The Reaction between K2CO3 and Ethylene Glycol in Deep Eutectic Solvents
Abstract
1. Introduction
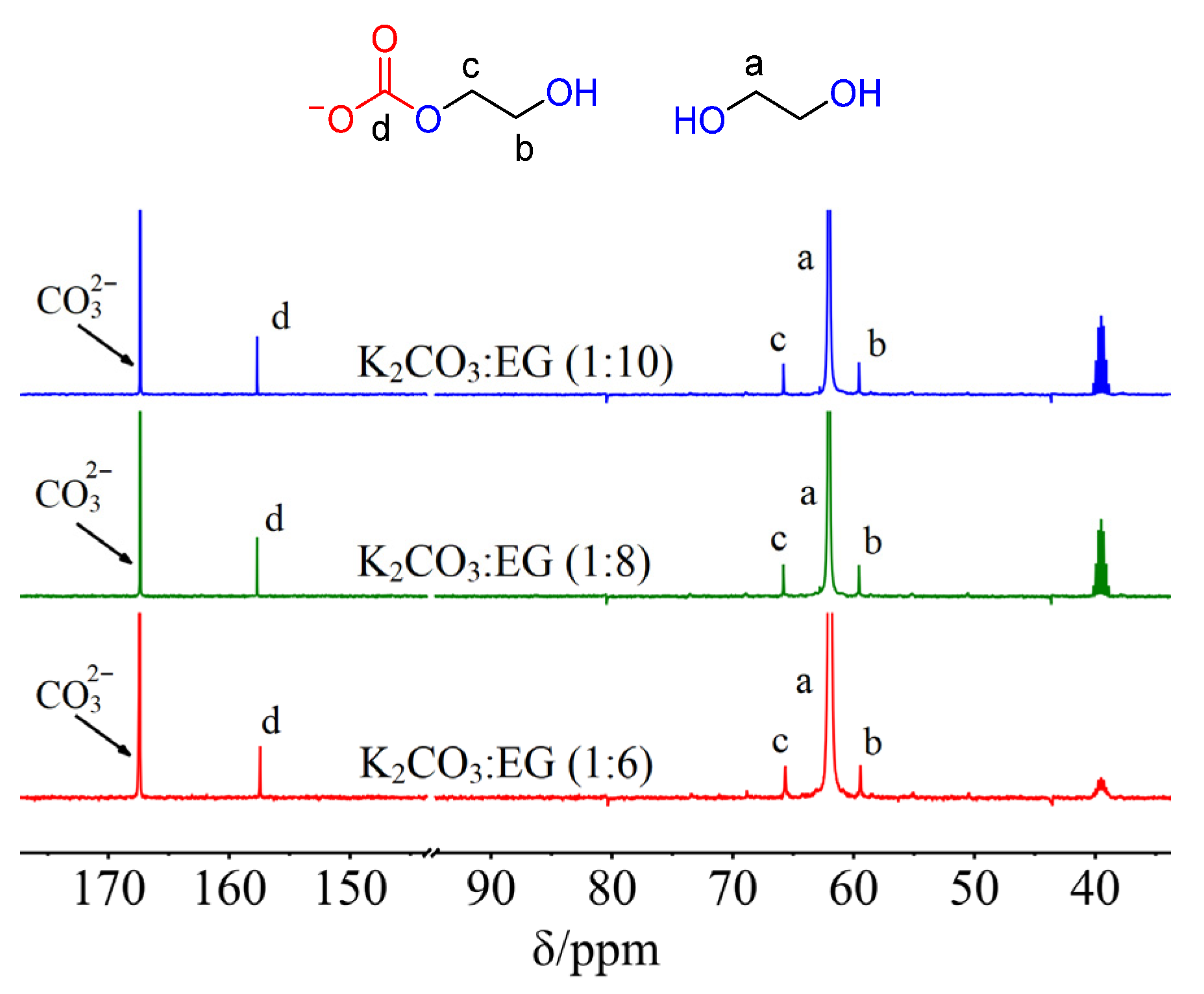
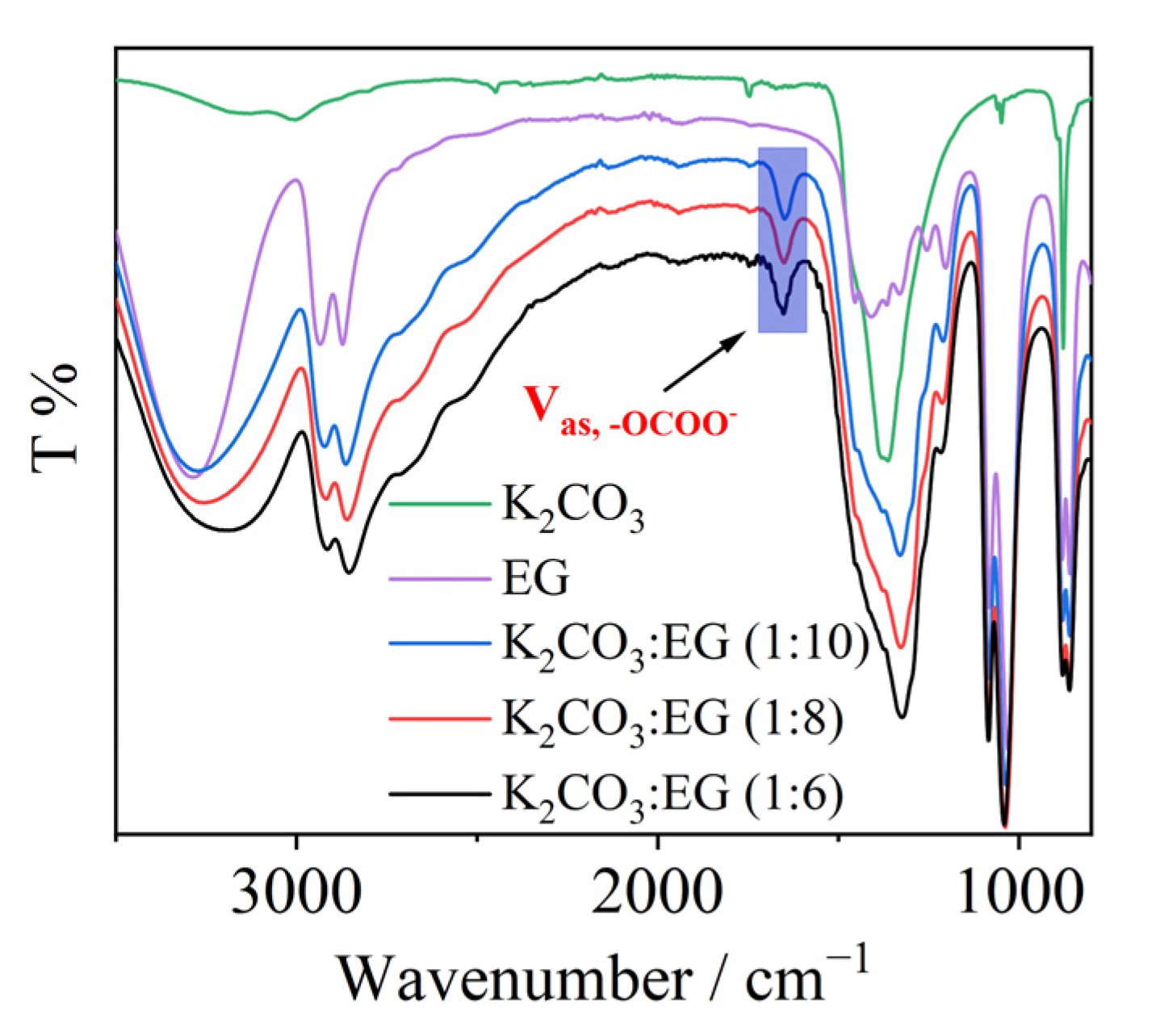
2. Results and Discussion

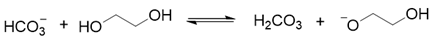
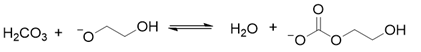
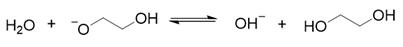
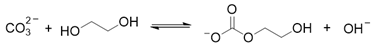

3. Materials and Methods
3.1. Materials and Characterizations
3.2. Synthesis of DESs
3.3. NMR and FTIR Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Database of deep eutectic solvents and their physical properties: A review. J. Mol. Liq. 2023, 384, 121899. [Google Scholar] [CrossRef]
- Yu, D.; Xue, Z.; Mu, T. Deep eutectic solvents as a green toolbox for synthesis. Cell Rep. Phys. Sci. 2022, 3, 100809. [Google Scholar] [CrossRef]
- Oyoun, F.; Toncheva, A.; Henríquez, L.C.; Grougnet, R.; Laoutid, F.; Mignet, N.; Alhareth, K.; Corvis, Y. Deep Eutectic Solvents: An Eco-friendly Design for Drug Engineering. ChemSusChem 2023, 16, e202300669. [Google Scholar] [CrossRef]
- Verma, S.; Saini, K.; Maken, S. Deep eutectic solvents: A long–term approach to chemical synthesis and separation. J. Mol. Liq. 2024, 393, 123605. [Google Scholar] [CrossRef]
- Makoś-Chełstowska, P. VOCs absorption from gas streams using deep eutectic solvents—A review. J. Hazard. Mater. 2023, 448, 130957. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, S.; Hou, Y.; Zhang, Y.; Li, Y.; Zhang, W.; Wu, W. Capture and Catalytic Reduction of SO2 with H2 to Elemental Sulfur by Novel Guanidinium-Based Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2023, 62, 14568–14576. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Ren, S.; Hou, Y.; Wu, W. Rapid Absorption and Desorption of CO2 by Deep Eutectic Solvents via Reversible CO2-Triggered Proton Transfer Process. ACS Sustain. Chem. Eng. 2024, 12, 3987–3995. [Google Scholar] [CrossRef]
- Deng, R.; Gao, M.; Zhang, B.; Zhang, Q. Solvent-Mediated Synthesis of Functional Powder Materials from Deep Eutectic Solvents for Energy Storage and Conversion: A Review. Adv. Energy Mater. 2024, 14, 2303707. [Google Scholar] [CrossRef]
- Cherniakova, M.; Varchenko, V.; Belikov, K. Menthol-Based (Deep) Eutectic Solvents: A Review on Properties and Application in Extraction. Chem. Rec. 2024, 24, e202300267. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lyu, Y.; Zeng, R.; Zhang, S.; Davey, K.; Mao, J.; Guo, Z. Green recycling of spent Li-ion battery cathodes via deep-eutectic solvents. Energy Environ. Sci. 2024, 17, 867–884. [Google Scholar] [CrossRef]
- Singh, K.; Mehra, S.; Kumar, A. Recent advances in catalytic conversion of lignin to value-added chemicals using ionic liquids and deep eutectic solvents: A critical review. Green Chem. 2024, 26, 1062–1091. [Google Scholar] [CrossRef]
- Naser, J.; Jibril, F.M.B.; Al-Hatmi, S.; Gano, Z. Potassium Carbonate as a Salt for Deep Eutectic Solvents. Int. J. Chem. Eng. Appl. 2013, 4, 114–118. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Naser, J.; Jibril, B.; Al-Hatmi, S.S.; Gano, Z.S. Ionic liquids analogues based on potassium carbonate. Thermochim. Acta 2014, 575, 135–143. [Google Scholar] [CrossRef]
- Suopajärvi, T.; Ricci, P.; Karvonen, V.; Ottolina, G.; Liimatainen, H. Acidic and alkaline deep eutectic solvents in delignification and nanofibrillation of corn stalk, wheat straw, and rapeseed stem residues. Ind. Crops Prod. 2020, 145, 111956. [Google Scholar] [CrossRef]
- Ghaedi, H.; Kalhor, P.; Zhao, M.; Clough, P.T.; Anthony, E.J.; Fennell, P.S. Potassium carbonate-based ternary transition temperature mixture (deep eutectic analogues) for CO2 absorption: Characterizations and DFT analysis. Front. Environ. Sci. Eng. 2021, 16, 92. [Google Scholar] [CrossRef]
- Saeed, U.; Khan, A.U.; Khan, A.L.; Gilani, M.A.; Bilad, M.R. Separation of Carbon Dioxide by Potassium Carbonate based Supported Deep Eutectic Liquid Membranes: Influence of Hydrogen Bond Donor. J. Membr. Sci. Res. 2022, 8, 526587. [Google Scholar]
- Olalere, O.A.; Gan, C.-Y. Process optimisation of defatted wheat germ protein extraction in a novel alkaline-based deep eutectic solvent (DES) via Box–Behnken experimental design (BBD). Food Chem. 2023, 409, 135224. [Google Scholar] [CrossRef]
- Zeng, P.; Wu, D.; Wang, T.; Liu, P.; Jia, D. Redefine the existence form and function of water in potassium carbonate-based deep eutectic electrolyte. Fuel 2024, 357, 129738. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, T.-X.; Qi, B.-K.; Li, H.; Zhao, Q.-S.; Zhao, B. Acidic and alkaline deep eutectic solvents (DESs) pretreatment of grapevine: Component analysis, characterization, lignin structural analysis, and antioxidant properties. Int. J. Biol. Macromol. 2023, 236, 123977. [Google Scholar] [CrossRef]
- Hua, Y.; Xu, Z.; Zhao, B.; Zhang, Z. Efficient separation of electrode active materials and current collector metal foils from spent lithium-ion batteries by a green deep eutectic solvent. Green Chem. 2022, 24, 8131–8141. [Google Scholar] [CrossRef]
- Ghaedi, H.; Ayoub, M.; Sufian, S.; Shariff, A.M.; Lal, B.; Wilfred, C.D. Density and refractive index measurements of transition-temperature mixture (deep eutectic analogues) based on potassium carbonate with dual hydrogen bond donors for CO2 capture. J. Chem. Thermodyn. 2018, 118, 147–158. [Google Scholar] [CrossRef]
- Cui, G.; Lv, M.; Yang, D. Efficient CO2 absorption by azolide-based deep eutectic solvents. Chem. Commun. 2019, 55, 1426–1429. [Google Scholar] [CrossRef]
- Nie, M.-N.; Wang, Z.; Niu, Q.-H.; Dai, J.-X.; Wang, Q.-Q.; Peng, J.-S.; Ji, P. Acidity Scale in a Choline Chloride- and Ethylene Glycol-Based Deep Eutectic Solvent and Its Implication on Carbon Dioxide Absorption. J. Org. Chem. 2023, 88, 5368–5376. [Google Scholar] [CrossRef]
- Sen, R.; Goeppert, A.; Kar, S.; Prakash, G.K.S. Hydroxide Based Integrated CO2 Capture from Air and Conversion to Methanol. J. Am. Chem. Soc. 2020, 142, 4544–4549. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yu, X.; Yang, Y.; Zhao, Z.K. Capturing CO2 for cellulose dissolution. Green Chem. 2014, 16, 2422–2427. [Google Scholar] [CrossRef]
- Im, J.; Hong, S.Y.; Cheon, Y.; Lee, J.; Lee, J.S.; Kim, H.S.; Cheong, M.; Park, H. Steric hindrance-induced zwitterionic carbonates from alkanolamines and CO2: Highly efficient CO2 absorbents. Energy Environ. Sci. 2011, 4, 4284–4289. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, C.; Wang, Z.; Zhang, S.; Yang, D. CO2 capture by 1,2,3-triazole-based deep eutectic solvents: The unexpected role of hydrogen bonds. Chem. Commun. 2022, 58, 7376–7379. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Chen, M.; Dong, X.; Yang, D. The Reaction between K2CO3 and Ethylene Glycol in Deep Eutectic Solvents. Molecules 2024, 29, 4113. https://doi.org/10.3390/molecules29174113
Zhou Y, Chen M, Dong X, Yang D. The Reaction between K2CO3 and Ethylene Glycol in Deep Eutectic Solvents. Molecules. 2024; 29(17):4113. https://doi.org/10.3390/molecules29174113
Chicago/Turabian StyleZhou, Yi, Mingzhe Chen, Xueling Dong, and Dezhong Yang. 2024. "The Reaction between K2CO3 and Ethylene Glycol in Deep Eutectic Solvents" Molecules 29, no. 17: 4113. https://doi.org/10.3390/molecules29174113
APA StyleZhou, Y., Chen, M., Dong, X., & Yang, D. (2024). The Reaction between K2CO3 and Ethylene Glycol in Deep Eutectic Solvents. Molecules, 29(17), 4113. https://doi.org/10.3390/molecules29174113







