Progress in Application of Silane Coupling Agent for Clay Modification to Flame Retardant Polymer
Abstract
:1. Introduction
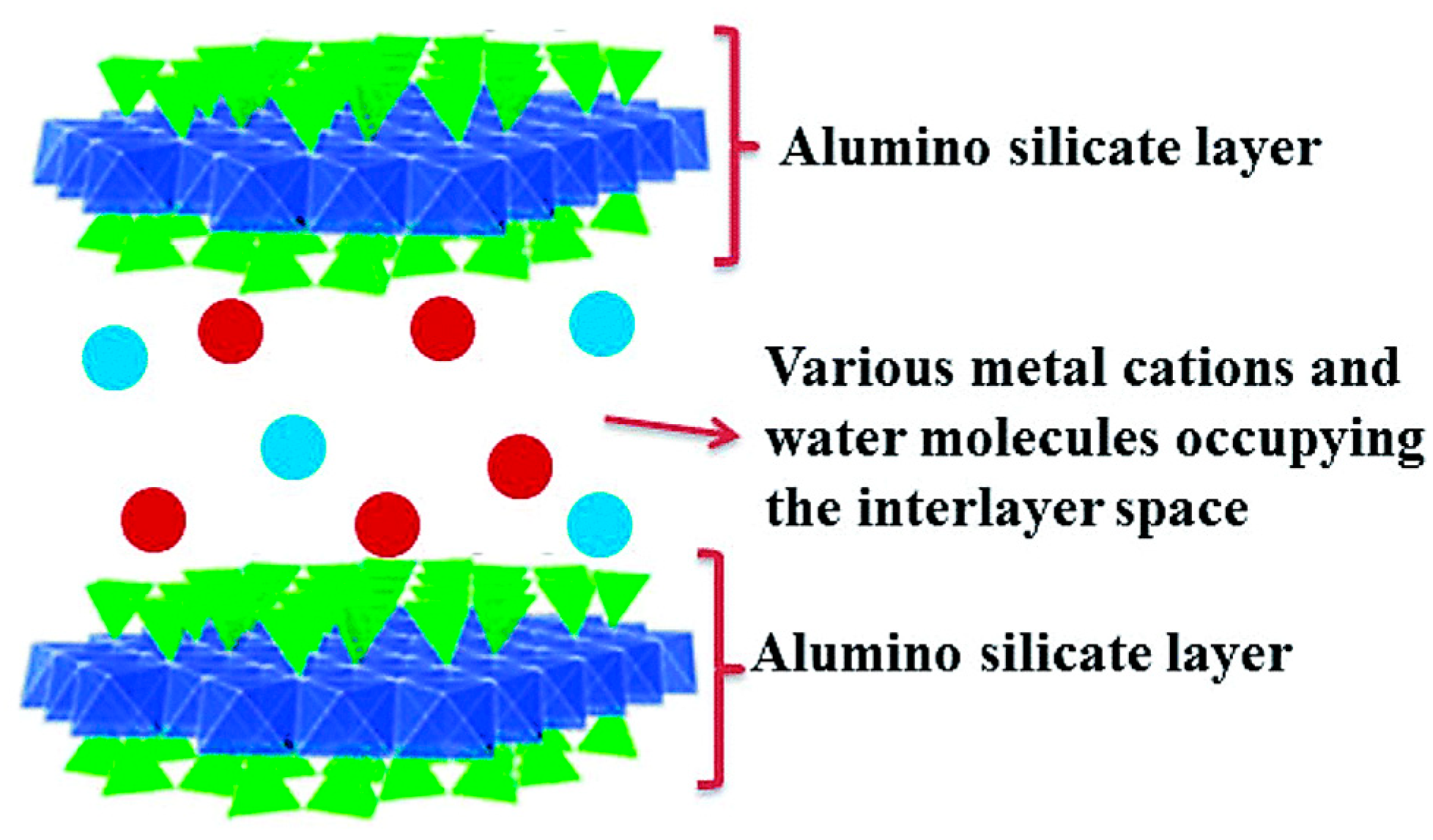
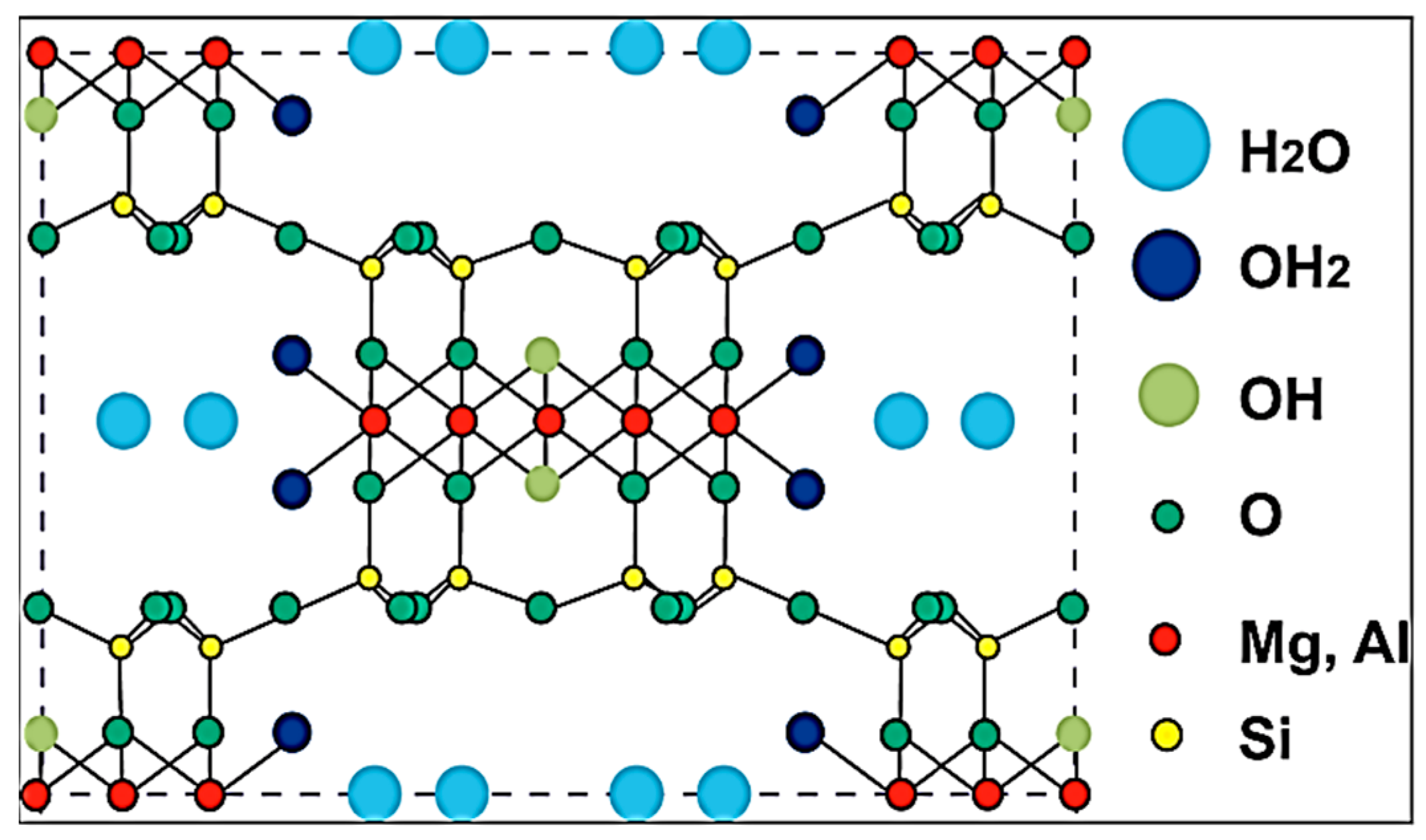
2. Overview of Silane Coupling Agent-Modified Clay
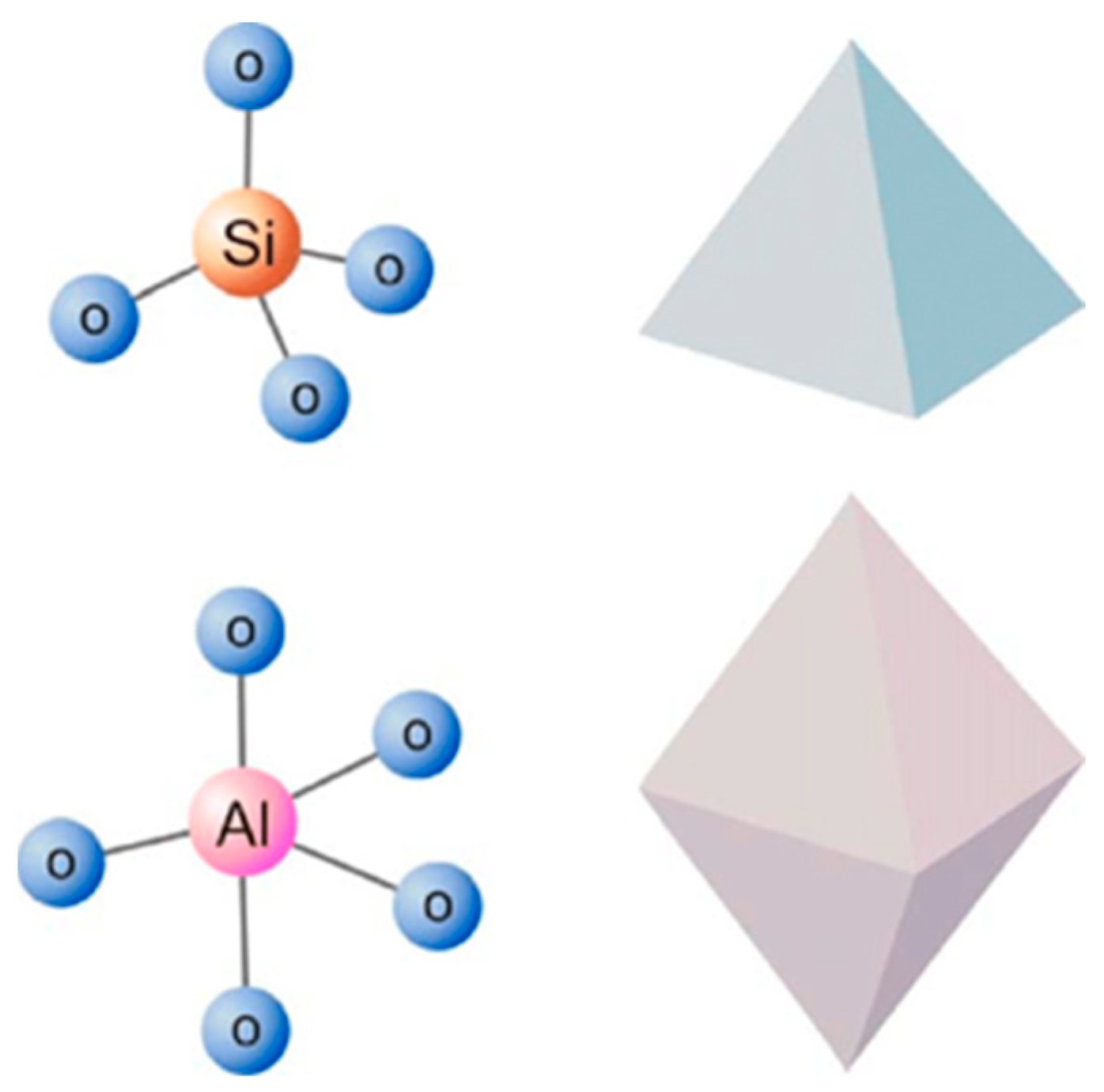
2.1. Classification of Silane Coupling Agents
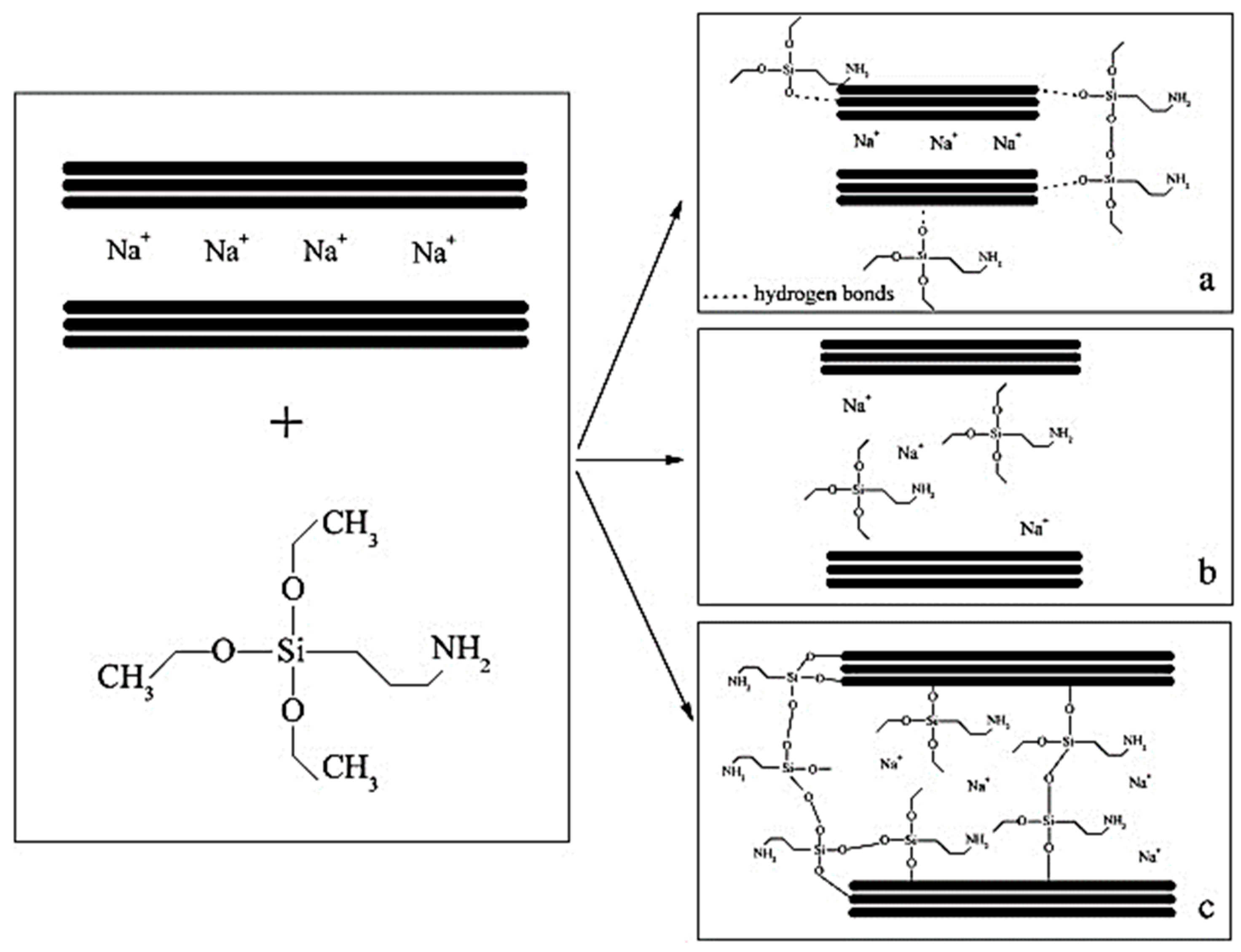
2.2. Mechanism of Silane Coupling Agent-Modified Clay
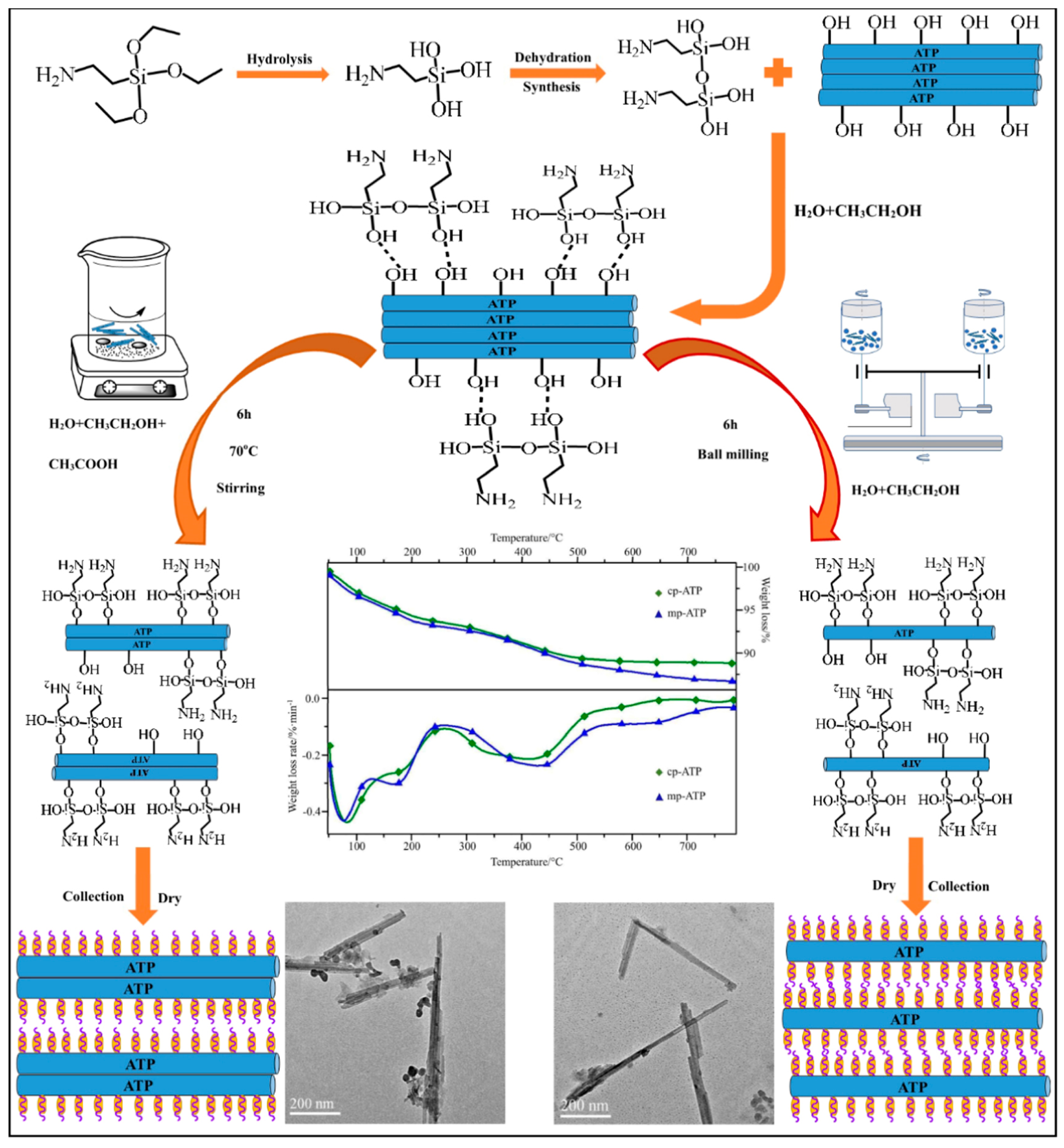
2.3. Silane Coupling Agent-Modified Clay Process
3. Silane Coupling Agent-Modified Clay Flame Retardant Polymer
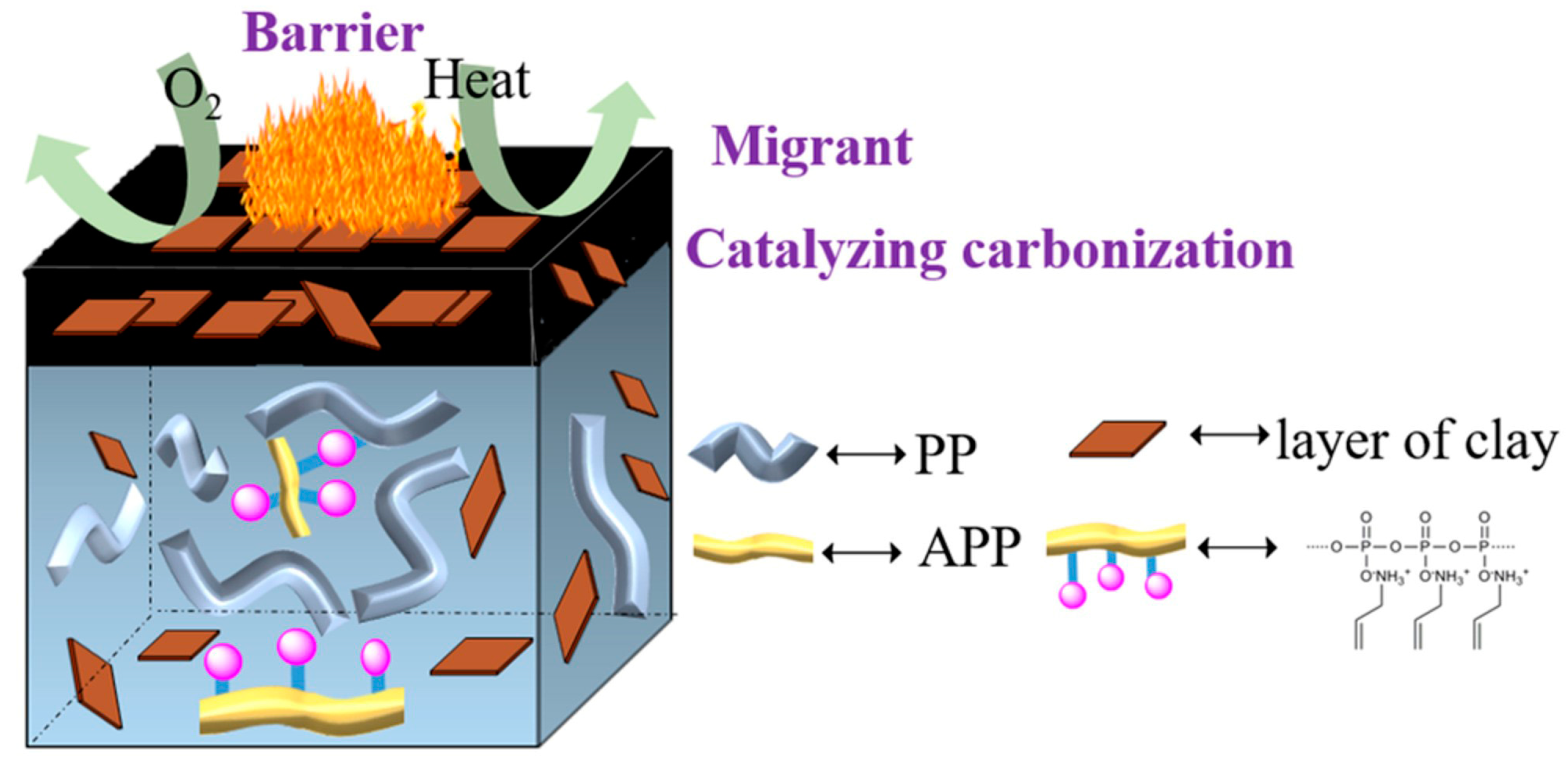
3.1. Mechanism of Silane Coupling Agent-Modified Clay Flame Retardant Polymer
3.2. Silane Coupling Agent-Modified Montmorillonite
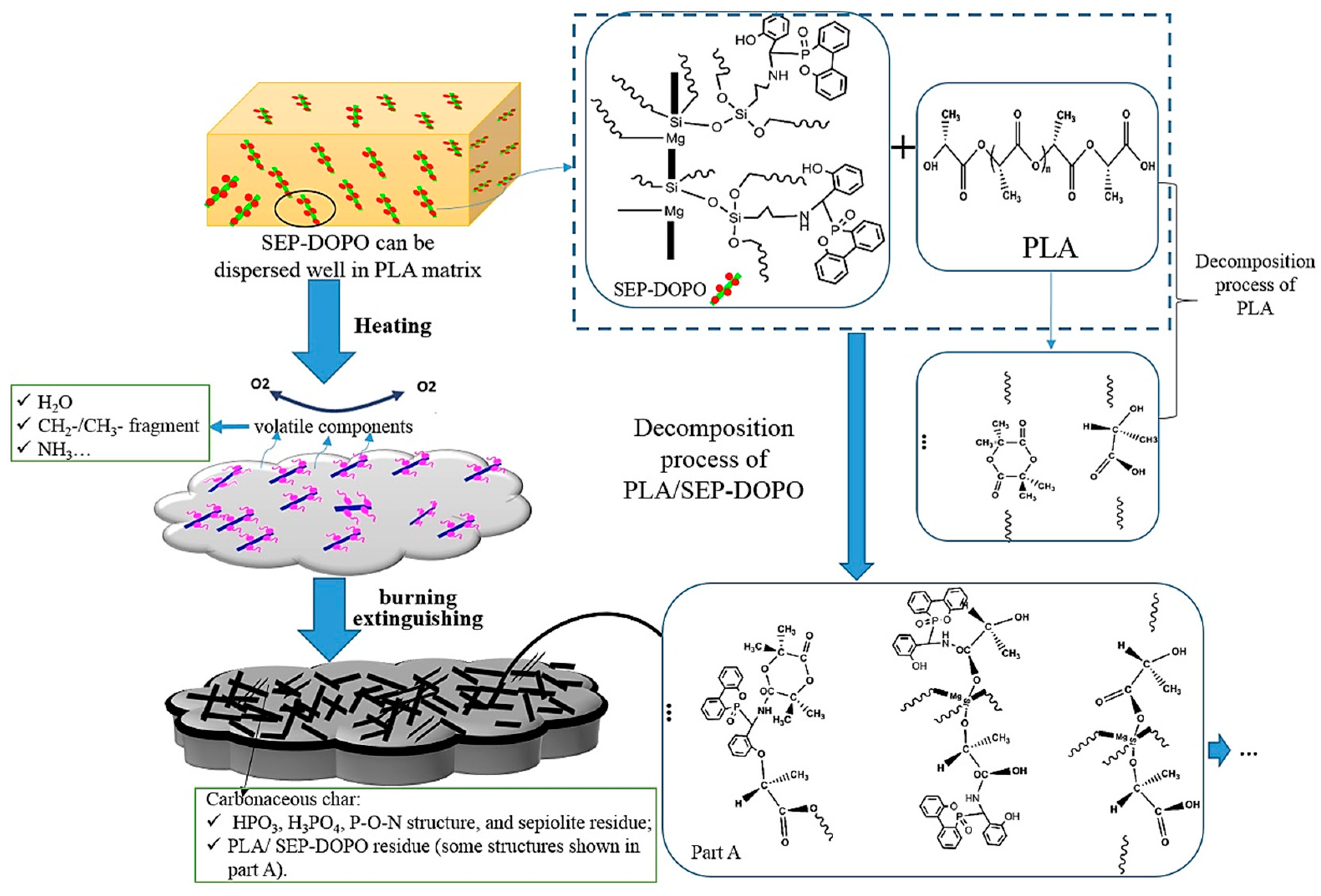
3.3. Silane Coupling Agent-Modified Sepiolite
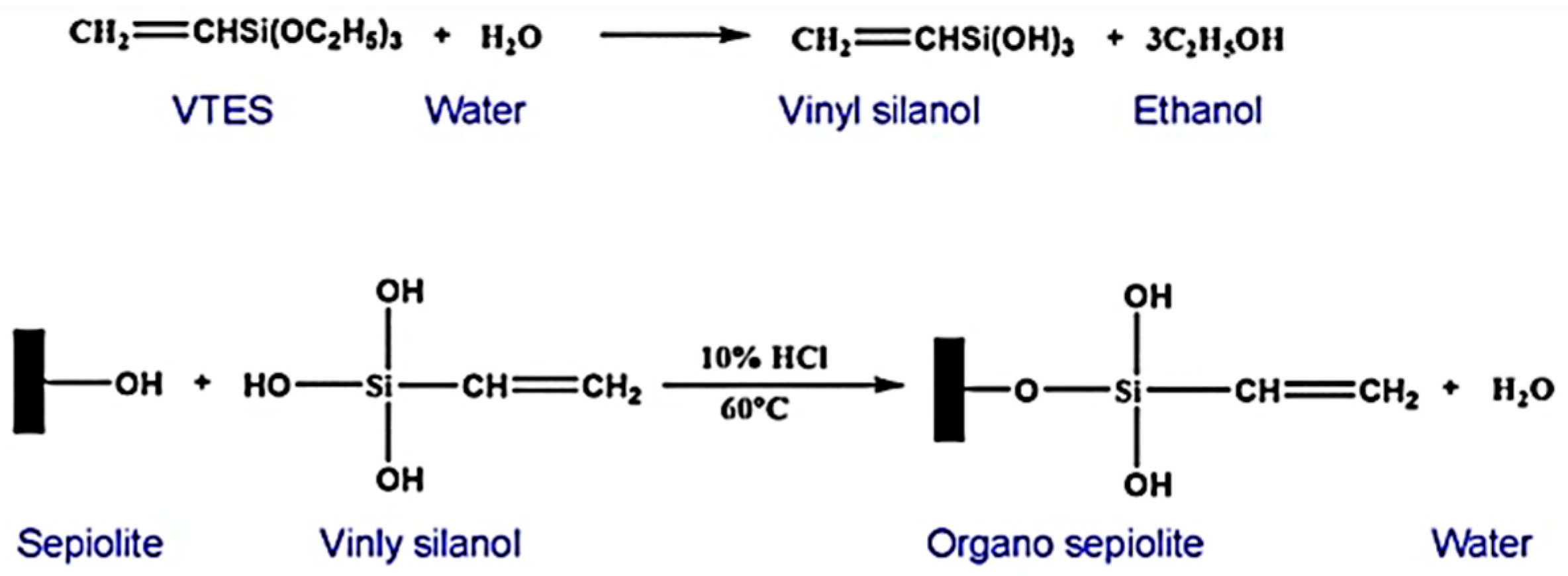
3.4. Silane Coupling Agent-Modified Attapulgite
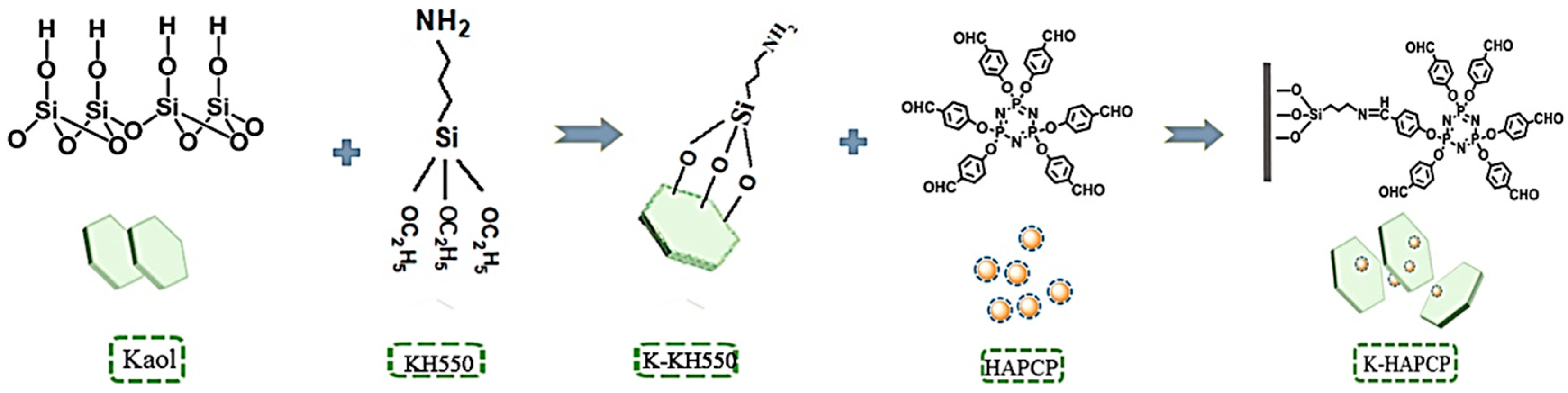
3.5. Silane Coupling Agent-Modified Kaolinite
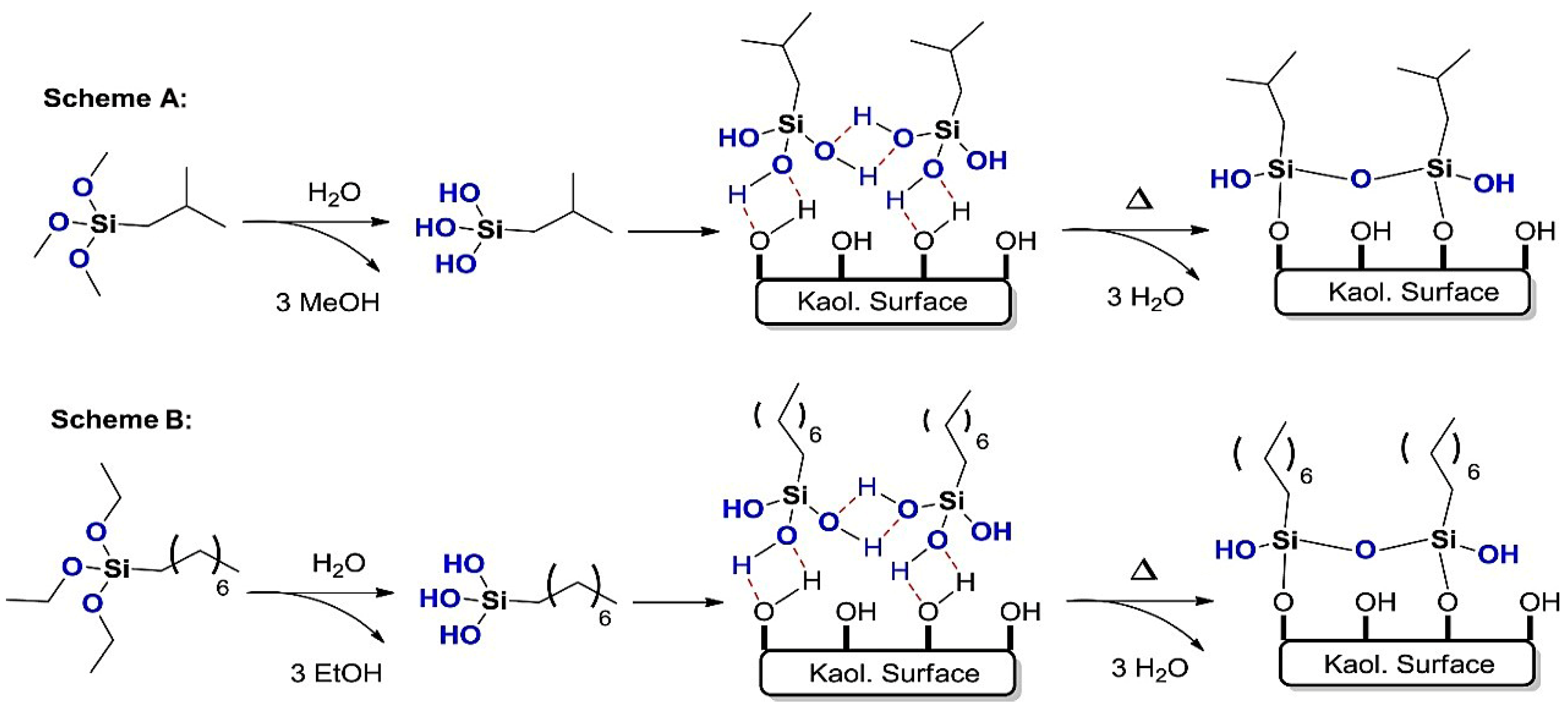
3.6. Silane Coupling Agent-Modification of Other Clays
4. Clay Grafted with Flame Retardant by Silane Coupling Agent
5. Current Challenges and Potential Future Trends
- (1)
- In order to give clay a better dispersion effect in the matrix and to enhance the compatibility of clay and organic polymer materials more effectively, it is necessary to develop silane coupling agents with better modification effects for different matrices. The key to developing silane coupling agents is to change the organic functional groups of silane coupling agents through organic synthesis reaction and other methods, so that the silane coupling agents can achieve a more efficient combination with a single-component specific organic matrices, or can be developed for multi-component organic matrices. Regardless of dry modification or wet modification, further optimization of the modification process is needed. It is recommended to study the optimal modification conditions such as modification temperature and modification time for different silane coupling agents and different clays, and it is also recommended to study the effect of the composite modification of silane coupling agents and other modifiers, as well as to optimize the ratio of the modifiers.
- (2)
- Research on silane coupling agent-modified clay for flame retardant polymers has focused on MMT, SEP, ATP, and Kaol, but few researchers have conducted comparative studies on the flame retardant effects of different silane coupling agent-modified clays in the same polymer matrix. A comparative study of the effects of different clays modified by silane coupling agents and the flame retardant effect of different clays in a polymer matrix after modification can be a future research direction.
- (3)
- While improving the flame retardant properties of clay composites by modifying the clay surface with silane coupling agents, it is also necessary to consider the effects on other properties of the composites, such as mechanical properties, water and corrosion resistance, processability, and degradability. The development of new silane coupling agents and the incorporation of specific functionalized components in composites are feasible solutions for this purpose.
- (4)
- Most silane coupling agent-modified clays for flame retardant composites can still only be realized in the laboratory. In order to meet the requirements of industrialized production, there is still a need for breakthroughs in production costs, facilities and equipment, and production processes. Especially for dry modification, which is less researched at present, it is recommended to explore low-cost and high-efficiency modifiers and modification environments, as well as more concise and efficient operational processes for modification under the premise of meeting the standards of industrial applications.
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, K. Preface: Special topic on fire risk assessment and safety protection. Saf. Environ. Eng. 2023, 30, 1–2. [Google Scholar]
- Xavier, J.R.; Vinodhini, S.P.; Beryl, J.R. Anti-corrosion and flame-retardant properties of environmentally benign smart functionalized WS2/rGO in epoxy coatings for enhanced steel structural protection in natural seawater. Mater. Today Commun. 2024, 38, 107842. [Google Scholar] [CrossRef]
- Xavier, J.R.; Vinodhini, S.P.; Bhaskar, R.; Beryl, J.R. Flame retardant and anticorrosion behavior of multifunctional epoxy nanocomposite coatings containing graphitic carbon nitride/silanized HfO2 nanofillers for the protection of steel surface in automobile industry. ACS Chem. Health Saf. 2023, 30, 428–450. [Google Scholar] [CrossRef]
- Beryl, J.R.; Xavier, J.R. Investigation of smart graphene oxide multilayer nanocoating for improved steel structural protection in natural seawater. J. Mater. Sci. 2024, 59, 458–490. [Google Scholar] [CrossRef]
- Xavier, J.R.; Vinodhini, S.P. High performance polyurethane nanocomposite coatings based on 2D materials for improved anticorrosion and flame-retardant applications. J. Adhes. Sci. Technol. 2024, 38, 1–35. [Google Scholar] [CrossRef]
- Xavier, J.R.; Bhaskar, R.; Subramanian, S. Multifunctional graphitic carbon nitride/manganese dioxide/epoxy nanocomposite coating on steel for enhanced anticorrosion, flame retardant, mechanical, and hydrophobic properties. J. Ind. Eng. Chem. 2024, 134, 514–536. [Google Scholar] [CrossRef]
- Xavier, J.R. Multifunctional nanocomposite coatings for superior anticorrosive, flame retardant and mechanical properties in aerospace components. Surf. Interfaces 2023, 38, 102832. [Google Scholar] [CrossRef]
- Xavier, J.R. Multilayered nanocomposite coatings for enhanced anticorrosive, flame retardant, and mechanical properties in automobile and aerospace industries. J. Appl. Polym. Sci. 2023, 140, e53943. [Google Scholar] [CrossRef]
- Xavier, J.R.; Vinodhini, S.P.; Beryl, J.R. Flame retardant and corrosion protection performance of multifunctional nanocomposites containing graphitic carbon nitride/silanized Ta2O5 nanofillers for the protection of steel surface for industrial applications. Colloids Surf. A 2024, 681, 132748. [Google Scholar] [CrossRef]
- Zhao, Q.; Fu, L.; Jiang, D.; Xi, Y.; Yang, H. A nanoclay-induced defective g-C3N4 photocatalyst for highly efficient catalytic reactions. Chem. Commun. 2018, 54, 8249. [Google Scholar] [CrossRef]
- Kuznetsov, V.; Ottermann, K.; Helfricht, N.; Kunz, D.; Loch, P.; Kalo, H.; Breu, J.; Papastavrou, G. Surface charge density and diffuse layer properties of highly defined 2:1 layered silicate platelets. Colloid Polym. Sci. 2020, 298, 907–920. [Google Scholar] [CrossRef]
- Nóra, H.; Szabolcs, P.; Richárd, T.; Béla, P. Coupling of PMMA to the surface of a layered silicate by intercalative polymerization: Processes, structure and properties. Colloids Surf. A 2020, 601, 124979. [Google Scholar]
- López-Quirós, A.; Sánchez-Navas, A.; Nieto, F.; Escutia, C. New insights into the nature of glauconite. Am. Miner. 2020, 105, 674–686. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; Medarević, D.; Đuriš, J.; El-Rahmany, M.M.; Ibrić, S.; Viseras, C. Flow and tableting behaviors of some egyptian kaolin powders as potential pharmaceutical excipients. Minerals 2020, 10, 23. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.D.; Abad, I.; Bentabol, M.; Cruz, M.D.R. Evidences of talc-white mica assemblage in low-grade metamorphic rocks from the internal zone of the Rif Cordillera (N Morocco). Appl. Clay Sci. 2020, 195, 105723. [Google Scholar] [CrossRef]
- VanRanst, E.; Kips, P.; Mbogoni, J.; Mees, F.; Dumon, M.; Delvaux, B. Halloysite-smectite mixed-layered clay in fluvio-volcanic soils at the southern foot of Mount Kilimanjaro, Tanzania. Geoderma 2020, 375, 114527. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D.; Pfaendner, R. Influence of accelerated aging on clay-reinforced polyamide 6. Polym. Degrad. Stab. 2009, 94, 389–396. [Google Scholar] [CrossRef]
- Al-Ani, T.; Sarapää, O. ResearchGate. Available online: https://www.researchgate.net/publication/292706105_Clay_and_clay_mineralogy (accessed on 1 January 2008).
- Shokoohi, S.; Arefazar, A.; Khosrokhavar, R. Silane coupling agents in polymer-based reinforced composites: A review. J. Reinf. Plast. Compos. 2008, 27, 473–485. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lung, C.Y.; Tsoi, J.K. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Ullah, A.; Fan, H.; Jamil, M.I.; Khan, F.U.; Ullah, R.; Iqbal, M.; Ali, A.; Ullah, B. Recent progress in silane coupling agent with its emerging applications. J. Polym. Environ. 2021, 29, 3427–3443. [Google Scholar] [CrossRef]
- Huskiĉ, M.; Žigon, M.; Ivankoviĉ, M. Comparison of the properties of clay polymer nanocomposites prepared by montmorillonite modified by silane and by quaternary ammonium salts. Appl. Clay Sci. 2013, 85, 109–115. [Google Scholar] [CrossRef]
- Piscitelli, F.; Posocco, P.; Toth, R.; Fermeglia, M.; Pricl, S.; Mensitieri, G.; Lavorgna, M. Sodium montmorillonite silylation: Unexpected effect of the aminosilane chain length. J. Colloid Interface Sci. 2010, 351, 108–115. [Google Scholar] [CrossRef]
- Takahashi, N.; Kuroda, K. Materials design of layered silicates through covalent modification of interlayer surfaces. J. Mater. Chem. 2011, 21, 14336–14353. [Google Scholar] [CrossRef]
- Khosravi, H.; Eslami-Farsani, R. Enhanced mechanical properties of unidirectional basalt fiber/epoxy composites using silane-modified Na+ -montmorillonite nanoclay. Polym. Test. 2016, 55, 135–142. [Google Scholar] [CrossRef]
- Shi, X. Application progress of siloxane compounds in solution polymerized styrene-butadiene rubber/silica composites. Petrochem. Technol. 2023, 52, 1602–1608. [Google Scholar]
- Sae-oui, P.; Sirisinha, C.; Thepsuwan, U.; Hatthapanit, K. Roles of silane coupling agents on properties of silica-filled polychloroprene. Eur. Polym. J. 2006, 42, 479–486. [Google Scholar] [CrossRef]
- Bee, S.-L.; Abdullah, M.A.A.; Bee, S.-T.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Ryu, C.; Yang, J.-K.; Park, W.; Seo, Y.; Kim, S.; Kim, D.; Park, S.; Seo, G. Reinforcement of styrene-butadiene/polybutadiene rubber compounds by modified silicas with different surface and networked states. J. Appl. Polym. Sci. 2017, 134, 44893. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, S.; Xie, T. Mechanism behind the improvement of coupling agent in interface bonding performance between organic transparent resin and inorganic cement matrix. Constr. Build. Mater. 2017, 143, 138–146. [Google Scholar] [CrossRef]
- Xavier, J.R.; Vinodhini, S.P.; Ramesh, B. Optimizing aluminum alloy performance for marine superstructures: Advanced nanocomposite coating for enhanced corrosion resistance, flame retardancy, and mechanical strength. Polym. Degrad. Stab. 2024, 227, 110847. [Google Scholar] [CrossRef]
- Xavier, J.R. A study on the influence of silanized clay on the barrier, hydrophobic and mechanical properties of epoxy coated steel in natural seawater. J. Adhes. 2023, 99, 1889–1915. [Google Scholar] [CrossRef]
- Xavier, J.R.; Vinodhini, S.P. Improved anticorrosion, flame retardant and mechanical behaviors of multifunctional polyurethane nanocomposite coatings for industrial applications. Polym. Degrad. Stab. 2023, 213, 110370. [Google Scholar] [CrossRef]
- Xavier, J.R. Investigation of anticorrosion, flame retardant and mechanical properties of polyurethane/GO nanocomposites coated AJ62 Mg alloy for aerospace/automobile components. Diamond Relat. Mater. 2023, 136, 110025. [Google Scholar] [CrossRef]
- Hooper, R.C.; Cunningham, J.A.; Harper, J.G. Electrical contacts to silicon. Solid-State Electron. 1965, 8, 831–833. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Interfaces in Polymer Matrix Composites; Academic Press: New York, NY, USA, 1974; pp. 217–284. [Google Scholar]
- Ishida, H. A review of recent progress in the studies of molecular and microstructure of coupling agents and their functions in composites, coatings and adhesive joints. Polym. Compos. 1984, 5, 101–123. [Google Scholar] [CrossRef]
- Arkles, B. Tailoring Surface with silanes. Chem. Technol. 1977, 7, 766–778. [Google Scholar]
- Zisman, W.A. Surface chemistry of plastics reinforced by strong fibers. Ind. Eng. Chem. Prod. Res. Dev. 1963, 8, 98–111. [Google Scholar] [CrossRef]
- Tian, Q.; Qin, S.; Yang, M.; Yin, X.; Wu, W.; He, W.; Xu, G. Surface modification of super-refined montmorillonite by silane coupling agent. Plastics 2015, 44, 35–37. [Google Scholar]
- Li, X.; Xia, C.; Li, J.; Zhou, X. Design and build an elastic crosslinked network to strengthen and toughen soybean-meal based bioadhesive using organo-sepiolite and greener crosslinker triglycidylamine. Polym. Test. 2020, 89, 106648. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; Zhang, L.; Liu, Y.; Zhou, Q. Preparation and characterization of starch/EVA composite foams with surface modified kaolin. Starch 2013, 65, 840–847. [Google Scholar] [CrossRef]
- Xu, J.; Ren, S.; Kang, C.; Cui, F.; Li, C.; Fan, J. Study on the ball milling modification of attapulgite. Mater. Res. Express 2020, 7, 115006. [Google Scholar] [CrossRef]
- Beryl, J.R.; Xavier, J.R. Halloysite for clay–polymer nanocomposites: Effects of nanofillers on the anti-corrosion, mechanical, microstructure, and flame-retardant properties—A review. J. Mater. Sci. 2023, 58, 10943. [Google Scholar] [CrossRef]
- Xiao, D.; Zheng, M.; Gohs, U.; Wagenknecht, U.; Voit, B.; Wang, D. Highly efficient flame retardant and smoke suppression mechanism of polypropylene nanocomposites based on clay and allylamine polyphosphate. J. Appl. Polym. Sci. 2022, 139, 52311. [Google Scholar] [CrossRef]
- Sai, T.; Ran, S.; Guo, Z.; Song, P.; Fang, Z. Recent advances in fire-retardant carbon-based polymeric nanocomposites through fighting free radicals. SusMat 2022, 2, 411–434. [Google Scholar] [CrossRef]
- Shen, F.; Li, Y.; Chen, Z.; Cao, C.; Shen, Y.; Li, L.; Pan, L.; Li, J.; Zhang, G.; Gao, J.; et al. Lightweight, surface hydrophobic and flame-retardant polydimethylsiloxane foam composites coated with graphene oxide via interface engineering. Prog. Org. Coat. 2024, 189, 108276. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate(clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Köklükaya, O.; Carosio, F.; Durán, V.L.; Wågberg, L. Layer-by-layer modified low density cellulose fiber networks: A sustainable and fireproof alternative to petroleum based foams. Carbohydr. Polym. 2020, 230, 115616. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Parida, K.; Nyamori, V.O. Transforming inorganic layered montmorillonite into inorganic–organic hybrid materials for various applications: A brief overview. Inorg. Chem. Front. 2016, 3, 1100–1111. [Google Scholar] [CrossRef]
- Bertuoli, P.T.; Piazza, D.; Scienza, L.C.; Zattera, A.J. Preparation and characterization of montmorillonite modified with 3-aminopropyltriethoxysilane. Appl. Clay Sci. 2014, 87, 46–51. [Google Scholar] [CrossRef]
- Sun, D. Study on the flame retardancy of flame-retardant montmorillonite nanocomposite paint. Electroplat. Finish. 2009, 28, 52–54. [Google Scholar]
- Li, S.; Yang, Z.; Xie, J.; Deng, Y.; Han, Q. Preparation and properties of silylated montmorillonite/epoxy composites. China Plast. Ind. 2015, 43, 42–45. [Google Scholar]
- Yu, J.; He, J.; Zhang, H. Preparation and properties of phenolic resin/montmorillonite modified by silane coupling agent. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2010, 32, 16–20. [Google Scholar]
- Wang, F.; Ma, G.; Wang, F.; Yu, P.; Zhou, Z.; Zhu, X. Preparation and properties of aramid-based epoxy resin/montmorillonite composites. Thermosetting Resin 2022, 37, 9–14. [Google Scholar]
- Yang, Z.; Li, B.; Tang, F. Influence of Cu2+-organic montmorillonites on thermal decomposition and smoke emission of poly(vinyl chloride) by cone calorimetric study. J. Vinyl Addit. Technol. 2007, 13, 31–39. [Google Scholar] [CrossRef]
- Zhou, T.; Tang, Y.; Tian, Y. The synergistic effect of microencapsulized red phosphorus flame retarded ABS composites. Polym. Mater. Sci. Eng. 2010, 26, 129–132. [Google Scholar]
- Xu, J.; Liu, X.; Ren, S.; Kang, C.; Niu, L. Synergistic effect of montmorillonite and nano-Sb2O3/brominated polystyrene on the flame retardancy of polypropylene matrix composites. Polym. Polym. Compos. 2022, 30, 09673911221083769. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Zheng, S.; Jin, Y. Clay Science and Applied Technology, 3rd ed.; Science Press: Beijing, China, 2017; pp. 29–52. [Google Scholar]
- Zhang, T.; Liu, Y.; Shi, S.; Geng, X. Research progress on flame retardancy of sepiolite composite. Plastics 2023, 52, 139–145. [Google Scholar]
- Lee, J.-I.; Hong, S.-H.; Lee, C.-G.; Park, S.-G. Experimental and model study for fluoride removal by thermally activated sepiolite. Chemosphere 2020, 241, 125094. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, F.; Tang, M.; Liang, J.; Ren, C. Study on pore distribution and formation rule of sepiolite mineral nanomaterials. J. Nanomater. 2012, 2012, 382603. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Fu, C.; Xie, J.; Xie, S.; Huang, W.; Yan, W. Effect of organic sepiolite on flame retardancy of epoxy resin. Plast. Sci. Technol. 2019, 47, 51–55. [Google Scholar]
- Bidsorkhi, H.C.; Adelnia, H.; Naderi, N.; Moazeni, N.; Mohamad, Z. Ethylene vinyl acetate copolymer nanocomposites based on (un)modified sepiolite: Flame retardancy, thermal, and mechanical properties. Polym. Compos. 2017, 38, 1302–1310. [Google Scholar] [CrossRef]
- Yan, W.; Xie, P.; Yang, Z.; Luo, G.; Huang, W.; Tian, Q.; Tu, C.; Zhang, C.; Yang, C.; Wang, K. Flame-retardant behaviors of aluminum phosphates coated sepiolite in epoxy resin. J. Fire Sci. 2021, 39, 3–18. [Google Scholar] [CrossRef]
- Altaf, F.; Batool, R.; Ahmad, M.A.; Raza, R.; Khan, M.A.; Abbas, G. Novel vinyl-modified sepiolite-based polymer nanocomposites: Synthesis and characterization. Iran. Polym. J. 2018, 27, 413–422. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Zhang, R.; Li, Z.; Huang, W.; Tian, Q.; Yan, W. Sepiolite fiber and nano SiO2 synergistic flame retardant EP. Eng. Plast. Appl. 2022, 50, 132–137. [Google Scholar]
- Liu, H.; Wu, Z.; Jing, B.; Dai, W. The research of flame-retardant properties of PA6/modified sepiolite composite materials. China Plast. Ind. 2011, 39, 54–57. [Google Scholar]
- Li, H.; Meng, D.; Qi, P.; Sun, J.; Li, H.; Gu, X.; Zhang, S. Fabrication of a hybrid from metal organic framework and sepiolite (ZIF-8@SEP) for reducing the fire hazards in thermoplastic polyurethane. Appl. Clay Sci. 2022, 216, 106376. [Google Scholar] [CrossRef]
- Gao, X. Clay Mineralogy, 3rd ed.; Chemical Industry Press: Beijing, China, 2017; pp. 13–57. [Google Scholar]
- Bradley, W.F. The structural scheme of attapulgite. Am. Miner. 1940, 25, 405–410. [Google Scholar]
- Gao, D.; Zhang, Y.; Lyu, B.; Wang, P.; Ma, J. Nanocomposite based on poly(acrylic acid)/ attapulgite towards flame retardant of cotton fabrics. Carbohydr. Polym. 2019, 206, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Gueye, R.S.; Davy, C.A.; Cazaux, F.; Ndiaye, A.; Diop, M.B.; Skoczylas, F.; Wele, A. Mineralogical and physico-chemical characterization of mbodiene palygorskite for pharmaceutical applications. J. Afr. Earth Sci. 2017, 135, 186–203. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Wang, Y.; Liu, W.; Deng, L.; Jiao, W.; Yang, F. Effects of modified attapulgite on the properties of attapulgite/epoxy nanocomposites. Polym. Compos. 2013, 34, 22–31. [Google Scholar] [CrossRef]
- Jesionowski, T.; Krysztafkiewicz, A. Influence of silane coupling agents on surface properties of precipitated silicas. Appl. Surf. Sci. 2001, 172, 18–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Chu, H.; Zhou, X.; Wei, Y. Effect of modified attapulgite addition on the performance of a PVDF ultrafiltration membrane. Desalination 2014, 344, 71–78. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, B.; Zhao, S.; Zhou, P.; Liu, Y. Preparation and kinetics of thermal degradation of the composite palygorskite/silane grafted crosslinked polyethylene. J. Northwest. Teachnol. Univ. Nat. Sci. 2013, 49, 63–69. [Google Scholar]
- Bao, Y.; Li, X.; Tang, P.; Liu, C.; Zhang, W.; Ma, J. Attapulgite modified cotton fabric and its flame retardancy. Cellulose 2019, 26, 9311–9322. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, Y.; Xu, J.; Wu, Z.; Ma, H.; Lei, Z. Structures and properties characterization of acrylonitrile-butadiene-styrene/organo-palygorskite clay composites. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2012, 27, 1068–1071. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Chen, H.; Ni, A.; Ding, A. Synergistic effect of intumescent flame retardant and attapulgite on mechanical properties and flame retardancy of glass fibre reinforced polyethylene composites. Compos. Struct. 2020, 246, 112404. [Google Scholar] [CrossRef]
- Zhou, P.; Huang, L.; Ma, D.; Huo, S.; Zhang, Z.; Wang, L. Preparation and performance of organo palygorskite intumscent flame retardant-type polypropylene composite. Fine Chem. 2015, 32, 961–967. [Google Scholar]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.F.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar] [CrossRef]
- Tang, W.; Fan, H.; Liu, F.; Liao, Y.; Xiao, Q.; Yan, J.; Zhang, S.; Gu, X. MoS2 supported on acid activated kaolinite used in the kaolinite-epoxy resin composites for suppressing smoke toxicity and degradation. Appl. Clay Sci. 2023, 233, 106822. [Google Scholar] [CrossRef]
- Wang, J.; Fu, L.; Yang, H.; Zuo, X.; Wu, D. Energetics, interlayer molecular structures, and hydration mechanisms of dimethyl sulfoxide(DMSO)-kaolinite nanoclay guest-host interactions. J. Phys. Chem. Lett. 2021, 12, 9973–9981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ge, X.; Xing, R.; Zhang, B. Effects of different silane coupling agents on structure and properties of starch–chitosan–kaolin composites. J. Appl. Polym. Sci. 2019, 136, 48050. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, J.; Zhang, J.; Li, H.; Zhang, S.; Sun, J.; Xin, F.; Liu, F.; Qin, Z.; Tang, W. TiO2/SiO2/kaolinite hybrid filler to improve the flame retardancy, smoke suppression and anti-aging. Mater. Chem. Phys. 2022, 277, 125576. [Google Scholar] [CrossRef]
- Albach, B.; Santos, P.H.V.D.; Rampon, D.D.S.; Barbosa, R.V. An evaluation of modified Kaolinite surface on the crystalline and mechanical behavior of polypropylene. Polym. Test. 2019, 75, 237–345. [Google Scholar] [CrossRef]
- Zeng, Z.; Gao, S.; Meng, Q. Flame retardant property of halogen-free compounding system on PPO/HIPS alloy. Plastics 2011, 40, 77–79. [Google Scholar]
- Zhang, Y.; Tan, J.; Xiang, J.; Liu, Q. Combustion properties and thermal stability of styrene butadiene rubber composites filled with kaolinite. New Chem. Mater. 2014, 42, 21–23. [Google Scholar]
- Martin, R.T.; Bailey, S.W.; Eberl, D.D.; Fanning, D.S.; Guggenheim, S.; Kodama, H.; Pevear, D.R.; Srodon, J.; Wicks, F.J. Report of the clay minerals society nomenclature committee: Revised classification of clay materials. Clays Clay Miner. 1991, 39, 333–335. [Google Scholar] [CrossRef]
- Schulze, D.G. An Introduction to Soil Mineralogy. In Soil Mineralogy with Environmental Applications, 1st ed.; Dixon, J.B., Schulze, D.G., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; Volume 8, pp. 1–35. [Google Scholar]
- Sun, J.; Peng, T.; Sun, H. Review on the preparation of organic vermiculite and its application. Mater. Rep. 2007, 3, 50–53. [Google Scholar]
- Song, W.; Wang, M.; Liu, S.; Chen, Y.; Gao, J. Preparation and properties of rigid polyurethane/expanded vermiculite composite thermal insulation material. Eng. Plast. Appl. 2019, 47, 22–27. [Google Scholar]
- Lyu, L.; Wang, J. Preparation and properties of heat-insulation flame-retardant composites made from waste fibers and thermoplastic polyurethane. Adv. Text. Technol. 2017, 25, 15–18. [Google Scholar]
- Yang, Y.; Li, Y.; Wang, P.; Kong, Y.; Li, X.; Zhang, Y. Effect of talc on flame retardant property of PA6/MCA composites. Plast. Sci. Technol. 2016, 44, 64–68. [Google Scholar]
- Seckin, T.; Yildirim, A.; Koytepe, S. Synthesis and properties of novel high thermally stable polyimide-chrysotile composites as fire retardant materials. J. Polym. Eng. 2014, 34, 793–802. [Google Scholar] [CrossRef]
- Wang, J.; Mao, Z. Modified montmorillonite and its application as a flame retardant for polyester. J. Appl. Polym. Sci. 2013, 131, 39625. [Google Scholar] [CrossRef]
- Bao, J.; Liu, Y.; Luo, Y.; Yu, J.; Huang, G.; Xiang, J. Preparation of intumescent flame retardant-functionalized montmorillonite and its modification on EPDM. Spec. Purp. Rubber Prod. 2016, 37, 1–5. [Google Scholar]
- Chen, Q.; Sai, T.; Fang, Z.; Guo, Z. Thermal stability and oxygen resistance of polypropylene composites with fullerene/montmorillonite hybrid fillers. J. Therm. Anal. Calorim. 2020, 146, 1383–1392. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y. Preparation of high-transparency phosphenanthrene-based flame retardants and studies of their flame-retardant properties. Polymers 2023, 15, 4665. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, S.; Bourbigot, S.; Chen, Z.; Duquesne, S.; Casetta, M. Surface grafting of sepiolite with a phosphaphenanthrene derivative and its flame-retardant mechanism on PLA nanocomposites. Polym. Degrad. Stab. 2019, 165, 68–79. [Google Scholar] [CrossRef]
- Peng, X.; Chen, S.; Tang, W.; Zhang, S.; Gu, X. Study on the flame retardancy of PP composites by introducing modified kaolinite with DOPO. Mod. Plast. Process. Appl. 2019, 31, 18–21. [Google Scholar]
- Zhang, S.; Li, Y.; Guo, J.; Gu, L.; Li, H.; Fei, B.; Sun, J.; Gu, X. Preparation of hexakis (4-aldehyde phenoxy) cyclotriphosphazene grafted kaolinite and its synergistic fire resistance in poly (butylene succinate). Polym. Compos. 2020, 44, 1024–1035. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, D.; Wang, W.; Li, H.; Gu, X.; Zhang, S.; Sun, J.; Xin, F.; Qin, Z.; Tang, W. Fabrication of phytic acid embellished kaolinite and its effect on the flame retardancy and thermal stability of ethylene vinyl acetate composites. J. Appl. Polym. Sci. 2021, 138, 51364. [Google Scholar] [CrossRef]





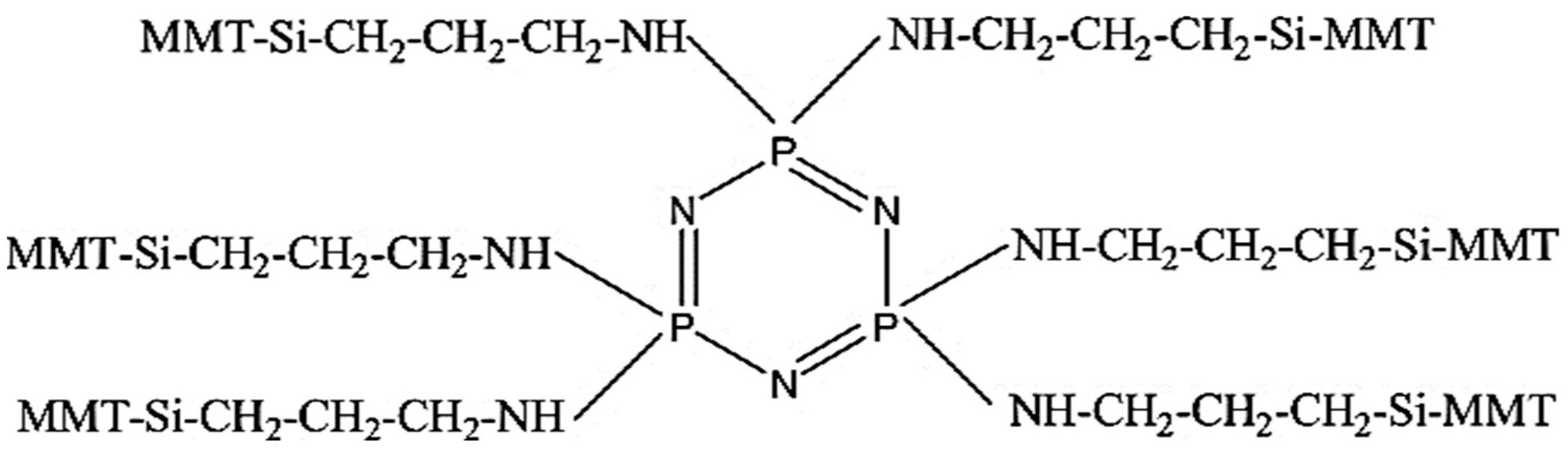
| Categorization | Name | Nickname | Chemical Formula |
|---|---|---|---|
| Vinyl | Trichlorovinylsilane | WD-26, A-150, VTCS | C2H3Cl3Si |
| Triethoxyvinylsilane | WD-20, A-151, VTEO | C8H18O3Si | |
| Vinyltrimethoxysilane | WD-21, A-171, VTMO | C5H12O3Si | |
| Vinyl tris(2-methoxyethoxy) silane | WD-27, A-172 | C11H24O6Si | |
| Vinyltriacetoxysilane | C8H12O6Si | ||
| Chlorocarbon | 3-Chloropropyltrichlorosilane | C3H6Cl4Si | |
| 3-Chloropropyl(trimethoxy)silane | WD-31, A-143 | C6H15ClO3Si | |
| 3-Chloropropyltriethoxysilane | WD-30 | C9H21ClO3Si | |
| Aminohydrocarbon | (3-Aminopropyl)triethoxysilane | KH-550, APTES, WD-50 | C9H23NO3Si |
| 3-(Trimethoxysilyl)-1-propanamine | KH-540, WD-56 | C6H17NO3Si | |
| N-[3-(Trimethoxysilyl)propyl]ethylenediamine | KH-791, WD-51 | C8H22N2O3Si | |
| Epoxy hydrocarbon | 3-Glycidoxypropyltrimethoxysilane | KH-560, WD-60, A-187 | C9H20O5Si |
| Triethoxy(3-glycidyloxypropyl)silane | WD-62 | C12H26O5Si | |
| 3-Glycidyloxypropyl(dimethoxy)methylsilane | WD-61 | C9H20O4Si | |
| 2-(3,4-Epoxycyclohexyl)ethyltriethoxysilane | A-186 | C14H28O4Si | |
| Methacryloxyalkyl | 3-(Trichlorosilyl)propyl methacrylate | C7H11Cl3O2Si | |
| 3-Methacryloxypropyltrimethoxysilane | KH-570, WD-70, A-174 | C10H20O5Si | |
| 3-Methacryloxypropylmethyldimethoxysilane | KH-571, WD-71 | C10H20O4Si | |
| Sulfur-containing hydrocarbons | Trimethoxysilylpropanethiol | KH-590, WD-80, A-189 | C6H16O3SSi |
| 3-Mercaptopropyltriethoxysilane | KH-580, WD-81 | C9H22O3SSi | |
| Bis[3-(Triethoxysilyl)propyl]tetrasulfide | WD-40,Si-69 | C18H42O6S4Si2 | |
| Bis(triethoxysilylpropyl)disulfide | WD-42,Si-75 | C18H42O6S2Si2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Liu, Y.; Zheng, S.; Kang, W. Progress in Application of Silane Coupling Agent for Clay Modification to Flame Retardant Polymer. Molecules 2024, 29, 4143. https://doi.org/10.3390/molecules29174143
Hu Y, Liu Y, Zheng S, Kang W. Progress in Application of Silane Coupling Agent for Clay Modification to Flame Retardant Polymer. Molecules. 2024; 29(17):4143. https://doi.org/10.3390/molecules29174143
Chicago/Turabian StyleHu, Yongwei, Yong Liu, Shihao Zheng, and Wendong Kang. 2024. "Progress in Application of Silane Coupling Agent for Clay Modification to Flame Retardant Polymer" Molecules 29, no. 17: 4143. https://doi.org/10.3390/molecules29174143
APA StyleHu, Y., Liu, Y., Zheng, S., & Kang, W. (2024). Progress in Application of Silane Coupling Agent for Clay Modification to Flame Retardant Polymer. Molecules, 29(17), 4143. https://doi.org/10.3390/molecules29174143







