Organic Heterostructures with Dendrimer Based Mixed Layer for Electronic Applications
Abstract
1. Introduction
2. Results
2.1. Optical Characterization
2.1.1. Transmission of Single and Mixed Layers
2.1.2. Photoluminescence Spectra of Single and Mixed Layers
2.2. Structural Properties of Single and Mixed Layers
2.3. Morphological Properties of Single and Mixed Layers
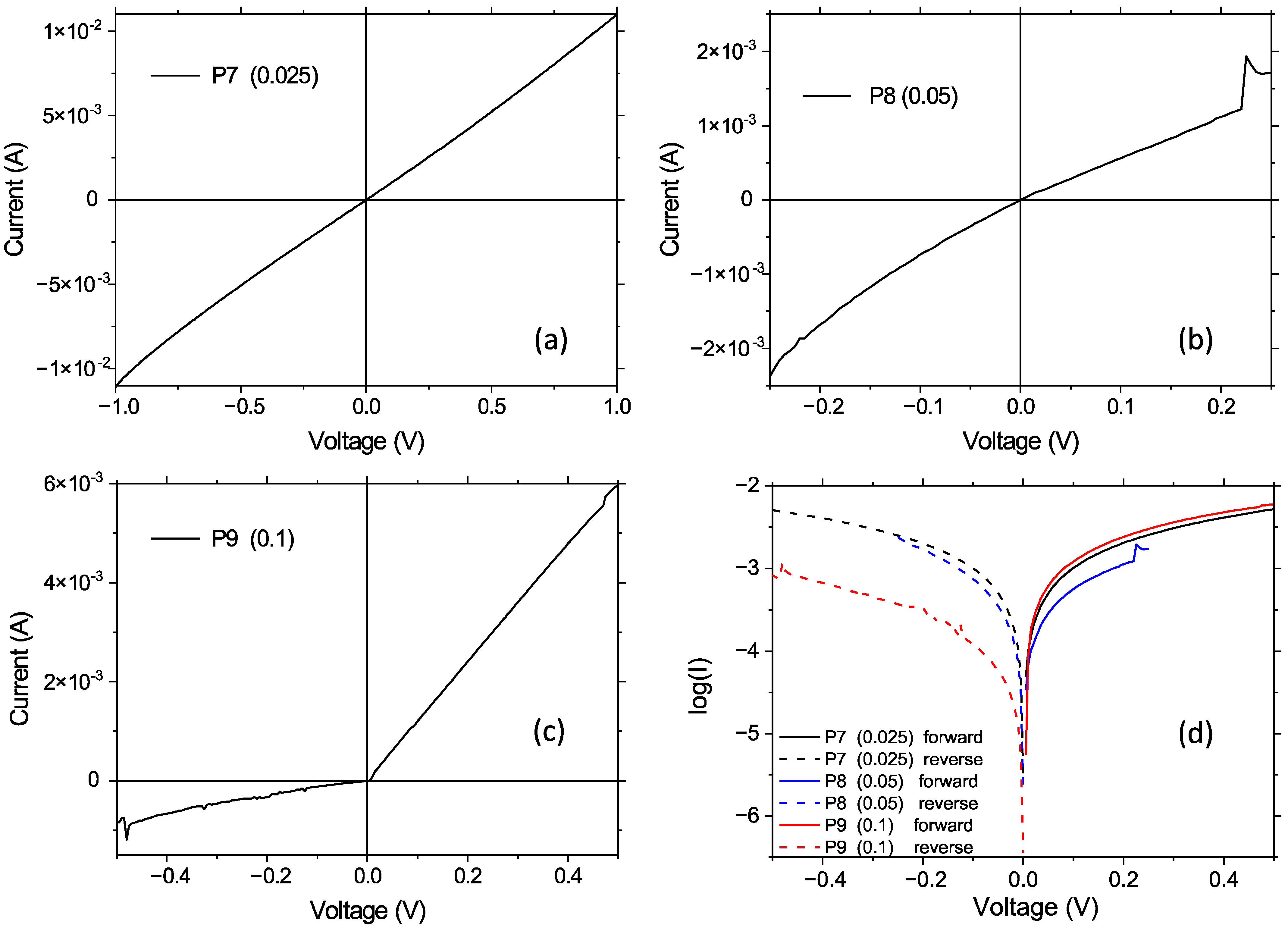
3. Electrical Characterization
4. Discussion
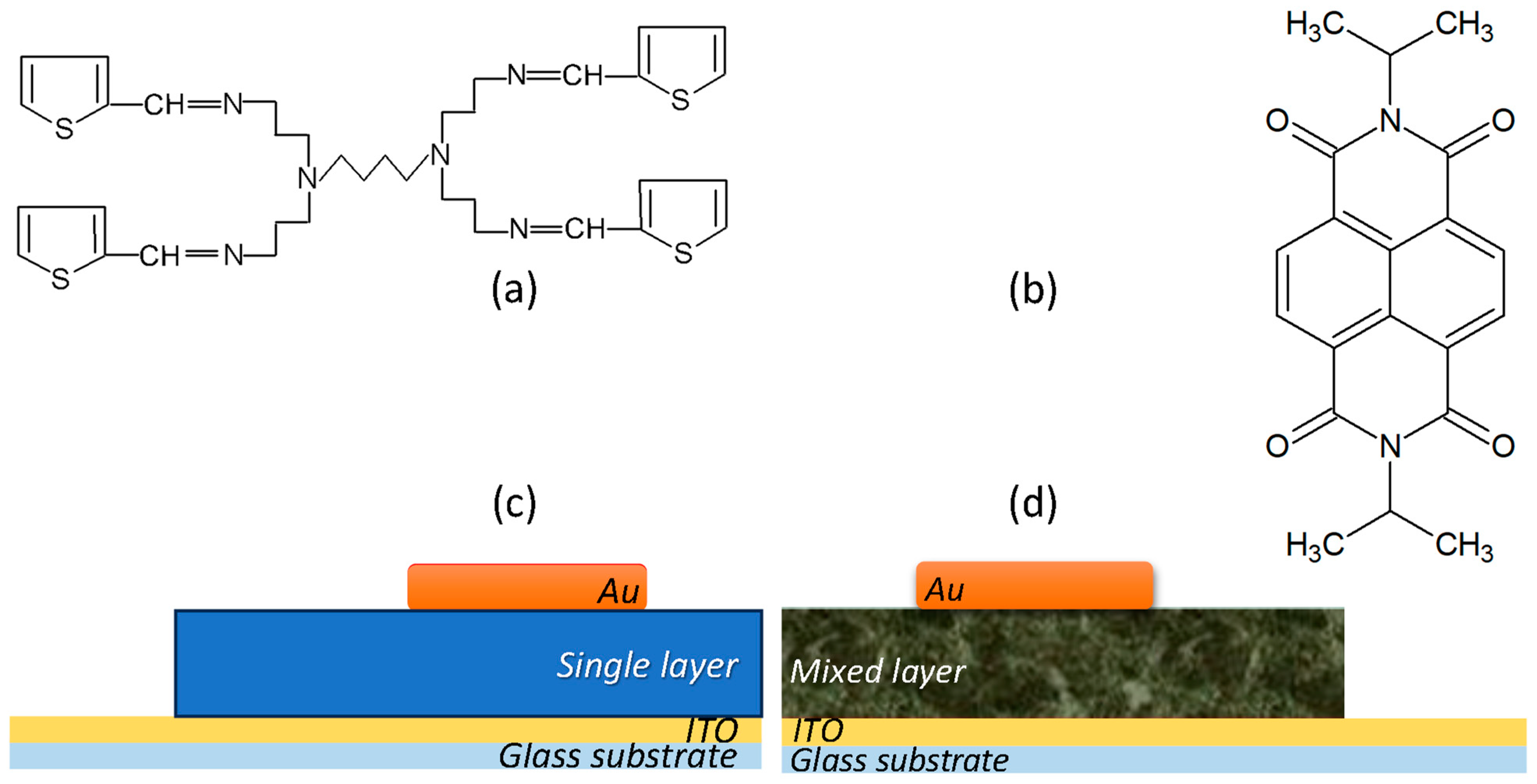
5. Materials and Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopidakis, N.; Mitchell, W.J.; Van de Lagemaat, J.; Ginley, D.S.; Rumbles, G.; Shaheen, S.E. Bulk heterojunction organic photovoltaic devices based on phenyl-cored thiophene dendrimers. Appl. Phys. Lett. 2006, 89, 103524. [Google Scholar] [CrossRef]
- Arotiba, O.A.; Owino, J.; Songa, E.; Hendricks, N.; Waryo, T.; Jahed, N.; Baker, P.; Iwuoha, E. An electrochemical DNA Biosensor developed on a nanocomposite platform of gold and poly(propyleneimine) dendrimer. Sensors 2008, 8, 6791–6809. [Google Scholar] [CrossRef]
- Arotiba, O.A.; Songa, E.A.; Parker, P.G.; Iwuoha, E.I. Dendrimeric gold-poly(propylene imine) electrochemical DNA nanobiosensor. Chim. Oggi (Chem. Today) 2009, 27, 55–58. [Google Scholar]
- Makelane, H.R.; Tovide, O.; Sunday, T.C.E.; Waryo, T.; Iwuoha, E.I. Electrochemical interrogation of G3-poly(propylene thiophenoimine) dendritic star polymer in phenanthrene sensing. Sensors 2015, 15, 22343–22363. [Google Scholar] [CrossRef] [PubMed]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About dendrimers: Structure, physical properties and applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2020, 13, 65. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Spallarossa, A.; Lusardi, M.; Zuccari, G. Successful dendrimer and liposome-based strategies to solubilize an antiproliferative pyrazole otherwise not clinically applicable. Nanomaterials 2022, 12, 233. [Google Scholar] [CrossRef]
- Saruchi; Singh, N.; Kumar, V. An overview on surface modified dendrimers: Current direction and future perspective. Adv. Chemicobiol. Res. 2023, 2, 238. [Google Scholar] [CrossRef]
- Caminade, A.-M. Dendrimers, an Emerging Opportunity in Personalized Medicine? J. Pers. Med. 2022, 12, 1334. [Google Scholar] [CrossRef]
- Jeong, W.-J.; Bu, J.; Mickel, P.; Han, Y.; Rawding, P.A.; Wang, J.; Kang, H.; Hong, H.; Král, P.; Hong, S. Dendrimer-peptide conjugates for effective blockade of the interactions between SARS-CoV-2 Spike protein and human ACE2 receptor. Biomolecules 2023, 24, 141. [Google Scholar] [CrossRef] [PubMed]
- Militello, M.P.; Porcal, G.V.; Bertolotti, S.G.; Previtali, C.M.; Arbeloa, E.M. The effect of PAMAM dendrimers on the excited states of little-explored xanthene dyes. In search of eco-friendly photoinitiating systems. J. Photoch. Photobio. A 2023, 434, 114234. [Google Scholar] [CrossRef]
- Lo, S.-C.; Burn, P.L. Development of dendrimers: Macromolecules for use in organic light-emitting diodes and solar cells. Chem. Rev. 2007, 107, 1097–1116. [Google Scholar] [CrossRef] [PubMed]
- Kose, M.E.; Mitchell, W.J.; Kopidakis, N.; Chang, C.H.; Shaheen, S.E.; Kim, K.; Rumbles, G. Theoretical studies on conjugated phenyl-cored thiophene dendrimers for photovoltaic applications. J. Am. Chem. Soc. 2007, 129, 14257–14270. [Google Scholar] [CrossRef]
- Rupert, B.; Rance, W.; Ferguson, A.; Kose, M.; Shaheen, S.; Kopidakis, N.M. π-conjugated dendrimers for solar-energy harvesting. SPIE Newsroom 2009, 1, 1–3. [Google Scholar] [CrossRef]
- Wang, J.-L.; Zhong, C.; Tang, Z.-M.; Wu, H.; Ma, Y.; Cao, Y.; Pei, J. Solution-processed bulk heterojunction photovoltaic cells from gradient π-conjugated thienylene vinylene dendrimers. Chem. Asian 2010, 5, 105–113. [Google Scholar] [CrossRef]
- Pedro-Hernandez, L.; Martinez-Garcia, M. Dendrimers Applications: A brief review. Curr. Org. Chem. 2021, 25, 1247–1269. [Google Scholar] [CrossRef]
- Yonkeu, A.L.D. Dendritic Poly(3-hexylthiophene) Star Copolymer Systems for Next Generation Bulk Heterojunction Organic Photovoltaic Cells. Ph.D. Thesis, University of the Western Cape, Cape Town, South Africa, 2019. Available online: http://etd.uwc.ac (accessed on 30 July 2024).
- Abd-El-Aziz, A.S.; Abdelghani, A.A.; Wagner, B.D.; Bissessur, R.T. Advances in light- emitting dendrimers. Macromol. Rapid Commun. 2019, 40, 1800711. [Google Scholar] [CrossRef]
- Russell, S.M.; Brewer, A.M.; Stoltzfus, D.M.; Saghaei, J.; Burn, P.L. Organic light-emitting diodes comprising highly luminescent red-emitting dendrimers with carbazole-based dendrons. J. Mater. Chem. C 2019, 7, 4681–4691. [Google Scholar] [CrossRef]
- Stoltzfus, D.M.; Jiang, W.; Brewer, A.M.; Burn, P.L. Twisted dendrons for highly luminescent green emissive phosphorescent dendrimers. J. Mater. Chem. C 2018, 6, 10315–10326. [Google Scholar] [CrossRef]
- Gao, M.; Jang, J.; Mai, V.T.N.; Ranasinghe, C.S.K.; Chu, R.; Burn, P.L.; Gentle, I.R.; Pivrikas, A.; Shaw, P.E. Effect of dendrimer surface groups on the properties of phosphorescent emissive films. Org. Electron. 2021, 99, 106321. [Google Scholar] [CrossRef]
- Wallwork, N.R.; Mamada, M.; Keto, A.B.; McGregor, S.K.M.; Shukla, A.; Adachi, C.; Krenske, E.H.; Namdas, E.B.; Lo, S.-C. Cibalackrot dendrimers for hyperfluorescent organic light-emitting diodes. Macromol. Rapid Comm. 2022, 2200118. [Google Scholar] [CrossRef] [PubMed]
- Matthews, O.A.; Shipway, A.N.; Stoddard, J.F. Dendrimers branching out from curiosities into new technologies. Prog. Polym. Sci. 1998, 23, 1–56. [Google Scholar] [CrossRef]
- Fréchet, J.M.J.; Hawker, C.J.; Gitsov, I.; Leon, J.W. Dendrimers and hyperbranched polymers: Two families of three dimensional macromolecules with similar but clearly distinct properties. J. Macromol. Sci. A 1996, 33, 1399–1425. [Google Scholar] [CrossRef]
- Li, J.C.; Han, N.; Wang, S.S.; Ba, D.C. Study of the charge transport characteristics of dendrimer molecular thin films. Thin Solis Films 2011, 519, 5234–5237. [Google Scholar] [CrossRef]
- Mizusaki, M.; Yamada, Y.; Obara, S.; Tada, K. Synthesis of π-conjugated two generation dendrimer composed of p-phenylenevinylene Dendron and triphenylamine surface group. Chem. Lett. 2012, 41, 516–517. [Google Scholar] [CrossRef]
- Stevenson, S.G.; Samuel, I.D.W.; Staton, S.V.; Knights, K.A.; Burn, P.I.; Williams, J.H.T.; Walker, A.B. Current-voltage characteristics of dendrimer light-emitting diodes. J. Phys. D Appl. Phys. 2010, 43, 3851056. [Google Scholar] [CrossRef]
- Kotadiya, N.B.; Mondal, A.; Blom, P.W.M.; Andrienko, D.; Wetzelaer, G.-J.A.H. A window to trap-free charge transport in organic semiconducting thin films. Nat. Mater. 2019, 18, 1182–1186. [Google Scholar] [CrossRef]
- Yonkeu, A.L.D. Novel Poly(propylene Thiophenoimine)-Co Poly(ethylenedioxythiophene) Composites of Naphthalene Diimide for Applications in Organic Photovoltaic Cells. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2013; pp. 45–46. Available online: https://etd.uwc.ac.za/handle/11394/3281 (accessed on 30 July 2024).
- Admassie, S.; Inganäs, O.; Mammo, W.; Perzon, E.; Andersson, M.R. Electrochemical and optical studies of the band gaps of alternating polyfluorene copolymers. Synthetic Met. 2006, 156, 614–623. [Google Scholar] [CrossRef]
- Ma, W.; Ying, Y.-L.; Qin, L.-X.; Gu, Z.; Zhou, H.; Li, D.-W.; Sutherland, T.C.; Chen, H.-Y.; Long, Y.-T. Investigating electron-transfer processes using a biomimetic hybrid bilayer membrane system. Nat. Protoc. 2013, 8, 439–450. [Google Scholar] [CrossRef]
- Liu, J.; Tu, G.; Zhou, Q.; Cheng, Y.; Geng, Y.; Wang, L.; Ma, D.; Jing, X.; Wang, F. Highly efficient green light emitting polyfluorene incorporated with 4-diphenylamino-1,8-naphthalimide as green dopant. J. Mater. Chem. 2006, 16, 1431–1438. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.A. Electrochemical Methods-Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001; pp. 1–43. [Google Scholar]
- Zhang, X.; Jiang, B.; Zhang, S.; Hou, J.; Yao, J.; Zhan, C. Solution-processable perylene diimide based star-shaped small molecule acceptor: Synthesis and photovoltaic properties. In Organic Photovoltaics XV, Proceedings of the SPIE, SPIE Organic Photonics+Electronics, San Diego, CA, USA, 6 October 2014; Kafafi, Z.H., Lane, P.A., Samuel, I.D.W., Eds.; SPIE: San Francisco, CA, USA, 2014; Volume 9184, p. 91840C. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Al Kobaisi, M.; Jadhav, R.W.; Mojrajkar, P.P.; Jones, L.A.; George, S. Naphthalene diimide: Perspectives and promise. Chem. Soc. Rev. 2021, 50, 9845–9998. [Google Scholar] [CrossRef] [PubMed]
- Suraru, S.-L.; Würthner, F. Strategies for the synthesis of functional naphthalene diimide. Angew. Chem. Int. Ed. 2014, 53, 7428–7448. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Minemawari, H.; Tsutsumi, J.; Chikamatsu, M.; Yamada, T.; Horiuchi, S.; Tanaka, M.; Kumai, R.; Yoneya, M.; Hasegawa, T. Effects of Substituted Alkyl Chain Length on Solution-Processable Layered Organic Semiconductor Crystals. Chem. Phys. Mater. 2015, 27, 3809–3812. [Google Scholar] [CrossRef]
- Takimiya, K.; Osaka, I.; Mori, T.; Nakano, M. Organic semiconductors based on [1]benzothieno [3,2-b][1]benzothiophene substructure. Acc. Chem. Res. 2014, 47, 1493–1502. [Google Scholar] [CrossRef]
- Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I. Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 2019, 48, 1596–1625. [Google Scholar] [CrossRef]
- Nicolai, H.T.; Kuik, M.; Wetzelaer, G.A.H.; De Boer, B.; Campbell, C.; Risko, C.; Brédas, J.L.; Blom, P.W.M. Unification of trap-limited electron transport in semiconducting polymers. Nat. Mater. 2012, 11, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Haneef, H.F.; Zeidell, A.M.; Jurchescu, A.D. Charge carrier traps in organic semiconductors: A review on the underlying physics and impact on electronic devices. J. Mater. Chem. C 2020, 8, 759–787. [Google Scholar] [CrossRef]
- Erten, S.; Posokhov, Y.; Alp, S.; Içli, S. The study of the solubility of naphthalene diimides with various bulky flanking substituents in different solvents by UV-vis spectroscopy. Dyes Pigments 2005, 64, 171–178. [Google Scholar] [CrossRef]
- Jung, J.; Selerowicz, A.; Maczugowska, P.; Halagan, K.; Rybakiewicz-Sekita, R.; Zagorska, M.; Stefaniuk-Grams, A. Electron transport in naphthalene diimide derivatives. Materials 2021, 14, 4026. [Google Scholar] [CrossRef]
- Gawrys, P.; Boudinet, D.; Zagorska, M.; Djurado, D.; Verilhac, J.-M.; Horowitz, G.; Pécaud, J.; Poget, S.; Pron, A. Solution processible naphthalene and perylene bisimides: Synthesis, electrochemical characterization and application to organic field effect transistors (OFETs) fabrication. Synthetic Met. 2009, 159, 1478–1485. [Google Scholar] [CrossRef]
- Olowu, R.A.; Nangili, P.M.; Baleg, A.A.; Ikpo, C.O.; Njomo, N.; Baker, P.; Iwuoha, E. Spectroelectrochemical dynamics of dendritic poly(propylene imine)-polythiophene star copolymer aptameric 17β-estradiol biosensor. Int. J. Electrochem. Sci. 2011, 6, 1686–1708. [Google Scholar] [CrossRef]
- Yonkeu, A.L.D.; Ndipingwi, M.M.; Ikpo, C.; Nwambaekwe, K.; Yussuf, S.; Tesfay, H.; Iwuoha, E. Photoluminescence quenching of a novel electroconductive poly(propylene thiophenoimine)-co-poly(ethylenedioxy thiophene) star copolymer. Polymers 2020, 12, 2894. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of electronic absorption of solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Brütting, W.; Berleb, S.; Mückl, A.G. Device physics of organic light-emitting diodes based on molecular materials. Org. Electron. 2001, 2, 1–36. [Google Scholar] [CrossRef]
- Coropceanu, V.; Cornil, J.; Da Silva Filho, D.A.; Olivier, Y.; Silbey, R.; Brédas, J.-L. Charge Transport in Organic Semiconductors. Chem. Rev. 2007, 107, 926–952. [Google Scholar] [CrossRef] [PubMed]
- Orhan, E.; Anter, A.; Ulusoy, M.; Polat, B.; Okuyucu, C.; Yildiz, M.; Altindal, S. Effect of Gadolinium on Electrical Properties of Polyethyleneimine. Adv. Electron. Mater. 2023, 2300261. [Google Scholar] [CrossRef]
- Wetzelaer, G.A.H.; Kuik, M.; Lenes, M.; Blom, P.W.M. Origin of the dark-current ideality factor in polymer. Appl. Phys. Lett. 2011, 99, 153506. [Google Scholar] [CrossRef]
- Malenfant, P.R.L.; Fréchet, J.M.J. Dendrimers as solubilizing groups for conducting polymers: Preparation and characterization of polythiophene functionalized exclusively with aliphatic ether convergent dendrons. Macromolecules 2000, 33, 3634–3640. [Google Scholar] [CrossRef]
- Valencia, D.; Whiting, G.T.; Bulo, R.E.; Weckhuysen, B.M. Protonated thiophene-based oligomers as formed within zeolites: Understanding their electron delocalization and aromaticity. Phys. Chem. Chem. Phys. 2016, 18, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Cervini, R.; Holmes, A.B.; Moratti, S.C.; Köhler, A.; Friend, R.H. Synthesis of new conjugated thiophene polymers. Synthetic Met. 1996, 76, 169–171. [Google Scholar] [CrossRef]
- Myhre, C.C.E.L.; Nielsen, C.J. Optical properties in the UV and visible spectral region of organic acids relevant to tropospheric aerosols. Atmos. Chem. Phys. 2004, 4, 1759–1769. [Google Scholar] [CrossRef]
- Freidel, R.A.; Orchin, M. Ultraviolet Spectra of Aromatic Compounds; Wiley: New York, NY, USA, 1951. [Google Scholar]
- Ghule, N.V.; La, D.D.; Bhosale, R.S.; Al Kobaisi, M.; Raynor, A.M.; Bhosale, S.V.; Bhosale, S.V. Effect of amine hydrogen bonding interaction on supramolecular self-assembly of naphthalene diimide amphiphiles with aggregation induced emission. ChemistryOpen 2016, 5, 157–163. [Google Scholar] [CrossRef]
- Gao, X.; Qiu, W.; Yang, X.; Liu, Y.; Wang, Y.; Zhang, H.; Qi, T.; Liu, Y.; Lu, K.; Du, C.; et al. First synthesis of 2,3,6,7-tetrabromonaphthalene diimide. Org. Lett. 2007, 9, 3917–3920. [Google Scholar] [CrossRef]
- Barros, T.C.; Brochsztain, S.; Toscano, V.G.; Filhoh, P.B.; Politi, M.J. Photophysical characterization of a 1,4,5,8-naphthalenediimide derivative. J. Photoch. Photobio. A 1997, 111, 97–104. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Awartani, O.; Zhao, J.; Han, H.; Ade, H.; Zhao, D.; Yan, H. A Vinylene-Bridged Perylenediimide-Based Polymeric Acceptor Enabling Efficient All-Polymer Solar Cells Processed under Ambient Conditions. Adv. Mater. 2016, 28, 8483–8489. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.P.J.; Anthopoulos, T.D.; Samuel, I.D.W. Nondispersive hole transport in a spin-coated dendrimer film measured by the charge-generation-layer time-of-flight method. Appl. Phys. Lett. 2002, 81, 3266–3268. [Google Scholar] [CrossRef]
- Yu, Z.G.; Smith, D.I.; Saxena, A.; Martin, R.L.; Bishop, A.R. Molecular Geometry Fluctuation Model for the Mobility of Conjugated Polymers. Phys. Rev. Lett. 2000, 84, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Bayan, S.; Mohanta, D. Significant Fowler-Nordheim tunneling across ZnO-Nanorod based nanojunctions for nanoelectronic device applications. Curr. Appl. Phys. 2013, 13, 705–709. [Google Scholar] [CrossRef]
- Ichikawa, M.; Iwasaki, K.; Ohyama, A.; Miyazawa, J. Comparative study of long alkyl chain substituted naphthalene diimide derivatives as n-type organic thin-film transistor materials. Jpn. J. Appl. Phys. 2017, 56, 111601. [Google Scholar] [CrossRef]
- Govor, L.V.; Reiter, G.; Parisi, J. Tunneling of charge carriers across a gold-squaraine interface. Phys. Lett. A. 2016, 380, 1493–1498. [Google Scholar] [CrossRef]
- Hu, Z.; Zhong, Z.; Zhang, K.; Hu, Z.; Song, C.; Huang, F.; Peng, J.; Wang, J.; Cao, Y. Dipole formation at organic/metal interfaces with pre-deposited and post-deposited metal. NPG Asia Mater. 2017, 9, e379. [Google Scholar] [CrossRef]
- Scott, J.C. Metal–organic interface and charge injection in organic electronic devices. J. Vac. Sci. Technol. A 2003, 21, 521–531. [Google Scholar] [CrossRef]
- Stanculescu, F.; Rasoga, O.; Catargiu, A.M.; Vacareanu, L.; Socol, M.; Breazu, C.; Preda, N.; Socol, G.; Stanculescu, A. MAPLE prepared heterostructures with arylene based polymer active layer for photovoltaic applications. Appl. Surf. Sci. 2015, 336, 240–248. [Google Scholar] [CrossRef]
- Stanculescu, A.; Socol, G.; Vacareanu, L.; Socol, M.; Rasoga, O.; Breazu, C.; Girtan, M.; Stanculescu, F. MAPLE preparation and characterization of mixed arylenevinylene based oligomers:C60 layers. Appl. Surf. Sci. 2016, 374, 278–289. [Google Scholar] [CrossRef]
- Stanculescu, A.; Rasoga, O.; Socol, M.; Vacareanu, L.; Grigoras, M.; Socol, G.; Stanculescu, F.; Breazu, C.; Matei, E.; Preda, N.; et al. MAPLE prepared heterostructures with oligoazomethine: Fullerene derivative mixed layer for photovoltaic appli-cations. Appl. Surf. Sci. 2017, 417, 183–195. [Google Scholar] [CrossRef]
- Stanculescu, A.; Socol, G.; Grigoras, M.; Ivan, T.; Vacareanu, L.; Socol, M.; Rasoga, O.; Breazu, C.; Mihailescu, I.N.; Iordache, I.; et al. Laser prepared organic heterostructures based on star-shaped, arylenevinylene compounds. Appl. Phys. A 2014, 117, 261–268. [Google Scholar] [CrossRef]
- Stanculescu, A.; Breazu, C.; Socol, M.; Rasoga, O.; Preda, N.; Petre, G.; Solonaru, A.M.; Grigoras, M.; Stanculescu, F.; Socol, G.; et al. Effect of ITO electrode patterning on the properties of organic heterostructures based on non-fullerene acceptor prepared by MAPLE. Appl. Surf. Sci. 2020, 509, 145351. [Google Scholar] [CrossRef]
- Petre, G.; Antohe, S.; Socol, M.; Iftimie, S.; Preda, N.; Socol, G.; Breazu, C.; Rasoga, O.; Stanculescu, A. Organic heterostructures based on thermal evaporated phthalocyanine and porphyrin as mixed (ZnPc:TPyP) or stacked (ZnPc/TPyP) films. Thin Solid Films 2023, 787, 140140. [Google Scholar] [CrossRef]
- Nazarova, B.; Krinichnyi, V.I.; Goldenberg, L.M. Schottky diodes based on poly(p-phenylene) and poly(1,4-dipyrrolobenzene). Syn. Met. 1993, 53, 399–402. [Google Scholar] [CrossRef]
- Yağlioğlu, E.; Tűzűn Őzmen, Ő. F4-TCNQ concentration dependence of the current-voltage characteristics in the Au/P3HT:PCBM:F4-TCNQ/n-Si (MPS) Schottky barrier diode. Chin. Phys. B 2014, 23, 117306. [Google Scholar] [CrossRef]
- Ferchichi, K.; Pecqueur, S.; Guerin, D.; Bourguiga, R.; Lmimouni, K. High Rectification Ratio in Polymer Diode Rectifier through Interface Engineering with Self-Assembled Monolayer. Electron. Mater. 2021, 2, 445–453. [Google Scholar] [CrossRef]
- Li, T.; Bandari, V.K.; Hantusch, M.; Xin, J.; Kuhrt, R.; Ravishankar, R.; Xu, L.; Zhang, J.; Knupfer, M.; Zhu, F.; et al. Integrated molecular diode as 10 MHz half-wave rectifier based on an organic nanostructure heterojunction. Nat. Commun. 2020, 11, 3592. [Google Scholar] [CrossRef] [PubMed]
- Memela, M.J.; Feleni, U.; Mdluli, S.; Ramoroka, M.E.; Ekwere, P.; Douman, S.; Iwuoha, E. Electro-photovoltaics of polymer-stabilized copper-indium-selenide quantum dots. Electroanalysis 2020, 32, 3086–3097. [Google Scholar] [CrossRef]
- Kobaisi, M.A.; Bhosale, S.V.; Latham, K.; Raynor, A.M.; Bhosale, S.V. Functional naphthalene diimides: Synthesis, properties and applications. Chem. Rev. 2016, 116, 11685–11796. [Google Scholar] [CrossRef]
- Kumari, N.; Naqvi, S.; Ahuja, M.; Bhardwaj, K.; Kumar, R. Facile synthesis of naphthalene diimide (NDI) derivatives: Aggregation-induced emission, photophysical and transport properties. J. Mater. Sci.-Mater. El. 2020, 31, 4310–4322. [Google Scholar] [CrossRef]
- Breazu, C.; Socol, M.; Preda, N.; Matei, E.; Rasoga, O.; Girtan, M.; Mallet, R.; Stanculescu, F.; Stanculescu, A. On the properties of organic heterostructures prepared with nano-patterned metallic electrode. Appl. Surf. Sci. 2018, 443, 592–602. [Google Scholar] [CrossRef]
- Stanculescu, A.; Socol, M.; Rasoga, O.; Breazu, C.; Preda, N.; Stanculescu, F.; Socol, G.; Vacareanu, L.; Girtan, M.; Doroshkevich, A.S. Arylenevinylene oligomer-based heterostructures on flexible AZO electrodes. Materials 2021, 14, 7688. [Google Scholar] [CrossRef]

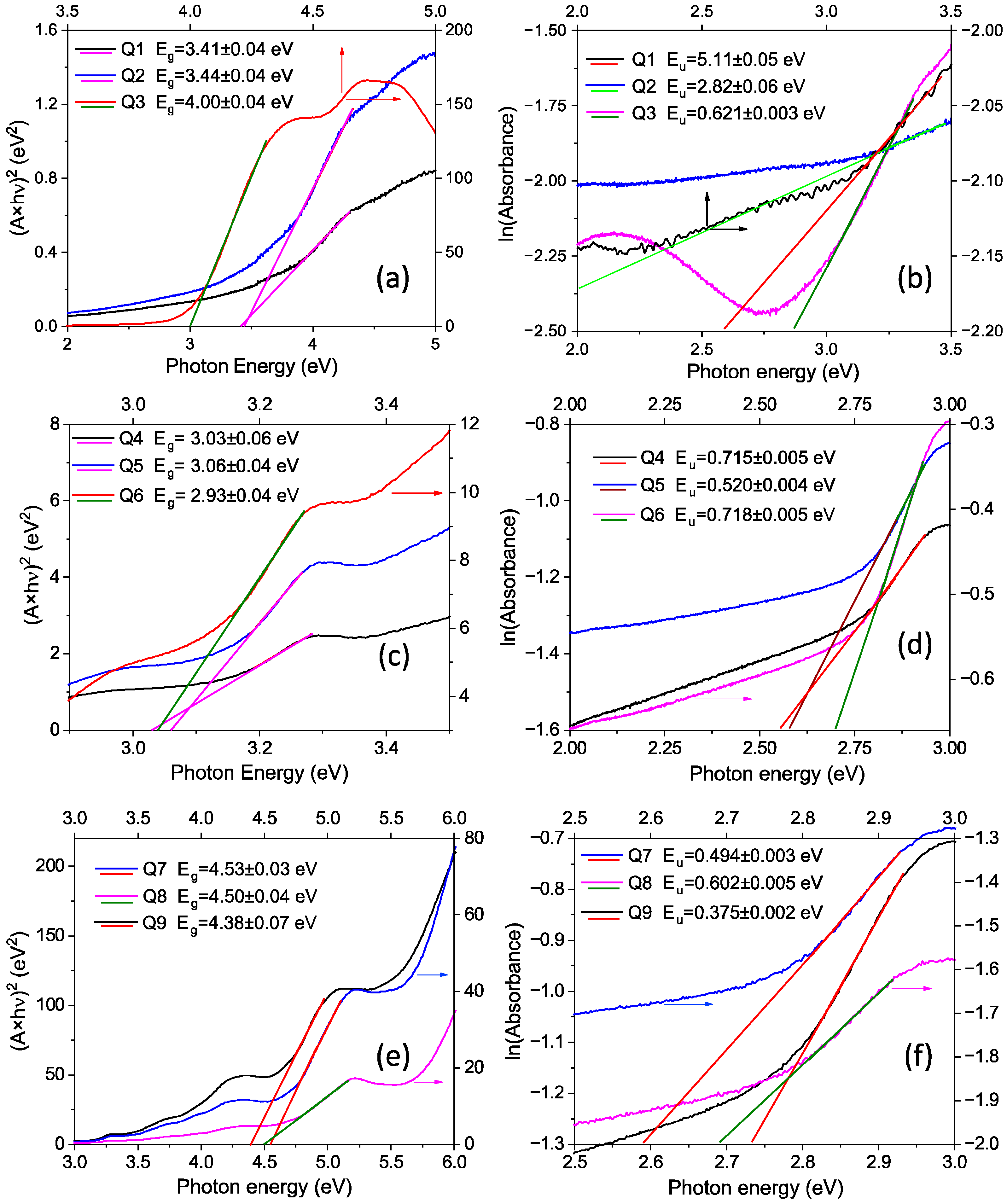
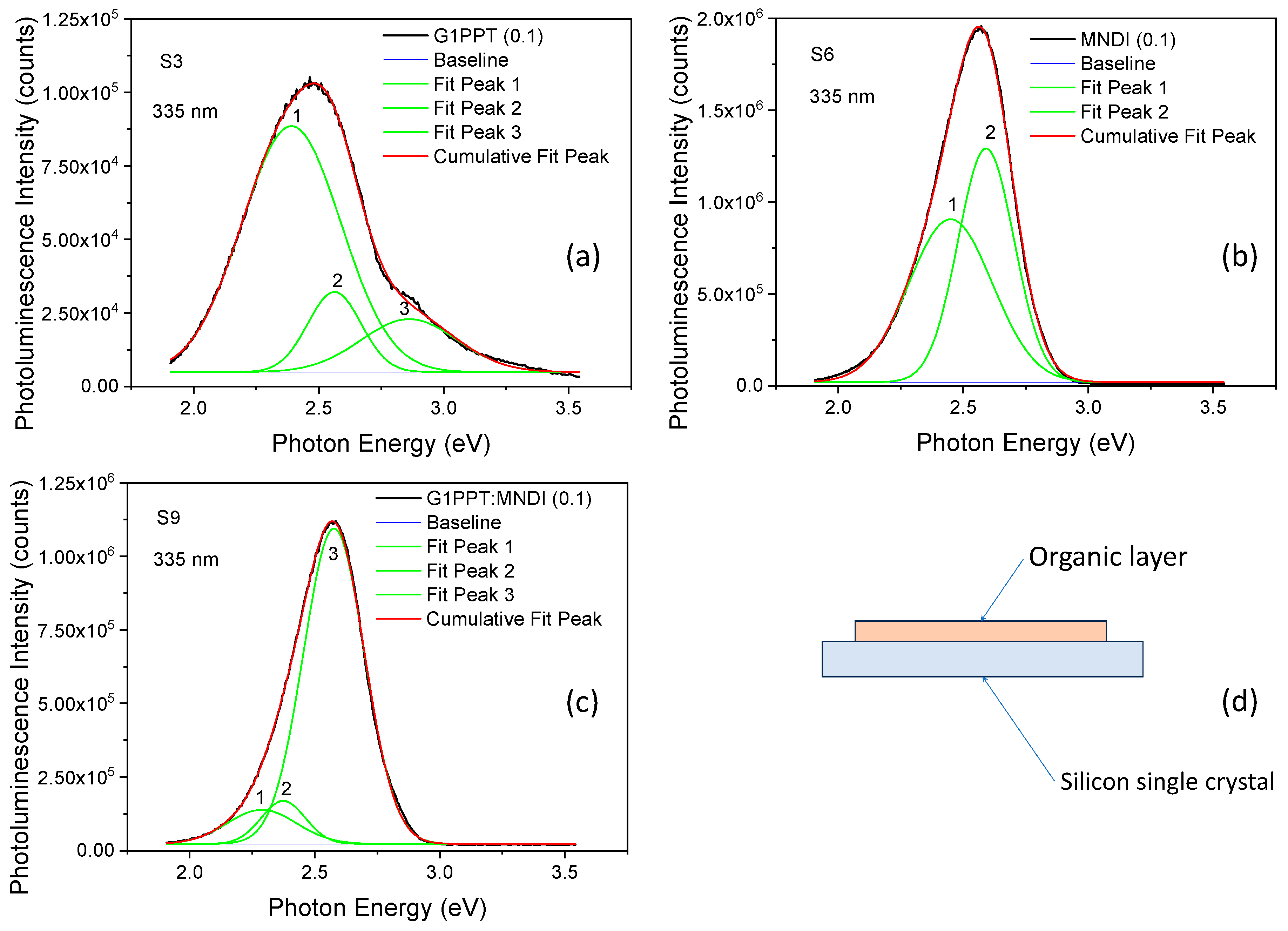

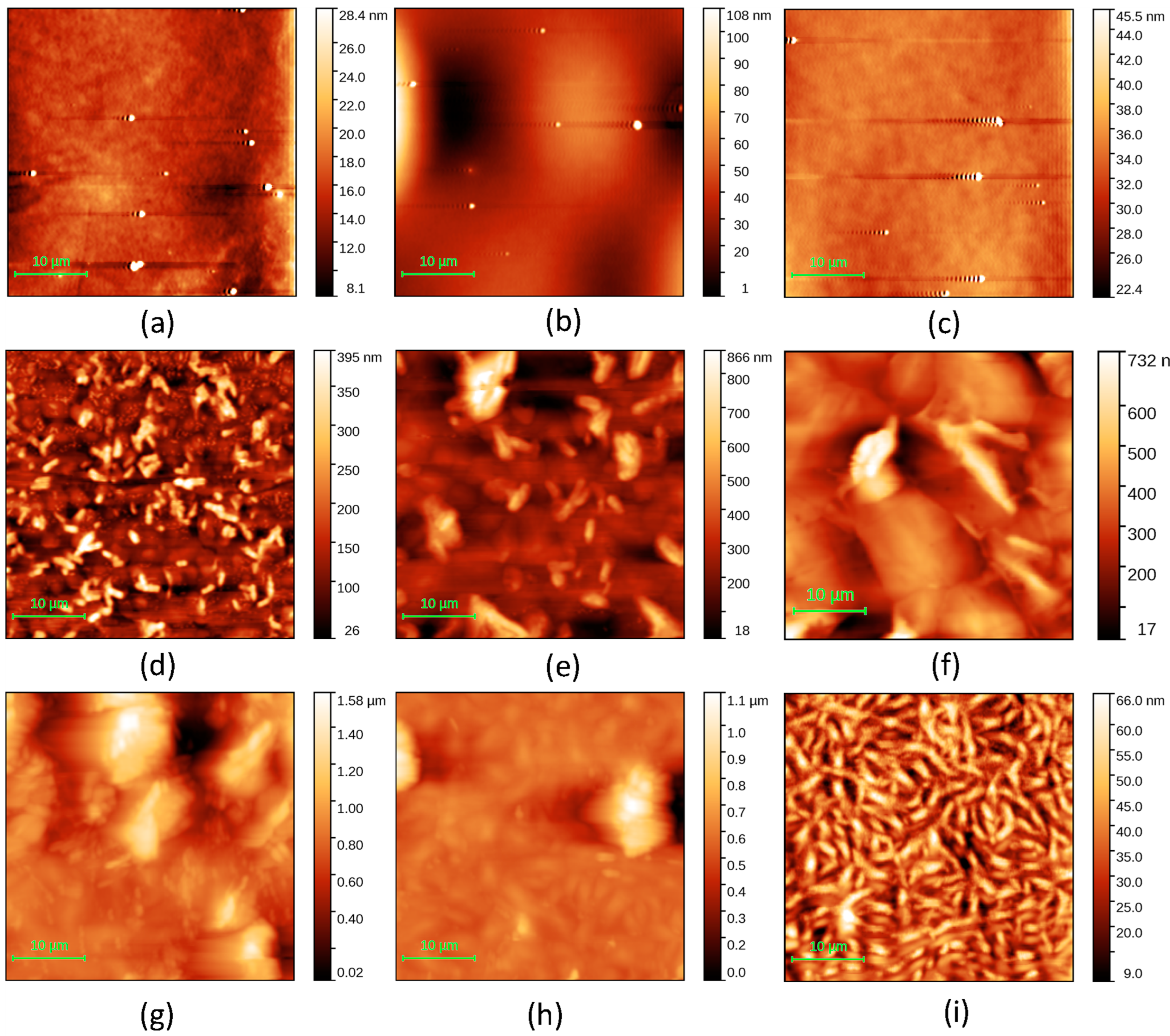



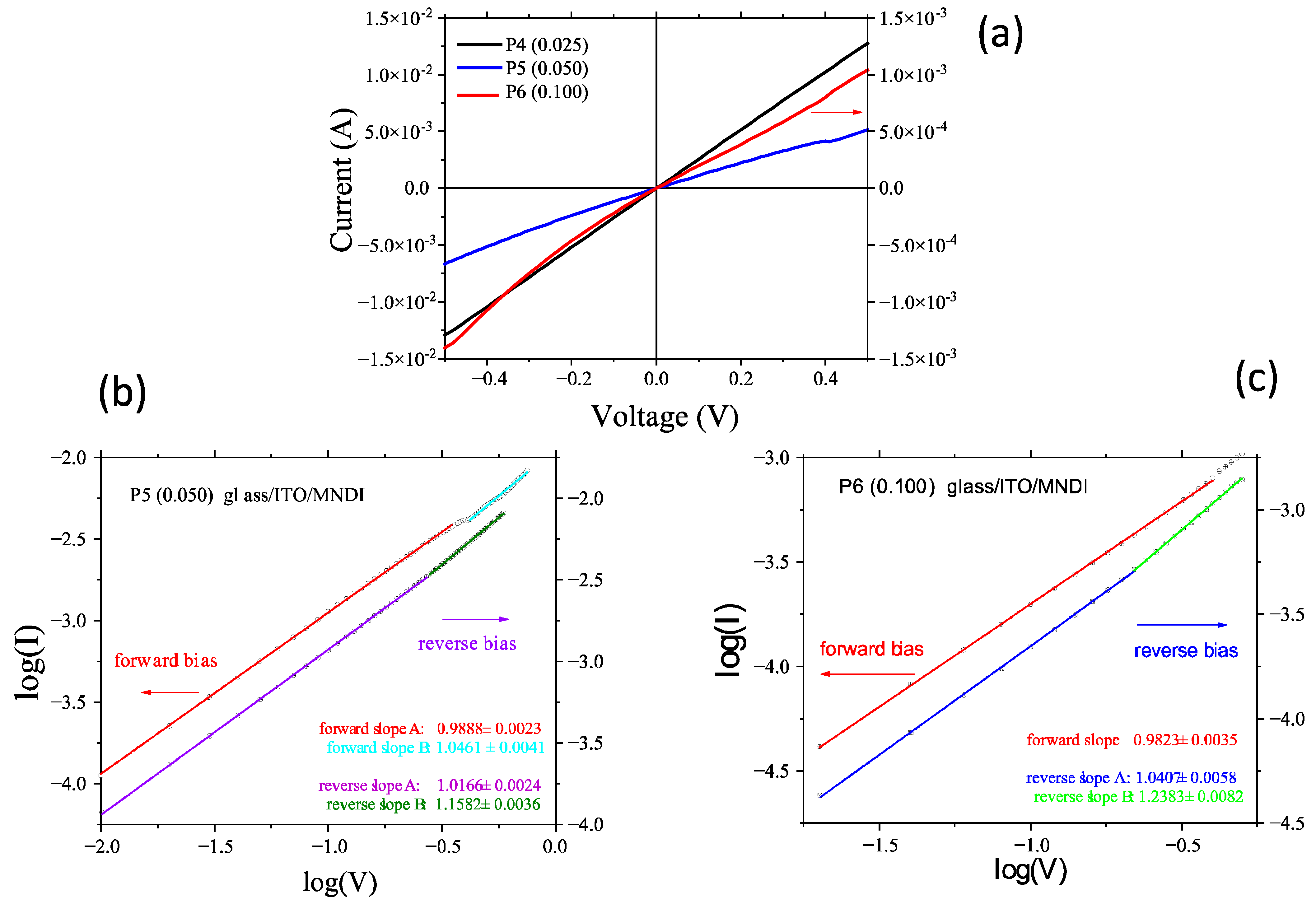

| Code | Structure | Eg (eV) | Eu (eV) |
|---|---|---|---|
| Q1 | Quartz/G1PPT (0.025 g/2 mL) | 3.41 ± 0.04 | 5.10 ± 0.05 |
| Q2 | Quartz/G1PPT (0.05 g/2 mL) | 3.44 ± 0.04 | 2.82 ± 0.06 |
| Q3 | Quartz/G1PPT (0.1 g/2 mL) | 4.00 ± 0.04 | 0.621 ± 0.003 |
| Q4 | Quartz/MNDI (0.025 g/2 mL) | 3.03 ± 0.06 | 0.715 ± 0.005 |
| Q5 | Quartz/MNDI (0.05 g/2 mL) | 3.06 ± 0.04 | 0.520 ± 0.004 |
| Q6 | Quartz/MNDI (0.1 g/2 mL) | 2.93 ± 0.04 | 0.718 ± 0.005 |
| Q7 | Quartz/G1PPT (0.025 g/2 mL):MNDI (0.025 g/2 mL) | 4.53 ± 0.03 | 0.494 ± 0.003 |
| Q8 | Quartz/G1PPT (0.05 g/2 mL):MNDI (0.05 g/2 mL) | 4.50 ± 0.04 | 0.602 ± 0.005 |
| Q9 | Quartz/G1PPT (0.1 g/2 mL):MNDI (0.1 g/2 mL) | 4.38 ± 0.07 | 0.375 ± 0.002 |
| Code | Structure | E (eV) | FWHM (eV) | Peak Intensity (Count) |
|---|---|---|---|---|
| S1 | G1PPT (0.025) | 2.41; 2.57; 2.88 | 0.47; 0.25; 0.43 | 2.26 × 104; 1.059 × 104; 0.991 × 104 |
| S2 | G1PPT (0.05) | 2.40; 2.57; 2.89 | 0.50; 0.25; 0.39 | 3.42 × 104; 1.115 × 104; 0.864 × 104 |
| S3 | G1PPT (0.1) | 2.39; 2.56; 2.86 | 0.47; 0.25; 0.45 | 8.36 × 104; 2.72 × 104; 1.799 × 104 |
| S4 | MNDI (0.025) | 2.42; 2.58 | 0.44; 0.26 | 2.09 × 105; 2.59 × 105 |
| S5 | MNDI (0.05) | 2.44; 2.58 | 0.44; 0.27 | 3.82 × 105; 5.59 × 105 |
| S6 | MNDI (0.1) | 2.46; 2.59 | 0.40; 0.26 | 8.87 × 105; 12.71 × 105 |
| S7 | G1PPT:MNDI (0.025) | 2.30; 2.37; 2.57 | 0.33; 0.21; 0.29 | 2.71 × 105; 3.12 × 105; 22.2 × 105 |
| S8 | G1PPT:MNDI (0.05) | 2.30; 2.37; 2.57 | 0.35; 0.21; 0.29 | 2.07 × 105; 2.22 × 105; 15.33 × 105 |
| S9 | G1PPT:MNDI (0.1) | 2.29; 2.37; 2.58 | 0.32; 0.21; 0.29 | 1.158 × 105; 1.461 × 105; × 10.71 × 105 |
| Code | Sample | RMS (nm) | RA (nm) |
|---|---|---|---|
| P1 | Glass/ITO/G1PPT (0.025 g/2 mL) | 3.77 | 1.83 |
| P2 | Glass/ITO/G1PPT (0.05 g/2 mL) | 37.4 | 23.1 |
| P3 | Glass/ITO/G1PPT (0.1 g/2 mL) | 4.89 | 2.16 |
| P4 | Glass/ITO/MNDI (0.025 g/2 mL) | 69.7 | 52.0 |
| P5 | Glass/ITO/MNDI (0.05 g/2 mL) | 132.4 | 92.8 |
| P6 | Glass/ITO/MNDI (0.1 g/2 mL) | 157.5 | 108.0 |
| P7 | Glass/ITO/G1PPT (0.025 g/2 mL):MNDI (0.025 g/2 mL) | 265 | 201 |
| P8 | Glass/ITO/G1PPT (0.05 g/2 mL):MNDI (0.05 g/2 mL) | 110.9 | 70.3 |
| P9 | Glass/ITO/G1PPT (0.1 g/2 mL):MNDI (0.1 g/2 mL) | 11.76 | 9.33 |
| Code | Heterostructure | Voltage (V) | Ideality Factor (n) |
|---|---|---|---|
| P7 | Glass/ITO/G1PPT:MNDI (0.025)/Au | <0.025 | n < 1 |
| 0.025–0.05 | 1 < n < 2 | ||
| 0.05–0.1 | 2 < n < 3 | ||
| P8 | Glass/ITO/G1PPT:MNDI (0.05)/Au | ≤0.025 | n < 1 |
| 0.025–0.05 | 1 < n < 1.5 | ||
| 0.05–0.1 | 1.5 < n < 3 | ||
| P9 | Glass/ITO/G1PPT:MNDI (0.1)/Au | ≤0.025 | n < 1 |
| 0.025–0.05 | 1 < n < 1.5 | ||
| 0.05–0.1 | 1.5 < n < 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasoga, O.; Yonkeu, A.L.D.; Breazu, C.; Socol, M.; Preda, N.; Stanculescu, F.; Stanculescu, A.; Iwuoha, E. Organic Heterostructures with Dendrimer Based Mixed Layer for Electronic Applications. Molecules 2024, 29, 4155. https://doi.org/10.3390/molecules29174155
Rasoga O, Yonkeu ALD, Breazu C, Socol M, Preda N, Stanculescu F, Stanculescu A, Iwuoha E. Organic Heterostructures with Dendrimer Based Mixed Layer for Electronic Applications. Molecules. 2024; 29(17):4155. https://doi.org/10.3390/molecules29174155
Chicago/Turabian StyleRasoga, Oana, Anne Lutgarde Djoumessi Yonkeu, Carmen Breazu, Marcela Socol, Nicoleta Preda, Florin Stanculescu, Anca Stanculescu, and Emmanuel Iwuoha. 2024. "Organic Heterostructures with Dendrimer Based Mixed Layer for Electronic Applications" Molecules 29, no. 17: 4155. https://doi.org/10.3390/molecules29174155
APA StyleRasoga, O., Yonkeu, A. L. D., Breazu, C., Socol, M., Preda, N., Stanculescu, F., Stanculescu, A., & Iwuoha, E. (2024). Organic Heterostructures with Dendrimer Based Mixed Layer for Electronic Applications. Molecules, 29(17), 4155. https://doi.org/10.3390/molecules29174155








