Investigation into the Synergistic Effect of the Zinc Peroxide/Peroxymonosulfate Double-Oxidation System for the Efficient Degradation of Tetracycline
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of ZnO2 Particles
2.3. Characterizations
2.4. Catalytic Degradation Experiment
3. Results and Discussion
3.1. Characterizations of ZnO2
3.2. Degradation Performance of ZnO2/PMS Double-Oxidation System
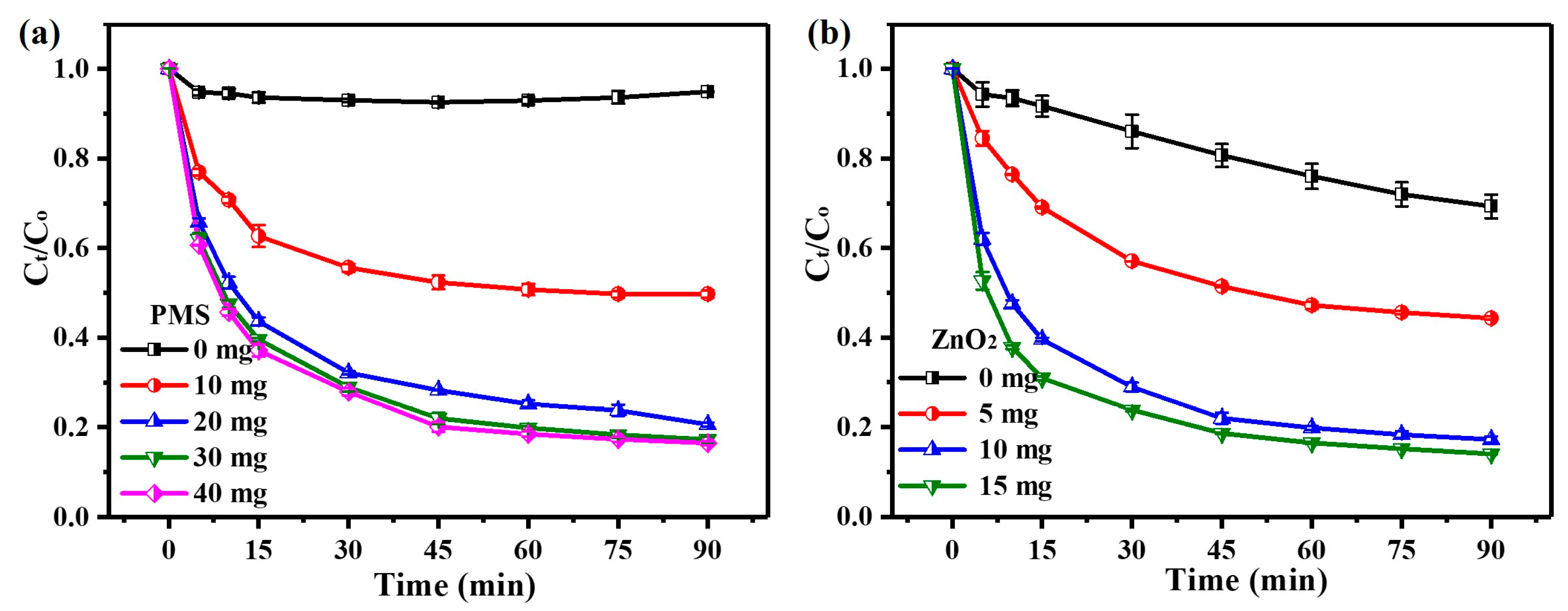
3.2.1. Effects of PMS and ZnO2 Dosages
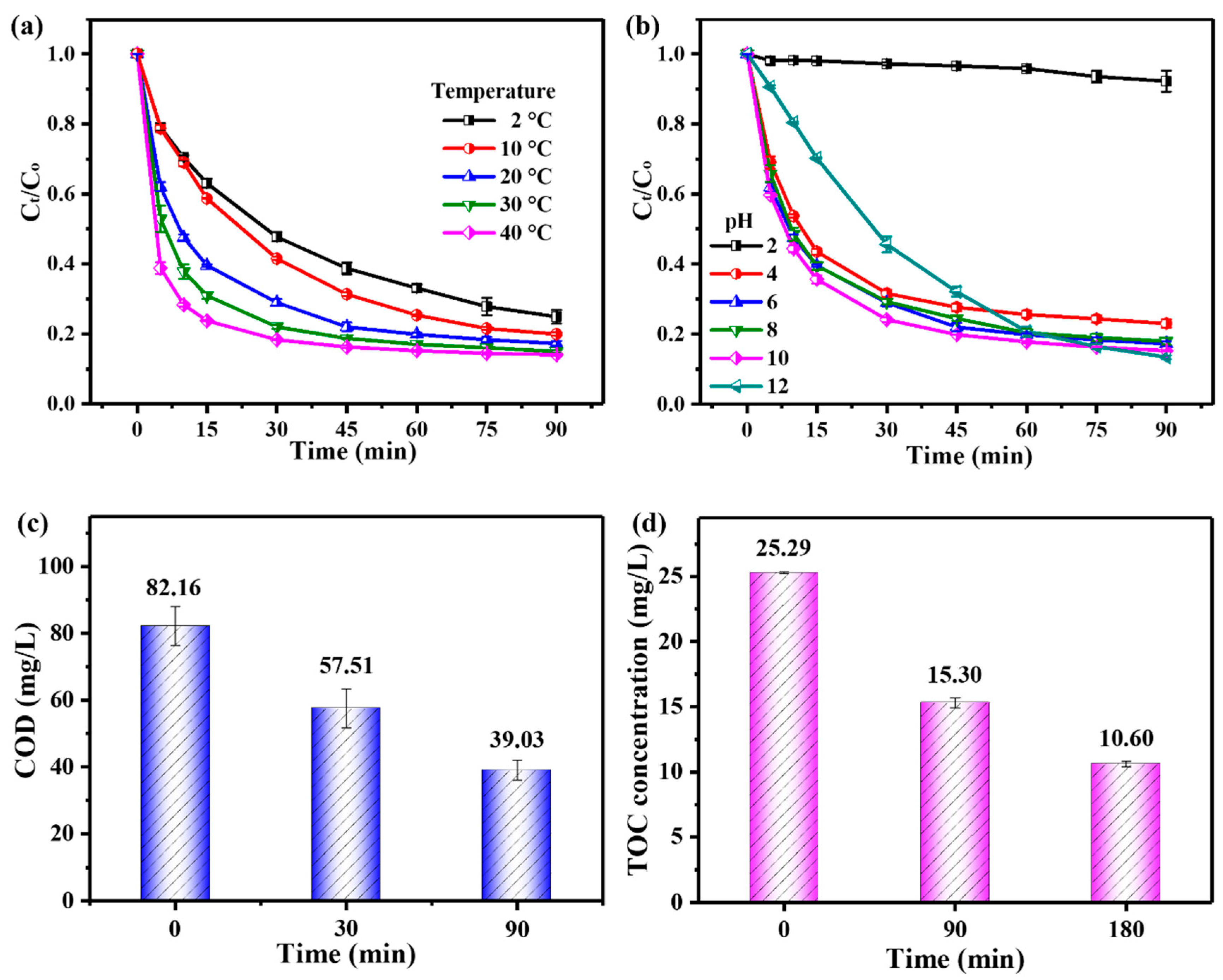
3.2.2. Effects of Temperature and Initial Solution pH
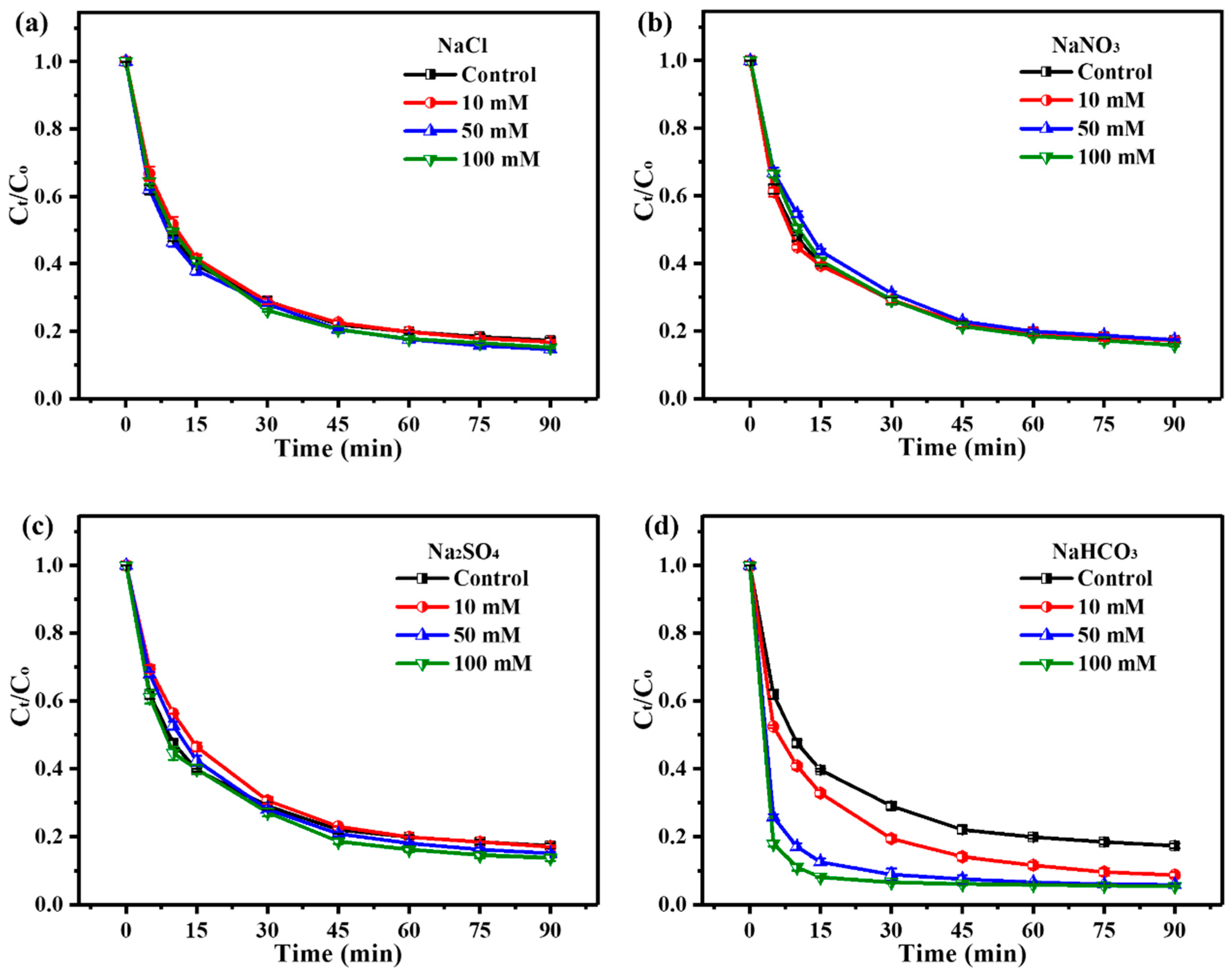
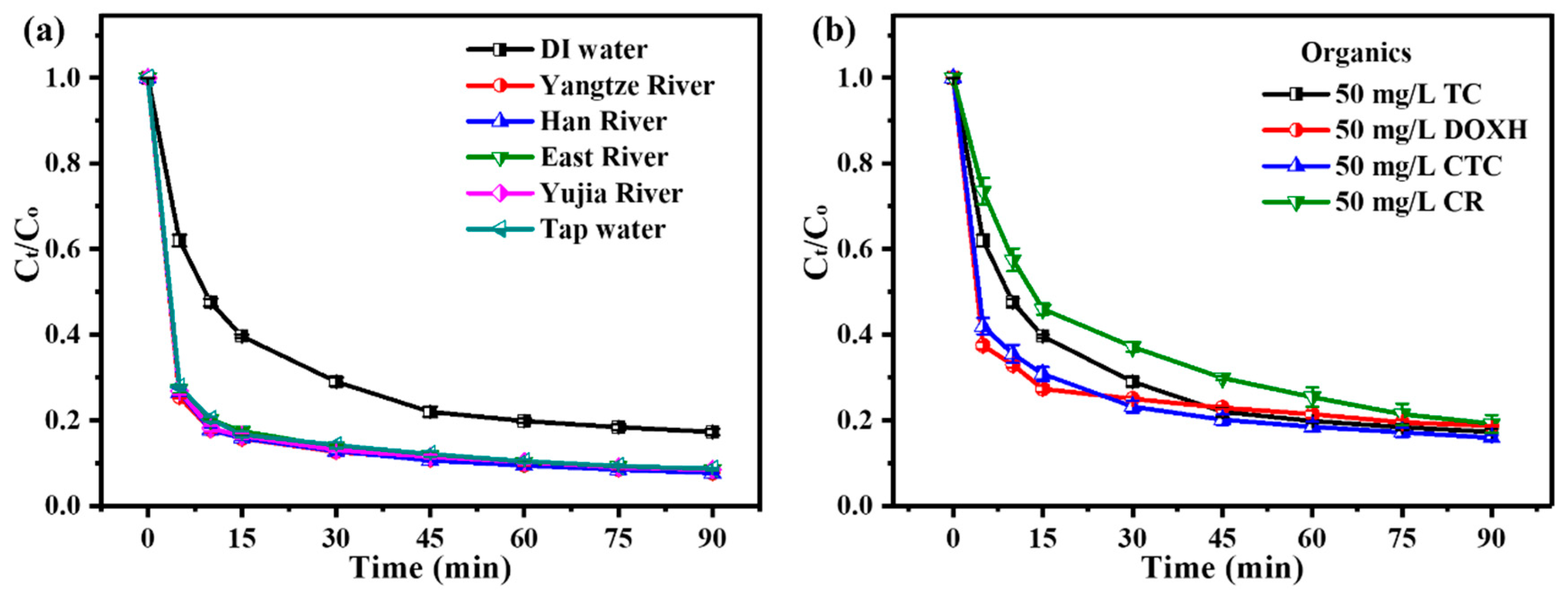
3.2.3. Adaptability of ZnO2/PMS Double-Oxidation System
3.3. Mechanism Analysis
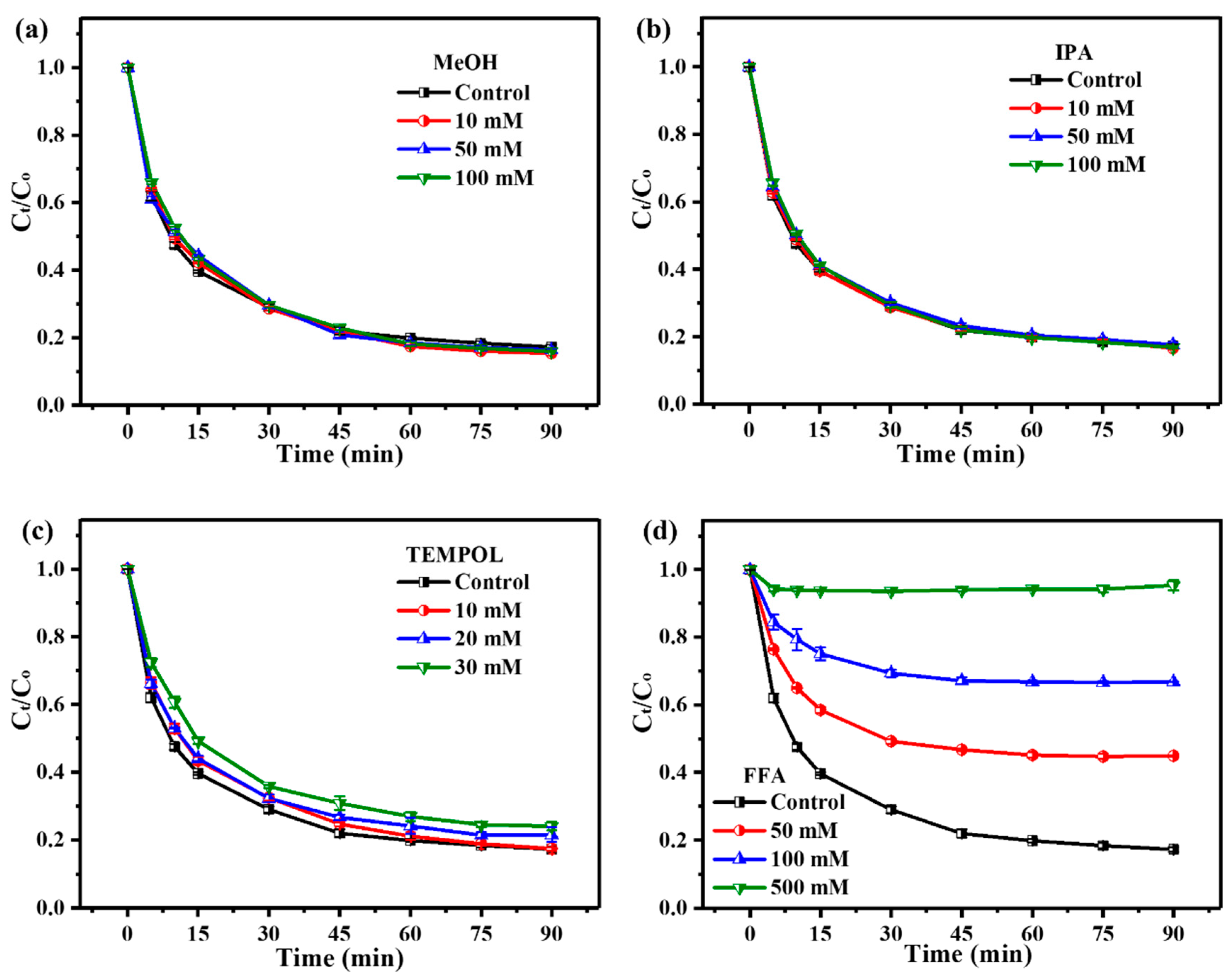
3.3.1. Quenching Experiments
3.3.2. Intermediate Identification and TC Degradation Pathway Investigation
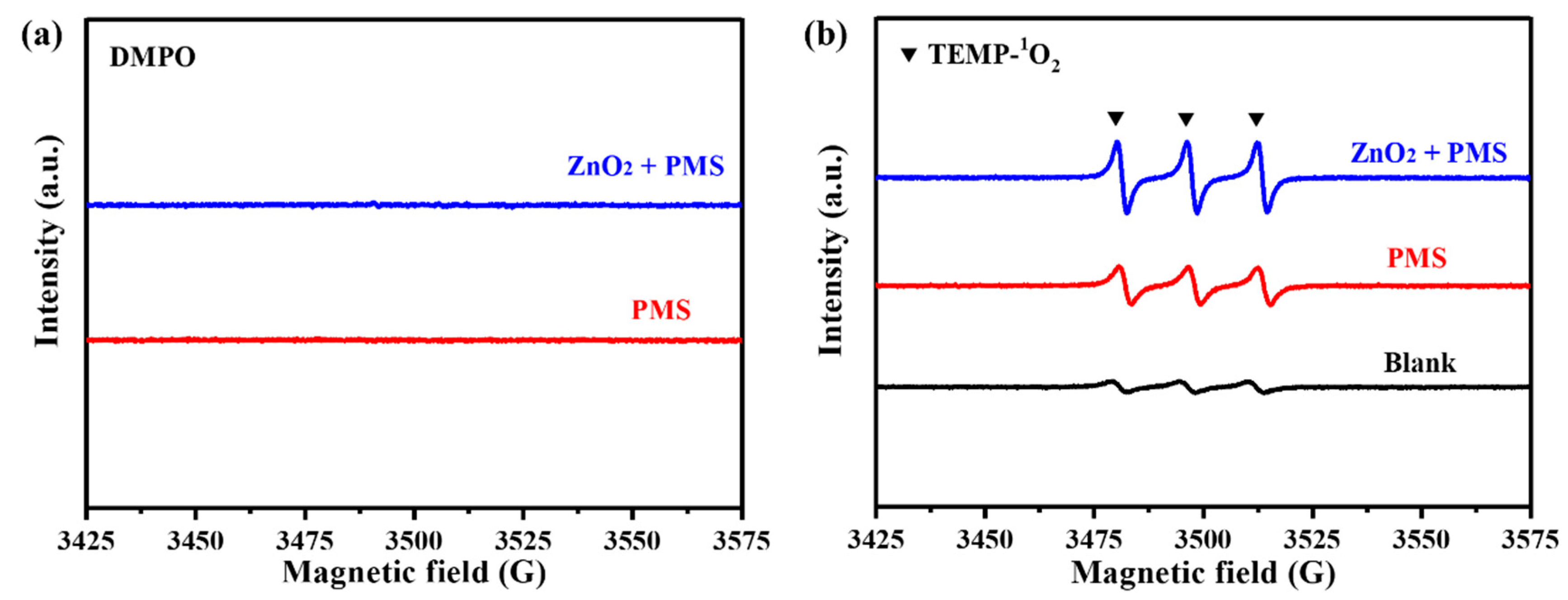
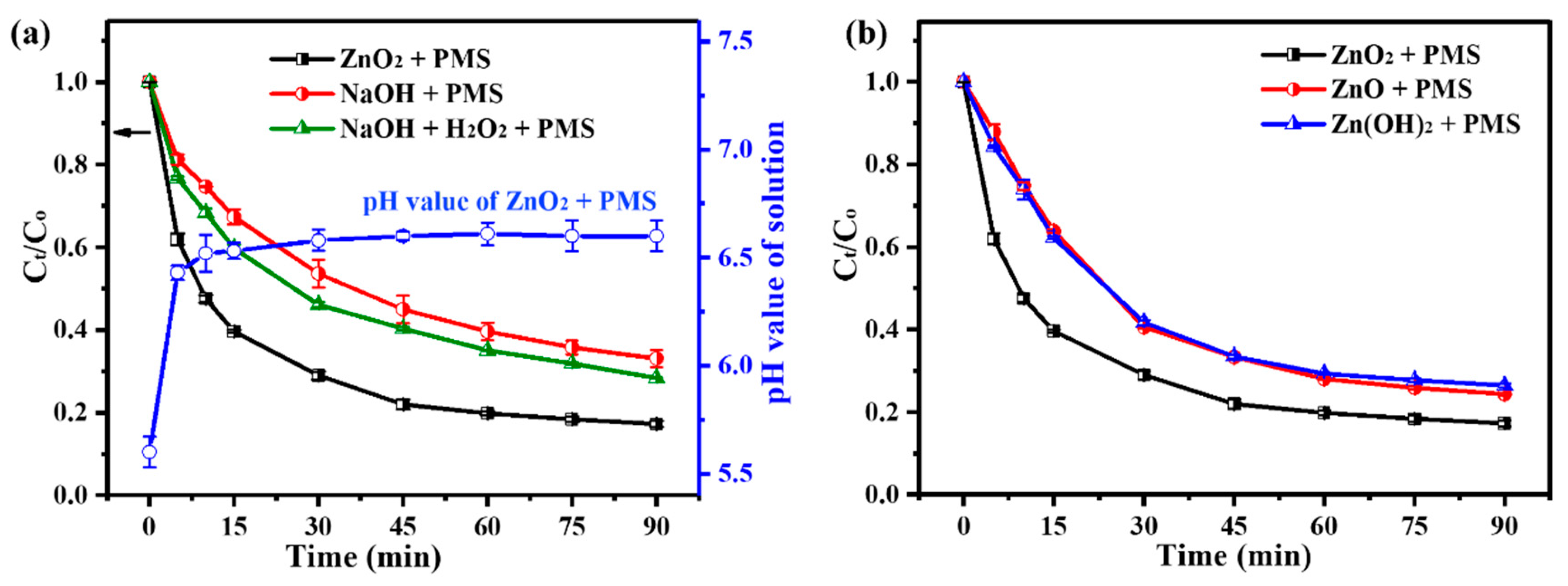
3.3.3. Activation Pathway Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Dong, Y.; Liu, Q.; Liu, W.; Lin, H. MoS2-based nanocomposites and aerogels for antibiotic pollutants removal from wastewater by photocatalytic degradation process: A review. Chemosphere 2024, 354, 141582. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Liu, Y.; Feng, L.; Yang, S.; Tan, X.; Liu, Z. Magnetic Fe3O4-C@MoS2 composites coordinated with peroxymonosulfate catalysis for enhanced tetracycline degradation. J. Alloys Compd. 2024, 989, 174318. [Google Scholar] [CrossRef]
- Zhao, H.; He, T.; Zhang, Q.; Feng, H.; Mi, H.; Liang, Z.; Zhou, D.; Dong, W.; Xue, X. Interfacial bonded K-doped-C3N4@Bi2WO6 heterostructure for efficient photocatalytic degradation of tetracycline. J. Alloys Compd. 2024, 972, 172822. [Google Scholar] [CrossRef]
- Xu, X.; Shao, W.; Tai, G.; Yu, M.; Han, X.; Han, J.; Wu, G.; Xing, W. Single-atomic Co-N site modulated exciton dissociation and charge transfer on covalent organic frameworks for efficient antibiotics degradation via peroxymonosulfate activation. Sep. Purif. Technol. 2024, 333, 125890. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, M.; Huo, B.; Wang, J.; Yang, L.; Ma, W.; Qi, J.; Wang, Y.; Zhu, Z.; Meng, F. A novel ZnO/CQDs/PVDF piezoelectric system for efficiently degradation of antibiotics by using water flow energy in pipeline: Performance and mechanism. Nano Energy 2023, 107, 108162. [Google Scholar] [CrossRef]
- Goudarzi, M.; Abdulhusain, Z.H.; Salavati-Niasari, M. Low-cost and eco-friendly synthesis of Mn-doped Tl2WO4 nanostructures for efficient visible light photocatalytic degradation of antibiotics in water. Sol. Energy 2023, 262, 111912. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Zang, J.; Zhao, X.; Jiang, F.; Jiang, L.; Xiong, C.; Wang, N.; Fu, C. Antibiotic residues of drinking-water and its human exposure risk assessment in rural Eastern China. Water Res. 2023, 236, 119940. [Google Scholar] [CrossRef]
- Yu, X.; Yu, F.; Li, Z.; Zhan, J. Occurrence, distribution, and ecological risk assessment of pharmaceuticals and personal care products in the surface water of the middle and lower reaches of the Yellow River (Henan section). J. Hazard. Mater. 2023, 443, 130369. [Google Scholar] [CrossRef]
- Chen, P.; Dong, N.; Zhang, J.; Wang, W.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. Investigation on visible-light photocatalytic performance and mechanism of zinc peroxide for tetracycline degradation and Escherichia coli inactivation. J. Colloid Interface Sci. 2022, 624, 137–149. [Google Scholar] [CrossRef]
- Li, S.; Zhu, L. Copper regulates degradation of typical antibiotics by microalgal-fungal consortium in simulated swine wastewater: Insights into metabolic routes and dissolved organic matters. Water Res. 2023, 245, 120654. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Huang, Y.; Xiang, Z.; Zeng, R.; Zeng, S.; Liu, S. Polystyrene nanoplastics foster Escherichia coli O157, H7 growth and antibiotic resistance with a stimulating effect on metabolism. Environ. Sci. Nano 2023, 10, 1341–1351. [Google Scholar] [CrossRef]
- Zhang, S.; Han, W.; Liu, T.; Feng, C.; Jiang, Q.; Zhang, B.; Chen, Y.; Zhang, Y. Tetracycline inhibits the nitrogen fixation ability of soybean (Glycine max (L.) Merr.) nodules in black soil by altering the root and rhizosphere bacterial communities. Sci. Total Environ. 2024, 908, 168047. [Google Scholar] [CrossRef] [PubMed]
- Míguez-González, A.; Cela-Dablanca, R.; Barreiro, A.; Rodríguez-López, L.; Rodríguez-Seijo, A.; Arias-Estévez, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Castillo-Ramos, V.; Álvarez-Rodríguez, E. Adsorption of antibiotics on bio-adsorbents derived from the forestry and agro-food industries. Environ. Res. 2023, 233, 116360. [Google Scholar] [CrossRef]
- Zhi, S.; Shen, S.; Zhou, J.; Ding, G.; Zhang, K. Systematic analysis of occurrence, density and ecological risks of 45 veterinary antibiotics: Focused on family livestock farms in Erhai Lake basin, Yunnan, China. Environ. Pollut. 2020, 267, 115539. [Google Scholar] [CrossRef]
- Ali, H.; Masar, M.; Yasir, M.; Machovsky, M.; Monteiro, O.C.; Kuritka, I. Current trends in environmental and energy photocatalysis and ISO standardization. J. Environ. Chem. Eng. 2023, 11, 111541. [Google Scholar] [CrossRef]
- Mutke, X.A.M.; Swiderski, P.; Drees, F.; Akin, O.; Lutze, H.V.; Schmidt, T.C. Efficiency of ozonation and sulfate radical-AOP for removal of pharmaceuticals, corrosion inhibitors, X-ray contrast media and perfluorinated compounds from reverse osmosis concentrates. Water Res. 2024, 255, 121346. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; You, X.; Li, Y.; Lin, H.; Chen, J.P. A novel bimetallic Fe-Cu-CNT catalyst for effective catalytic wet peroxide oxidation: Reaction optimization and mechanism investigation. Chem. Eng. J. 2024, 479, 147320. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, W.; Guo, Y.; Zhao, T.; Zhou, H.; Wang, Q.; Li, H.; Guo, Z.; Xu, B.B.; Gu, H. Magnetic field facilitated electrocatalytic degradation of tetracycline in wastewater by magnetic porous carbonized phthalonitrile resin. Appl. Catal. B Environ. 2024, 340, 123225. [Google Scholar] [CrossRef]
- Xie, Z.H.; He, C.S.; Zhou, H.Y.; Li, L.L.; Liu, Y.; Du, Y.; Liu, W.; Mu, Y.; Lai, B. Effects of molecular structure on organic contaminants’ degradation efficiency and dominant ROS in the advanced oxidation process with multiple ROS. Environ. Sci. Technol. 2022, 56, 8784–8795. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Meng, C.; Zhang, Z. Nanoconfined catalytic membranes assembled by cobalt-functionalized graphitic carbon nitride nanosheets for rapid degradation of pollutants. Appl. Catal. B Environ. 2023, 322, 122098. [Google Scholar] [CrossRef]
- Tang, R.; Zeng, H.; Deng, Y.; Xiong, S.; Li, L.; Zhou, Z.; Wang, J.; Tang, L. Dual modulation on peroxymonosulfate activation site and photocarrier separation in carbon nitride for efficient photocatalytic organics degradation: Efficacy and mechanism evaluation. Appl. Catal. B Environ. 2023, 336, 122918. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal-and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, H.; Yao, B.; Gao, X.; Yang, X.; Zhou, Y. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review. Chem. Eng. J. 2021, 414, 128800. [Google Scholar] [CrossRef]
- Ahn, Y.Y.; Choi, J.; Kim, M.; Kim, M.S.; Lee, D.; Bang, W.H.; Yun, E.T.; Lee, H.; Lee, J.H.; Lee, C.; et al. Chloride-mediated enhancement in heat-induced activation of peroxymonosulfate: New reaction pathways for oxidizing radical production. Environ. Sci. Technol. 2021, 55, 5382–5392. [Google Scholar] [CrossRef] [PubMed]
- Alayande, A.B.; Hong, S. Ultraviolet light-activated peroxymonosulfate (UV/PMS) system for humic acid mineralization: Effects of ionic matrix and feasible application in seawater reverse osmosis desalination. Environ. Pollut. 2022, 307, 119513. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.; Cui, M.; Ren, Y.; Park, B.; Ma, J.; Han, Z.; Khim, J. Activation of peroxodisulfate and peroxymonosulfate by ultrasound with different frequencies: Impact on ibuprofen removal efficient, cost estimation and energy analysis. Chem. Eng. J. 2021, 413, 127487. [Google Scholar] [CrossRef]
- Wang, W.; Chen, M.; Wang, D.; Yan, M.; Liu, Z. Different activation methods in sulfate radical-based oxidation for organic pollutants degradation: Catalytic mechanism and toxicity assessment of degradation intermediates. Sci. Total Environ. 2021, 772, 145522. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, T.; Yang, C.; Ma, C.; Zhao, Z.; Wu, Z.; Cao, S.; Geng, W.; Wang, Y.; Yao, Y.; et al. Activity trends and mechanisms in peroxymonosulfate-assisted catalytic production of singlet oxygen over atomic metal-N-C catalysts. Angew. Chem. Int. Ed. 2021, 60, 22513–22521. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, B.; Wang, N.; Zhang, N.; Ma, Y.; Zang, L.; Li, Z.; Xue, R. Refractory organics removal in PMS and H2O2/PMS oxidation system activated by biochar/nZVI/MoS2 composite: Synthesis, performance, mechanism and dosing methods. J. Environ. Chem. Eng. 2023, 11, 109134. [Google Scholar] [CrossRef]
- Zhong, D.; Zhou, Z.; Ma, W.; Ma, J.; Lv, W.; Feng, W.; Du, X.; He, F. Study on degradation of chloramphenicol by H2O2/PMS double-oxidation system catalyzed by pipe deposits from water networks. J. Environ. Chem. Eng. 2022, 10, 107529. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, S.; Duan, X.; Zheng, W.; Shao, C.; Wu, W.; Jiang, Z.; Lai, W. MnO2/UIO-66 improves the catalysed degradation of oxytetracycline under UV/H2O2/PMS system. J. Solid State Chem. 2021, 300, 122231. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Liu, C.; Xia, D.; Ou, X.; Cai, Y.; Zhou, Y.; Jiang, J.; Han, B. Sulfone-Modified Covalent Organic Frameworks Enabling Efficient Photocatalytic Hydrogen Peroxide Generation via One-Step Two-Electron O2 Reduction. Angew. Chem. Int. Ed. 2023, 62, e202305355. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, W.; Dong, N.; Chen, P.; Ge, L.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. A dual-oxidant advanced oxidation process system containing CaO2 and peroxymonosulfate for organic pollutant degradation: High adaptability and synergistic effect. Sep. Purif. Technol. 2023, 308, 122909. [Google Scholar] [CrossRef]
- Abbas, Q.; Shakoor, A.; Naushad, M.; Naushad, M.; Yousaf, B. In-situ oxidative degradation of sulfamethoxazole by calcium peroxide/persulfate dual oxidant system in water and soil. Process Saf. Environ. Prot. 2022, 164, 696–705. [Google Scholar]
- Wolanov, Y.; Prikhodchenko, P.V.; Medvedev, A.V.; Pedahzur, R.; Lev, O. Zinc dioxide nanoparticulates: A hydrogen peroxide source at moderate pH. Environ. Sci. Technol. 2013, 47, 8769–8774. [Google Scholar] [CrossRef]
- Chen, P.; Wang, W.; Dong, N.; Zhang, J.; Yang, T.; Tan, F.; Tan, S.; Wang, X.; Qiao, X.; Wong, P.K. Facile in-situ fabrication of ZnO2/CQD composites with promoted visible-light photocatalytic activities for organic degradation and bacterial inactivation. Appl. Surf. Sci. 2022, 604, 154629. [Google Scholar] [CrossRef]
- Wang, B.; Hu, J.; Liu, K.; Zhang, L.; Jiang, H.; Li, C. Reinforcement mechanism of silica surface hydroxyl: The opposite effect. Appl. Surf. Sci. 2023, 13, 157000. [Google Scholar] [CrossRef]
- Nie, Y.; Zhou, H.; Tian, S.; Tian, X.; Yang, C.; Li, Y.; Tian, Y.; Wang, Y. Anionic ligands driven efficient ofloxacin degradation over LaMnO3 suspended particles in water due to the enhanced peroxymonosulfate activation. Chem. Eng. J. 2022, 427, 130998. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, K.; He, J.; Li, N.; You, H.; Jiang, J. Droplet spray ionization mass spectrometry for real-time monitoring of activation of peroxymonosulfate by 1,4-benzoquinone. Microchem. J. 2018, 139, 437–442. [Google Scholar] [CrossRef]
- Liang, S.; Zheng, W.; Zhu, L.; Duan, W.; Wei, C.; Feng, C. One-Step treatment of phosphite-laden wastewater: A single electrochemical reactor integrating superoxide radical-induced oxidation and electrocoagulation. Environ. Sci. Technol. 2019, 53, 5328–5336. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Li, W.; Zhong, R.; Lan, Y.; Guo, J. The efficient degradation of sulfisoxazole by singlet oxygen (1O2) derived from activated peroxymonosulfate (PMS) with Co3O4–SnO2/RSBC. Environ. Res. 2020, 187, 109665. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yan, L.; Li, X.; Yan, T.; Song, W.; Hou, T.; Tong, C.; Mu, J.; Xu, M. Goethite/biochar-activated peroxymonosulfate enhances tetracycline degradation: Inherent roles of radical and non-radical processes. Sci. Total Environ. 2021, 783, 147102. [Google Scholar] [CrossRef]
- Su, X.; Guo, Y.; Yan, L.; Wang, Q.; Zhang, W.; Li, X.; Song, W.; Li, Y.; Liu, G. MoS2 nanosheets vertically aligned on biochar as a robust peroxymonosulfate activator for removal of tetracycline. Sep. Purif. Technol. 2022, 282, 120118. [Google Scholar] [CrossRef]
- Luo, X.; Shen, M.; Liu, J.; Ma, Y.; Gong, B.; Liu, H.; Huang, Z. Resource utilization of piggery sludge to prepare recyclable magnetic biochar for highly efficient degradation of tetracycline through peroxymonosulfate activation. J. Clean. Prod. 2021, 294, 126372. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, X.; Yan, Y.; Sun, C.; Wu, H.; He, J.; Wang, D. Heterogeneous activation of peroxymonosulfate by different ferromanganese oxides for tetracycline degradation: Structure dependence and catalytic mechanism. Chem. Eng. J. 2018, 348, 263–270. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Wang, Y.; Jia, F.; Song, S. Construction of 3D-sized Mn (II)-doped MoS2@activated alumina beads as PMS activator for tetracycline degradation under light irradiation. Chem. Phys. Lett. 2022, 806, 139996. [Google Scholar] [CrossRef]
- Yan, X.; Yao, Y.; Zhang, H.; Xie, J.; Xiao, C.; Zhang, S.; Qi, J.; Sun, X.; Li, J. Zeolitic imidazolate framework (ZIF-8)/polyacrylonitrile derived millimeter-sized hierarchical porous carbon beads for peroxymonosulfate catalysis. Environ. Res. 2022, 206, 112618. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Lin, Q.; Huang, R.; Fu, H.; Zhang, X.; Luo, H.; Xiao, R. Oxidative degradation of tetracycline using persulfate activated by N and Cu codoped biochar. Chem. Eng. J. 2020, 380, 122608. [Google Scholar] [CrossRef]
- Wang, H.; Chen, T.; Chen, D.; Zou, X.; Li, M.; Huang, F.; Sun, F.; Wang, C.; Shu, D.; Liu, H. Sulfurized oolitic hematite as a heterogeneous Fenton-like catalyst for tetracycline antibiotic degradation. Appl. Catal. B Environ. 2020, 260, 118203. [Google Scholar] [CrossRef]
- Ge, L.; Yue, Y.; Wang, W.; Tan, F.; Zhang, S.; Wang, X.; Qiao, X.; Wong, P.K. Efficient degradation of tetracycline in wide pH range using MgNCN/MgO nanocomposites as novel H2O2 activator. Water Res. 2021, 198, 117149. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zhang, R.; Ding, Y.; Ren, Z.; Fu, M.; Cao, X.; Zeng, G. Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway. J. Hazard. Mater. 2021, 419, 126495. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Xu, T.; Faheem, M.; Xi, Y.; Zhou, T.; Moryani, H.T.; Bao, J.; Du, J. Evolution of singlet oxygen by activating peroxydisulfate and peroxymonosulfate: A review. Int. J. Environ. Res. 2021, 18, 3344. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Deng, Y.; Nie, S.; Yan, C.; Ding, M.; Dong, W.; Dai, Y.; Zhang, Y. Simultaneous removal of bisphenol A and phosphate from water by peroxymonosulfate combined with calcium hydroxide. Chem. Eng. J. 2019, 369, 35–45. [Google Scholar] [CrossRef]
- Cai, H.; Zou, J.; Lin, J.; Li, J.; Huang, Y.; Zhang, S.; Yuan, B.; Ma, J. Sodium hydroxide-enhanced acetaminophen elimination in heat/peroxymonosulfate system: Production of singlet oxygen and hydroxyl radical. Chem. Eng. J. 2022, 429, 132438. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Ma, J.; Lin, C.; Li, X.; Zhang, H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 2016, 151, 280–288. [Google Scholar] [CrossRef]


| PMS-Based Systems | PMS Dosage (g/L) | Catalyst Dosage (g/L) | Concentration of TC (mg/L) | Reaction Time (min) | Degradation Rate (%) | Reference |
|---|---|---|---|---|---|---|
| ZnO2 + PMS | 0.3 | 0.1 | 50 | 60 | 80.2 | This work |
| Goethite/biochar composite + PMS | 0.62 | 0.1 | 30 | 60 | ~73% | [42] |
| MoS2/biochar + PMS | 0.62 | 0.5 | 20 | 120 | 78% | [43] |
| Piggery sludge-derived magnetic biochar + PMS | 0.2 | 0.5 | 10 | 120 | 77.23% | [44] |
| Ferromanganese oxide + PMS | 0.4 | 0.4 | 50 | 80 | ~79% | [45] |
| Mn(II)-doped MoS2@ alumina + PMS + light | 0.4 | 1.9 | 20 | 60 | 82.4 | [46] |
| ZIF-8/PAN-derived porous carbon + PMS | 0.5 | 0.5 | 50 | 120 | 85.1% | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, Y.; Ding, S.; Li, X.; Wang, W.; Dong, N.; Nie, M.; Chen, P. Investigation into the Synergistic Effect of the Zinc Peroxide/Peroxymonosulfate Double-Oxidation System for the Efficient Degradation of Tetracycline. Molecules 2024, 29, 4120. https://doi.org/10.3390/molecules29174120
Li S, Zhang Y, Ding S, Li X, Wang W, Dong N, Nie M, Chen P. Investigation into the Synergistic Effect of the Zinc Peroxide/Peroxymonosulfate Double-Oxidation System for the Efficient Degradation of Tetracycline. Molecules. 2024; 29(17):4120. https://doi.org/10.3390/molecules29174120
Chicago/Turabian StyleLi, Shefeng, Yong Zhang, Siyu Ding, Xuli Li, Wei Wang, Ningning Dong, Miaomiao Nie, and Pei Chen. 2024. "Investigation into the Synergistic Effect of the Zinc Peroxide/Peroxymonosulfate Double-Oxidation System for the Efficient Degradation of Tetracycline" Molecules 29, no. 17: 4120. https://doi.org/10.3390/molecules29174120




