Comparative Investigation of Water-Based CMC and LA133 Binders for CuO Anodes in High-Performance Lithium-Ion Batteries
Abstract
:1. Introduction
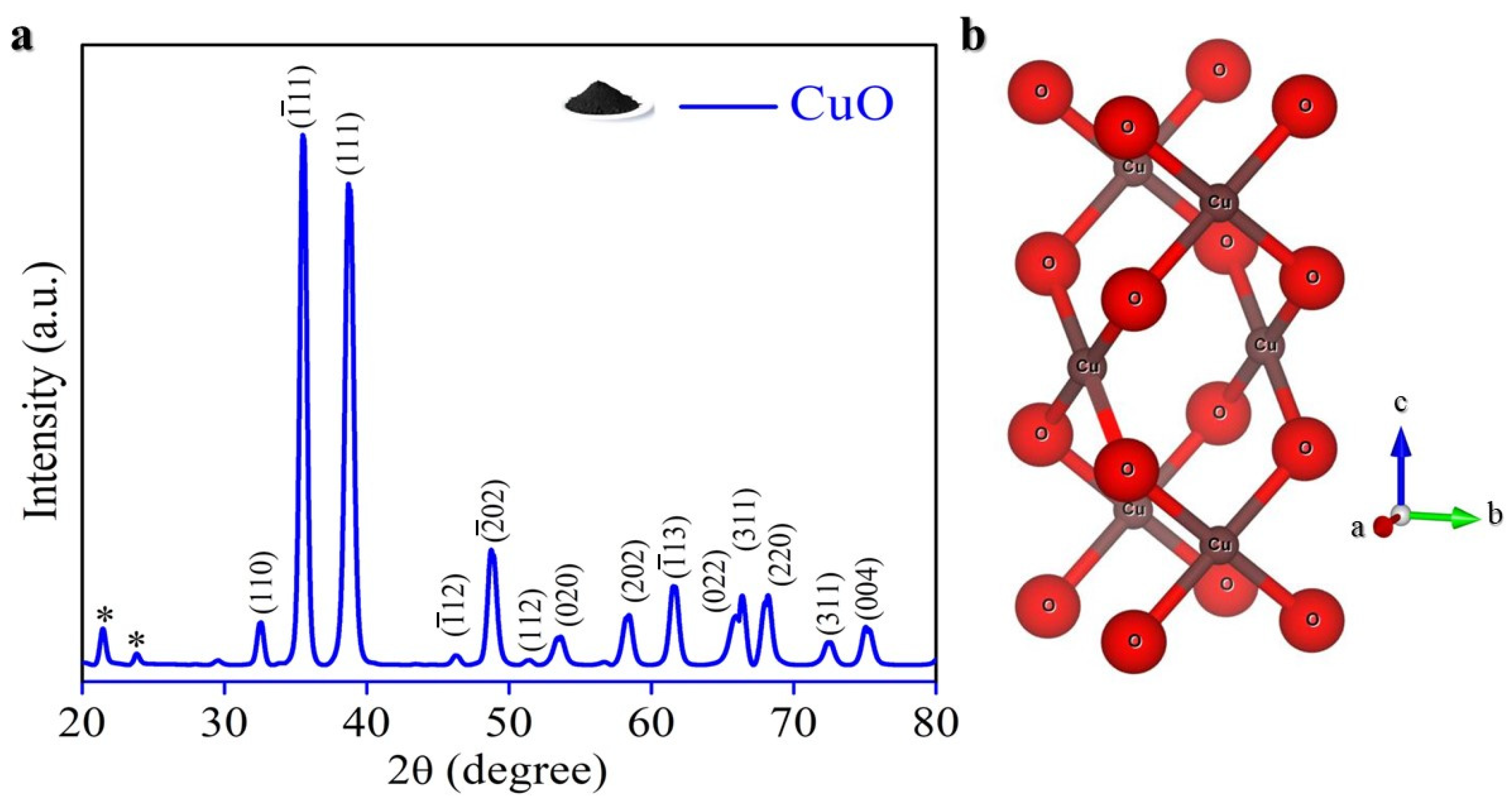
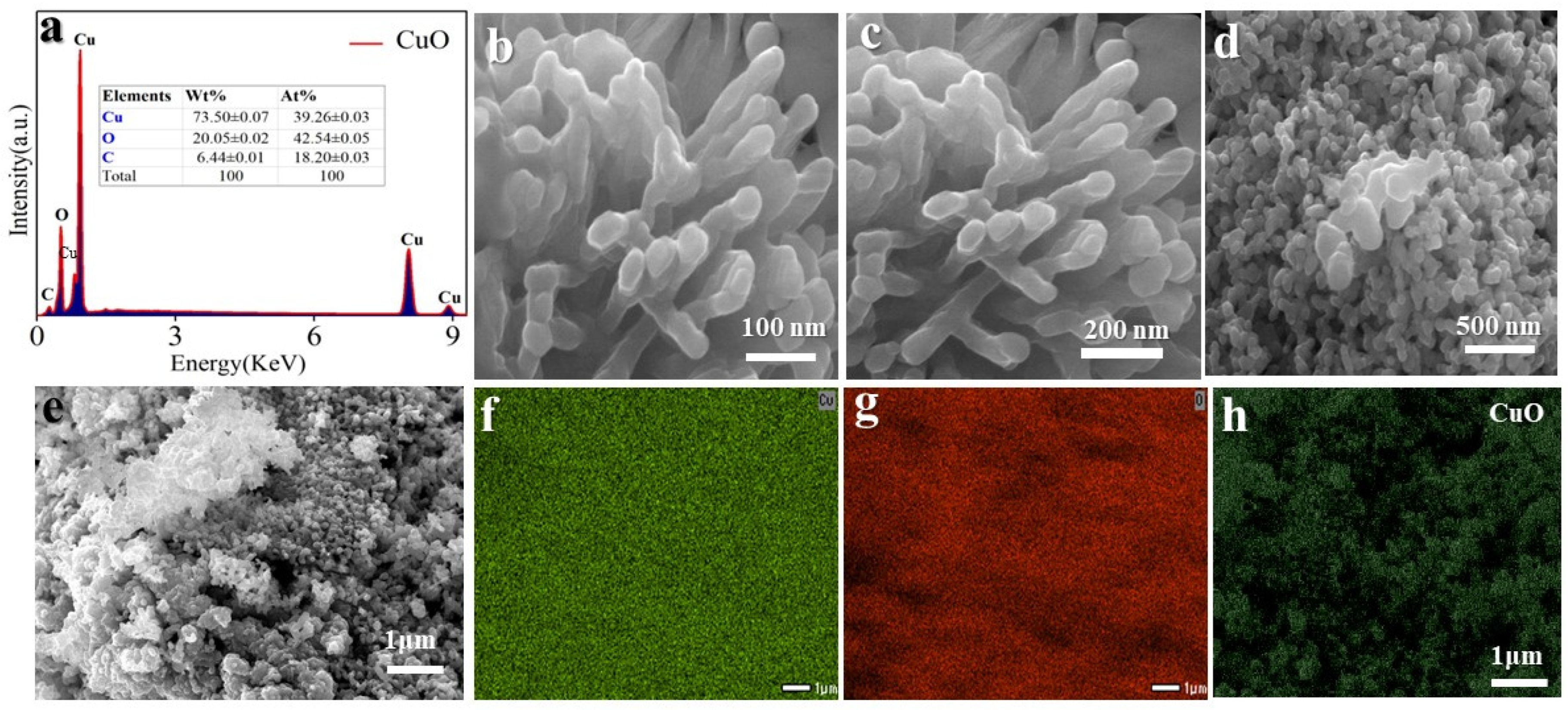
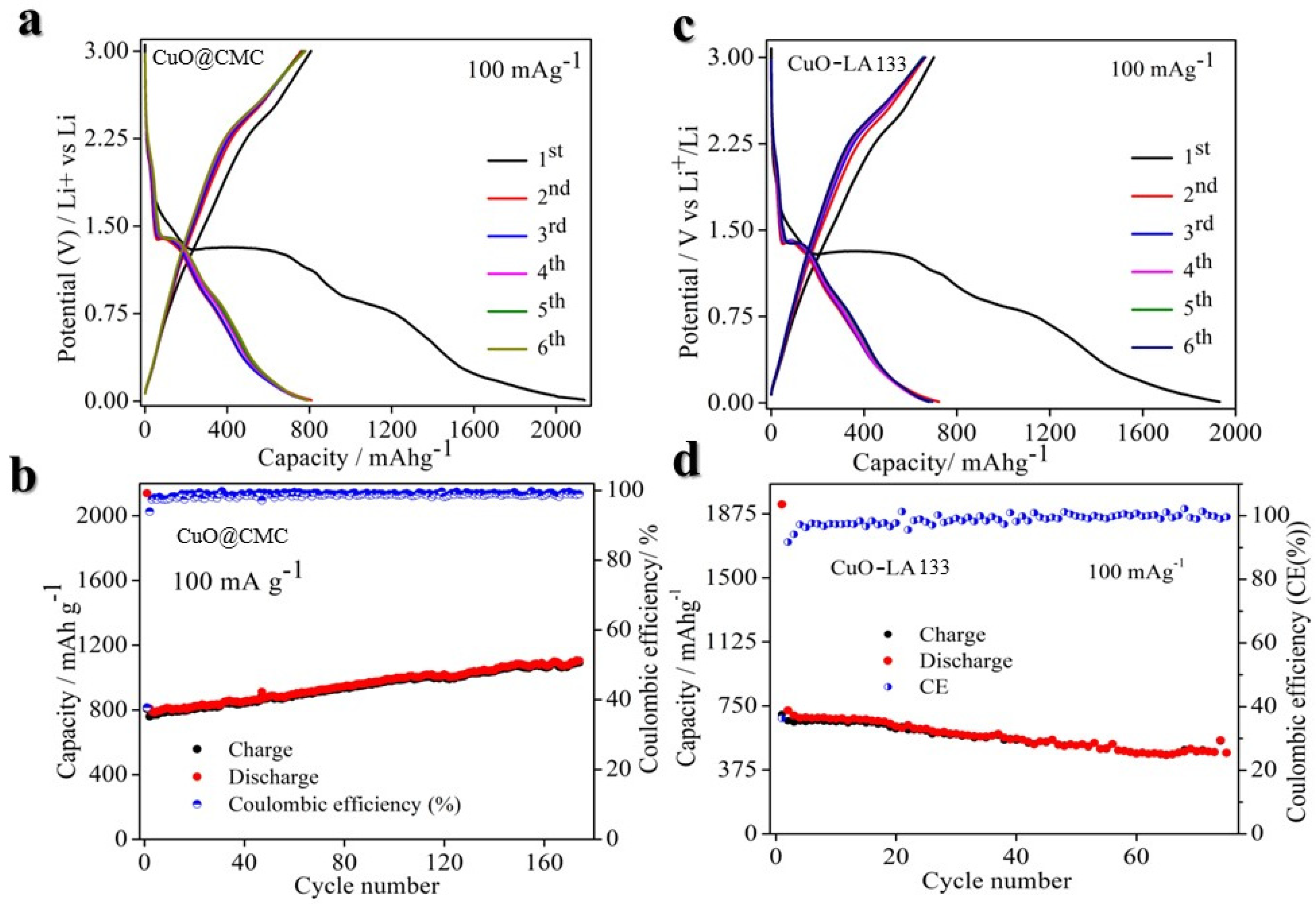
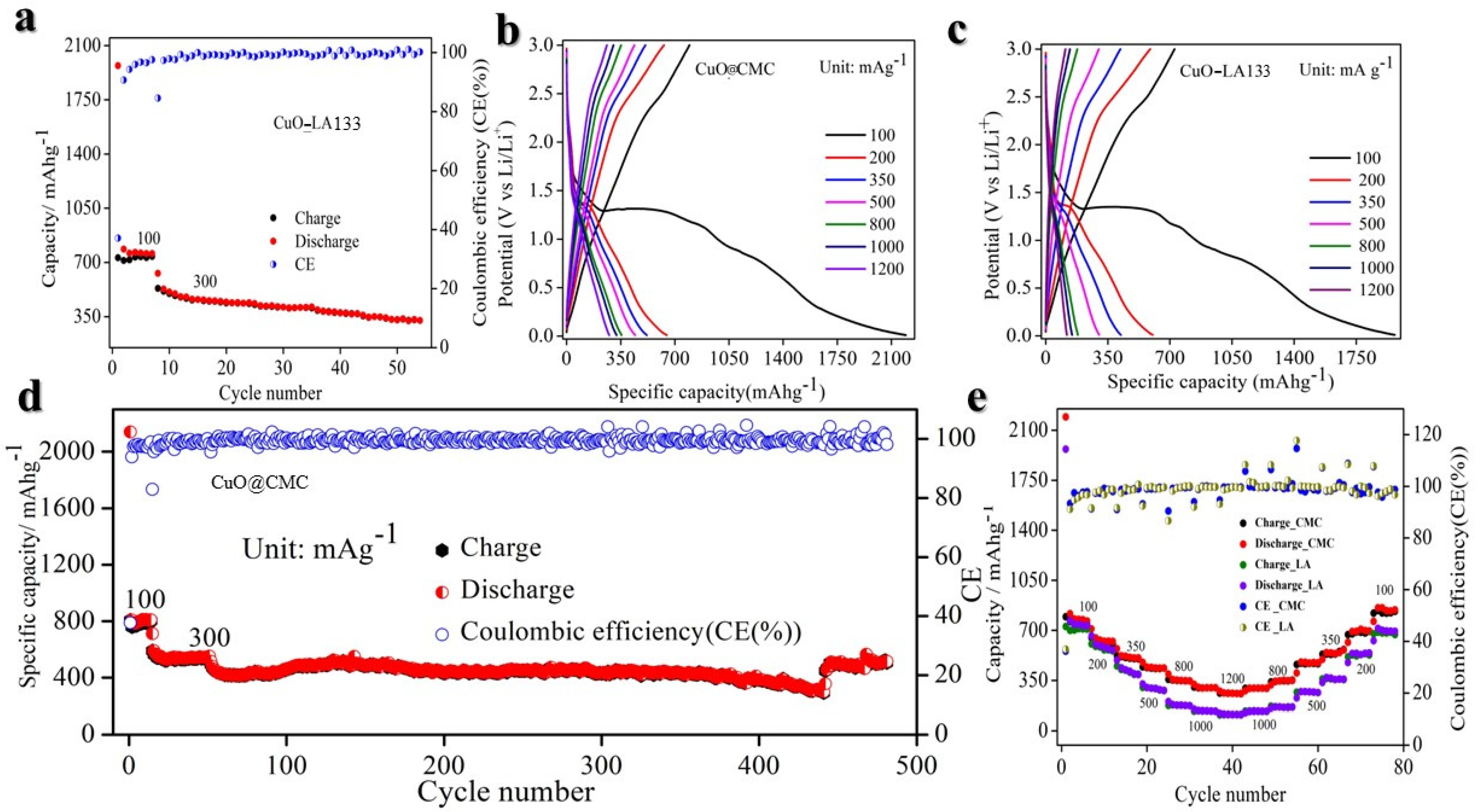
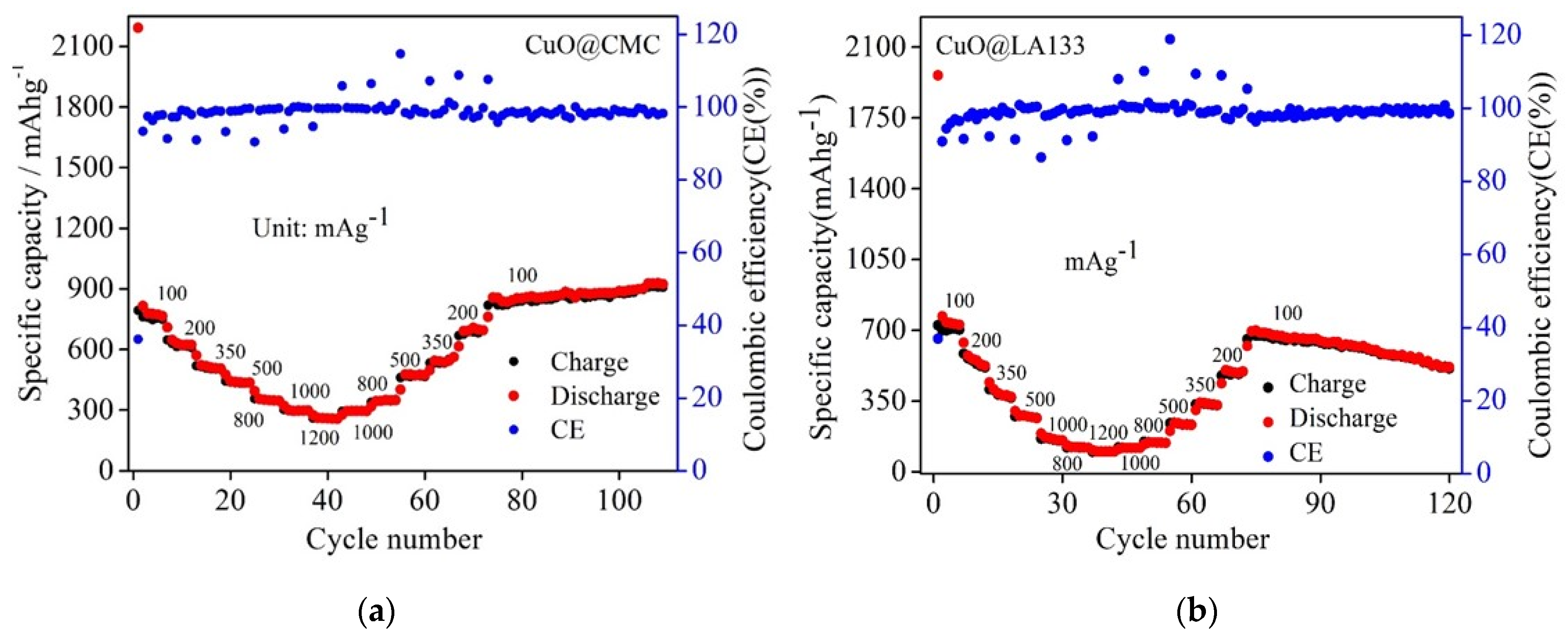
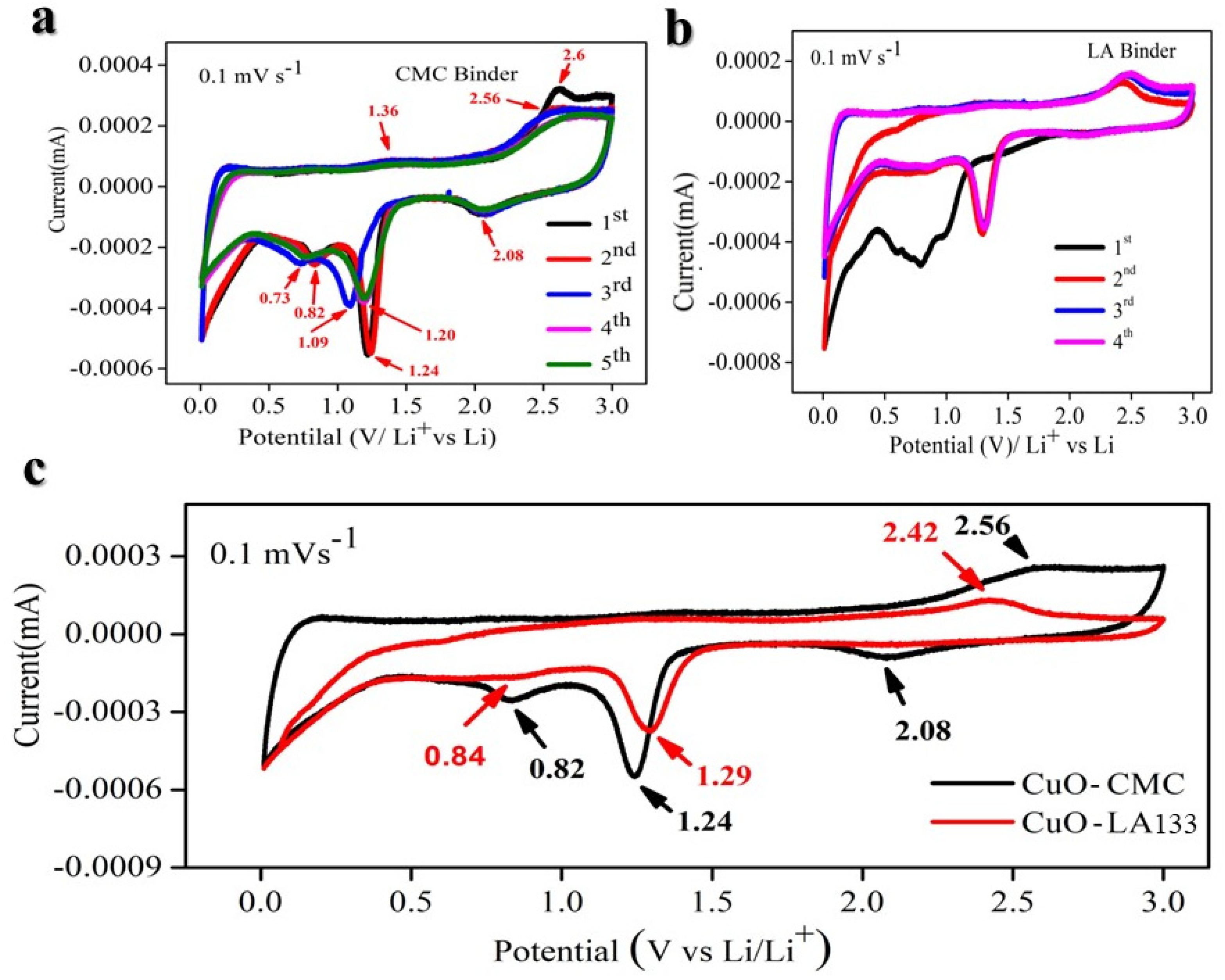
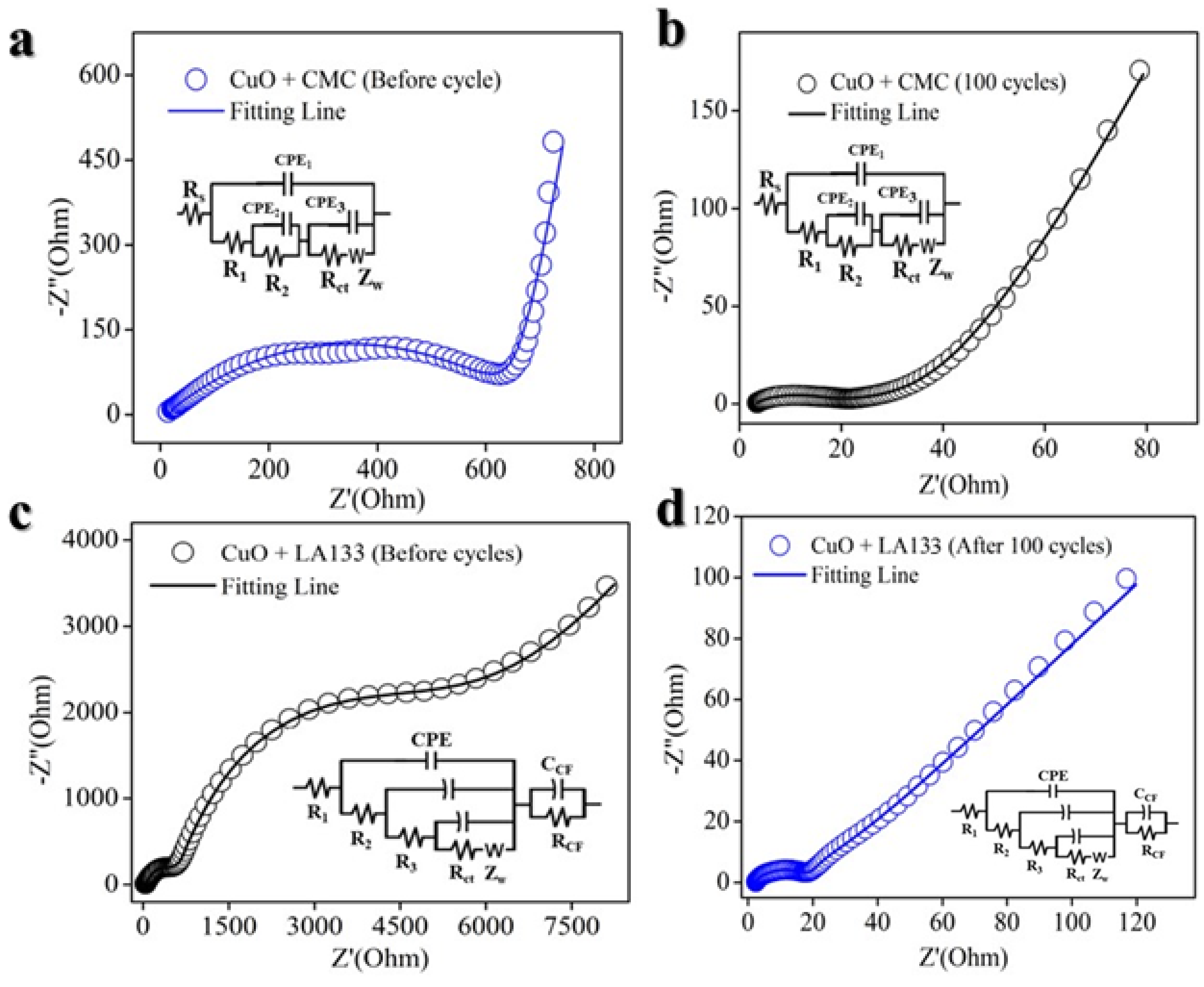
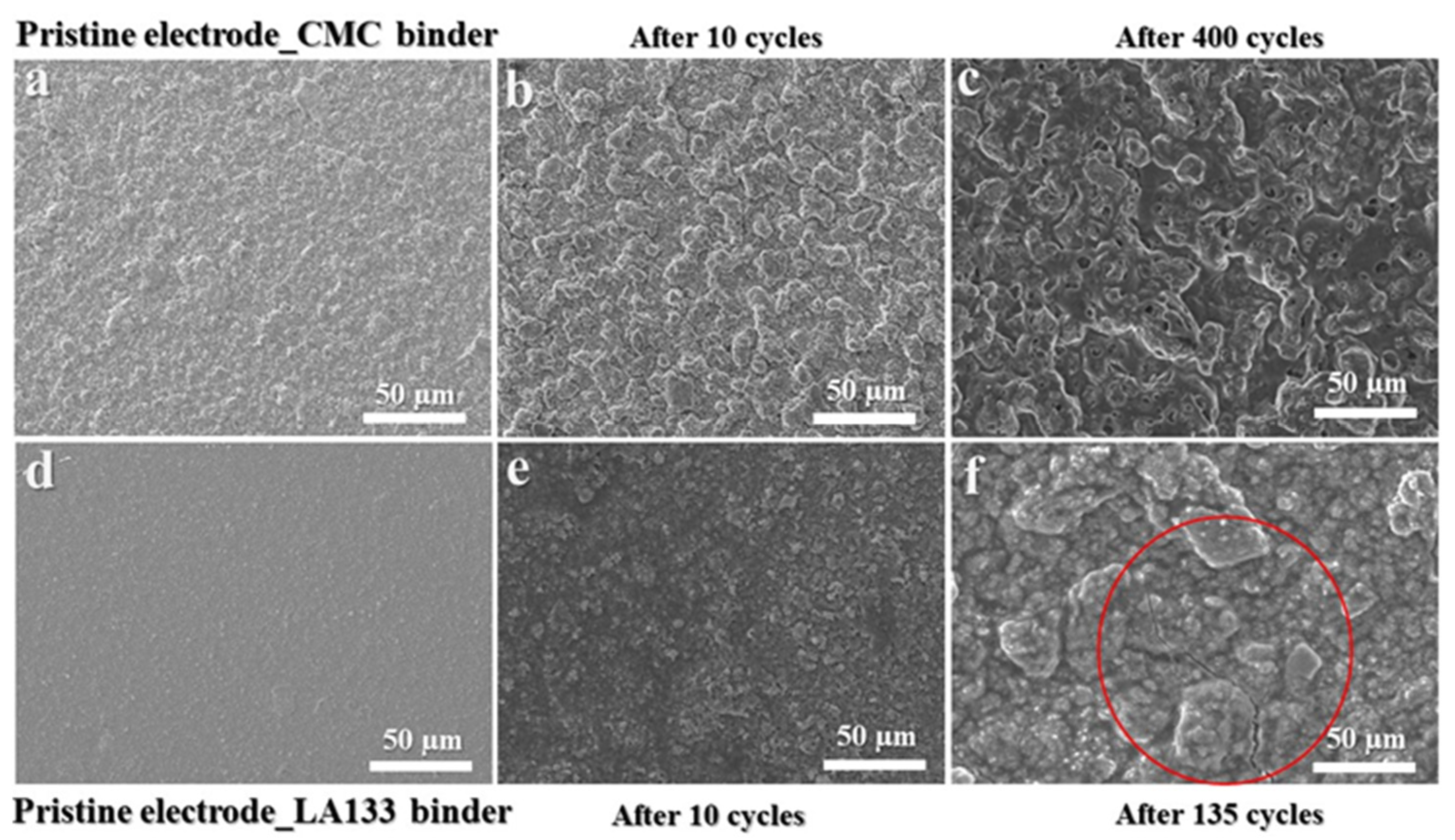
2. Result and Discussion
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
3.3. Cell Fabrication & Electrochemical Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Goodenough, J.B. Evolution of Strategies for Modern Rechargeable Batteries. Acc. Chem. Res. 2013, 46, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Goodenough, J.B.; Zaghib, K. Tribute to Michel Armand: From Rocking Chair—Li-Ion to Solid-State Lithium Batteries. J. Electrochem. Soc. 2020, 167, 070507. [Google Scholar] [CrossRef]
- Fang, C.; Wang, X.; Meng, Y.S. Key Issues Hindering a Practical Lithium-Metal Anode. Trends Chem. 2019, 1, 152–158. [Google Scholar] [CrossRef]
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and Optimization of Lithium-Ion Battery as an Efficient Energy Storage Device for Electric Vehicles: A Comprehensive Review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Zou, F.; Manthiram, A. A Review of the Design of Advanced Binders for High-Performance Batteries. Adv. Energy Mater. 2020, 10, 2002508. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Oli, N.; Gomez, J.F.F.; Qiu, S.; Tian, H.; Qiu, Q.; Sun, W.; Li, K.; Liu, Z.; et al. A Non-Volatile, Thermo-Reversible, and Self-Protective Gel Electrolyte Providing Highly Precise and Reversible Thermal Protection for Lithium Batteries. Adv. Energy Mater. 2023, 13, 2300143. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, W.; Chen, X.; Shan, R.; Han, Q.; Qiu, X.; Oli, N.; Florez Gomez, J.F.; Zhu, L.; Wu, X.; et al. Bimetallic Cobalt-Nickel Selenide Nanocubes Embedded in a Nitrogen-Doped Carbon Matrix as an Excellent Li-Ion Battery Anode. ACS Appl. Mater. Interfaces 2023, 15, 25536–25549. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, B.; Shen, Y.; Wu, J.; Zhao, Z.; Zhong, C.; Hu, W. Confronting the Challenges in Lithium Anodes for Lithium Metal Batteries. Adv. Sci. 2021, 8, 2101111. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, N.; Chiluwal, S.; Parajuli, P.; Rowland, A.; Podila, R. Insights into the Pseudocapacitive Behavior of Sulfurized Polymer Electrodes for Li–S Batteries. Adv. Sci. 2023, 10, 2206901. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.G. High Rate and Stable Cycling of Lithium Metal Anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.K.; Niu, Y.; Hussain, T.; Tabassum, H.; Tang, W.; Xu, M.; Ahuja, R. How to Avoid Dendrite Formation in Metal Batteries: Innovative Strategies for Dendrite Suppression. Nano Energy 2021, 86, 106142. [Google Scholar] [CrossRef]
- Peled, E.; Menachem, C.; Bar-Tow, D.; Melman, A. Improved Graphite Anode for Lithium-Ion Batteries Chemically: Bonded Solid Electrolyte Interface and Nanochannel Formation. J. Electrochem. Soc. 1996, 143, L4–L7. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as Anode Materials: Fundamental Mechanism, Recent Progress and Advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Ren, D.; Wang, L.; Ren, Y.; Xu, W.; Lapidus, S.; Wang, H.; He, X.; Chen, Z.; et al. In Situ Observation of Thermal-Driven Degradation and Safety Concerns of Lithiated Graphite Anode. Nat. Commun. 2021, 12, 4235. [Google Scholar] [CrossRef]
- Aurbach, D.; Zinigrad, E.; Cohen, Y.; Teller, H. A Short Review of Failure Mechanisms of Lithium Metal and Lithiated Graphite Anodes in Liquid Electrolyte Solutions. Solid. State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Chen, G.; Yan, L.; Luo, H.; Guo, S. Nanoscale Engineering of Heterostructured Anode Materials for Boosting Lithium-Ion Storage. Adv. Mater. 2016, 28, 7580–7602. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Mu, G.; Ma, C.; Mu, D.; Wu, F. Recent Progress and Perspectives on Silicon Anode: Synthesis and Prelithiation for LIBs Energy Storage. J. Energy Chem. 2022, 64, 615–650. [Google Scholar] [CrossRef]
- Ying, H.; Han, W.Q. Metallic Sn-Based Anode Materials: Application in High-Performance Lithium-Ion and Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1700298. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Xie, Z.; Liu, Z.; Zheng, X.; Zheng, J.; Li, X. Aluminum: An Underappreciated Anode Material for Lithium-Ion Batteries. Energy Storage Mater. 2020, 25, 93–99. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, R. High-Capacity Graphene-Confined Antimony Nanoparticles as a Promising Anode Material for Potassium-Ion Batteries. J. Alloys Compd. 2020, 834, 155191. [Google Scholar] [CrossRef]
- Fugattini, S.; Gulzar, U.; Andreoli, A.; Carbone, L.; Boschetti, M.; Bernardoni, P.; Gjestila, M.; Mangherini, G.; Camattari, R.; Li, T.; et al. Binder-Free Nanostructured Germanium Anode for High Resilience Lithium-Ion Battery. Electrochim. Acta 2022, 411, 139832. [Google Scholar] [CrossRef]
- Devina, W.; Setiadi Cahyadi, H.; Albertina, I.; Chandra, C.; Park, J.H.; Yoon Chung, K.; Chang, W.; Kyu Kwak, S.; Kim, J. High-Energy–Density Carbon-Coated Bismuth Nanodots on Hierarchically Porous Molybdenum Carbide for Superior Lithium Storage. Chem. Eng. J. 2022, 432, 134276. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Figgemeier, E. Confronting the Challenges of Next-Generation Silicon Anode-Based Lithium-Ion Batteries: Role of Designer Electrolyte Additives and Polymeric Binders. ChemSusChem 2019, 12, 2515–2539. [Google Scholar] [CrossRef]
- Imashuku, S.; Taguchi, H.; Fujieda, S.; Suzuki, S.; Wagatsuma, K. Three-Dimensional Lithium Mapping of Graphite Anode Using Laser-Induced Breakdown Spectroscopy. Electrochim. Acta 2019, 293, 78–83. [Google Scholar] [CrossRef]
- He, Y.; Jiang, L.; Chen, T.; Xu, Y.; Jia, H.; Yi, R.; Xue, D.; Song, M.; Genc, A.; Bouchet-Marquis, C.; et al. Progressive Growth of the Solid–Electrolyte Interphase towards the Si Anode Interior Causes Capacity Fading. Nat. Nanotechnol. 2021, 16, 1113–1120. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Yao, T.; Liu, T.; Tian, Y.; Li, C.; Li, F.; Meng, L.; Cheng, Y. Porous N-Doped Carbon Nanoflakes Supported Hybridized SnO2/Co3O4 Nanocomposites as High-Performance Anode for Lithium-Ion Batteries. J. Colloid. Interface Sci. 2020, 560, 546–554. [Google Scholar] [CrossRef]
- Revathi, J.; Jyothirmayi, A.; Rao, T.N.; Deshpande, A.S.; Revathi, J.; Jyothirmayi, A.; Rao, T.N.; Deshpande, A.S. Wood-Derived Carbon Fibers Embedded with SnOx Nanoparticles as Anode Material for Lithium-Ion Batteries. Glob. Chall. 2020, 4, 1900048. [Google Scholar] [CrossRef]
- Shin, Y.; Ding, H.; Persson, K.A. Revealing the Intrinsic Li Mobility in the Li2MnO3 Lithium-Excess Material. Chem. Mater. 2016, 28, 2081–2088. [Google Scholar] [CrossRef]
- Abouimrane, A.; Cui, Y.; Chen, Z.; Belharouak, I.; Yahia, H.B.; Wu, H.; Assary, R.; Curtiss, L.A.; Amine, K. Enabling High Energy Density Li-Ion Batteries through Li2O Activation. Nano Energy 2016, 27, 196–201. [Google Scholar] [CrossRef]
- Zhu, J.; Ding, Y.; Ma, Z.; Tang, W.; Chen, X.; Lu, Y. Recent Progress on Nanostructured Transition Metal Oxides As Anode Materials for Lithium-Ion Batteries. J. Electron. Mater. 2022, 51, 3391–3417. [Google Scholar] [CrossRef]
- Muchuweni, E.; Mombeshora, E.T.; Muiva, C.M.; Sathiaraj, T.S. Lithium-Ion Batteries: Recent Progress in Improving the Cycling and Rate Performances of Transition Metal Oxide Anodes by Incorporating Graphene-Based Materials. J. Energy Storage 2023, 73, 109013. [Google Scholar] [CrossRef]
- Ortiz, W.; Malca, C.; Barrionuevo, D.; Aldalbahi, A.; Pacheco, E.; Oli, N.; Feng, P. Two-Dimensional Tungsten Disulfide Nanosheets and Their Application in Self-Powered Photodetectors with Ultra-High Sensitivity and Stability. Vacuum 2022, 201, 111092. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-Sized Transition-Metal Oxides as Negative-Electrode Materials for Lithium-Ion Batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; O’Mullane, A.P.; Kalantar-Zadeh, K. Nanostructured Copper Oxide Semiconductors: A Perspective on Materials, Synthesis Methods and Applications. J. Mater. Chem. C Mater. 2014, 2, 5247–5270. [Google Scholar] [CrossRef]
- Mai, Y.J.; Wang, X.L.; Xiang, J.Y.; Qiao, Y.Q.; Zhang, D.; Gu, C.D.; Tu, J.P. CuO/Graphene Composite as Anode Materials for Lithium-Ion Batteries. Electrochim. Acta 2011, 56, 2306–2311. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, K.; Li, Z.; Xu, S.; Yao, G.; Niu, P.; Zheng, F. Porous CuO@C Composite as High-Performance Anode Materials for Lithium-Ion Batteries. Dalton Trans. 2020, 49, 11597–11604. [Google Scholar] [CrossRef]
- Chai, J.; Wang, K.; Li, Q.; Du, J.; Jiang, L.; Han, N.; Zhang, W.; Tang, B.; Rui, Y. Highly Dispersed CuO Nanoparticle on ZIF-4 Framework as Anode Material for LIBs. J. Alloys Compd. 2022, 914, 165316. [Google Scholar] [CrossRef]
- Pu, F.; Bai, Y.; Lv, J.; Zhao, X.; Wu, G.; Kong, C.; Lei, B.; Zhang, X.; Jin, H.; Yang, Z. Yolk–Shell Cu2O@CuO-Decorated RGO for High-Performance Lithium-Ion Battery Anode. Energy Environ. Mater. 2022, 5, 253–260. [Google Scholar] [CrossRef]
- Ezhyeh, Z.N.; Khodaei, M.; Torabi, F. Review on Doping Strategy in Li4Ti5O12 as an Anode Material for Lithium-Ion Batteries. Ceram. Int. 2023, 49, 7105–7141. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.L.; Shu, C.Y.; Guo, Y.G.; Wang, C.R. Synthesis of CuO/Graphene Nanocomposite as a High-Performance Anode Material for Lithium-Ion Batteries. J. Mater. Chem. 2010, 20, 10661–10664. [Google Scholar] [CrossRef]
- Hu, L.; Yang, Z.; Li, H.; Yang, X.; Li, J.; Wang, P.; Jin, C.; Zhang, C. Three-Dimensional Clusters of Peony-Shaped CuO Nanosheets as a High-Rate Anode for Li-Ion Batteries. RSC Adv. 2021, 11, 10760–10766. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Yue, W.C.; Zheng, S.M.; Li, X.; Gao, N.; Zhu, T.; Zhang, Y.J.; Xia, G.M.; Wang, B. Facile Fabrication of Hollow CuO Nanocubes for Enhanced Lithium/Sodium Storage Performance. CrystEngComm 2021, 23, 6107–6116. [Google Scholar] [CrossRef]
- Xu, Q.; Jiu, H.; Zhang, L.; Song, W.; Wei, H.; Wang, C.; Yang, J.; Guo, F.; Gao, T. Structure Design of CuO/Cu2O@C Heterostructure Polyhedron Accumulated by Hollow Microspheres for High-Performance Lithium Storage. J. Alloys Compd. 2021, 887, 161417. [Google Scholar] [CrossRef]
- Lingappan, N.; Kong, L.; Pecht, M. The Significance of Aqueous Binders in Lithium-Ion Batteries. Renew. Sustain. Energy Rev. 2021, 147, 111227. [Google Scholar] [CrossRef]
- Trivedi, S.; Pamidi, V.; Pinto Bautista, S.; Nur, F.; Shamsudin, A.; Weil, M.; Barpanda, P.; Bresser, D.; Fichtner, M.; Trivedi, S.; et al. Water-Soluble Inorganic Binders for Lithium-Ion and Sodium-Ion Batteries. Adv. Energy Mater. 2024, 14, 2303338. [Google Scholar] [CrossRef]
- Kukay, A.; Polizos, G.; Bott, E.; Ielvev, A.; Tao, R.; Sharma, J.; Li, J. Mechanical and Electrochemical Implications of Drying Temperature on Lithium-Ion Battery Electrodes. Batter. Supercaps 2024, 7, e202400113. [Google Scholar] [CrossRef]
- David, M.; Gerofke, A.; Lange, R.; Kolossa-Gehring, M.; Apel, P. The European Human Biomonitoring Initiative (HBM4EU): Human Biomonitoring Guidance Values (HBM-GVs) for the Aprotic Solvents N-Methyl-2-Pyrrolidone (NMP) and N-Ethyl-2-Pyrrolidone (NEP). Int. J. Hyg. Environ. Health 2021, 238, 113856. [Google Scholar] [CrossRef]
- Salini, P.S.; Gopinadh, S.V.; Kalpakasseri, A.; John, B.; Thelakkattu Devassy, M. Toward Greener and Sustainable Li-Ion Cells: An Overview of Aqueous-Based Binder Systems. ACS Sustain. Chem. Eng. 2020, 8, 4003–4025. [Google Scholar] [CrossRef]
- Chou, S.L.; Pan, Y.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Small Things Make a Big Difference: Binder Effects on the Performance of Li and Na Batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef]
- Li, J.-T.; Wu, Z.-Y.; Lu, Y.-Q.; Zhou, Y.; Huang, Q.-S.; Huang, L.; Sun, S.-G.; Li, J.-T.; Wu, Z.-Y.; Lu, Y.-Q.; et al. Water Soluble Binder, an Electrochemical Performance Booster for Electrode Materials with High Energy Density. Adv. Energy Mater. 2017, 7, 1701185. [Google Scholar] [CrossRef]
- Lestriez, B. Functions of Polymers in Composite Electrodes of Lithium Ion Batteries. Comptes Rendus Chim. 2010, 13, 1341–1350. [Google Scholar] [CrossRef]
- Mazouzi, D.; Karkar, Z.; Hernandez, C.R.; Manero, P.J.; Guyomard, D.; Roué, L.; Lestriez, B. Critical Roles of Binders and Formulation at Multiscales of Silicon-Based Composite Electrodes. J. Power Sources 2015, 280, 533–549. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward Efficient Binders for Li-Ion Battery Si-Based Anodes: Polyacrylic Acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef]
- Parikh, P.; Sina, M.; Banerjee, A.; Wang, X.; D’Souza, M.S.; Doux, J.M.; Wu, E.A.; Trieu, O.Y.; Gong, Y.; Zhou, Q.; et al. Role of Polyacrylic Acid (PAA) Binder on the Solid Electrolyte Interphase in Silicon Anodes. Chem. Mater. 2019, 31, 2535–2544. [Google Scholar] [CrossRef]
- Wang, R.; Feng, L.; Yang, W.; Zhang, Y.; Zhang, Y.; Bai, W.; Liu, B.; Zhang, W.; Chuan, Y.; Zheng, Z.; et al. Effect of Different Binders on the Electrochemical Performance of Metal Oxide Anode for Lithium-Ion Batteries. Nanoscale Res. Lett. 2017, 12, 575. [Google Scholar] [CrossRef]
- Parekh, Z.R.; Chaki, S.H.; Hirpara, A.B.; Patel, G.H.; Kannaujiya, R.M.; Khimani, A.J.; Deshpande, M.P. CuO Nanoparticles – Synthesis by Wet Precipitation Technique and Its Characterization. Physica B Condens. Matter 2021, 610, 412950. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Ren, Z.; Chen, Y.; Huang, J.; Lei, Z.; Qian, X.; Lai, Y.; Zhang, S. A Quadruple Biomimetic Hydrophilic/Hydrophobic Janus Composite Material Integrating Cu(OH)2 Micro-Needles and Embedded Bead-on-String Nanofiber Membrane for Efficient Fog Harvesting. Chem. Eng. J. 2023, 455, 140863. [Google Scholar] [CrossRef]
- Iqbal, T.; ur Rehman, A.; Khan, M.A.; Shafique, M.; Ahmad, P.; Mahmood, H.; Naeem, M.; Iqbal, J. Copper Oxide Nanosheets Prepared by Facile Microplasma Electrochemical Technique with Photocatalytic and Bactericidal Activities. J. Mater. Sci. Mater. Electron. 2020, 31, 16649–16660. [Google Scholar] [CrossRef]
- Musa, A.M.M.; Farhad, S.F.U.; Gafur, M.A.; Jamil, A.T.M.K. Effects of Withdrawal Speed on the Structural, Morphological, Electrical, and Optical Properties of CuO Thin Films Synthesized by Dip-Coating for CO2 Gas Sensing. AIP Adv. 2021, 11, 115004. [Google Scholar] [CrossRef]
- Asbrink, S.; Waskowska, A. CuO: X-Ray Single-Crystal Structure Determination at 196 K and Room Temperature. J. Phys. Condens. Matter 1991, 3, 8173. [Google Scholar] [CrossRef]
- Kaushik, H.K.; Kaur, A.; Garg, V.; Abida, K.; Kumar, S.; Singh, K.; Singh, S.P.; Khan, S. Effect of CuO on Physical, Structural and Optical Properties of Lithium Borosilicate Glasses. Mater. Today Commun. 2023, 35, 106208. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Guo, H.; Tong, X. Synthesis of CuO Nanowire Arrays as High-Performance Electrode for Lithium Ion Batteries. Mater. Lett. 2015, 139, 55–58. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, W.; Zhou, G.; Huang, Z.; Zhang, Y.; Lyu, R.; Wu, H.; Yun, Q.; Kang, F.; Yang, Q.-H.; et al. Vertically Aligned Lithiophilic CuO Nanosheets on a Cu Collector to Stabilize Lithium Deposition for Lithium Metal Batteries. Adv. Energy Mater. 2018, 8, 1703404. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, G.; Gu, H.; Yang, Z.; Cheng, H. A New Lithium-Ion Battery: CuO Nanorod Array Anode versus Spinel LiNi0.5Mn1.5O4 Cathode. J. Power Sources 2015, 273, 561–565. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, N.; Sun, K. Facile Fabrication of CuO Mesoporous Nanosheet Cluster Array Electrodes with Super Lithium-Storage Properties. J. Mater. Chem. 2012, 22, 13637–13642. [Google Scholar] [CrossRef]
- Yuan, S.; Huang, X.-L.; Ma, D.-L.; Wang, H.-G.; Meng, F.-Z.; Zhang, X.-B.; Yuan, S.; Huang, X.-L.; Ma, D.-L.; Wang, H.-G.; et al. Engraving Copper Foil to Give Large-Scale Binder-Free Porous CuO Arrays for a High-Performance Sodium-Ion Battery Anode. Adv. Mater. 2014, 26, 2273–2279. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, X.; Li, J.; Liu, J.H.; Huang, X.J. ZnO/CuO Hetero-Hierarchical Nanotrees Array: Hydrothermal Preparation and Self-Cleaning Properties. Langmuir 2011, 27, 6193–6200. [Google Scholar] [CrossRef]
- Yuan, W.; Luo, J.; Pan, B.; Qiu, Z.; Huang, S.; Tang, Y. Hierarchical Shell/Core CuO Nanowire/Carbon Fiber Composites as Binder-Free Anodes for Lithium-Ion Batteries. Electrochim. Acta 2017, 241, 261–271. [Google Scholar] [CrossRef]
- Deng, C.; Hu, H.; Ge, X.; Han, C.; Zhao, D.; Shao, G. One-Pot Sonochemical Fabrication of Hierarchical Hollow CuO Submicrospheres. Ultrason. Sonochem 2011, 18, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cai, X.; Shi, W. Free-Standing Graphene/Carbon Nanotubes/CuO Aerogel Paper Anode for Lithium Ion Batteries. Mater. Lett. 2016, 172, 72–75. [Google Scholar] [CrossRef]
- Pu, F.; Kong, C.; Lv, J.; BoMa; Zhang, W.; Zhang, X.; Yang, S.; Jin, H.; Yang, Z. CuO Ultrathin Nanosheets Decorated Reduced Graphene Oxide as a High Performance Anode for Lithium-Ion Batteries. J. Alloys Compd. 2019, 805, 355–362. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, X.; Ni, J.; Zhang, S.; Yang, J.; Liu, P.; Wang, Z.; Lei, J. Evaporation Induced Uniform Polypyrrole Coating on CuO Arrays for Free-Standing High Lithium Storage Anode. J. Solid. State Electrochem. 2019, 23, 1829–1836. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Marwani, H.M.; Asiri, A.M.; Alamry, K.A. Selective Iron(III) Ion Uptake Using CuO-TiO2 Nanostructure by Inductively Coupled Plasma-Optical Emission Spectrometry. Chem. Cent. J. 2012, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.S.; Lin, L.; Ma, Y.T.; Yang, J.R.; Huang, J.; Wang, L.S.; Peng, D.L. Facile Fabrication of ZnO–CuO Porous Hybrid Microspheres as Lithium Ion Battery Anodes with Enhanced Cyclability. Rare Met. 2017, 36, 403–410. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, H.; Yin, J.; Zhu, H.; Zhou, Y.; Tang, Y.; Wu, P.; Lu, T. Facile Preparation of CuO@SnO2 Nanobelts as a High-Capacity and Long-Life Anode for Lithium-Ion Batteries. RSC Adv. 2014, 4, 34417–34420. [Google Scholar] [CrossRef]
- Di Lecce, D.; Verrelli, R.; Campanella, D.; Marangon, V.; Hassoun, J. A New CuO-Fe2O3-Mesocarbon Microbeads Conversion Anode in a High-Performance Lithium-Ion Battery with a Li1.35Ni0.48Fe0.1Mn1.72O4 Spinel Cathode. ChemSusChem 2017, 10, 1607–1615. [Google Scholar] [CrossRef]
- Dong, Y.; Jiang, X.; Mo, J.; Zhou, Y.; Zhou, J. Hollow CuO Nanoparticles in Carbon Microspheres Prepared from Cellulose-Cuprammonium Solution as Anode Materials for Li-Ion Batteries. Chem. Eng. J. 2020, 381, 122614. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, R.; Zhang, X.; Zong, P.; Bai, Y.; Jin, H.; Xu, H.; Lian, K.; Ma, F. Cotton as a Sustainable Source of CuxO/C Anode for High-Performance Li-Ion Battery. Ionics 2019, 25, 2519–2524. [Google Scholar] [CrossRef]
- Chen, Z.; Hou, Z.; Xu, W.; Chen, Y.; Li, Z.; Chen, L.; Wang, W. Ultrafine CuO Nanoparticles Decorated Activated Tube-like Carbon as Advanced Anode for Lithium-Ion Batteries. Electrochim. Acta 2019, 296, 206–213. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Chen, Y.; Zhang, W.; Li, K.; Zhang, H. In Situ Fabrication of Hierarchical CuO@Cu Microspheres Composed of Nanosheets as High-Performance Anode Materials for Lithium-Ion Batteries. ChemistrySelect 2019, 4, 13569–13575. [Google Scholar] [CrossRef]
- Su, J.; Gao, Z.; Xie, Y.; Zhang, Z.; Wang, H. Boosting Li-Storage Properties of Conversion-Type Anodes for Lithium-Ion Batteries via Steric Effect of Intercalation-Type Materials: A Case of MnCO3. Compos. B Eng. 2021, 212, 108733. [Google Scholar] [CrossRef]
- Oli, N.; Ortiz Lago, W.; Tripathi, B.; Bhattarai, M.; Weiner, B.R.; Morell, G.; Katiyar, R.S. Enhanced Electrochemical Performance of Bi2O3 via Facile Synthesis as Anode Material for Ultra-Long Cycle Lifespan Lithium-Ion Batteries. Electrochem. Commun. 2024, 159, 107656. [Google Scholar] [CrossRef]
- Song, Z.; Wang, L.; Yang, K.; Gong, Y.; Yang, L.; Liu, X.; Pan, F. Intermolecular Chemistry for Designing Functional Binders in Silicon/Carbon Composite Anodes. Mater. Today Energy 2022, 30, 101153. [Google Scholar] [CrossRef]
- Chung, G.J.; Tran, Y.H.T.; Han, J.; Kim, K.; Lee, Y.S.; Song, S.W. Novel Additives-Package to Mitigate the Failure Modes of High-Capacity LiNi0.82Co0.11Mn0.07O2-Based Lithium-Ion Battery. Chem. Eng. J. 2022, 446, 137288. [Google Scholar] [CrossRef]
- Su, Y.; Liu, T.; Zhang, P.; Zheng, P. CuO Nanowire Arrays Synthesized at Room Temperature as a High-Performance Anode Material for Li/Na-Ion Batteries. Thin Solid. Films 2019, 690, 137522. [Google Scholar] [CrossRef]
- Yoon, D.H.; Marinaro, M.; Axmann, P.; Wohlfahrt-Mehrens, M. Study of the Binder Influence on Expansion/Contraction Behavior of Silicon Alloy Negative Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 160537. [Google Scholar] [CrossRef]
- Huang, W.; Wang, W.; Wang, Y.; Qu, Q.; Jin, C.; Zheng, H. Overcoming the Fundamental Challenge of PVDF Binder Use with Silicon Anodes with a Super-Molecular Nano-Layer. J. Mater. Chem. A Mater. 2021, 9, 1541–1551. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.H.; Ahn, K.H. Role of Carboxymethyl Cellulose Binder and Its Effect on the Preparation Process of Anode Slurries for Li-Ion Batteries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131130. [Google Scholar] [CrossRef]
- Akhlaq, M.; Mushtaq, U.; Naz, S.; Uroos, M. Carboxymethyl Cellulose-Based Materials as an Alternative Source for Sustainable Electrochemical Devices: A Review. RSC Adv. 2023, 13, 5723–5743. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhao, L.; Wan, J.; Shao, Z.; Wang, F.; Lv, S. The Electrochemical Performance of Carboxymethyl Cellulose Lithium as a Binding Material for Anthraquinone Cathodes in Lithium Batteries. J. Electrochem. Soc. 2012, 159, A499–A505. [Google Scholar] [CrossRef]
- Lestriez, B.; Bahri, S.; Sandu, I.; Roué, L.; Guyomard, D. On the Binding Mechanism of CMC in Si Negative Electrodes for Li-Ion Batteries. Electrochem. Commun. 2007, 9, 2801–2806. [Google Scholar] [CrossRef]

| Ref. | Samples | Rate | Cycles | Capacity | Notes |
|---|---|---|---|---|---|
| [80] | Hollow CuO nanoparticles | 100 | 100 | 630 | Time and energy consuming and expensive synthesis route |
| [46] | CuO/Cu2O/C composites | 200 | 600 | 260 | Energy consuming method |
| [81] | CuxO/C anode | 100 | 100 | 335 | Time and energy consuming |
| [82] | CuO/tube-like carbon | 100 | 100 | 650 | Complicated and expensive synthesis approach |
| [83] | CuO@Cu microspheres | 100 | 100 | 876 | Sensitive route and time consuming |
| [65] | CuO nanowire arrays | 300 | 100 | 550 | Complicated synthesis approach |
| [74] | CuO nanosheets | 100 | 100 | 600 | Poor performance and expensive method |
| [39] | CuO@C | 100 500 | 100 700 | 1024 | Toxic and energy consuming process |
| [44] | Peony shaped CuO nanosheets | 100 1000 | 80 100 | 780 441 | Time consuming |
| This work | Commercial CuO nano powder | 100 300 | 170 500 | 800 450 | Safe, simple, cost-effective, scalable, and financially sustainable strategy for battery production. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oli, N.; Choudhary, S.; Weiner, B.R.; Morell, G.; Katiyar, R.S. Comparative Investigation of Water-Based CMC and LA133 Binders for CuO Anodes in High-Performance Lithium-Ion Batteries. Molecules 2024, 29, 4114. https://doi.org/10.3390/molecules29174114
Oli N, Choudhary S, Weiner BR, Morell G, Katiyar RS. Comparative Investigation of Water-Based CMC and LA133 Binders for CuO Anodes in High-Performance Lithium-Ion Batteries. Molecules. 2024; 29(17):4114. https://doi.org/10.3390/molecules29174114
Chicago/Turabian StyleOli, Nischal, Sunny Choudhary, Brad R. Weiner, Gerardo Morell, and Ram S. Katiyar. 2024. "Comparative Investigation of Water-Based CMC and LA133 Binders for CuO Anodes in High-Performance Lithium-Ion Batteries" Molecules 29, no. 17: 4114. https://doi.org/10.3390/molecules29174114





