Application of PLGA-PEG-PLGA Nanoparticles to Percutaneous Immunotherapy for Food Allergy
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Physical Properties of Prepared Nanoparticles
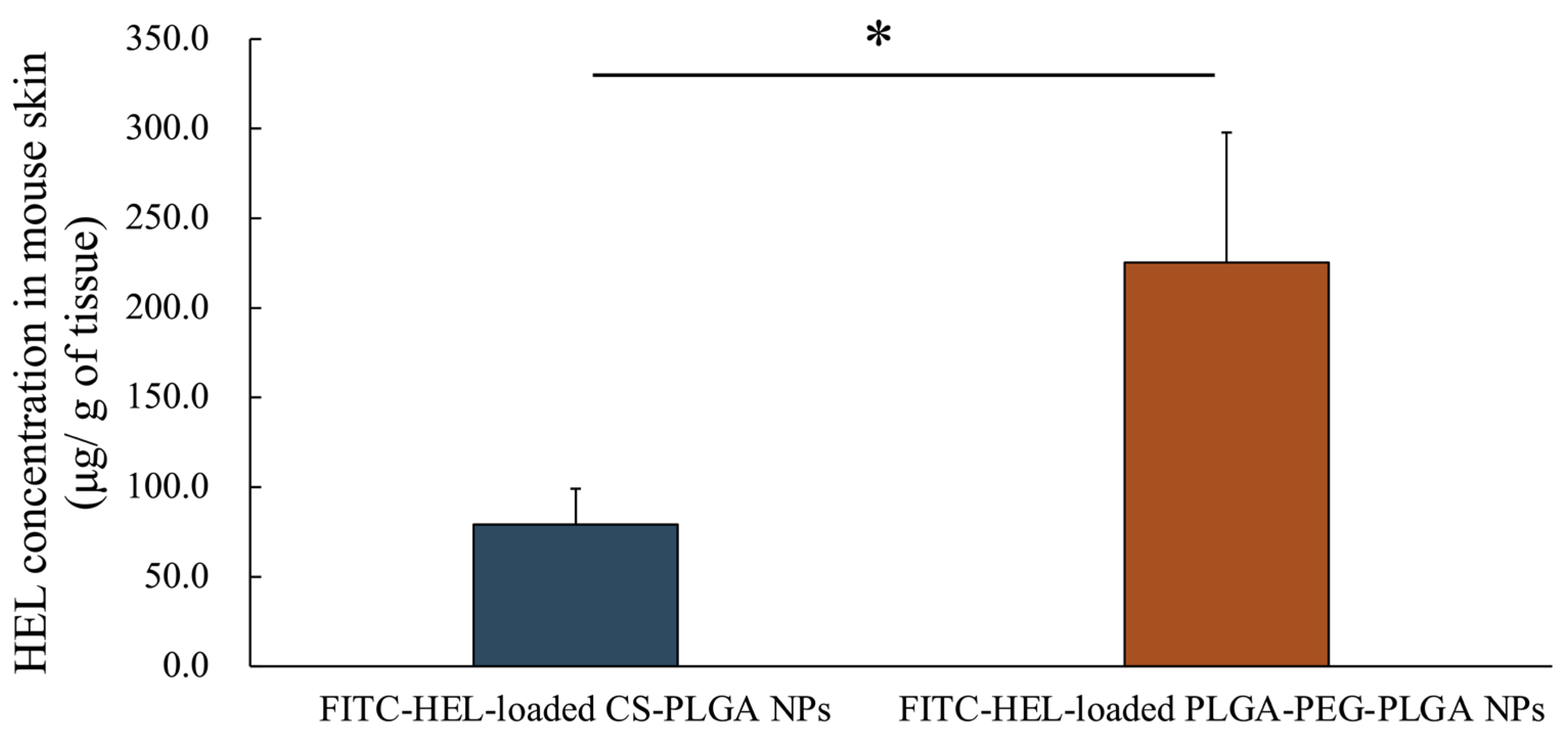
2.2. Ex Vivo Measurement of Intracutaneous HEL Accumulation
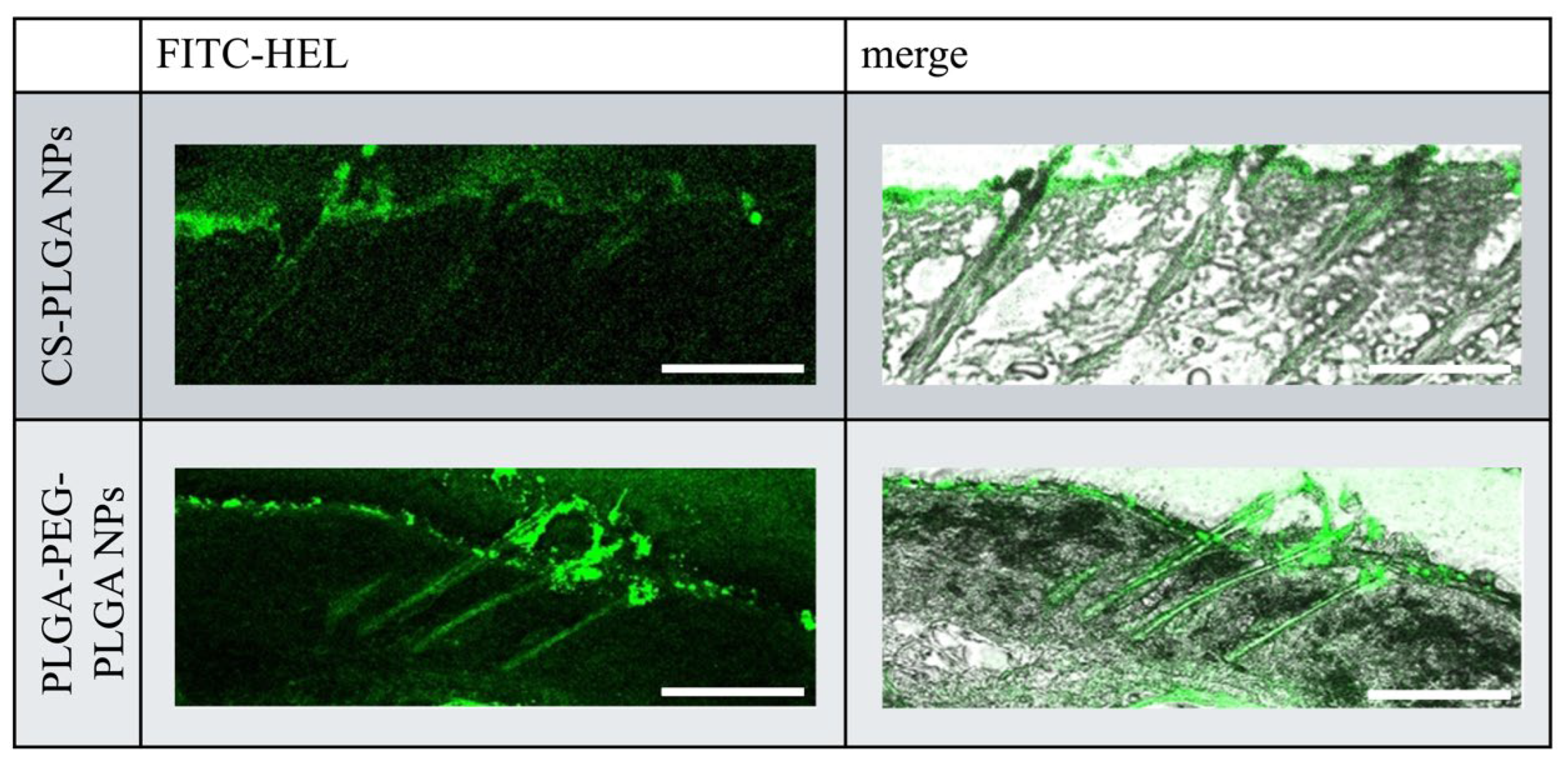
2.3. Ex Vivo Observation of the Intradermal Permeation Pathway of FITC-HEL
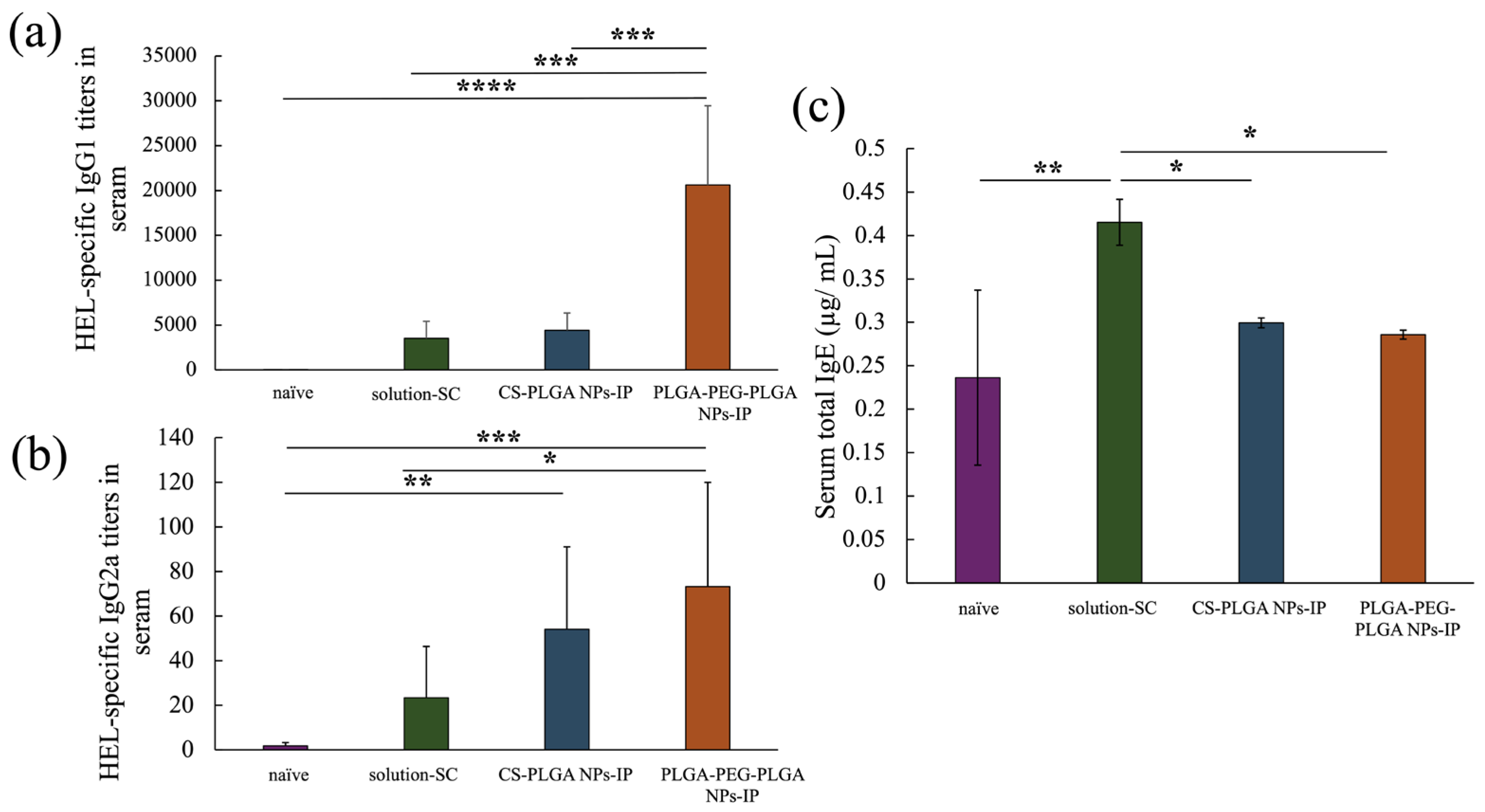
2.4. Results of the Measurement of Antibody Titer in Blood after in Vivo Percutaneous Immunization Experiment
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Preparation of HEL-Loaded PLGA and PLGA-PEG-PLGA Nanoparticle Formulations
3.4. Preparation of FITC-HEL-Loaded PLGA and PLGA-PEG-PLGA Nanoparticle Formulations
3.5. Evaluation of Physical Properties of PLGA and PLGA-PEG-PLGA Nanoparticle Formulations Loaded with HEL and FITC-HEL
3.6. Drug Skin Retention Study of FITC-HEL-Loaded PLGA and PLGA-PEG-PLGA Nanoparticle Formulations
3.7. Observation of Intradermal Permeation Pathway of FITC-HEL
3.8. In Vivo Percutaneous Immunization Experiments
3.9. Data Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loh, W.; Tang, M.L.K. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Borges, M.S.; et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef] [PubMed]
- Longo, G.; Berti, I.; Burks, A.W.; Krauss, B.; Barbi, E. IgE-mediated food allergy in children. Lancet 2013, 382, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Greenhawt, M. Food allergy quality of life and living with food allergy. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 284–290. [Google Scholar] [CrossRef]
- Polloni, L.; Muraro, A. Anxiety and food allergy: A review of the last two decades. Clin. Exp. Allergy 2020, 50, 420–441. [Google Scholar] [CrossRef]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi, S.; Roberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; et al. EAACI guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy 2018, 73, 799–815. [Google Scholar] [CrossRef]
- Virkud, Y.V.; Burks, A.W.; Steele, P.H.; Edwards, L.J.; Berglund, J.P.; Jones, S.M.; Scurlock, A.M.; Perry, T.T.; Pesek, R.D.; Vickery, B.P. Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy. J. Allergy Clin. Immunol. 2017, 139, 882–888.e5. [Google Scholar] [CrossRef]
- Muraro, A.; Tropeano, A.; Giovannini, M. Allergen immunotherapy for food allergy: Evidence and outlook. Allergol. Sel. 2022, 6, 285. [Google Scholar] [CrossRef]
- Mondoulet, L.; Dioszeghy, V.; Ligouis, M.; Dhelft, V.; Dupont, C.; Benhamou, P. Benhamou. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin. Exp. Allergy 2010, 40, 659–667. [Google Scholar] [CrossRef]
- Senti, G.; von Moos, S.; Kündig, T.M. Kündig. Epicutaneous immunotherapy for aeroallergen and food allergy. Curr. Treat. Options Allergy 2014, 1, 68–78. [Google Scholar] [CrossRef]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Chaulagain, B.; Jain, A.; Tiwari, A.; Verma, A.; Jain, S.K. Passive delivery of protein drugs through percutaneous route. Artif. Cells Nanomed. Biotechnol. 2018, 46, 472–487. [Google Scholar] [CrossRef]
- Jones, S.M.; Agbotounou, W.K.; Fleischer, D.M.; Burks, A.W.; Pesek, R.D.; Harris, M.W.; Martin, L.; Thebault, C.; Ruban, C.; Benhamou, P.H. Safety of epicutaneous immunotherapy for the treatment of peanut allergy: A phase 1 study using the Viaskin patch. J. Allergy Clin. Immunol. 2016, 137, 1258–1261.e10. [Google Scholar] [CrossRef]
- Ito, S.; Hirobe, S.; Kuwabara, Y.; Nagao, M.; Saito, M.; Quan, Y.-S.; Kamiyama, F.; Fujisawa, T.; Okada, N. Immunogenicity of milk protein-containing hydrophilic gel patch for epicutaneous immunotherapy for milk allergy. Pharm. Res. 2020, 37, 35. [Google Scholar] [CrossRef]
- Rattanapak, T.; Birchall, J.; Young, K.; Ishii, M.; Meglinski, I.; Rades, T.; Hook, S. Transcutaneous immunization using microneedles and cubosomes: Mechanistic investigations using optical coherence tomography and two-photon microscopy. J. Control. Release 2013, 172, 894–903. [Google Scholar] [CrossRef]
- Weiss, R.; Hessenberger, M.; Kitzmüller, S.; Bach, D.; Weinberger, E.E.; Krautgartner, W.D.; Hauser-Kronberger, C.; Malissen, B.; Boehler, C.; Kalia, Y.N.; et al. Transcutaneous vaccination via laser microporation. J. Control. Release 2012, 162, 391–399. [Google Scholar] [CrossRef]
- Scheiblhofer, S.; Thalhamer, J.; Weiss, R. Laser microporation of the skin: Prospects for painless application of protective and therapeutic vaccines. Expert. Opin. Drug Deliv. 2013, 10, 761–773. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of nanoparticles as permeation enhancers and targeted delivery options for skin: Advantages and disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Mönkäre, J.; Pontier, M.; van Kampen, E.E.M.; Du, G.; Leone, M.; Romeijn, S.; Nejadnik, M.R.; O’Mahony, C.; Slütter, B.; Jiskoot, W.; et al. Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur. J. Pharm. Biopharm. 2018, 129, 111–121. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, L.; Song, W.; Liu, J. Influencing factors and drug application of iontophoresis in percutaneous drug delivery: An overview of recent progress. Drug Deliv. Transl. Res. 2021, 12, 1–23. [Google Scholar]
- Blasi, P. Poly (lactic acid)/poly (lactic-co-glycolic acid)-based microparticles: An overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef]
- Takeuchi, I.; Hidaka, Y.; Oshizaka, T.; Takei, C.; Mori, K.; Sugibayashi, K.; Makino, K. Chitosan-coated PLGA nanoparticles for transcutaneous immunization: Skin distribution in lysozyme-sensitized mice. Colloids Surf. B Biointerfaces 2022, 220, 112916. [Google Scholar] [CrossRef]
- Takeuchi, I.; Kagawa, A.; Makino, K. Skin permeability and transdermal delivery route of 30-nm cyclosporin A-loaded nanoparticles using PLGA-PEG-PLGA triblock copolymer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124866. [Google Scholar] [CrossRef]
- Heine, S.; Aguilar-Pimentel, A.; Russkamp, D.; Alessandrini, F.; Gailus-Durner, V.; Fuchs, H.; Ollert, M.; Bredehorst, R.; Ohnmacht, C.; Zissle, U.M. Thermosensitive PLGA–PEG–PLGA hydrogel as depot matrix for allergen-specific immunotherapy. Pharmaceutics 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Kadlecová, Z.; Sevriugina, V.; Lysáková, K.; Rychetský, M.; Chamradová, I.; Vojtová, L. Liposomes Affect Protein Release and Stability of ITA-Modified PLGA-PEG-PLGA Hydrogel Carriers for Controlled Drug Delivery. Biomacromolecules 2023, 25, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cui, Y.; Liu, M.; An, Z.; Li, K.; Gu, X.; Li, P.; Fan, Y. Enhanced hydrophilicity of one-step electrosprayed red blood cell-like PLGA microparticles by block polymer PLGA-PEG-PLGA with excellent magnetic-luminescent bifunction and affinity to HUVECs. Eur. Polym. J. 2023, 191, 112040. [Google Scholar] [CrossRef]
- Tomoda, K.; Yabuki, N.; Terada, H.; Makino, K. Surfactant free preparation of PLGA nanoparticles: The combination of antisolvent diffusion with preferential solvation. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 88–93. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Baspinar, Y.; Borchert, H.-H. Penetration and release studies of positively and negatively charged nanoemulsions—Is there a benefit of the positive charge? Int. J. Pharm. 2012, 430, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Hoeller, S.; Sperger, A.; Valenta, C. Lecithin based nanoemulsions: A comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int. J. Pharm. 2009, 370, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, L.; Bai, S.; Sun, Y. Analysis of hydrophobic charge induction displacement chromatography by visualization with confocal laser scanning microscopy. Sep. Purif. Technol. 2011, 82, 138–147. [Google Scholar] [CrossRef]
- Brinkley, M. A brief survey of methods for preparing protein conjugates with dyes, haptens, and crosslinking reagents. Bioconjugate Chem. 1992, 3, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for transcutaneous immunization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125607. [Google Scholar] [CrossRef]
- Kawamoto, T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch. Histol. Cytol. 2003, 66, 123–143. [Google Scholar] [CrossRef]
- Takatani-Nakase, T.; Tokuyama, E.; Komai, M.; Takahashi, K. Transcutaneous immunization system using a hydrotropic formulation induces a potent antigen-specific antibody response. PLoS ONE 2012, 7, e47980. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lv, X.; Le, Y. Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef]
- Pamujula, S.; Hazari, S.; Bolden, G.; Graves, R.A.; Chinta, D.D.; Dash, S.; Kishore, V.; Mandal, T.K. Cellular delivery of PEGylated PLGA nanoparticles. J. Pharm. Pharmacol. 2012, 64, 61–67. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Mares, A.G.; Pacassoni, G.; Marti, J.S.; Pujals, S.; Albertazzi, L. Formulation of tunable size PLGA-PEG nanoparticles for drug delivery using microfluidic technology. PLoS ONE 2021, 16, e0251821. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Chanda, N. Formulation of PLGA nano-carriers: Specialized modification for cancer therapeutic applications. Mater. Adv. 2022, 3, 837–858. [Google Scholar] [CrossRef]
- Muraoka, T.; Adachi, K.; Ui, M.; Kawasaki, S.; Sadhukhan, N.; Obara, H.; Tochio, H.; Shirakawa, M.; Kinbara, K. A structured monodisperse PEG for the effective suppression of protein aggregation. Angewandte chemie 2013, 52, 2430–2434. [Google Scholar] [CrossRef] [PubMed]
- Burton, O.T.; Tamayo, J.M.; Stranks, A.J.; Koleoglou, K.J.; Oettgen, H.C. Allergen-specific IgG antibody signaling through FcγRIIb promotes food tolerance. J. Allergy Clin. Immunol. 2018, 141, 189–201. [Google Scholar] [CrossRef]
- Oettgen, H.C. Mast cells in food allergy: Inducing immediate reactions and shaping long-term immunity. J. Allergy Clin. Immunol. 2023, 151, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Mahé, B.; Costagliola, D.; Bonduelle, O.; Hadam, S.; Schaefer, G.; Schaefer, H.; Katlama, C.; Sterry, W.; Autran, B.; et al. Transcutaneous anti-influenza vaccination promotes both CD4 and CD8 T cell immune responses in humans. J. Immunol. 2008, 180, 1482–1489. [Google Scholar] [CrossRef]
- Rattanapak, T.; Birchall, J.C.; Young, K.; Kubo, A.; Fujimori, S.; Ishii, M.; Hook, S. Dynamic visualization of dendritic cell-antigen interactions in the skin following transcutaneous immunization. PLoS ONE 2014, 9, e89503. [Google Scholar] [CrossRef]
- Larsen, S.T.; Nielsen, G.D.; Thygesen, P. Investigation of the adjuvant effect of polyethylene glycol (PEG) 400 in BALB/c mice. Int. J. Pharm. 2002, 231, 51–55. [Google Scholar] [CrossRef]
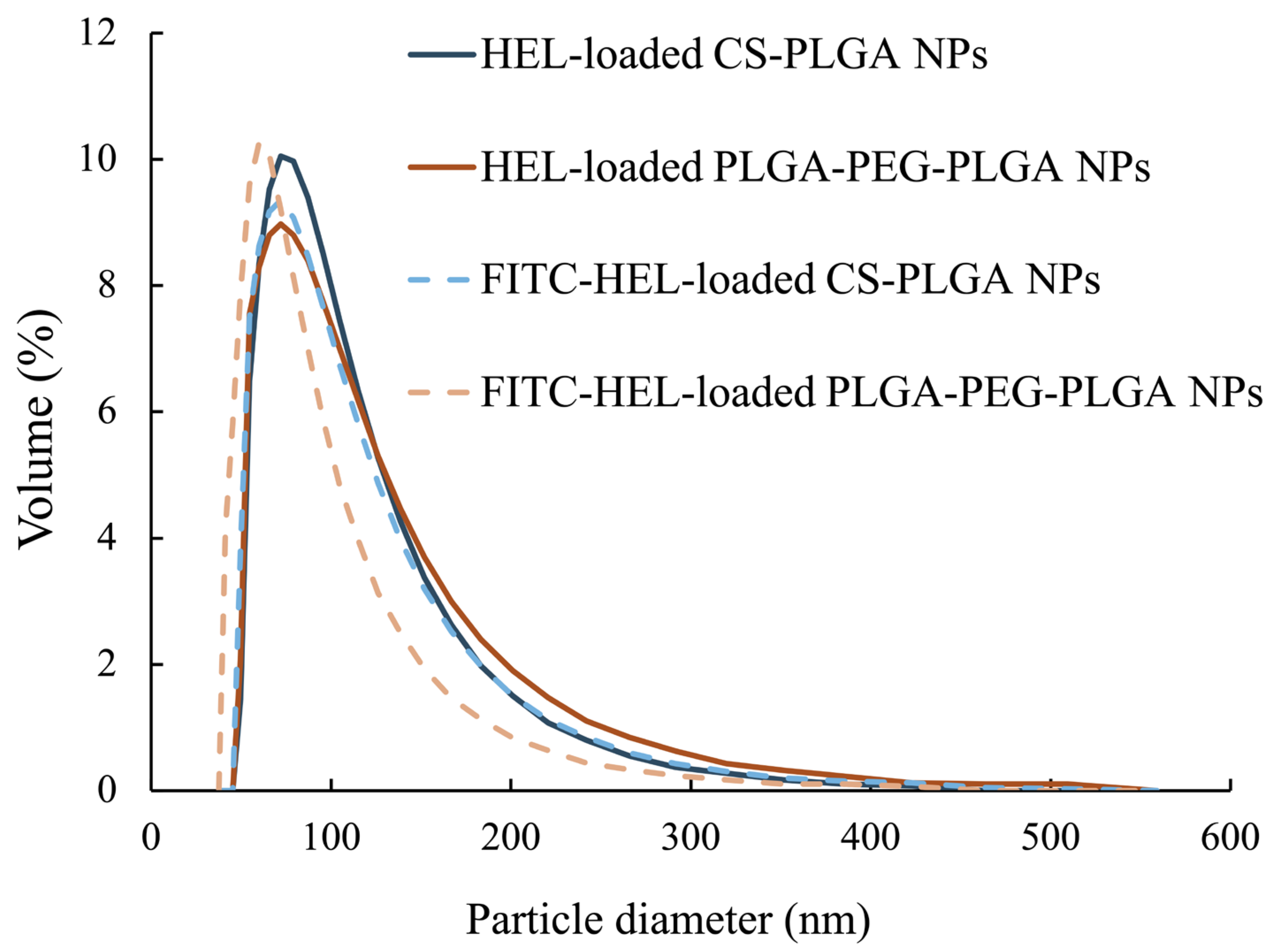
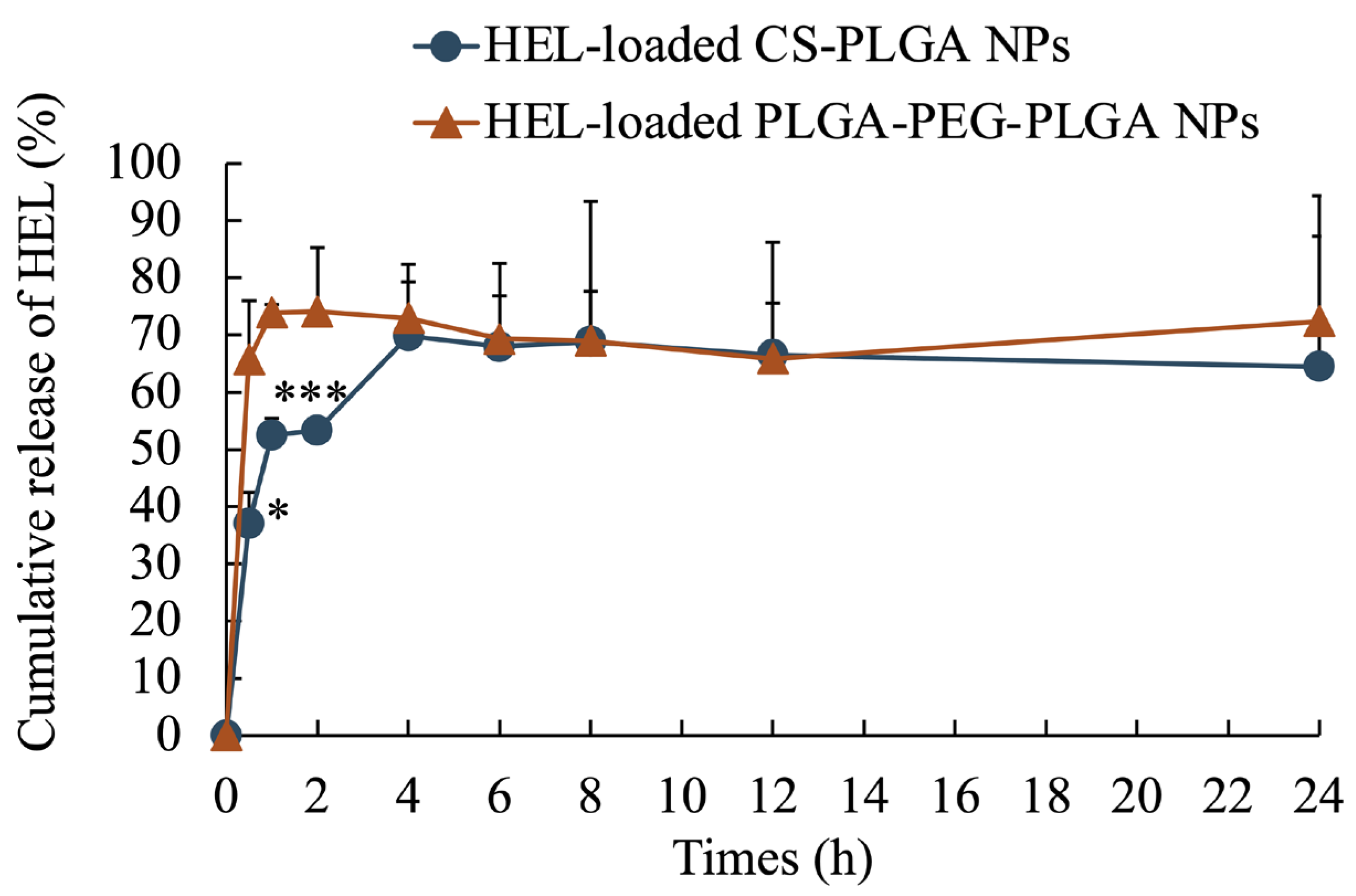
| HEL-Loaded CS-PLGA NPs | FITC-HEL-Loaded CS-PLGA NPs | HEL-Loaded PLGA-PEG-PLGA NPs | FITC-HEL-Loaded PLGA-PEG-PLGA NPs | |
|---|---|---|---|---|
| Mean diameter (nm) | 100 ± 47 | 99 ± 49 | 105 ± 54 | 87 ± 43 |
| Zeta potential (mV, I = 5 mM) | 38.4 ± 1.9 | −7.2 ± 0.1 | ||
| Yield (%) | 82.9 ± 12.0 | 67.7 ± 2.7 | ||
| HEL content in NPs (%) | 8.0 ± 0.8 | 7.9 ± 0.7 | 3.4 ± 0.4 | 3.4 ± 0.9 |
| HEL entrapment efficiency (w/w%) | 66.7 ± 7.0 | 65.5 ± 6.1 | 28.7 ± 3.4 | 28.1 ± 7.8 |
| Polydispersity index | 0.23 ± 0.01 | 0.25 ± 0.02 | 0.27 ± 0.01 | 0.30 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakurai, R.; Iwata, H.; Gotoh, M.; Ogino, H.; Takeuchi, I.; Makino, K.; Itoh, F.; Saitoh, A. Application of PLGA-PEG-PLGA Nanoparticles to Percutaneous Immunotherapy for Food Allergy. Molecules 2024, 29, 4123. https://doi.org/10.3390/molecules29174123
Sakurai R, Iwata H, Gotoh M, Ogino H, Takeuchi I, Makino K, Itoh F, Saitoh A. Application of PLGA-PEG-PLGA Nanoparticles to Percutaneous Immunotherapy for Food Allergy. Molecules. 2024; 29(17):4123. https://doi.org/10.3390/molecules29174123
Chicago/Turabian StyleSakurai, Ryuse, Hanae Iwata, Masaki Gotoh, Hiroyuki Ogino, Issei Takeuchi, Kimiko Makino, Fumio Itoh, and Akiyoshi Saitoh. 2024. "Application of PLGA-PEG-PLGA Nanoparticles to Percutaneous Immunotherapy for Food Allergy" Molecules 29, no. 17: 4123. https://doi.org/10.3390/molecules29174123






