditioner on Sulfur-Containing Gases during Sewage Sludge Pyrolysis. Molecules. 2024; 29(17):4110. https://doi.org/10.3390/molecules29174110
Chicago/Turabian StyleCheng, Shan, Lianghui Chen, Shaoshuo Wang, Kehui Yao, and Hong Tian. 2024. "Insights into the Synergistic Effect and Inhibition Mechanism of Composite Conditioner on Sulfur-Containing Gases during Sewage Sludge Pyrolysis" Molecules 29, no. 17: 4110. https://doi.org/10.3390/molecules29174110





