Study on the Bioactive Constituent and Mineral Elements of the Tibetan Medicine E’seguo from Different Regions of Ganzi Prefecture, China
Abstract
1. Introduction
2. Results
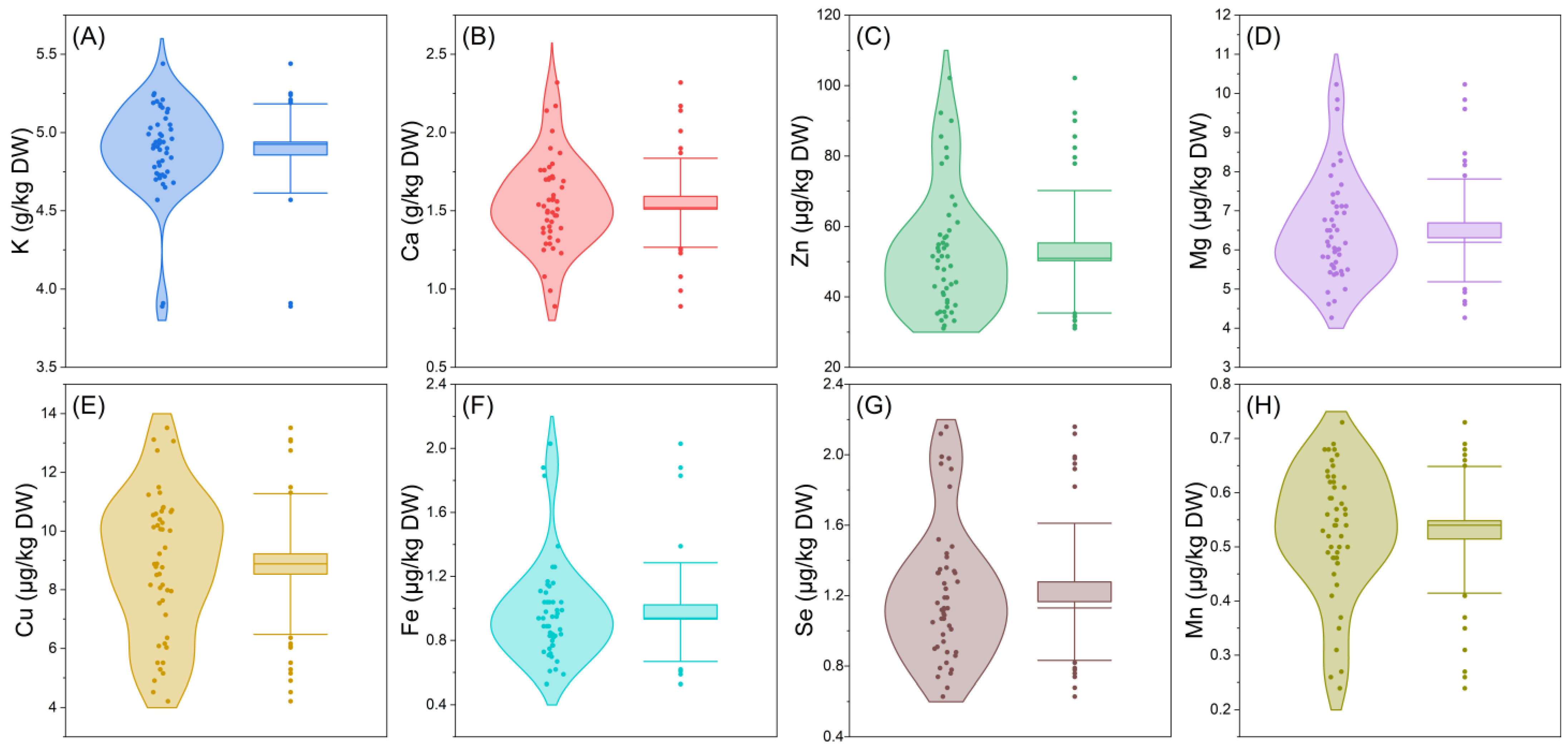
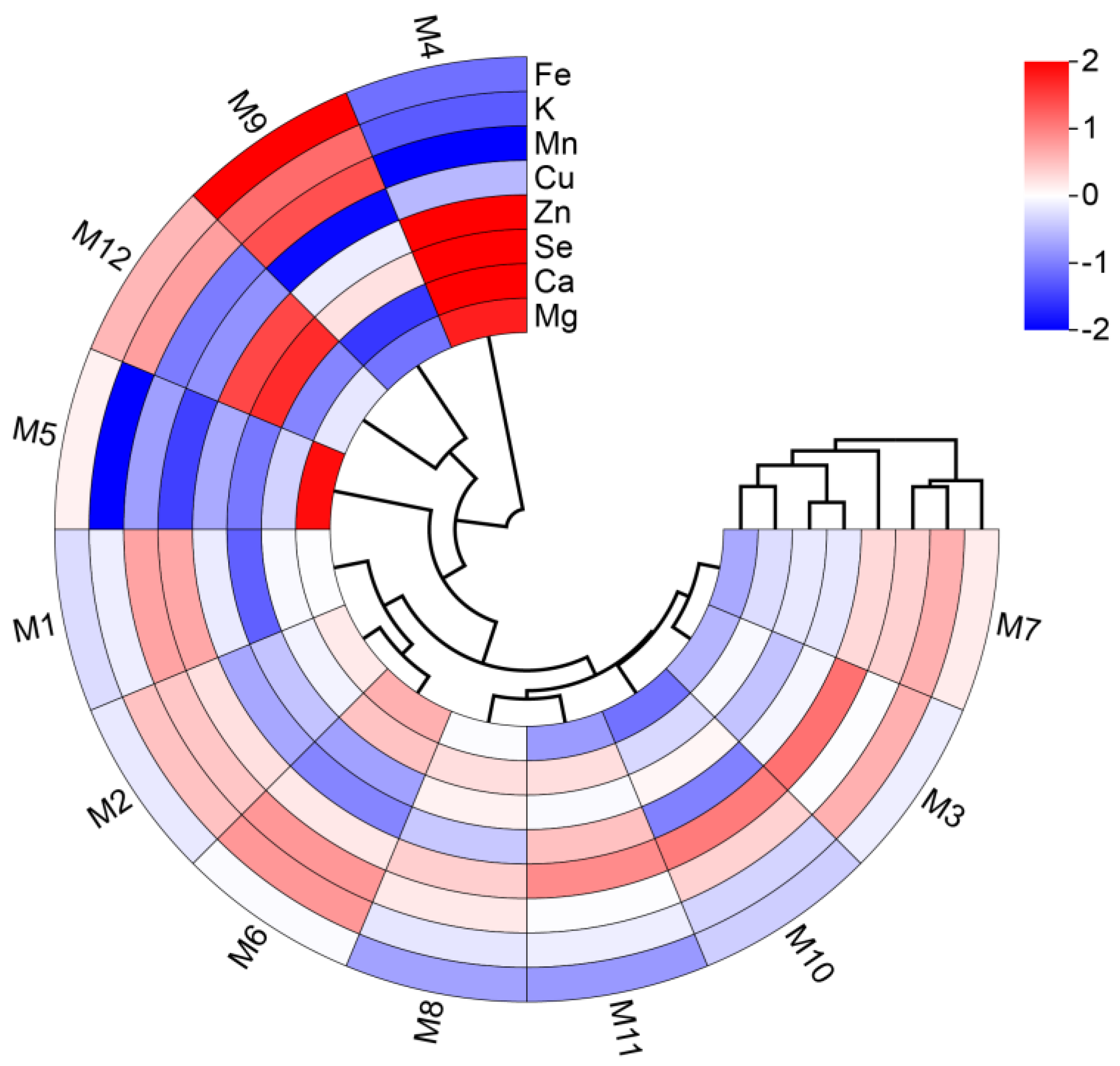
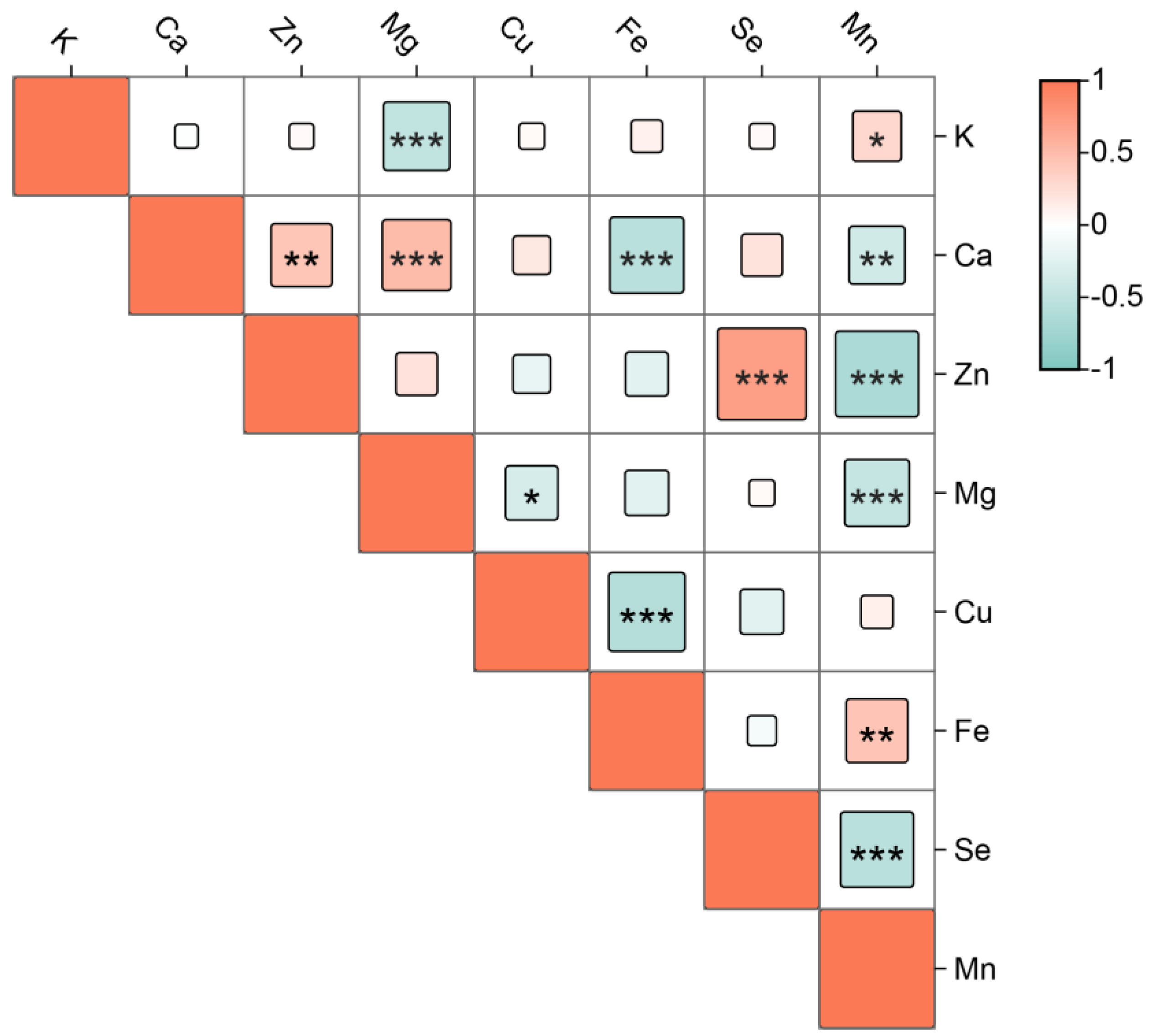
2.1. Mineral Elements Analysis of E’seguo
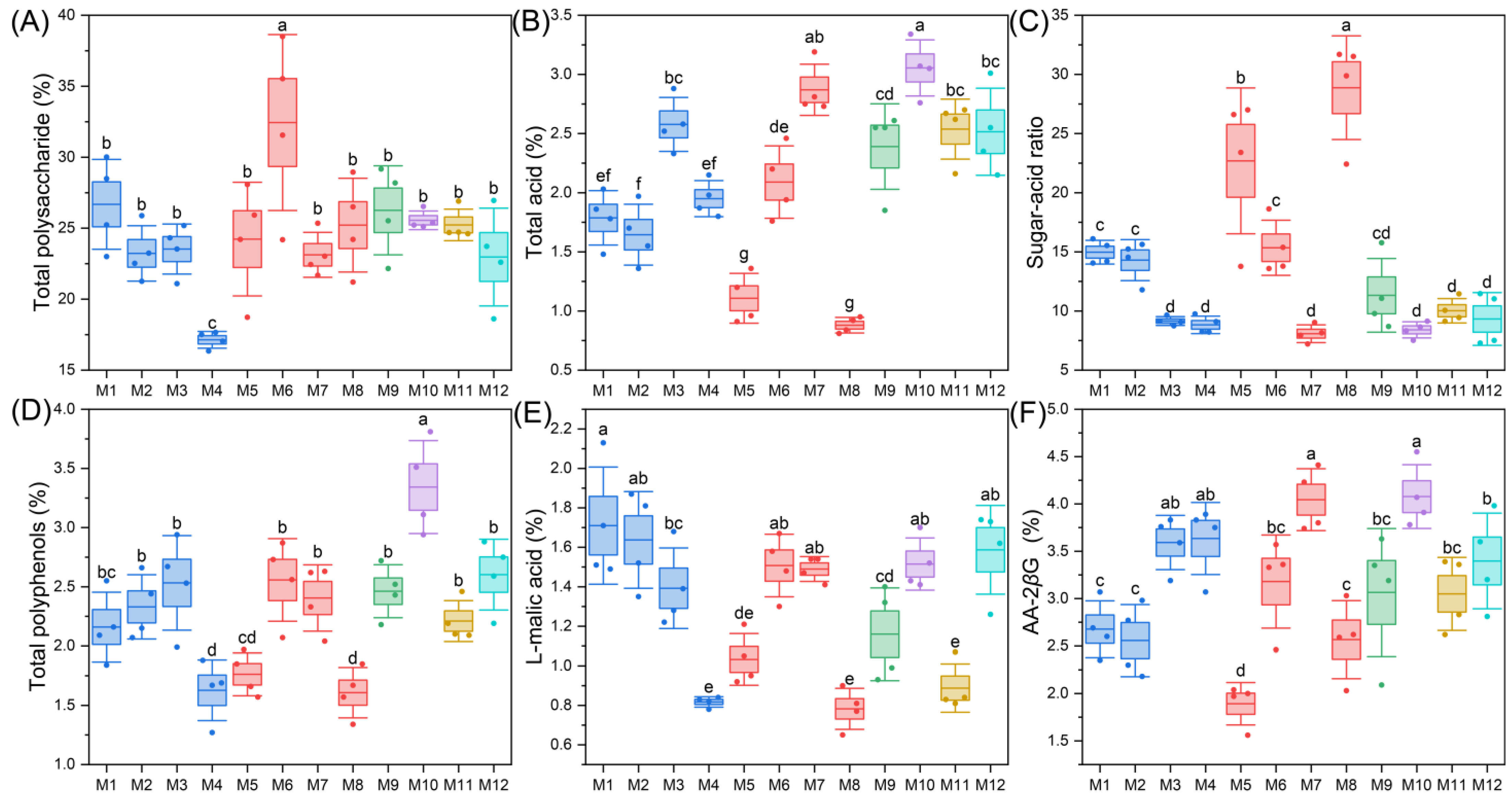
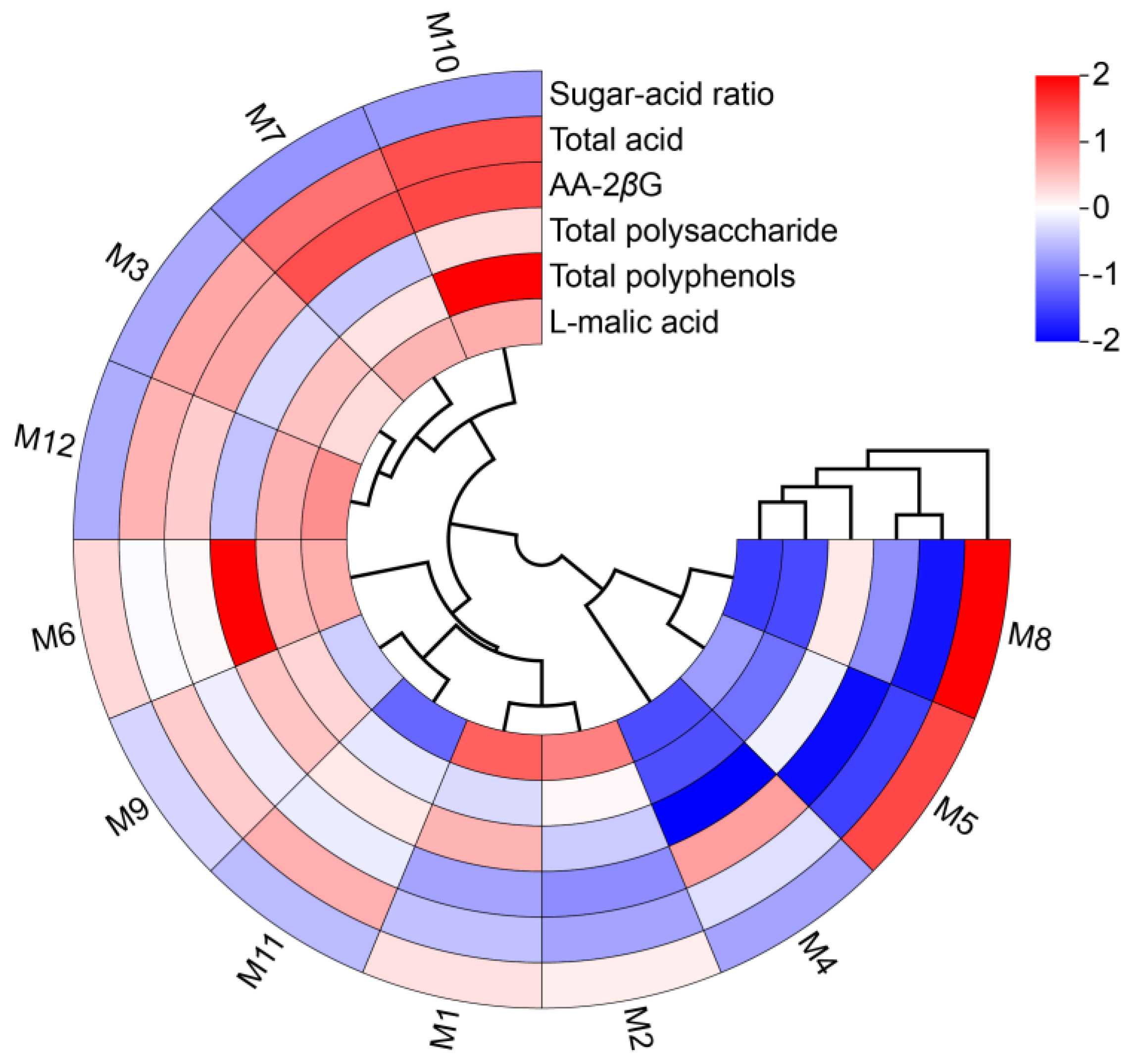
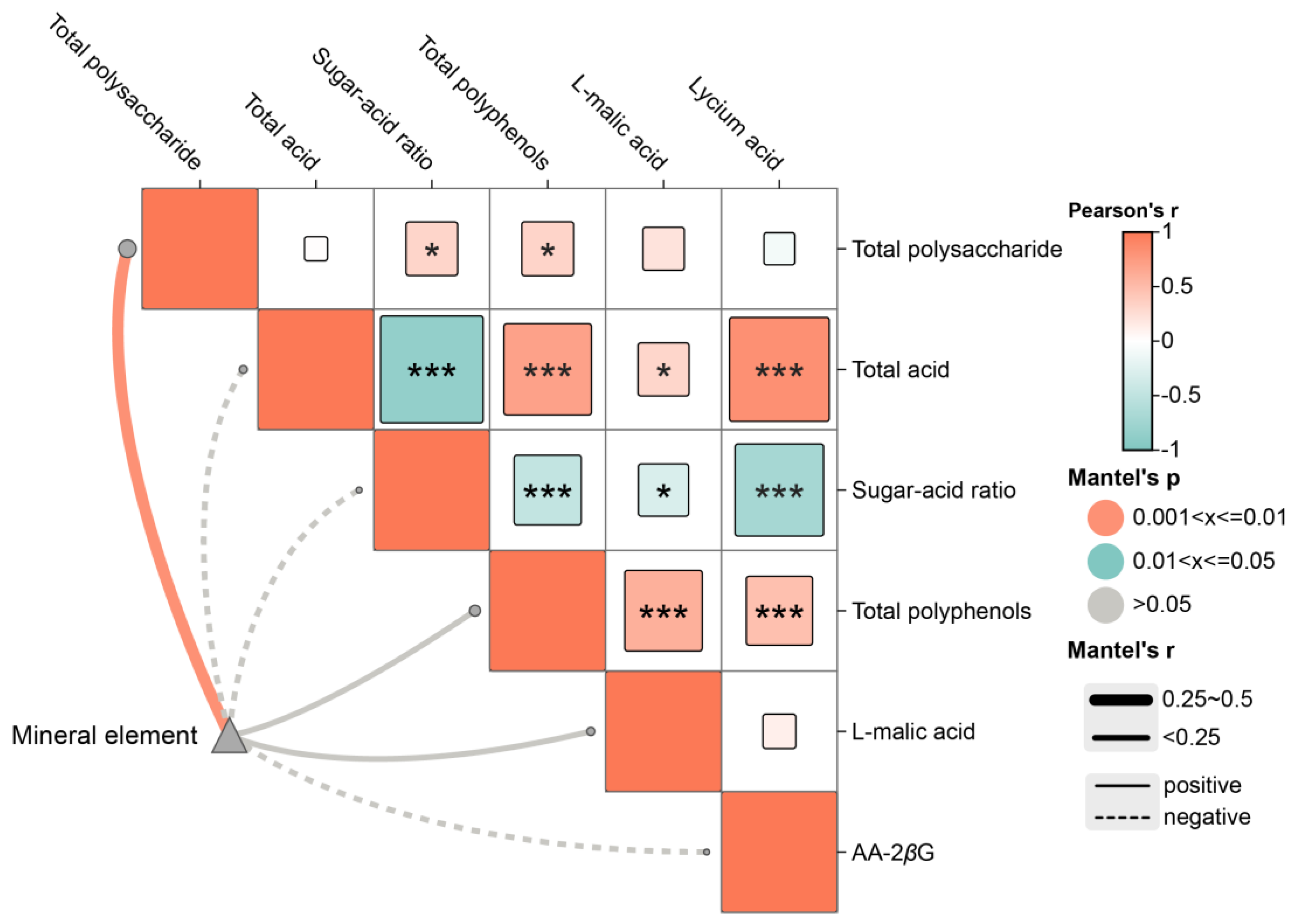
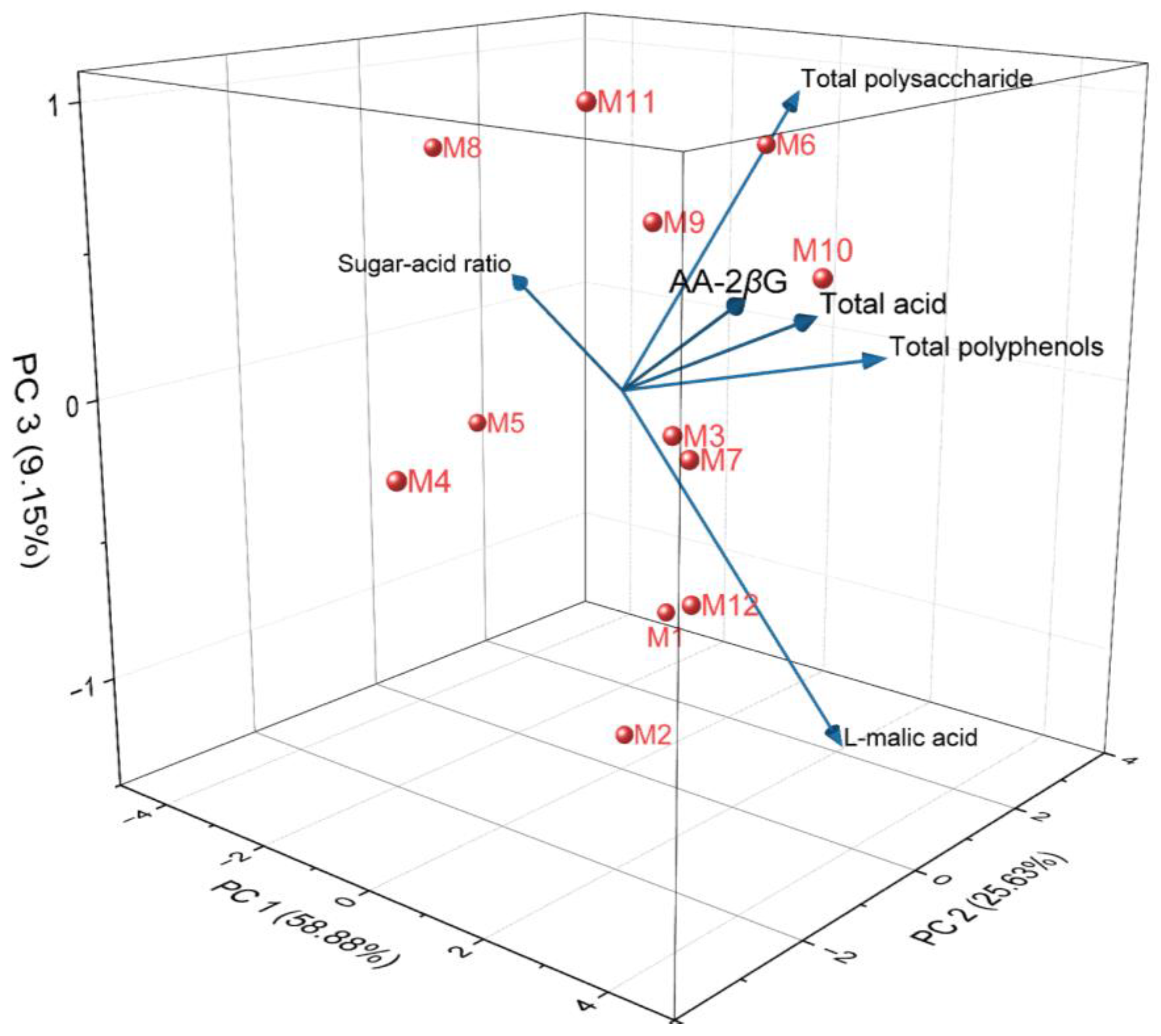
2.2. Analysis of Quality Indicators and Medicinal Properties of E’seguo
3. Materials and Methods
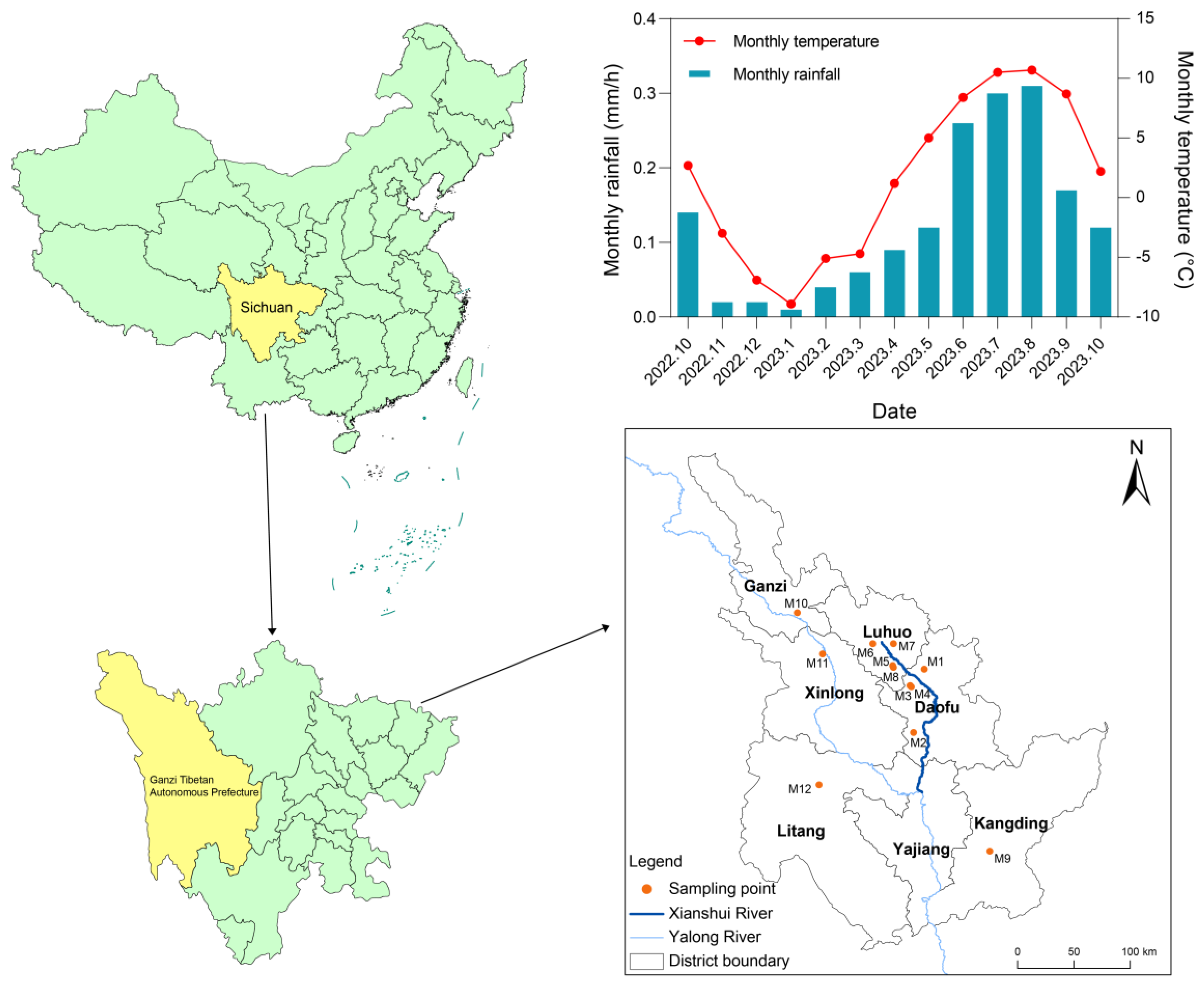
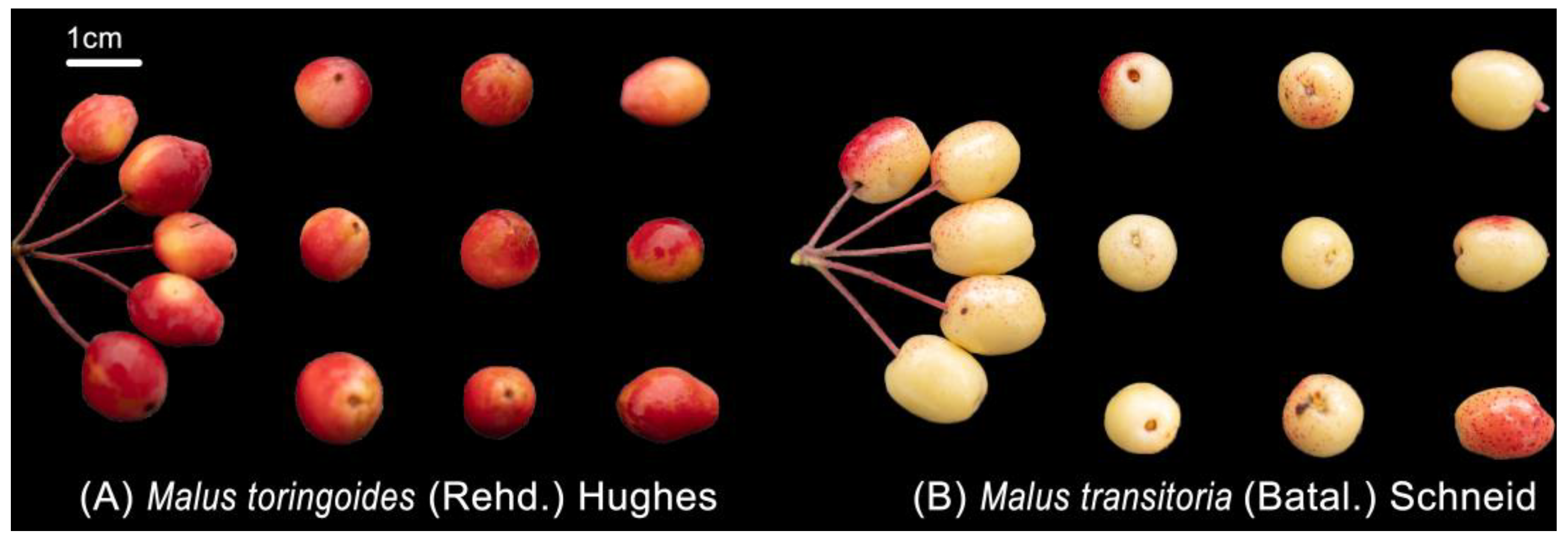
3.1. Plant Material
3.2. Determination of Mineral Elements, Polysaccharides, Total Acids, Sugar-Acid Ratio, and Polyphenols
3.3. Determination of L-malic Acid and AA-2βG
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renzhen, W.J.; Wencheng, D.Z.; He, Q.X.; Chu, Q.Q.; Gadeng, N.M.; Zhang, Y.; Yu, J. Analysis of Varieties and Characteristics of Plant Tibetan Medicine in The Stainless Crystal Minrror: A Tibetan Materia Medic. Chin. J. Exp. Tradit. Med. Formulae 2022, 28, 163–171. [Google Scholar]
- Qian, G.Z. Taxonomic Studies of Malus Mill. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2005. [Google Scholar]
- Deng, H.P. Studies on the Oringinof Genetic Diversity in Malus toringoides. Ph.D. Thesis, Southwest University, Chongqin, China, 2002. [Google Scholar]
- Hua, H.; Sun, H.P.; Liu, L.; Jiang, S.Y.; Zeng, A.Q.; Zhao, J.N. Potential geographical distribution and habitat characteristics of Malus toringoides (Rehd.) Hughes and its sympatric Relative Species. Shizhen Guoyi Guoyao 2020, 31, 203–206. [Google Scholar]
- Toyoda-Ono, Y.; Maeda, M.; Nakao, M.; Yoshimura, M.; Sugiura-Tomimori, N.; Fukami, H. 2-O-(β-d-glucopyranosyl)ascorbic acid, a novel ascorbic acid analogue isolated from Lycium fruit. J. Agric. Food Chem. 2004, 52, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; XU, J.; Cai, X.Y.; Chen, R.; Jing, Y.; Kang, J.J.; Li, M. Study on hypolipidemic activity of Tibet medicine “Malus Fructus”. Chin. J. New Drugs 2021, 30, 1709–1714. [Google Scholar]
- Zhang, X.K.; Cao, G.H.; Bi, Y.; Liu, X.H.; Yin, H.M.; Zuo, J.F.; Xu, W.; Li, H.D.; He, S.; Zhou, X.H. Comprehensive analysis of 34 edible flowers by the determination of nutritional composition and antioxidant capacity planted in yunnan province China. Molecules 2023, 28, 5260. [Google Scholar] [CrossRef]
- Kang, C.Y.; Yao, J.L.; Liu, Z.C.; Han, Y.P. Editorial: Rosaceae fruit development and quality. Front. Plant Sci. 2022, 12. [Google Scholar] [CrossRef]
- Gowman, A.C.; Picard, M.C.; Rodriguez-Uribe, A.; Misra, M.; Khalil, H.; Thimmanagari, M.; Mohanty, A.K. Physicochemical analysis of apple and grape pomaces. Bioresources 2019, 14, 3210–3230. [Google Scholar] [CrossRef]
- Sun, L.J.; Meng, Y.H.; Sun, J.J.; Guo, Y.R. Characterization, antioxidant activities and hepatoprotective effects of polysaccharides from pre-pressing separation Fuji apple peel. Cyta-J. Food 2017, 15, 307–319. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Yue, Z.G.; Li, Q.; Meng, J.; Liu, J.J.; Hekong, X.; Jiang, F.L.; Mi, M.; Liu, L.; et al. Apple polysaccharides induce apoptosis in colorectal cancer cells. Int. J. Mol. Med. 2012, 30, 100–106. [Google Scholar]
- Nunes, P.C.; Aquino, J.D.; Rockenbach, I.I.; Stamford, T.L.M. Physico-chemical characterization, bioactive compounds and antioxidant activity of Malay apple [Syzygium malaccense (L.) Merr. & LM Perry]. PLoS ONE 2016, 11, e0158134. [Google Scholar]
- Skoko, A.M.G.; Sarkanj, B.; Lores, M.; Celeiro, M.; Babojelic, M.S.; Kamenjak, D.; Flanjak, I.; Jozinovic, A.; Kovac, T.; Loncaric, A. Identification and quantification of polyphenols in croatian traditional apple varieties. Plants 2022, 11, 3540. [Google Scholar] [CrossRef] [PubMed]
- Schiavano, G.F.; De Santi, M.; Brandi, G.; Fanelli, M.; Bucchini, A.; Giamperi, L.; Giomaro, G. Inhibition of breast cancer cell proliferation and In Vitro tumorigenesis by a new red apple cultivar. PLoS ONE 2015, 10, e0135840. [Google Scholar]
- Kara, K.; Güçlü, B.K.; Baytok, E. Comparison of nutrient composition and anti-methanogenic properties of different Rosaceae species. J. Anim. Feed Sci. 2015, 24, 308–314. [Google Scholar] [CrossRef]
- Bai, Q.; Shen, Y.Y.; Huang, Y. Advances in mineral nutrition transport and signal transduction in rosaceae fruit quality and postharvest storage. Front. Plant Sci. 2021, 12, 620018. [Google Scholar] [CrossRef]
- Zhang, J.; Sivakumar, D.; Netzel, M.E.; Sultanbawa, Y. Minerals and trace elements in broad-leaved geebung (Persoonia stradbrokensis), an underutilised native australian fruit. Proc. Nutr. Soc. 2024, 83, E56. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.L.; Ren, A.H.; Chen, G.Y.; Ye, W.J.; Wu, Y.X.; Ma, P.; Yu, W.Q.; He, T.M. A comparison of the mineral element content of 70 different varieties of pear fruit (Pyrus ussuriensis) in china. PeerJ 2023, 11, e15328. [Google Scholar] [CrossRef]
- Bons, H.K.; Sharma, A. Impact of foliar sprays of potassium, calcium, and boron on fruit setting behavior, yield, and quality attributes in fruit crops: A review. J. Plant Nutr. 2023, 46, 3232–3246. [Google Scholar] [CrossRef]
- Shuai, W. Breeding and Cultivation Techniques of Superior Varieties of Malus toringoides in the Xiushuihe River Basin in the plateau of Western Sich. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2017. [Google Scholar]
- Todea, D.; Cadar, O.; Simedru, D.; Roman, C.; Tanaselia, C.; Suatean, I.; Naghiu, A. Determination of major-to-trace minerals and polyphenols in different apple cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 523–529. [Google Scholar] [CrossRef]
- Xia, Y.G.; Liang, J.; Yang, B.Y.; Wang, Q.H.; Kuang, H.X. A new method for quantitative determination of two uronic acids by cze with direct uv detection. Biomed. Chromatogr. 2011, 25, 1030–1037. [Google Scholar] [CrossRef]
- Llamas, N.E.; Di Nezio, M.S.; Band, B.S.F. Flow-injection spectrophotometric method with on-line photodegradation for determination of ascorbic acid and total sugars in fruit juices. J. Food Compos. Anal. 2011, 24, 127–130. [Google Scholar] [CrossRef]
- Tang, L.; Li, J.; Tan, S.; Li, M.X.; Ma, X.; Zhou, Z.Q. New insights into the hybrid origin of Malus toringoides and its close relatives based on a single-copy nuclear gene Sbei and three chloroplast fragments. J. Syst. Evol. 2014, 52, 477–486. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; El-Ansary, D.O.; Ekiert, H.; Al-Mana, F.A. Malusbaccata var. gracilis and Malus toringoides bark polyphenol studies and antioxidant, antimicrobial and anticancer activities. Processes 2020, 8, 283. [Google Scholar] [CrossRef]
- Li, M.Z.; Xiao, Y.W.; Mount, S.; Liu, Z.C. An atlas of genomic resources for studying rosaceae fruits and ornamentals. Front. Plant Sci. 2021, 12, 644881. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, D.Y.; Fan, Y.; Sun, J.; Cheng, J.X.; Shi, Y.J. Experimental study on the antioxidant activity of Malus hupehensis (Pamp.) rehd extracts In Vitro and In Vivo. J. Cell. Biochem. 2019, 120, 11878–11889. [Google Scholar] [CrossRef]
- Li, P.C.; Tan, J.Q.; Xiao, M.; Cai, X.; Xue, H.K.; Yu, H.S. Bioactive substances and biological functions in Malus hupehensis: A review. Molecules 2023, 28, 658. [Google Scholar] [CrossRef]
- Alrashidi, A.A.; Alhaithloul, H.A.S.; Soliman, M.H.; Attia, M.S.; Elsayed, S.M.; Ali, M.M.; Sadek, A.M.; Fakhr, M.A. Role of calcium and magnesium on dramatic physiological and anatomical responses in tomato plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12614. [Google Scholar] [CrossRef]
- Kuzin, A.; Solovchenko, A. Essential role of potassium in apple and its implications for management of orchard fertilization. Plants 2021, 10, 2624. [Google Scholar] [CrossRef]
- Valizadehkaji, B.; Naeini, M.R. Physicochemical characteristics of pomegranate fruits as affected by foliar application of potassium and zinc. J. Plant Nutr. 2024, 47, 2038–2055. [Google Scholar] [CrossRef]
- Shi, L.T.; He, Q.; Li, J.; Liu, Y.L.; Cao, Y.L.; Liu, Y.Q.; Sun, C.D.; Pan, Y.J.; Li, X.; Zhao, X.Y. Polysaccharides in fruits: Biological activities, structures, and structure-activity relationships and influencing factors—A review. Food Chem. 2024, 451, 139408. [Google Scholar] [CrossRef]
- Mu, Q.; Zhang, D.D.; Yang, Y.F.; Zhang, J.; Hu, X.X.; Zhang, W.X. Determination and cluster analysis of sugar and acid components in different cultivars of crabapple (Malus spp.) fruits. Nonwood For. Res. 2018, 36, 135–144. [Google Scholar]
- Ma, B.Q.; Chen, J.; Zheng, H.Y.; Fang, T.; Ogutu, C.; Li, S.H.; Han, Y.P.; Wu, B.H. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef]
- Hameed, A.; Liu, Z.Y.; Wu, H.J.; Zhong, B.M.; Ciborowski, M.; Suleria, H.A.R. A comparative and comprehensive characterization of polyphenols of selected fruits from the rosaceae family. Metabolites 2022, 12, 271. [Google Scholar] [CrossRef]
- Miao, T.Y.; Song, G.M.; Yang, J. Protective effect of apple polyphenols on H2O2-induced oxidative stress damage in human colon adenocarcinoma caco-2 cells. Chem. Pharm. Bull. 2023, 71, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-L.; Wu, Q.-P.; Yang, X.-F.; Wei, M.-K.; Zhang, J.-M.; Huang, Q.; Zhou, X.-Y. L-malate reverses oxidative stress and antioxidative defenses in liver and heart of aged rats. Physiol. Res. 2008, 57, 261–268. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Liu, X.M.; Zhang, X.; Liu, J.H.; Hao, Y.F.; Yang, X.Y.; Wang, Y.J. Comparative evaluation of the antioxidant effects of the natural vitamin c analog 2-O-β-D-glucopyranosyl-l-ascorbic acid isolated from Goji berry fruit. Arch. Pharmacal Res. 2011, 34, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.L.; Wang, R.; Yang, L.; Wang, Z.T.; Shi, Y.H. The quality of Lycium barbarum was evaluated based on entropy weight method and grey correlation degree method. Chin. Tradit. Pat. Med. 2024, 46, 919–926. [Google Scholar]

| Analyte | Equation | R2 | Range (mg/mL) |
|---|---|---|---|
| L-malic acid | y = 17.734x − 3.4596 | 0.9990 | 0.626~1.565 |
| AA-2βG | y = 395.97x + 2.2132 | 0.9991 | 0.031~0.760 |
| Traits | PC 1 | PC 2 | PC 3 | |
|---|---|---|---|---|
| Eigenvalue | 3.533 | 1.538 | 0.549 | |
| Variability (%) | 58.880 | 25.628 | 9.147 | |
| Cumulative (%) | 58.880 | 84.508 | 93.655 | |
| Total polysaccharide | Factor loadings | 0.041 | 0.906 | 0.386 |
| Total acid | 0.963 | −0.124 | 0.172 | |
| Sugar-acid ratio | −0.888 | 0.333 | 0.097 | |
| Total polyphenols | 0.865 | 0.389 | 0.070 | |
| L-malic acid | 0.590 | 0.566 | −0.560 | |
| AA-2βG | 0.848 | −0.345 | 0.206 | |
| Total polysaccharide | Component Score Coefficient Matrix (CSC) | 0.022 | 0.730 | 0.521 |
| Total acid | 0.512 | −0.100 | 0.233 | |
| Sugar-acid ratio | −0.472 | 0.269 | 0.131 | |
| Total polyphenols | 0.460 | 0.314 | 0.094 | |
| L-malic acid | 0.314 | 0.456 | −0.756 | |
| AA-2βG | 0.451 | −0.279 | 0.278 | |
| Varieties | H1 | H2 | H3 | H0 |
|---|---|---|---|---|
| M1 | −0.415 | 1.213 | −0.936 | −0.021 |
| M2 | −0.498 | 0.537 | −1.358 | −0.299 |
| M3 | 1.276 | −0.387 | −0.077 | 0.689 |
| M4 | −0.572 | −3.020 | −0.134 | −1.199 |
| M5 | −3.073 | 0.282 | −0.273 | −1.882 |
| M6 | 0.362 | 2.166 | 0.774 | 0.896 |
| M7 | 1.870 | −0.698 | −0.109 | 0.974 |
| M8 | −3.601 | 0.054 | 0.739 | −2.177 |
| M9 | 0.342 | 0.173 | 0.586 | 0.320 |
| M10 | 2.922 | 0.415 | 0.472 | 1.997 |
| M11 | 0.050 | −0.642 | 1.008 | −0.046 |
| M12 | 1.336 | −0.092 | −0.692 | 0.747 |
| Sample Number | Variety | Sampling Location | River Basin |
|---|---|---|---|
| M1 | Malus toringoides (Rehd.) Hughes | Kongse Township, Daofu County | Xianshui river |
| M2 | Malus toringoides (Rehd.) Hughes | Genji Village, Wari Town, Daofu County | Xianshui river |
| M3 | Malus toringoides (Rehd.) Hughes | Mazi Township, Daofu County | Xianshui river |
| M4 | Malus transitoria (Batal.) Schneid | Mazi Township, Daofu County | Xianshui river |
| M5 | Malus toringoides (Rehd.) Hughes | Renda Township, Luhuo County | Xianshui river |
| M6 | Malus toringoides (Rehd.) Hughes | Keke, Xiala Tuo Town, Luhuo County | Xianshui river |
| M7 | Malus toringoides (Rehd.) Hughes | Yade Township, Luhuo County | Xianshui river |
| M8 | Malus transitoria (Batal.) Schneid | Renda Township, Luhuo County | Xianshui river |
| M9 | Malus toringoides (Rehd.) Hughes | Jiagenba Town, Kangding City | Yalong river |
| M10 | Malus toringoides (Rehd.) Hughes | Tuoba Township, Ganzi County | Yalong river |
| M11 | Malus toringoides (Rehd.) Hughes | Dagai Township, Xinlong County | Yalong river |
| M12 | Malus toringoides (Rehd.) Hughes | Junba Town, Litang County | Yalong river |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Fan, H.; Chen, Y.; Zou, Y.; Cai, X.; Wang, H.; Li, M. Study on the Bioactive Constituent and Mineral Elements of the Tibetan Medicine E’seguo from Different Regions of Ganzi Prefecture, China. Molecules 2024, 29, 4154. https://doi.org/10.3390/molecules29174154
Jiang M, Fan H, Chen Y, Zou Y, Cai X, Wang H, Li M. Study on the Bioactive Constituent and Mineral Elements of the Tibetan Medicine E’seguo from Different Regions of Ganzi Prefecture, China. Molecules. 2024; 29(17):4154. https://doi.org/10.3390/molecules29174154
Chicago/Turabian StyleJiang, Menglian, Heling Fan, Yixuan Chen, Yulin Zou, Xiaoyang Cai, Haohan Wang, and Min Li. 2024. "Study on the Bioactive Constituent and Mineral Elements of the Tibetan Medicine E’seguo from Different Regions of Ganzi Prefecture, China" Molecules 29, no. 17: 4154. https://doi.org/10.3390/molecules29174154
APA StyleJiang, M., Fan, H., Chen, Y., Zou, Y., Cai, X., Wang, H., & Li, M. (2024). Study on the Bioactive Constituent and Mineral Elements of the Tibetan Medicine E’seguo from Different Regions of Ganzi Prefecture, China. Molecules, 29(17), 4154. https://doi.org/10.3390/molecules29174154






