Chemical Constituents, Anti-Tumor Mechanisms, and Clinical Application: A Comprehensive Review on Scutellaria barbata
Abstract
1. Introduction
2. Chemical Constituents
2.1. Flavanoids
2.2. Terpenoids
2.2.1. Diterpenoids
2.2.2. Triterpenoids
2.3. Polysaccharides
2.4. Volatile Compounds
2.5. Trace Elements
2.6. Steroids
2.7. Phenylpropanoid and Lignan
2.8. Phenolic Acids
2.9. Others
3. Anti-Tumor Mechanism
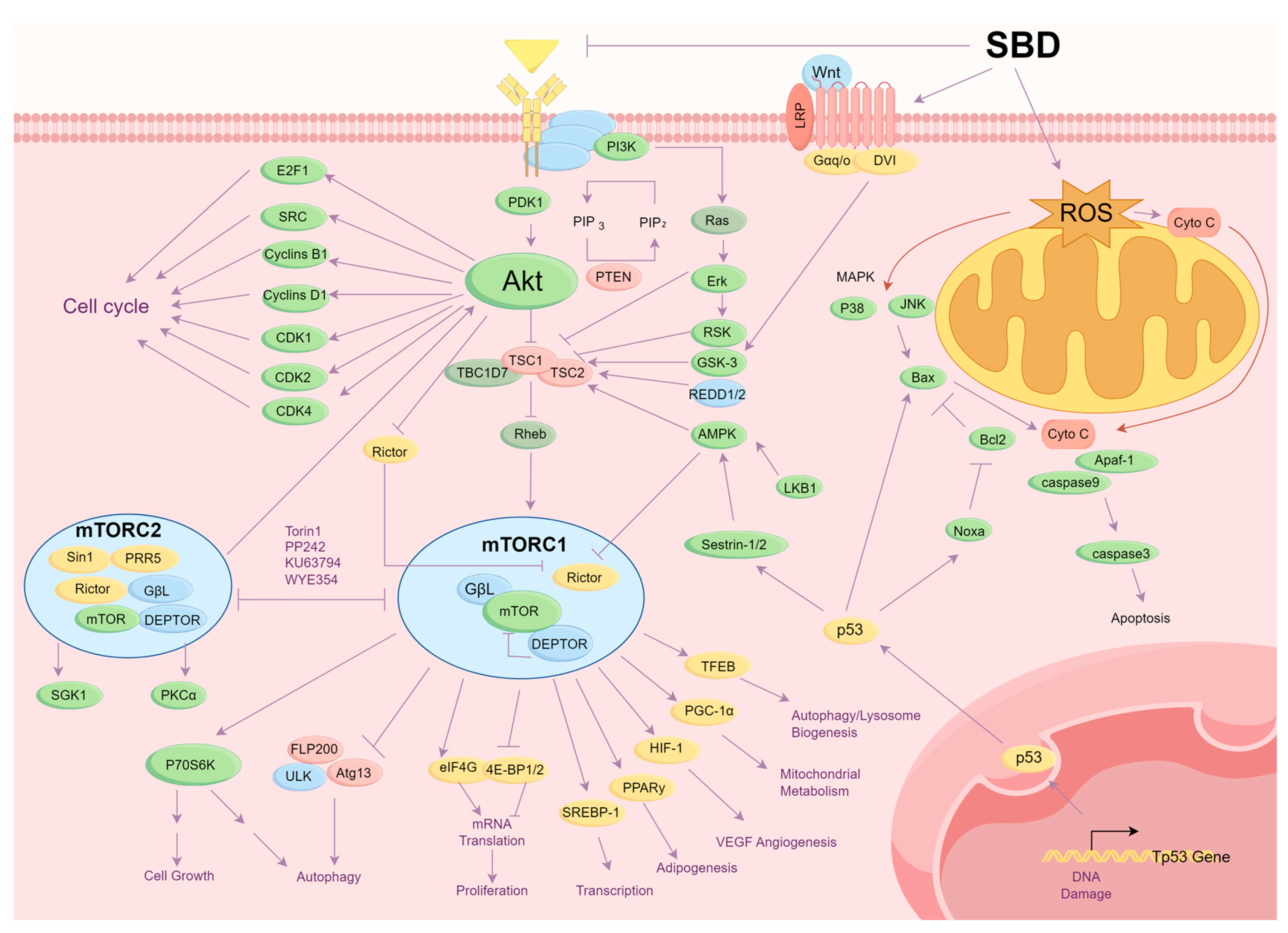
3.1. Inhibition of Tumor Cell Proliferation
3.2. Induction of Apoptosis in Cancer Cells
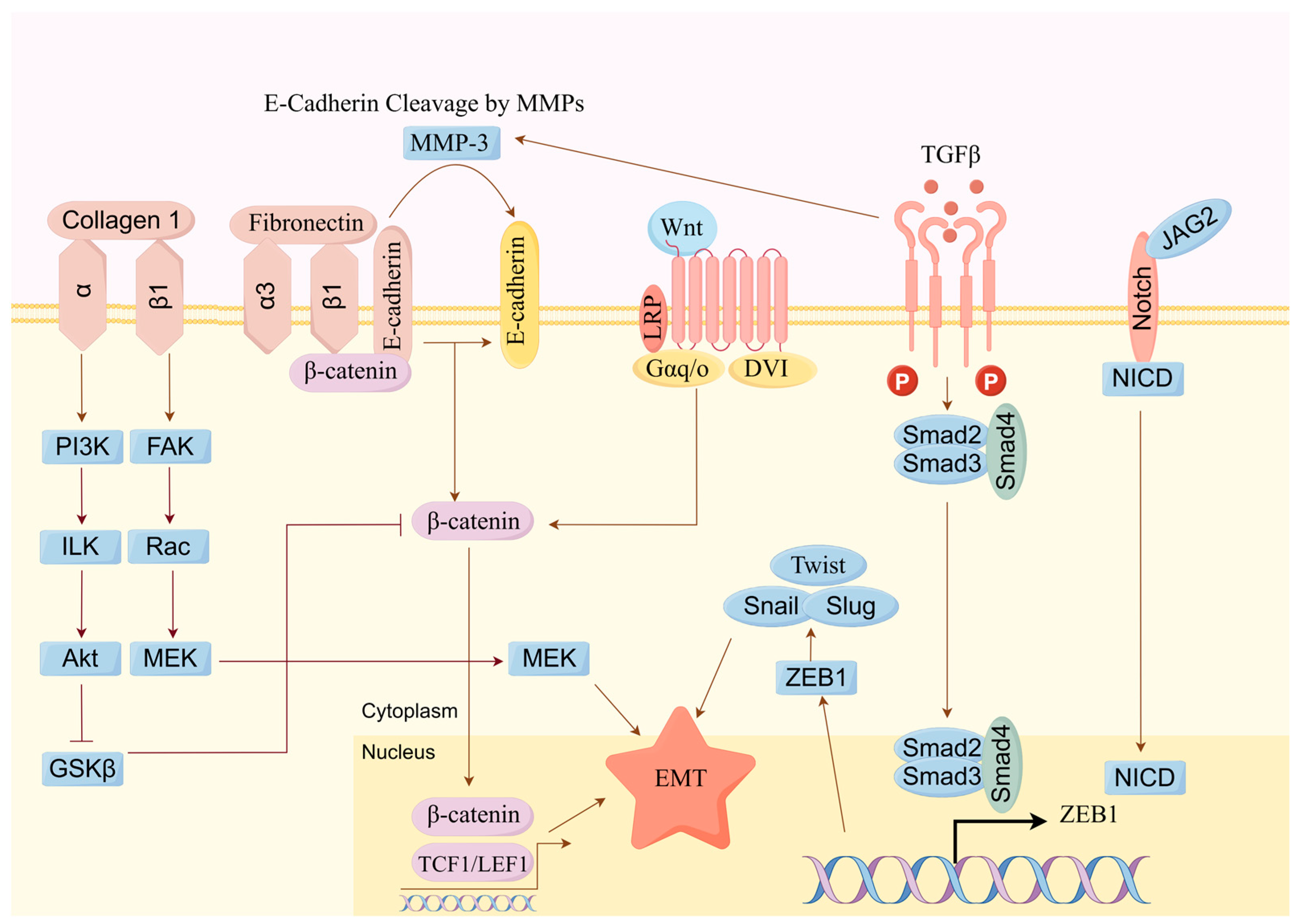
3.3. Inhibition of Cell Migration and Invasion
3.4. Inhibition of Angiogenesis
3.5. Reduction of Cancer Cell Drug Resistance
3.6. Improvement of Tumor Microenvironment
4. Clinical Application
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PI3K | Phosphatidylinositol3-kinase |
| AKT | Protein kinase B |
| mTOR | Mechanistic target of rapamycin |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular-regulated protein kinases |
| JNK | C-Jun N-terminal kinase |
| NFκB | Nuclear factor kappa-B |
| SBP | Scutellaria barbata polysaccharide |
| GC-MS | Gas chromatography–mass spectrometry |
| PLK1 | Polo-like kinase 1 |
| TP53 | Tumor protein p53 |
| BAX | BCL2-associated X |
| P21 | Protein 21 |
| HepG2 | Human hepatocellular carcinomas |
| Bax | BCL2-associated X |
| Bcl | B-cell lymphoma-2 |
| CNE | Human nasopharyngeal cancer cell |
| SBD | Scutellaria barbata D. Don |
| VEGFA | Vascular endothelial growth factor A |
| p-EGFR | p-Epidermal growth factor receptor |
| PDE7B | cAMP-specific 3′,5′-cyclic phosphodiesterase 7B |
| PTHrP | Parathyroid-hormone-related protein |
| HD-SB | Hedyotis diffusa–Scutellaria barbata |
| Smad | Drosophila mothers against decapentaplegic protein |
| MMP | Matrix metalloproteinase |
| EMT | Epithelial–mesenchymal transition |
| EGFR | Epidermal growth factor receptor |
| HUVEC | Human umbilical vein endothelial cells |
| ROS | Reactive oxygen species |
| LMO1 | LIM domain only 1 |
| PFS | Progression-free survival |
| OS | Overall survival |
References
- Banerjee, J.; Tiwari, A.K.; Banerjee, S. Drug repurposing for cancer. Prog. Mol. Biol. Transl. Sci. 2024, 207, 123–150. [Google Scholar] [CrossRef]
- Yu, Q.; Xu, C.; Song, J.; Jin, Y.; Gao, X. Mechanisms of Traditional Chinese medicine/natural medicine in HR-positive Breast Cancer: A comprehensive Literature Review. J. Ethnopharmacol. 2024, 319, 117322. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Textual research on herbs of Scutellaria barbata. TCM Res. 2006, 19, 21–23. [Google Scholar]
- Lu, L.; Zhan, S.; Liu, X.; Zhao, X.; Lin, X.; Xu, H. Antitumor Effects and the Compatibility Mechanisms of Herb Pair Scleromitrion diffusum (Willd.) R. J. Wang-Sculellaria barbata D. Don. Front. Pharmacol. 2020, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, W.; Li, M.; Zhang, F.; Chen, W. A review of the ethnopharmacology, phytochemistry, pharmacology, and quality control of Scutellaria barbata D. Don. J. Ethnopharmacol. 2019, 254, 112260. [Google Scholar] [CrossRef]
- Tang, T.T.; Li, S.M.; Pan, B.W.; Xiao, J.W.; Pang, Y.X.; Xie, S.X.; Zhou, Y.; Yang, J.; Wei, Y. Identification of Flavonoids from Scutellaria barbata D. Don as Inhibitors of HIV-1 and Cathepsin L Proteases and Their Structure-Activity Relationships. Molecules 2023, 28, 4476. [Google Scholar] [CrossRef]
- Su, W.; Wu, L.; Liang, Q.; Lin, X.; Xu, X.; Yu, S.; Lin, Y.; Zhou, J.; Fu, Y.; Gao, X.; et al. Extraction Optimization, Structural Characterization, and Anti-Hepatoma Activity of Acidic Polysaccharides from Scutellaria barbata D. Don. Front. Pharmacol. 2022, 13, 827782. [Google Scholar] [CrossRef]
- Gong, B.; Kao, Y.; Zhang, C.; Sun, F.; Zhao, H. Systematic Investigation of Scutellariae barbatae Herba for Treating Hepatocellular Carcinoma Based on Network Pharmacology. Evid. Based Complement. Altern. Med. 2018, 2018, 4365739. [Google Scholar] [CrossRef]
- Yang, A.; Liu, H.; Yang, Y. Study on the mechanism of action of Scutellaria barbata on hepatocellular carcinoma based on network pharmacology and bioinformatics. Front. Pharmacol. 2022, 13, 1072547. [Google Scholar] [CrossRef]
- Shao, H.; Chen, J.; Li, A.; Ma, L.; Tang, Y.; Chen, H.; Chen, Y.; Liu, J. Salvigenin Suppresses Hepatocellular Carcinoma Glycolysis and Chemoresistance Through Inactivating the PI3K/AKT/GSK-3β Pathway. Appl. Biochem. Biotechnol. 2023, 195, 5217–5237. [Google Scholar] [CrossRef]
- Huang, C.; Luo, H.; Huang, Y.; Fang, C.; Zhao, L.; Li, P.; Zhong, C.; Liu, F. AURKB, CHEK1 and NEK2 as the Potential Target Proteins of Scutellaria barbata on Hepatocellular Carcinoma: An Integrated Bioinformatics Analysis. Int. J. Gen. Med. 2021, 14, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Kang, W.; Liu, H.; Guo, S. Inhibition effects of total flavonoids from Sculellaria barbata D. Don on human breast carcinoma bone metastasis via downregulating PTHrP pathway. Int. J. Mol. Med. 2018, 41, 3137–3146. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Liu, X.; Sun, Z.; Sun, X. Ethyl Acetate Fraction from Hedyotis Diffusa Plus Scutellaria barbata Inhibits the Progression of Breast Cancer via Targeting LMO1 and AKT/Mtor Signaling Pathway. Comb. Chem. High Throughput Screen. 2024, 27, 27–1735. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, T.; Cao, Y.L.; Lv, Y.X.; Chang, Q.Q.; Zhang, D.D. Ethyl Acetate Fraction from Hedyotis diffusa plus Scutellaria barbata Exerts Anti-Breast Cancer Effect via miR-200c-PDE7B/PD-L1-AKT/MAPK Axis. Evid. Based Complement. Altern. Med. 2020, 2020, 3587095. [Google Scholar] [CrossRef]
- Shi, L.; Wu, Y.; Lv, D.L.; Feng, L. Scutellarein selectively targets multiple myeloma cells by increasing mitochondrial superoxide production and activating intrinsic apoptosis pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Li, L.; Xue, X. Flavonoids from Scutellaria barbata inhibit activation of tumor-associated macrophages by blocking the Toll-like receptor 4/myeloid differentiation factor 88/nuclear factor-κB signaling pathway. J. Tradit. Chin. Med. 2019, 39, 160–165. [Google Scholar] [PubMed]
- Liu, L.; Liu, T.; Tao, W.; Liao, N.; Yan, Q.; Li, L.; Tan, J.; Shen, W.; Cheng, H.; Sun, D. Flavonoids from Scutellaria barbata D. Don exert antitumor activity in colorectal cancer through inhibited autophagy and promoted apoptosis via ATF4/sestrin2 pathway. Phytomed. Int. J. Phytother. Phytopharm. 2022, 99, 154007. [Google Scholar] [CrossRef]
- Sheng, D.; Zhao, B.; Zhu, W.; Wang, T.; Peng, Y. Scutellaria barbata D.Don (SBD) extracts suppressed tumor growth, metastasis and angiogenesis in Prostate cancer via PI3K/Akt pathway. BMC Complement. Med. Ther. 2022, 22, 120. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Wang, C.-S.; Wei, M.-C. Kinetics and mass transfer considerations for an ultrasound-assisted supercritical CO2 procedure to produce extracts enriched in flavonoids from Scutellaria barbata. J. CO2 Util. 2019, 32, 219–231. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; Yin, X.; Wang, M.; Zhao, J.; Ren, Y. A strategy integrating parent ions list-modified mass defect filtering-diagnostic product ions for rapid screening and systematic characterization of flavonoids in Scutellaria barbata using hybrid quadrupole-orbitrap high-resolution mass spectrometry. J. Chromatogr. A 2022, 1674, 463149. [Google Scholar] [CrossRef]
- Kim, D.I.; Lee, T.K.; Lim, I.S.; Kim, H.; Lee, Y.C.; Kim, C.H. Regulation of IGF-I production and proliferation of human leiomyomal smooth muscle cells by Scutellaria barbata D. Don in vitro: Isolation of flavonoids of apigenin and luteolin as acting compounds. Toxicol. Appl. Pharmacol. 2005, 205, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Suzaki, S.; Nishikawa, T.; Kihara, M.; Shibata, H.; Higuti, T. Phytochemical flavones isolated from Scutellaria barbata and antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2000, 72, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Wei, M.C. Development and characterization of a green procedure for apigenin extraction from Scutellaria barbata D. Don. Food Chem. 2018, 252, 381–389. [Google Scholar] [CrossRef]
- Yuan, Y.Q.; Han, L.W.; Wang, X.M.; Hou, H.R.; Liu, K.C. Study on Anti-Angiogenic Activity of the Chemical Constituents from Scutellaria barbata. Chin. Pharm. J. 2012, 47, 1032–1035. [Google Scholar]
- Lee, S.R.; Kim, M.S.; Kim, S.; Hwang, K.W.; Park, S.Y. Constituents from Scutellaria barbata Inhibiting Nitric Oxide Production in LPS-Stimulated Microglial Cells. Chem. Biodivers. 2017, 14, e1700231. [Google Scholar] [CrossRef]
- Li, N.; Wang, P.; Sun, T.F.; Han, L.; Hu, Y.N.; Du, H.T.; Liu, H. Research Progress on Chemical Constituents of Scutellaria barbata. China J. Chin. Mater. Med. 2020, 45, 5117–5128. [Google Scholar] [CrossRef]
- Wang, C.Y.; Duan, X.C.; Li, Y.; Wang, Y.Z. Studies on chemical constituents of flavonoids from Scutellaria barbata. Anhui Med. J. 2019, 40, 848–851. [Google Scholar] [CrossRef]
- Zhu, P.Y.; Liu, G.Q. Isolation and identification of the diterpenold and flavone in Scutellaria barbata D. Don. J. Plant Resour. Environ. 1993, 2, 63–64. [Google Scholar]
- Yao, H.; Li, S.; Hu, J.; Chen, Y.; Huang, L.; Lin, J.; Li, G.; Lin, X. Chromatographic fingerprint and quantitative analysis of seven bioactive compounds of Scutellaria barbata. Planta Med. 2011, 77, 388–393. [Google Scholar] [CrossRef]
- Wang, Z. Chemical Constituents of Scutellaria barbata. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 84–86. [Google Scholar] [CrossRef]
- Yu, Q.Y.; Zhang, D.W.; Dai, S.J. Isolation and Identification of Chemical Consistents from the Whole herb of Scutellaria Barbata. Mod. Chin. Med. 2011, 13, 25–28. [Google Scholar] [CrossRef]
- Wang, F.; Ren, F.C.; Li, Y.J.; Liu, J.K. Scutebarbatines W-Z, new neo-clerodane diterpenoids from Scutellaria barbata and structure revision of a series of 13-spiro neo-clerodanes. Chem. Pharm. Bull. 2010, 58, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yang, G.C.; Li, D.H.; Hu, X.X.; Sun, L.X. Chemical constituents from Scutellaria barbata. Chin. Tradit. Herb. Herbal. Drugs 2016, 47, 4322–4325. [Google Scholar] [CrossRef]
- Fu, Q.; Tong, C.; Guo, Y.; Xu, J.; Shi, F.; Shi, S.; Xiao, Y. Flavonoid aglycone–oriented data-mining in high-performance liquid chromatography–quadrupole time-of-flight tandem mass spectrometry: Efficient and targeted profiling of flavonoids in Scutellaria barbata. Anal. Bioanal. Chem. 2020, 412, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, F.; Liu, J.K. Two New Phenols from Scutellaria barbata. Molecules 2011, 16, 1402–1408. [Google Scholar] [CrossRef]
- Zhong, H.; Xue, X.X.; Yao, Q.Q. Research of chemical constituents of Scutellaria barbata. Chin. Tradit. Herb. Herbal. Drugs 2008, 39, 21–23. [Google Scholar]
- Wang, W.S.; Zhou, Y.W.; Ye, Y.H.; Du, N. Studies on the flavonoids in herb from Scutellaria barbata. China J. Chin. Mater. Med. 2004, 29, 957–959. [Google Scholar]
- Xiao, H.; Li, X. Chemical constituents of Scutellaria barbata D. Don. J. Shenyang Pharm. Univ. 2006, 23, 637–640. [Google Scholar]
- Lin, J.; Chen, Y.; Cai, Q.; Wei, L.; Zhan, Y.; Shen, A.; Sferra, T.J.; Peng, J. Scutellaria barbata D Don Inhibits Colorectal Cancer Growth via Suppression of Multiple Signaling Pathways. Integr. Cancer Ther. 2014, 13, 240–248. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, Y.; Wang, H.; Guo, X.; Li, Z. Studies on the Flavonoids in Scutellaria barbata with LC-MS. J. Cap. Capital Norm. Univ. 2009, 30, 32–34. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Ge, D.; Wang, T.; Hu, L.; Gao, X. Isolation and identification of flavonoids of whole plant of Scutellaria barbata D.Don. J. Shenyang Pharm. Univ. 2011, 28, 182–184. [Google Scholar] [CrossRef]
- Li, H.Y.; Wei, W.J.; Ma, K.L.; Zhang, J.Y.; Li, Y.; Gao, K. Phytotoxic neo-clerodane diterpenoids from the aerial parts of Scutellaria barbata. Phytochemistry 2020, 171, 112230. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.C.; Hu, J.H.; Li, B.L.; Liu, H.; Wang, J.Y.; Sun, L.X. Six New neo-Clerodane Diterpenoids from Aerial Parts of Scutellaria barbata and Their Cytotoxic Activities. Planta Med. 2018, 84, 1292–1299. [Google Scholar] [CrossRef]
- do Thao, T.; Phuong, D.T.; Hanh, T.T.; Thao, N.P.; Cuong, N.X.; Nam, N.H.; Minh, C.V. Two new neoclerodane diterpenoids from Scutellaria barbata D. Don growing in Vietnam. J. Asian Nat. Prod. Res. 2014, 16, 364–369. [Google Scholar] [CrossRef]
- Dai, S.J.; Sun, J.Y.; Ren, Y.; Liu, K.; Shen, L. Bioactive ent-clerodane diterpenoids from Scutellaria barbata. J. Planta Med. 2007, 73, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.C.; Liang, C.; Li, S.G.; Liu, M.; Jia, M.J.; Xu, X.N.; Wang, X.B.; Hua, H.M.; Sun, L.X. Neoclerodane diterpenoids from aerial parts of Scutellaria barbata. Phytochem. Lett. 2017, 19, 1–6. [Google Scholar] [CrossRef]
- Zhu, F.; Di, Y.T.; Li, X.Y.; Liu, L.L.; He, H.P. Neoclerodane Diterpenoids from Scutellaria barbata. Planta Med. 2011, 77, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, Q.; Jiang, C.; Morris-Natschke, S.L.; Cui, H.; Wang, Y.; Yan, Y.; Xu, J.; Lee, K.H.; Gu, Q. Neo-clerodane diterpenoids from Scutellaria barbata with activity against Epstein-Barr virus lytic replication. J. Nat. Prod. 2015, 78, 500–509. [Google Scholar] [CrossRef]
- Wang, M.; Ma, C.; Chen, Y.; Li, X.; Chen, J. Cytotoxic Neo-Clerodane Diterpenoids from Scutellaria barbata D.Don. Chem. Biodivers. 2019, 16, e1800499. [Google Scholar] [CrossRef]
- Dai, S.J.; Tao, J.Y.; Liu, K.; Jiang, Y.T.; Shen, L. neo-Clerodane diterpenoids from Scutellaria barbata with cytotoxic activities. Phytochemistry 2006, 67, 1326–1330. [Google Scholar] [CrossRef]
- Dai, S.J.; Shen, L.; Ren, Y. Two new neo-clerodane diterpenoids from Scutellaria barbata. J. Integr. Plant Biol. 2008, 50, 699–702. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Hu, P.; Ji, J.; Li, X.; Chen, J. Neoclerodane diterpenoids from Scutellaria barbata with cytotoxic activities. Nat. Prod. Res. 2020, 34, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.J.; Peng, W.B.; Zhang, D.W.; Shen, L.; Wang, W.Y.; Ren, Y. Cytotoxic neo-clerodane diterpenoid alkaloids from Scutellaria barbata. J. Nat. Prod. 2009, 72, 1793–1797. [Google Scholar] [CrossRef]
- Yeon, E.T.; Lee, J.W.; Lee, C.; Jin, Q.; Jang, H.; Lee, D.; Ahn, J.S.; Hong, J.T.; Kim, Y.; Lee, M.K. neo-Clerodane Diterpenoids from Scutellaria barbata and Their Inhibitory Effects on LPS-Induced Nitric Oxide Production. J. Nat. Prod. 2015, 78, 2292–2296. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.M.; Xia, Y.Z.; Wang, Z.M.; Li, L.N.; Luo, J.G.; Kong, L.Y. neo-Clerodane diterpenoids from Scutellaria barbata mediated inhibition of P-glycoprotein in MCF-7/ADR cells. Eur. J. Med. Chem. 2016, 121, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Xu, F.M.; Yan, X.Z.; Zhu, Y. Scutebarsatine a, a new neoclerodane-type diterpenoid alkaloid from Scutellaria barbata. Chin. Chem. Lett. 1996, 56, 333–334. [Google Scholar]
- Dai, S.J.; Liang, D.D.; Ren, Y.; Liu, K.; Shen, L. New neo-clerodane diterpenoid alkaloids from Scutellaria barbata with cytotoxic activities. Chem. Pharm. Bull. 2008, 56, 207–209. [Google Scholar] [CrossRef]
- Nie, X.P.; Qu, G.W.; Yue, X.D.; Li, G.S.; Dai, S.J. Scutelinquanines A–C, three new cytotoxic neo-clerodane diterpenoid from Scutellaria barbata. Phytochem. Lett. 2010, 3, 190–193. [Google Scholar] [CrossRef]
- Li, S.; Xu, D.; Jia, J.; Zou, W.; Liu, J.; Wang, Y.; Zhang, K.; Zheng, X.; Ma, Y.Y.; Zhang, X.; et al. Structure and anti-inflammatory activity of neo-clerodane diterpenoids from Scutellaria barbata. Phytochemistry 2023, 213, 113771. [Google Scholar] [CrossRef]
- Hanh, T.T.H.; Quang, T.H.; Trung, N.Q.; Cuong, N.T.; An, N.T.; Cuong, N.X.; Nam, N.H.; Van Kiem, P.; Van Minh, C. Scutebarbatolides A-C, new neo -clerodane diterpenoids from Scutellaria barbata D. Don with cytotoxic activity. Phytochem. Lett. 2019, 29, 65–69. [Google Scholar] [CrossRef]
- Dai, S.J.; Qu, G.W.; Yu, Q.Y.; Zhang, D.W.; Li, G.S. New neo-clerodane diterpenoids from Scutellaria barbata with cytotoxic activities. Fitoterapia 2010, 81, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.J.; Chen, M.; Liu, K.; Jiang, Y.T.; Shen, L. Four new neo-clerodane diterpenoid alkaloids from Scutellaria barbata with cytotoxic activities. Chem. Pharm. Bull. 2006, 54, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.J.; Peng, W.B.; Shen, L.; Zhang, D.W.; Ren, Y. Two new neo-clerodane diterpenoid alkaloids from Scutellaria barbata with cytotoxic activities. J. Asian Nat. Prod. Res. 2009, 11, 451–456. [Google Scholar] [CrossRef]
- Kizu, H.; Imoto, Y.; Tomimori, T.; Kikuchi, T.; Kadota, S.; Tsubono, K. Studies on the Constituents of Scutellaria Species. XVIII. Structures of Neoclerodane-Type Diterpenoids from the Whole Herb of Scutellaria rivularis WALL. Chem. Pharm. Bull. 1997, 45, 152–160. [Google Scholar] [CrossRef]
- Kikuchi, T.; Tsubono, K.; Kadota, S.; Kizu, H.; Imoto, Y.; Tomimori, T. Structures of scuterivulactone C1 and C2 by two-dimensional NMR spectroscopy. New clerodane type diterpenoids from Scutellaria rivularis Wall. Chem. Lett. 1987, 16, 987–990. [Google Scholar] [CrossRef]
- Lin, Y.L.; Kuo, Y.H.; Cheng, M.C.; Wang, Y. Structures of Scutellones D and E Determined from X-Ray Diffraction, Spectral and Chemical Evidence. Neoclerodane-Type Diterpenoids from Scutellaria rivularis WALL. Chem. Pharm. Bull. 1988, 36, 2642–2646. [Google Scholar] [CrossRef]
- Lin, Y.; Kuo, Y.H.S.C. Two New Neoclerodane Type Diterpenoids from Scutellaria rivularis. Heterocycles 1988, 27, 779–783. [Google Scholar] [CrossRef]
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Zhang, Q.; Jia, Z. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef]
- Lin, Y.L.; Kuo, Y.H. Four New Neoclerodane-Type Diterpenoids, Scutellones B, G, H, and I, from Aerial Part of Scutellaria Rivularis. Chem. Pharm. Bull. 1989, 37, 582–585. [Google Scholar] [CrossRef]
- Yuan, Q.Q.; Song, W.B.; Wang, W.Q.; Xuan, L.J. Scubatines A-F, new cytotoxic neo-clerodane diterpenoids from Scutellaria barbata D. Don. Fitoterapia 2017, 119, 40–44. [Google Scholar] [CrossRef]
- Zhu, F.; Di, Y.T.; Liu, L.L.; Zhang, Q.; He, H.P. Cytotoxic neoclerodane diterpenoids from Scutellaria barbata. J. Nat. Prod. 2010, 73, 233–236. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.; Choi, I.; Min, B.S.; Shim, S.H. Two novel neo-clerodane diterpenoids from Scutellaria barbata. Bioorg. Med. Chem. Lett. 2010, 20, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.W.; Yue, X.D.; Li, G.S.; Yu, Q.Y.; Dai, S.J. Two new cytotoxic ent-clerodane diterpenoids from Scutellaria barbata. J. Asian Nat. Prod. Res. 2010, 12, 859–864. [Google Scholar] [CrossRef]
- Feng, X.S.; Yan, W.; Bai, L.H.; Wang, K.; Chen, X.Q. neo-Clerodane Diterpenoids from the Aerial Parts of Scutellaria barbata with Anti-Inflammatory Activity. Chem. Biodivers. 2021, 18, e2100693. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Tang, X.L.; Jiang, T.; Li, P.F.; Li, P.L.; Li, G.Q. Bioassay-guided isolation of neo-clerodane diterpenoids from Scutellaria barbata. J. Asian Nat. Prod. Res. 2013, 15, 941–949. [Google Scholar] [CrossRef]
- Shim, S.H. A New Diterpenoid from Aerial Parts of Scutellaria barbata. Chem. Nat. Compd. 2014, 50, 291–292. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Pham, V.C.; Nguyen, T.T.H.; Tran, V.H.; Doan, T.M.H. Novel Antioxidant neo-Clerodane Diterpenoids from Scutellaria barbata. Eur. J. Org. Chem. 2009, 2009, 5810–5815. [Google Scholar] [CrossRef]
- Dai, S.J.; Wang, G.F.; Chen, M.; Liu, K.; Shen, L. Five new neo-clerodane diterpenoid alkaloids from Scutellaria barbata with cytotoxic activities. Chem. Pharm. Bull. 2007, 55, 1218–1221. [Google Scholar] [CrossRef]
- Liang, C.X.; Cao, Y.X.; Zhang, D.; Zhang, K.; Yang, L. A new neoclerodane diterpenoid from Scutellaria barbata. Chin. Tradit. Herb. Herbal. Drugs 2015, 46, 2843–2845. [Google Scholar] [CrossRef]
- Feng, Z.; Ling-Li, L.; Ying-Tong, D.I.; Xiao-Jiang, H.; Hong-Ping, H.E. Scutellin A, a New Neoclerodane Diterpenoid from Scutellaria barbata (Labiatae). Plant Divers. 2009, 31, 474. [Google Scholar] [CrossRef]
- Kizu, H.; Imoto, Y.; Tomimori, T.; Tsubono, K.; Kadota, S.; Kikuchi, T.J.C.; Bulletin, P. Structure of scuterivulactone d determined by two-dimensional nmr spectroscopy. a new diterpenoid from a chinese crude drug “ban zhi lian” (scutellaria rivularis wall). Chem. Pharm. Bull. 1987, 35, 1656–1659. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.J.; Peng, W.B.; Shen, L.; Zhang, D.W.; Ren, Y. New norditerpenoid alkaloids from Scutellaria barbata with cytotoxic activities. Nat. Prod. Res. 2011, 25, 1019–1024. [Google Scholar] [CrossRef]
- Li, P.; Zuo, T.T.; Wang, X.Q.; Zhu, L.X.; Zhang, G.G. Chemical constituents of Scutellaria barbata D. Don (II). Chin. J. Med. Chem. 2008, 18, 374–376. [Google Scholar] [CrossRef]
- Yi, Z.; Shu-Heng, H.E.; Dan-Dan, G.E.; Peng, Z.; Xiu-Mei, G.; Tao, W. Isolation and identification of chemical constituents from whole plant of Scutellaria barbata D.Don (II). J. Shenyang Pharm. Univ. 2011, 28, 425–428. [Google Scholar] [CrossRef]
- Jiang, X.G.; Gu, Z.L. Chemical Constituents and Pharmacological Effects of Scutellaria barbata. Chin. Wild Plant Resour. 2004, 23, 3–5. [Google Scholar]
- Xu, Y.M.; Guo, L.W.; Chen, J.W. Isolation and physico-chemical properties of polysaccharide from the herb of Scutellaria barbata D. Don. Nat. Prod. Product. Res. Dev. 1992, 4, 1–5. [Google Scholar] [CrossRef]
- Meng, Y.F.; Li, Z.X.; Wang, B.F.; Meng, X.Q.; Chen, Y.Z. Study on Polysaccharide from Scutellaria Barbata. J. Lanzhou Univ. (Nat. Sci.) 1992, 28, 112–116. [Google Scholar] [CrossRef]
- Kong, M.L.; Zhang, J. Extraction, separation and preliminary structure research of polysaccharide from Scutellaria barbata. Chin. Tradit. Pat. Med. 2008, 30, 1694–1697. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, H.P.; Wang, J.B. Anti-complement activity of polysaccharide B3-PS2 purified from Herba Scutellariae barbatae. Acta Pharm. Sin. 2009, 44, 615–619. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Dai, L.; Zhu, Y.Y.; Liao, H.M.; Zhang, K. Purification, properties and antioxidant activity of acidic polysaccharide SBPs from Scutellaria barbata. Chin. Tradit. Herb. Herbal. Drugs 2009, 40, 728–731. [Google Scholar]
- Sun, P.; Sun, D.; Wang, X. Effects of Scutellaria barbata polysaccharide on the proliferation, apoptosis and EMT of human colon cancer HT29 Cells. Carbohydr. Polym. 2017, 167, 90–96. [Google Scholar] [CrossRef]
- Lin, J.Y.; Liu, S.S.; Ming, Y.L. Advances on Chemical Constituents and Pharmacological Activities of Scutellaria barbata. Subtrop. Plant Sci. 2015, 44, 77–82. [Google Scholar] [CrossRef]
- Wu, X.; Xu, N.; Ye, Z.; Zhao, Q.; Liu, J.; Li, J.; Wu, M.; Zheng, Y.; Li, X.; Li, W.; et al. Polysaccharide from Scutellaria barbata D. Don attenuates inflammatory response and microbial dysbiosis in ulcerative colitis mice. Int. J. Biol. Macromol. 2022, 206, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lei, J.; Yu, H.; Cai, X.; Zou, G. Chemical composition and antimicrobial activity of the essential oil Scutellaria barbata. Phytochemistry 2004, 65, 881–884. [Google Scholar] [CrossRef]
- Zhang, F.W.; Hui, R.H.; Hou, D.Y. Analysis of Volatile Components in Scutellaria barbata D. Don. J. Chin. Mass. Spectrom. Soc. 2009, 30, 175–178. [Google Scholar]
- Wang, Z.Y.; Wang, T.S.; Chen, F.L.; Lin, J.M. GC-MS analysis of volatile oil from Scutellaria barbata. J. South. Med. Univ. 2009, 29, 1482–1483. [Google Scholar]
- Yang, W.W.; Hu, J.F.; Yang, S.; Liu, Y.Q.; Yin, W.J.; Lv, Q.T.; Rong, R. Optimization of the supercritical carbon dioxide extraction conditions for Scutellaria barbata D. Don by orthogonal test and GC-MS analysis. Mod. Instrum. 2012, 18, 11–24. [Google Scholar]
- Fan, J.; Wang, Y.; Zhang, J.; Qin, R. Quantitative changes analysis between contents of active ingredients and trace elements in Scutellaria barbata D. Don. Chem. Res. Appl. 2017, 29, 1164–1170. [Google Scholar]
- Bei, Z.Y.; Luo, X.B.; Li, Y.B. Determination of Microelements in Herba Scutellariae Barbatae by Microwave-assisted Sample Digestion-flame Atom ic Absorption Spectrametric. Lishizhen Med. Mater. Med. Res. 2008, 19, 709–710. [Google Scholar]
- Yang, S.L. Studies on extraction of Scutellaria barbata D. Don by supercritical fluid of CO2. Pharm. J. Chin. PLA 2004, 20, 46–48. [Google Scholar]
- Li, N.; Xiao, H.T.; Meng, D.L.; Wang, J.H. Chemical constituents of Scutellaria barbata. Mod. Chin. Med. 2009, 11, 16–20. [Google Scholar] [CrossRef]
- Ducki, S.; Hadfield, J.A.; Lawrence, N.J.; Liu, C.Y.; Zhang, X. Isolation of E-1-(4′-Hydroxyphenyl)-but-1-en-3-one from Scutellaria barbata. Planta Med. 1996, 62, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, G.; Zuo, T.; Wang, S. Chemical constituents of the whole plant of Scutellaria barbata D. Don. J. Shenyang Pharm. Univ. 2008, 27, 549–552. [Google Scholar] [CrossRef]
- Sonoda, M.; Nishiyama, T.; Matsukawa, Y.; Moriyasu, M. Cytotoxic activities of flavonoids from two Scutellaria plants in Chinese medicine. J. Ethnopharmacol. 2004, 91, 65–68. [Google Scholar] [CrossRef]
- Tomimori, T.; Miyaichi, Y.; Imoto, Y.; Kizu, H. Studies on the constitutents of Scutellaria species (VIII). On the flavonoid constituents of “Ban Zhi Lian”, the whole herb of Scutellaria rivularis. J. Pharmacogn. 1986, 40, 432–433. [Google Scholar]
- Yan, C.; Guo-Gang, Z.; De-Shuang, M.; Ying-Na, L.I. Chemical constituents of Scutellaria barbata D.Don (I). Chin. J. Med. Chem. 2008, 18, 48. [Google Scholar] [CrossRef]
- Li, Y.; Tang, X.; Li, P.; Li, G. Studies on Chemical Consisutents of Scutellaria barbata D. Don. Period. Ocean Univ. China 2013, 43, 77–80. [Google Scholar] [CrossRef]
- Hou, C.; Wen, X.; Yan, S.; Gu, X.; Jiang, Y.; Chen, F.; Liu, Y.; Zhu, Y.; Liu, X. Network-based pharmacology-based research on the effect and mechanism of the Hedyotis diffusa-Scutellaria barbata pair in the treatment of hepatocellular carcinoma. Sci. Rep. 2024, 14, 963. [Google Scholar] [CrossRef]
- Xu, X.; Chen, F.; Zhang, L.; Liu, L.; Zhang, C.; Zhang, Z.; Li, W. Exploring the mechanisms of anti-ovarian cancer of Hedyotis diffusa Willd and Scutellaria barbata D. Don through focal adhesion pathway. J. Ethnopharmacol. 2021, 279, 114343. [Google Scholar] [CrossRef]
- Hnit, S.S.T.; Yao, M.; Xie, C.; Ge, G.; Bi, L.; Jin, S.; Jiao, L.; Xu, L.; Long, L.; Nie, H.; et al. Transcriptional regulation of G(2)/M regulatory proteins and perturbation of G(2)/M Cell cycle transition by a traditional Chinese medicine recipe. J. Ethnopharmacol. 2020, 251, 112526. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, C.; Li, H.; Ding, C.; Wang, L.; Wu, H.; Dai, W.; Wang, C. Exploration of the effect and mechanism of Scutellaria barbata D. Don in the treatment of ovarian cancer based on network pharmacology and in vitro experimental verification. Medicine 2023, 102, e36656. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, J.; Fan, J.; He, L.; Wang, X.; Tang, F.; Tian, D.; He, Y. Molecular Assessment of Scutellaria barbata D. Don in the Treatment of Nasopharyngeal Carcinoma Based on Network Pharmacology and Experimental Verification. Evid. Based Complement. Altern. Med. 2022, 2022, 1988378. [Google Scholar] [CrossRef]
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Li, Z. Baicalein: A review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed. Pharmacother. 2017, 93, 1285–1291. [Google Scholar] [CrossRef]
- Li, Y.; Duan, S.; Jia, H.; Bai, C.; Zhang, L.; Wang, Z. Flavonoids from tartary buckwheat induce G2/M cell cycle arrest and apoptosis in human hepatoma HepG2 cells. Acta Biochim. Biophys. Sin. 2014, 46, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Gravina, G.L.; Senapedis, W.; McCauley, D.; Baloglu, E.; Shacham, S.; Festuccia, C. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J. Hematol. Oncol. 2014, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Xu, H.; Zhang, P.; Chen, G.; Chen, Z.; Fang, C.; Lin, L. Investigating the Mechanism of Scutellariae barbata Herba in the Treatment of Colorectal Cancer by Network Pharmacology and Molecular Docking. Evid. Based Complement. Altern. Med. 2021, 2021, 3905367. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.T.; Zhang, G.L.; Dai, C.F.; Zhang, B.R.; Cao, K.X.; Wang, C.G.; Yang, G.W.; Wang, X.M. Scutellaria barbata and Hedyotis diffusa herb pair for breast cancer treatment: Potential mechanism based on network pharmacology. J. Ethnopharmacol. 2020, 259, 112929. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, Z.; Wang, S.; Li, T.; Mastriani, E.; Li, Q.H.; Bao, H.X.; Zhou, Y.J.; Wang, X.; Liu, Y.; et al. Main components of pomegranate, ellagic acid and luteolin, inhibit metastasis of ovarian cancer by down-regulating MMP2 and MMP9. Cancer Biol. Ther. 2017, 18, 990–999. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.; Liu, K. Study on Inhibition of Reversal of Epithelial-mesenchymal Transition by Extracts from Scutellaria Barbata for Cell Migration and Invasion of Hepatoma Cells. J. New Chin. Med. 2019, 51, 17–21. [Google Scholar] [CrossRef]
- Yang, J.; Yang, G.; Hou, G.; Liu, Q.; Hu, W.; Zhao, P.; He, Y. Scutellaria barbata D. Don polysaccharides inhibit the growth of Calu-3 xenograft tumors via suppression of the HER2 pathway and angiogenesis. Oncol. Lett. 2015, 9, 2721–2725. [Google Scholar] [CrossRef]
- Shiau, A.L.; Shen, Y.T.; Hsieh, J.L.; Wu, C.L.; Lee, C.H. Scutellaria barbata inhibits angiogenesis through downregulation of HIF-1α in lung tumor. Environ. Toxicol. 2014, 29, 363–370. [Google Scholar] [CrossRef]
- Wei, L.; Lin, J.; Xu, W.; Cai, Q.; Shen, A.; Hong, Z.; Peng, J. Scutellaria barbata D. Don Inhibits Tumor Angiogenesis via Suppression of Hedgehog Pathway in a Mouse Model of Colorectal Cancer. Int. J. Mol. Sci. 2012, 13, 9419–9430. [Google Scholar] [CrossRef]
- Wang, T.; Lyu, C.Y.; Jiang, Y.H.; Dong, X.Y.; Wang, Y.; Li, Z.H.; Wang, J.X.; Xu, R.R. A drug-biomarker interaction model to predict the key targets of Scutellaria barbata D. Don in adverse-risk acute myeloid leukaemia. Mol. Divers. 2021, 25, 2351–2365. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Feng, J.; Yang, H.; Yan, Z.; Li, Q.; Wei, L.; Lai, Z.; Jin, Y.; Peng, J. Scutellaria barbata D. Don inhibits 5-fluorouracil resistance in colorectal cancer by regulating PI3K/AKT pathway. Oncol. Rep. 2017, 38, 2293–2300. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Yan, W.; Chen, X.; Li, H.; Li, R.; Liu, D. Reversal of multi-drug resistance in HepG2/ADR cells by neo-clerodane diterpenoids from Scutellaria barbata. Nat. Prod. Res. Dev. 2020, 32, 365–372. [Google Scholar]
- Liang, H.; Xie, S.; Wei, Z.; Wei, L.; Xiaoping, L.; Jiao, S.; Chun, Y. Clinical study on the treatment of 30 cases of advanced refractory colorectal cancer with “Intestinal Compound”. Jiangsu J. Tradit. Chin. Med. 2017, 49, 36–39. [Google Scholar]
- Tian, J.; Liu, L.; Wang, L. Clinical observation of Compound Banmao Capsules combined with DP regiment in treatment of advanced ovarian cancer. Drugs Clin. 2018, 33, 2050–2054. [Google Scholar] [CrossRef]
- Guan, Q.; Jiao, J.; Ye, Z.; Wang, K. Clinical Analysis of Yipi Fuzheng Recipe combined with Chemotherapy in the Treatment of Advanced Esophageal Cancer. J. Shaanxi Univ. Chin. Med. 2019, 42, 150–158. [Google Scholar] [CrossRef]
- Xu, H.; Cao, L.; Gao, S.; Li, R.; Wang, A.; Li, L.; Wang, G. Clinical Effect of Yangzheng Xiaoji Capsule Combined with Endostar in Treatment of Advanced Malignant Tumor of Digestive Tract. Chin. Arch. Tradit. Chin. Med. 2018, 36, 4. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.; Liu, Y.; Zhao, T. Application effect of Ankangxin Capsule combined with Cisplatin in the adjuvant chemotherapy for non-small cell lung cancer. China Pr. Prac. Med. 2018, 13, 2. [Google Scholar] [CrossRef]
- Chen, F.; Luo, J.; Du, M.; Zhang, X. Effect of Anti-cancer Pill Combined with Trastuzumab and Chemotherapy on Short Term Efficacy and Long-term Efficacy of HER2 Positive Patients with Advanced Gastric Cancer. Pract. J. Cancer 2019, 34, 4. [Google Scholar] [CrossRef]
- Zhang, J. Clinical analysis of Ban zhi Qin lian decoction joint with gefitinib in the treatment of elderly non-small cell lung cancer. Captial Food Med. 2020, 27, 186. [Google Scholar]
- Zhang, Y.; Luo, X.; Guo, Y. Clinical study of oldenlandia diffusa-scutellaria barbata for the maintenance treatment of malignant tumors. J. Gannan Med. Univ. 2022, 42, 583–586. [Google Scholar] [CrossRef]
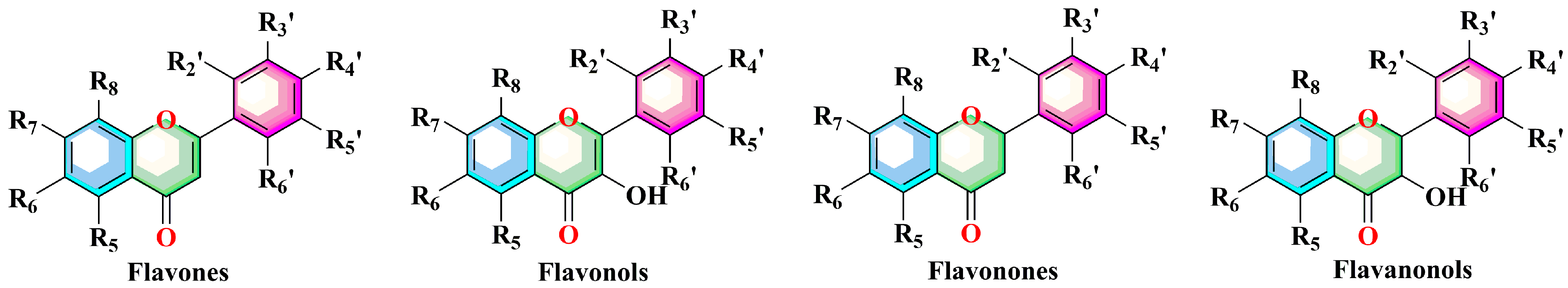

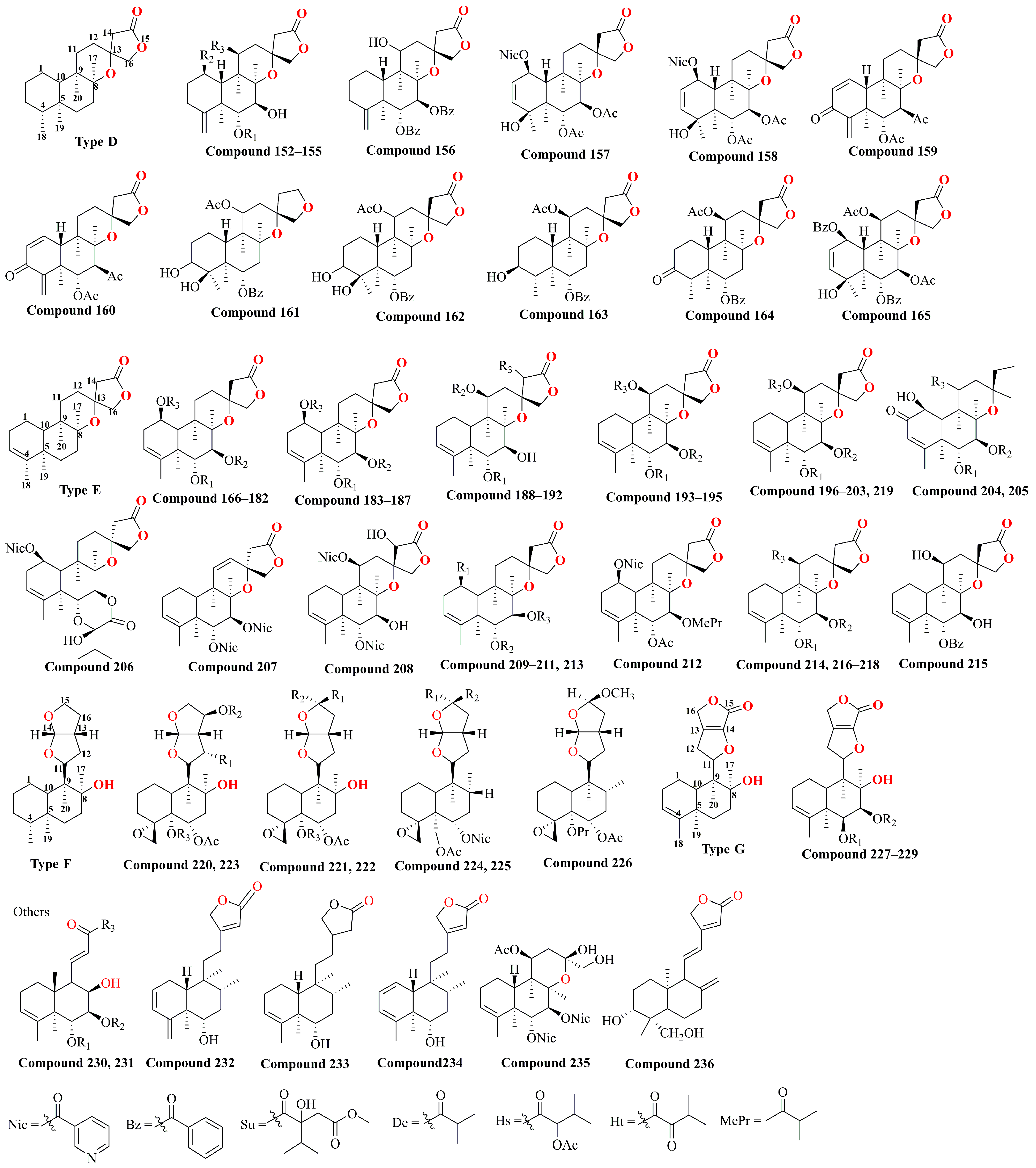
| No. | Compound | R5 | R6 | R7 | R8 | R2’ | R3’ | R4’ | R5’ | R6’ | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flavones | |||||||||||
| 1 | luteolin | OH | H | OH | H | H | H | OH | OH | H | [21] |
| 2 | apigenin | OH | H | OH | H | H | H | OH | H | H | [22,23] |
| 3 | scutellarein | OH | OH | OH | H | H | H | OH | H | H | [21] |
| 4 | wogonin | OH | H | OH | OMe | H | H | H | H | H | [24] |
| 5 | 6-O-methylscutellarein | OH | OMe | OH | H | H | H | OH | H | H | [25] |
| 6 | 4′,5-dihydroxy-3′,5′,6,7-tetramethoxyflavone | OH | OMe | OMe | H | H | OMe | OH | OMe | H | [26,27] |
| 7 | 5-hydroxy-7,8-dimethoxyflavone | OH | H | OMe | OMe | H | H | H | H | H | [28] |
| 8 | rivularin | OH | H | OMe | OMe | OMe | H | H | H | OH | [28] |
| 9 | 4′-hydroxy-wogonin | OH | H | OH | OMe | H | H | OH | H | H | [27,29] |
| 10 | 5,7,3′,4′,5′-pentamethoxyflavone | OMe | H | OMe | H | H | OMe | OMe | OMe | H | [30] |
| 11 | 5-hydroxy-7,3′,4′,5′-tetramethoxyflavone | OH | H | OMe | H | H | OMe | OMe | OMe | H | [30] |
| 12 | 5-hydroxy-7,4′-dimethoxy-flavone | OH | H | OMe | H | H | H | OMe | H | H | [31] |
| 13 | 5-hydroxy-7,8,4′-trimethoxyflavone | OH | H | OMe | OMe | H | H | OMe | H | H | [31] |
| 14 | baicalein | OH | OH | OH | H | H | H | H | H | H | [32] |
| 15 | isoscutellarein | OH | H | OH | OH | H | H | OH | H | H | [33] |
| 16 | 6-hydroxyluteolin | OH | OH | OH | H | H | H | OH | OH | H | [33] |
| 17 | 5-hydroxy-6,7,3′,4′-tetramethoxyflavone | OH | OMe | OMe | H | H | H | H | H | H | [33] |
| 18 | 5,6,7,3′,4′,5′-hexahydroxyflavone | OH | OH | OH | H | H | OH | OH | OH | H | [34] |
| 19 | 6-methoxyluteolin | OH | OMe | OH | H | H | H | OH | OH | H | [34] |
| 20 | chrysoeriol | OH | H | OH | H | H | H | OH | OMe | H | [34] |
| 21 | cirsiliol | OH | OMe | OMe | H | H | OH | OH | H | H | [34] |
| 22 | tenaxin | OH | OMe | OMe | OMe | H | H | H | H | OH | [34] |
| 23 | wogonoside | OH | H | OGlcA | H | H | H | H | H | H | [34] |
| 24 | apigenin-5-O-β-d-glucopyranoside | Glu | H | OH | H | H | H | OH | H | H | [35] |
| 25 | apigenin-7-O-β-d-glucoside | OH | H | O-Glu | H | H | H | OH | H | H | [36] |
| 26 | apigenin-7-O-neohesperidoside | OH | H | O-Neo | H | H | H | OH | H | H | [37] |
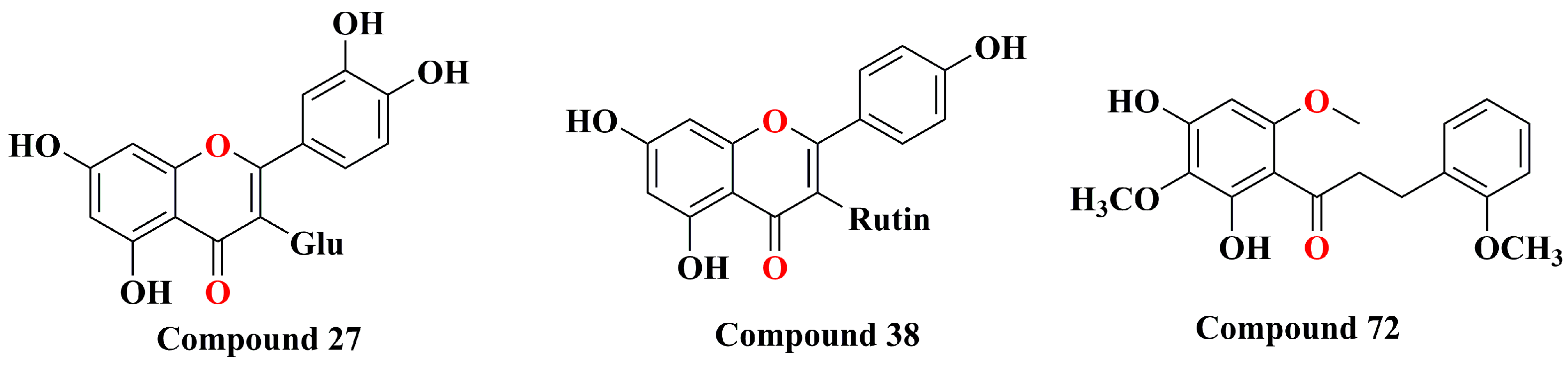
| 27 | hyperoside | Refer to Figure 2 | [30] | ||||||||
| 28 | baicalin | OH | OH | O-GlcA | H | H | H | H | H | H | [24] |
| 29 | salvigenin | OH | OMe | OMe | H | H | H | OMe | H | H | [31] |
| 30 | luteolin-7-O-β-d-glucopyranoside | OH | H | O-Glu | H | H | OH | OH | H | H | [36] |
| 31 | scutellarein-7-O-glucoside | OH | OH | O-Glu | H | H | H | H | H | H | [34] |
| 32 | isoscutellarein-8-O-β-d-glucuronide-6″-methylester | OH | H | OH | Glu-Me ester | H | H | OH | H | H | [33] |
| 33 | apigenin-7-O-β-d-glucuronide-6″-methylester | OH | H | O-GlcA-Me ester | H | H | H | OH | H | H | [33] |
| 34 | 5-hydroxy-4′-methoxyflavone-7-O-α-Lrhamnosyl-(1→6)-β-d-glucopyranoside | OH | H | O-Rha-Glu | H | H | H | OMe | H | H | [38] |
| 35 | scutellarin | OH | OH | O-GlcA | H | H | H | OMe | H | H | [39] |
| 36 | 5,8,2′-trihydroxy-7-O-flavonoid glucuronide | OH | H | O-GlcA | OH | OH | H | H | H | H | [40] |
| 37 | apigenin-7-O-β-d-glucuronide | OH | H | O-GlcA | H | H | H | OH | H | H | [37] |
| 38 | kaempferol-3-O-β-d-rutinoside | Refer to Figure 2 | [41] | ||||||||
| 39 | isoscutellarein-8-O-glucuronide | OH | H | OH | O-GlcA | H | H | OH | H | H | [33] |
| 40 | scutellarein-7-O-glucoside | OH | OH | O-Glu | H | H | H | OH | H | H | [34] |
| 41 | luteolin-7-O-glucuronide | OH | H | O-GlcA | H | H | OH | OH | H | H | [34] |
| 42 | isoscutellarein-7-O-glucuronide | OH | H | O-GlcA | OH | H | H | OH | H | H | [34] |
| 43 | hispidulin-7-O-β-d-methylgluzcuronide | OH | OMe | O-GlcA | H | H | H | OH | H | H | [33] |
| 44 | scutellarein-7-O-β-d-glucuronide methyl ester | OH | OH | O-GlcA Me ester | H | H | H | OH | H | H | [33] |
| 45 | 4-hydroxy-wogonin-7-O-glucuronide | OH | H | O-GlcA | OMe | H | H | OH | H | H | [34] |
| 46 | 8-hydroxyluteolin-7-O-glucuronide | OH | H | O-GlcA | OH | H | OH | OH | H | H | [34] |
| 47 | 6-hydroxyluteolin-7-O-glucuronide | OH | OH | O-GlcA | H | H | OH | OH | H | H | [34] |
| Flavonols | |||||||||||
| 48 | quercetin | OH | H | OH | H | H | OH | OH | OH | H | [39] |
| 49 | isorhamnetin | OH | H | OH | H | H | H | OH | OMe | H | [32] |
| 50 | quercetin-4′-O-glucuronide | OH | H | OH | H | H | OH | GlcA | H | H | [39] |
| Flavonones | |||||||||||
| 51 | carthamidin | OH | OH | OH | H | H | H | OH | H | H | [35] |
| 52 | naringenin | OH | H | OH | H | H | H | OH | H | H | [25] |
| 53 | 6-methoxynaringenin | OH | OMe | OH | H | H | H | OH | H | H | [25] |
| 54 | 5,7,4′-trihydroxy-8-methoxyflavanone | OH | H | OH | OMe | H | H | OH | H | H | [25] |
| 55 | dihydrooroxylin A | OH | OMe | OH | H | H | H | H | H | H | [25] |
| 56 | isocarthamidin | OH | H | OH | OH | H | H | OH | H | H | [25] |
| 57 | eriodictyol | OH | H | OH | H | H | OH | OH | H | H | [27] |
| 58 | naringenin-4′-O-glucuronide | OH | H | OH | H | H | H | O-GlcA | H | H | [34] |
| 59 | 8-methoxynaringenin-7-O-glucuronide | OH | H | O-GlcA | OMe | H | H | OH | H | H | [34] |
| 60 | naringenin-7-O-glucuronide | OH | H | O-GlcA | H | H | H | OH | H | H | [34] |
| 61 | 6-methoxynaringenin-7-O-glucuronide | OH | OMe | O-GluA | H | H | H | OH | H | H | [34] |
| 62 | isocarthamidin-7-O-glucuronide | OH | H | O-GluA | OH | H | H | OH | H | H | [34] |
| 63 | carthamidin-7-O-glucuronide | OH | OH | O-GluA | H | H | H | OH | H | H | [34] |
| 64 | 5,6,7,3′,4′-pentahydroxyflavanone-3-O-glucuronide | OH | OH | O-GlcA | H | H | OH | OH | H | H | [34] |
| Flavanonols | |||||||||||
| 65 | 7-hydroxy-2′,5,8-trimethoxyflavanone | OMe | H | OH | OMe | OMe | H | H | H | H | [27] |
| 66 | 2(S)-2′,7-dihydroxy-5,8-dimethoxyflavanone | OMe | H | OH | OMe | OH | H | H | H | H | [35] |
| 67 | 3,5,6,7,4′-pentahydroxyflavanone | OH | H | H | OH | H | OH | OH | H | H | [34] |
| 68 | 3,5,7,8,4′-pentahydroxyflavanone) | OH | H | OH | OH | H | H | OH | H | H | [34] |
| 69 | 3,5,7,4′-tetrahydroxy-6-methoxyflavanone | OH | OMe | OH | H | H | H | OH | H | H | [34] |
| 70 | 3,5,7,4′-tetrahydroxy-8-methoxyflavanone) | OH | H | OH | OMe | H | H | OH | H | H | [34] |
| 71 | 3,5,8,3′,4′-pentahydroxyflavanone-3-O-glucuronide | OH | H | O-GlcA | OH | H | OH | OH | H | H | [34] |
| Chalcones | |||||||||||
| 72 | 2′,4′-dihydroxy-2,3′,6′-trimethoxy-chalcone | Refer to Figure 2 | [35] | ||||||||
| No. | Compound | R1 | R2 | R3 | Reference |
|---|---|---|---|---|---|
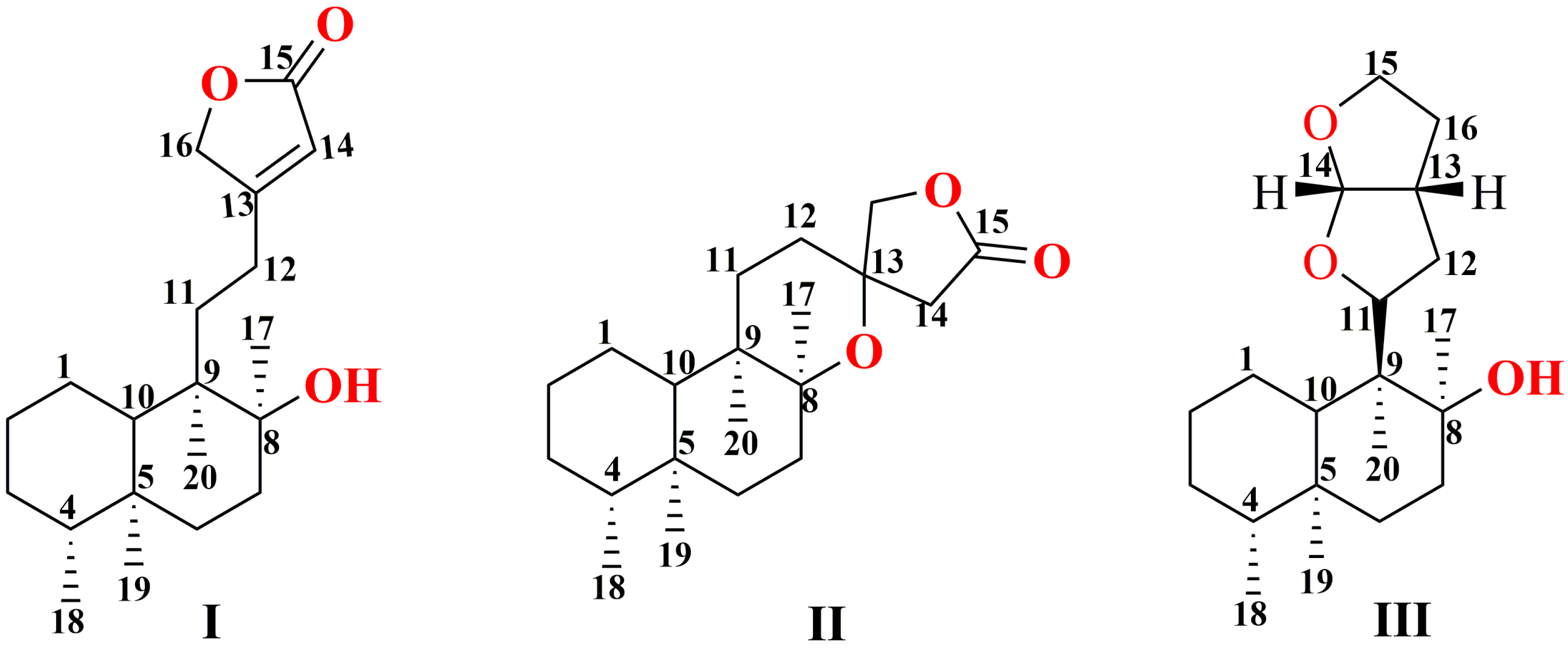
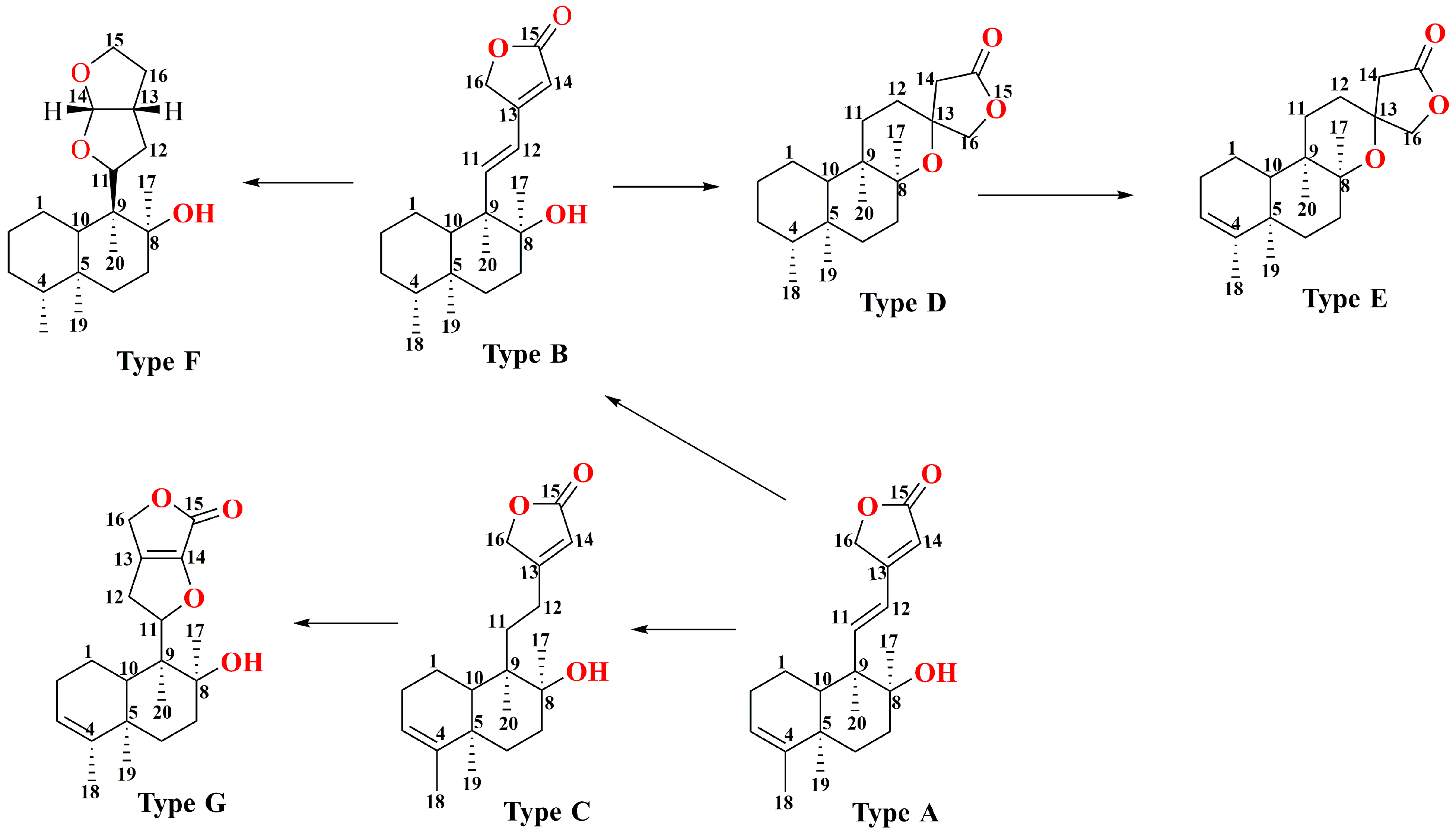
| Type A | |||||
| 73 | scutebata X | Nic | Bz | O | [32,42,43,44] |
| 74 | scutebata Y | Nic | Bz | β-OH, H | [43] |
| 75 | 2-carbonylscutebartatin A | Nic | Nic | O | [45] |
| 76 | scutebata I | Ac | H | [42,46] | |
| 77 | scutebata J | Bz | H | [47,48] | |
| 78 | scutebata K | Su | H | [42,43,46,47] | |
| 79 | scutebata T | Ac | De | [44,48] | |
| 80 | barbatin C | H | H | [42,49,50] | |
| 81 | barbatin D | Bz | Bz | [43,48,51] | |
| 82 | barbatin E | Hs | Ht | [51] | |
| 83 | barbatin F | Ht | Bz | [52] | |
| 84 | 6-O-nicotinoylbarbatin A | Nic | H | [45,53] | |
| 85 | 6, 7-di-O-acetoxybarbatin A | Ac | Ac | [45,49] | |
| 86 | scutebarbatine A | Nic | Nic | [48,54,55,56] | |
| 87 | scutebarbatine B | Nic | Bz | [50,55] | |
| 88 | scutebarbatine K | Nic | Ac | [48,57] | |
| 89 | scutebarbatine L | Nic | Hs | [43,48,55,57] | |
| 90 | scutebarbatine Y | Bz | Nic | [32,48,54] | |
| 91 | scutebartine F | Nic | Bz | Z(RΔ11–12) | [42,55] |
| 92 | scutebartine G | Nic | Nic | Z(RΔ11–12) | [42,55] |
| 93 | scutolide A | Ac | De | Z(RΔ11–12) | [44,48] |
| 94 | scutolide C | Bz | De | [48] | |
| 95 | scutolide D | Bz | Hs | [48] | |
| 96 | scutolide E | Ac | Bz | [48] | |
| 97 | scutehenanine A | H | Nic | [42,53] | |
| 98 | 6-O-(2-carbonyl-3-methylbutanoyl)-scutehenanine A | Ht | Nic | [53] | |
| 99 | 6-O-acetylScutehenanine A | Ac | Nic | [42] | |
| 100 | scutelinquanine C | Nic | Ht | [58] | |
| 101 | barbaolide M | Ht | Mepr | [59] | |
| 102 | scutebarbatolide A | Bz | Ac | [60] | |
| 103 | scutebarbolide J | Su | OH | [42] | |
| 104 | scutebarbolide K | OH | Su | [42] | |
| 105 | 8-O-nicotinoylbarbatin A | [45] | |||
| 106 | 6-(2, 3-epoxy-2isopropyl-n-propoxyl) -barbatin C | [61] | |||
| 107 | scutolide B | [42,48,55] | |||
| 108 | scutebarbolide I | [42] | |||
| Type B | |||||
| 109 | scutebata L | Bz | Bz | H | [46,47] |
| 110 | scutebarbatine D | Nic | Bz | OH | [62] |
| 111 | scutebarbatine O | Nic | Nic | OH | [63] |
| 112 | scutehenanine A | Bz | Bz | OH | [42,53] |
| 113 | scutebarbatine E | Nic | Bz |  | [62] |
| 114 | scutellone D | OH | [64,65,66] | ||
| 115 | scutellone E | [66] | |||
| 116 | scutellone F | [67,68] | |||
| 117 | scutellone H | OEt | [68,69] | ||
| 118 | scutellone I | OMe | [68] | ||
| 119 | scutebarbatine C | [62] | |||
| Type C | |||||
| 120 | scutebata A | Bz | Bz | OH | [70,71] |
| 121 | scutebata B | Nic | Bz | OH | [25,54,70] |
| 122 | scutebata C | Nic | H | OH | [54,70] |
| 123 | scutebata W | Su | H | H | [42,71] |
| 124 | barbatin G | H | Nic | OH | [52] |
| 125 | scutebarbatine X | Nic | Nic | OH | [32,49,54,55] |
| 126 | scutebarbolide D | Bz | Nic | OH | [25,32,42] |
| 127 | scutebarbolide E | Bz | De | OH | [42] |
| 128 | scutolide F | Bz | H | H | [48] |
| 129 | scutolide G | Ac | De | H | [48] |
| 130 | scutolide H | Nic | De | H | [48] |
| 131 | scutolide I | Ac | Ac | H | [48] |
| 132 | scutolide J | Nic | Nic | H | [48] |
| 133 | barbatellarine B | Nic | Bz | H | [48,72] |
| 134 | scutebata M | Nic | Hs | OH | [46,55,72] |
| 135 | scutebarbatine Z | Nic | H | H | [32,55] |
| 136 | 6-acetoxybarbatin C | Ac | H | OH | [73] |
| 137 | scutebarbolide A | Ac | H | [42] | |
| 138 | scutebarbolide C | H | OH | [42] | |
| 139 | scutebarbolide B | [42] | |||
| 140 | scutebarbolide F | [42] | |||
| 141 | scutebarbolide G | [42] | |||
| 142 | scutebarbolide H | [42] | |||
| 143 | barbaolide J | Nic | H | OH | [59] |
| 144 | barbaolide K | [59] | |||
| 145 | barbaolide L | Nic | Ac | H | [59] |
| 146 | scutebarbatolide B | Nic | [60] | ||
| 147 | scutebarbatolide C | Bz | [60] | ||
| 148 | scuttenline A | Nic | OB | [74] | |
| 149 | scuttenline B | [74] | |||
| 150 | scuttenline C | Bz | H | [74] | |
| 151 | scuttenline D | [74] | |||
| Type D | |||||
| 152 | scutebata P | Bz | H | OBz(13S) | [49,70,75] |
| 153 | scutebata Q | Bz | OBz | H(13R) | [43,75] |
| 154 | scutehenanine C | Nic | H | Nic | [53] |
| 155 | scutebata N | Bz | H | Bz | [46,47] |
| 156 | barbatin B | [50] | |||
| 157 | barbatellarine C | [74] | |||
| 158 | barbatellarine D | [74] | |||
| 159 | barbatellarine E | [74] | |||
| 160 | barbatellarine F | [72,76] | |||
| 161 | scuterivulactone C1 | [64,65] | |||
| 162 | scuterivulactone C2 | [64,65] | |||
| 163 | scutellone C | [67,68] | |||
| 164 | scutellone G | [68,69] | |||
| 165 | barbatellarine A | [72] | |||
| Type E | |||||
| 166 | scutebata D | Ac | Ac | Bz | [44,48,54,71] |
| 167 | scutebata E | Ac | Ac | MePr | [49,70,71] |
| 168 | scutebata F | Ac | Ac | Nic | [48,49,71] |
| 169 | scutebata G | Nic | Bz | Bz | [43,54,71] |
| 170 | scutebata O | Bz | H | H | [42,46,47] |
| 171 | scutebata R | Ac | Ac | De | [43,75] |
| 172 | scutebata S | Ac | De | Bz | [44] |
| 173 | scutebata V | Nic | Nic | H | [46] |
| 174 | scutebata B1 | Ac | Nic | Nic | [43] |
| 175 | scutebata C1 | Bz | H | Bz | [43] |
| 176 | scutebartine A | Nic | H | Bz | [55] |
| 177 | scubatine E | Ac | Bz | Bz | [70] |
| 178 | scubatine F | Bz | Ac | Bz | [70] |
| 179 | barbatine C | Ac | Ac | Nic | [42,55] |
| 180 | barbatine D | Nic | Ac | Nic | [55,71] |
| 181 | scutebatin A | Nic | Bz | Nic | [43,54,63] |
| 182 | scutebatin B | Nic | Nic | Bz | [54,63] |
| 183 | barbatin H | Ac | Nic | Nic | [48] |
| 184 | scutebarbatine W | Nic | H | OBz | [32,54,55] |
| 185 | scutebartine B | Ac | Ac | Nic | [55] |
| 186 | scutebatin C | Nic | H | Nic | [54] |
| 187 | scutebarbolide L | Ac | Ac | Ac | [42] |
| 188 | scutebartine C | Nic | Nic | H | [42,55] |
| 189 | scutebartine D | Nic | Nic | OH | [55] |
| 190 | scutolide K | Bz | Bz | H | [48,70] |
| 191 | scutolide L | Bz | Bz | OH | [48] |
| 192 | scutelinquanine D | H | Nic | H | [73] |
| 193 | scubatine D | Ac | Ac | Ac | [70] |
| 194 | barbatine A | Nic | Ac | Nic | [42,55,77] |
| 195 | barbatine B | Nic | Nic | Nic | [48,77] |
| 196 | barbatin A | Bz | H | Bz | [32,50] |
| 197 | scutebarbatine F | Nic | Ac | Ac | [32,54] |
| 198 | scutebarbatine G | H | H | Nic | [32,54,78] |
| 199 | 6-O-nicotinoyl-7-O-acetylscutebarbatine G | Nic | Ac | Nic | [32,78] |
| 200 | 6-O-nicotinoylscutebarbatine G | Nic | H | Nic | [32,54,55] |
| 201 | 6,7-di-O-nicotinoylscutebarbatine G | Nic | Nic | Nic | [32,78] |
| 202 | scutehenanine B | Nic | H | Nic | [32,56] |
| 203 | scutebarbolide M | Ac | Ac | Ac | [42] |
| 204 | scutebata Z | Ac | Nic | H(13S) | [43,55] |
| 205 | scutebata A1 | Ac | Nic | H(13R) | [43] |
| 206 | scutebartine E | [55] | |||
| 207 | scutebata U | [46] | |||
| 208 | scutehenanine H | [61] | |||
| 209 | barbaolide A | H | Nic | Ac | [59] |
| 210 | barbaolide B | ONic | Nic | MePr | [59] |
| 211 | barbaolide C | ONic | Ac | MePr | [59] |
| 212 | barbaolide D | [59] | |||
| 213 | barbaolide E | H | Ac | MePr | [59] |
| 214 | barbaolide F | Ac | MePr | H | [59] |
| 215 | barbaolide G | [59] | |||
| 216 | barbaolide H | Bz | Ac | OH | [59] |
| 217 | barbaolide I | Ac | Ac | ONic | [59] |
| 218 | scutellone J | Ac | H | Ac | [79] |
| 219 | scutelinquanine A | Ac | H | Nic | [58] |
| Type F | |||||
| 220 | scutebata H | OH | Bz | Ac | [45,46] |
| 221 | scutebartine H | OMe | H | Nic | [55] |
| 222 | scutebartine I | H | OMe | Nic | [55] |
| 223 | scutebartine J | OH | Nic | Ac | [55] |
| 224 | scutebarbatine I | H | OEt | [57] | |
| 225 | scutebarbatine J | OEt | H | [57] | |
| 226 | scutellin A | [80] | |||
| Type G | |||||
| 227 | scutebarbatine H | Nic | H | [78,81] | |
| 228 | 7-O-nicotinoylscutebarbatine H | Nic | Nic | [78,81] | |
| 229 | scutehenanine D | Nic | Bz | [53] | |
| Others | |||||
| 230 | scutebarbatine M | Nic | Nic | OH | [82] |
| 231 | scutebarbatine N | Nic | Nic | CH2OH | [82] |
| 232 | scubatine A | [55,70] | |||
| 233 | scubatine B | [70] | |||
| 234 | scubatine C | [70] | |||
| 235 | scutelinquanine B | [58] | |||
| 236 | 14-deoxy-11,12-didehydroandrographolide | [60] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Cao, Y.; Liu, Q.; Zhou, Z.; Xu, Y.; Liu, C. Chemical Constituents, Anti-Tumor Mechanisms, and Clinical Application: A Comprehensive Review on Scutellaria barbata. Molecules 2024, 29, 4134. https://doi.org/10.3390/molecules29174134
Sun J, Cao Y, Liu Q, Zhou Z, Xu Y, Liu C. Chemical Constituents, Anti-Tumor Mechanisms, and Clinical Application: A Comprehensive Review on Scutellaria barbata. Molecules. 2024; 29(17):4134. https://doi.org/10.3390/molecules29174134
Chicago/Turabian StyleSun, Jiagui, Yuqi Cao, Qiqi Liu, Zhengshu Zhou, Yanan Xu, and Chenggang Liu. 2024. "Chemical Constituents, Anti-Tumor Mechanisms, and Clinical Application: A Comprehensive Review on Scutellaria barbata" Molecules 29, no. 17: 4134. https://doi.org/10.3390/molecules29174134
APA StyleSun, J., Cao, Y., Liu, Q., Zhou, Z., Xu, Y., & Liu, C. (2024). Chemical Constituents, Anti-Tumor Mechanisms, and Clinical Application: A Comprehensive Review on Scutellaria barbata. Molecules, 29(17), 4134. https://doi.org/10.3390/molecules29174134






