Neuroprotective Effect of Flavonoid Agathisflavone in the Ex Vivo Cerebellar Slice Neonatal Ischemia
Abstract
1. Introduction
2. Results
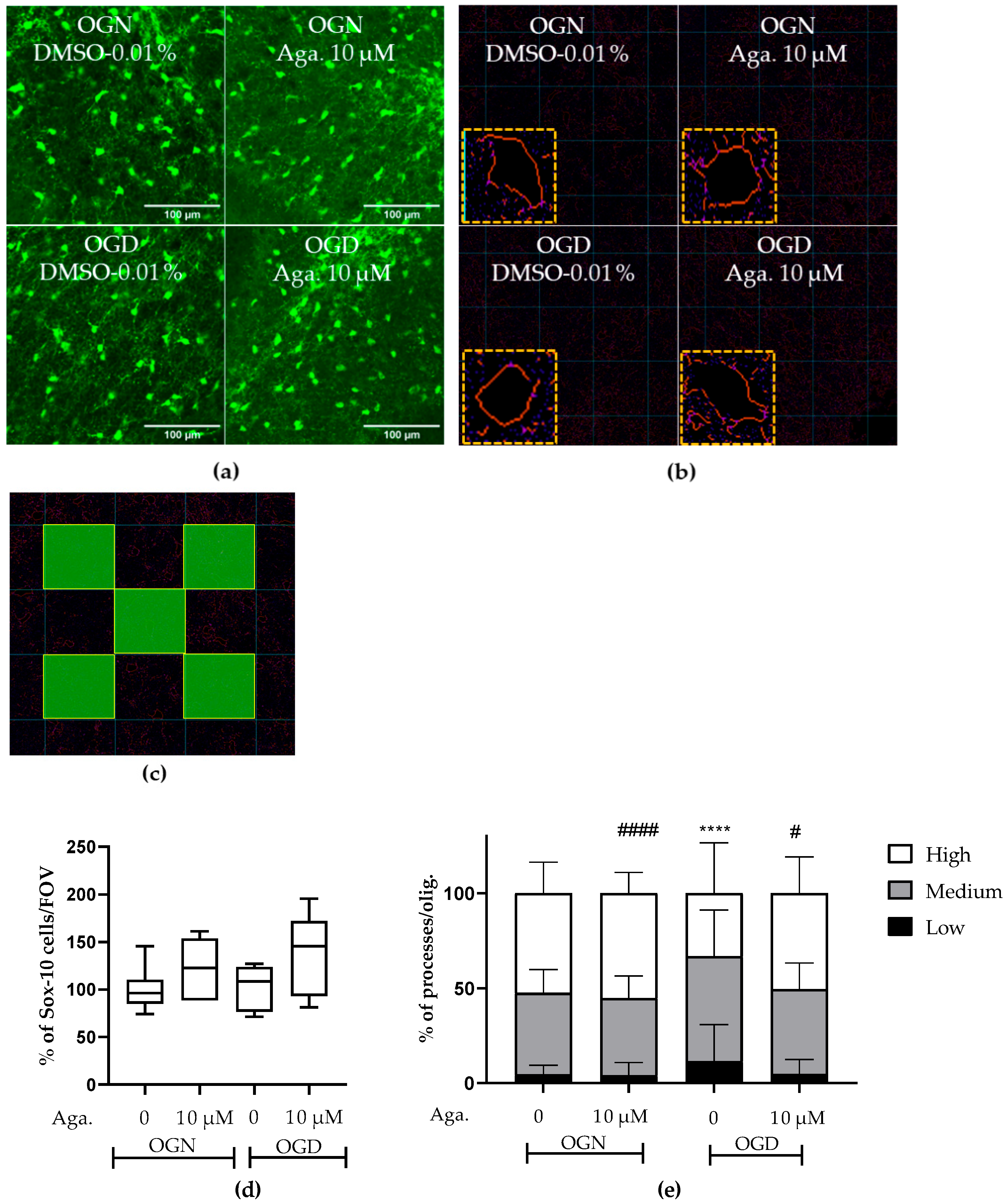
2.1. The Flavonoid Agathisflavone Prevented the Loss of Oligodendrocyte Processes
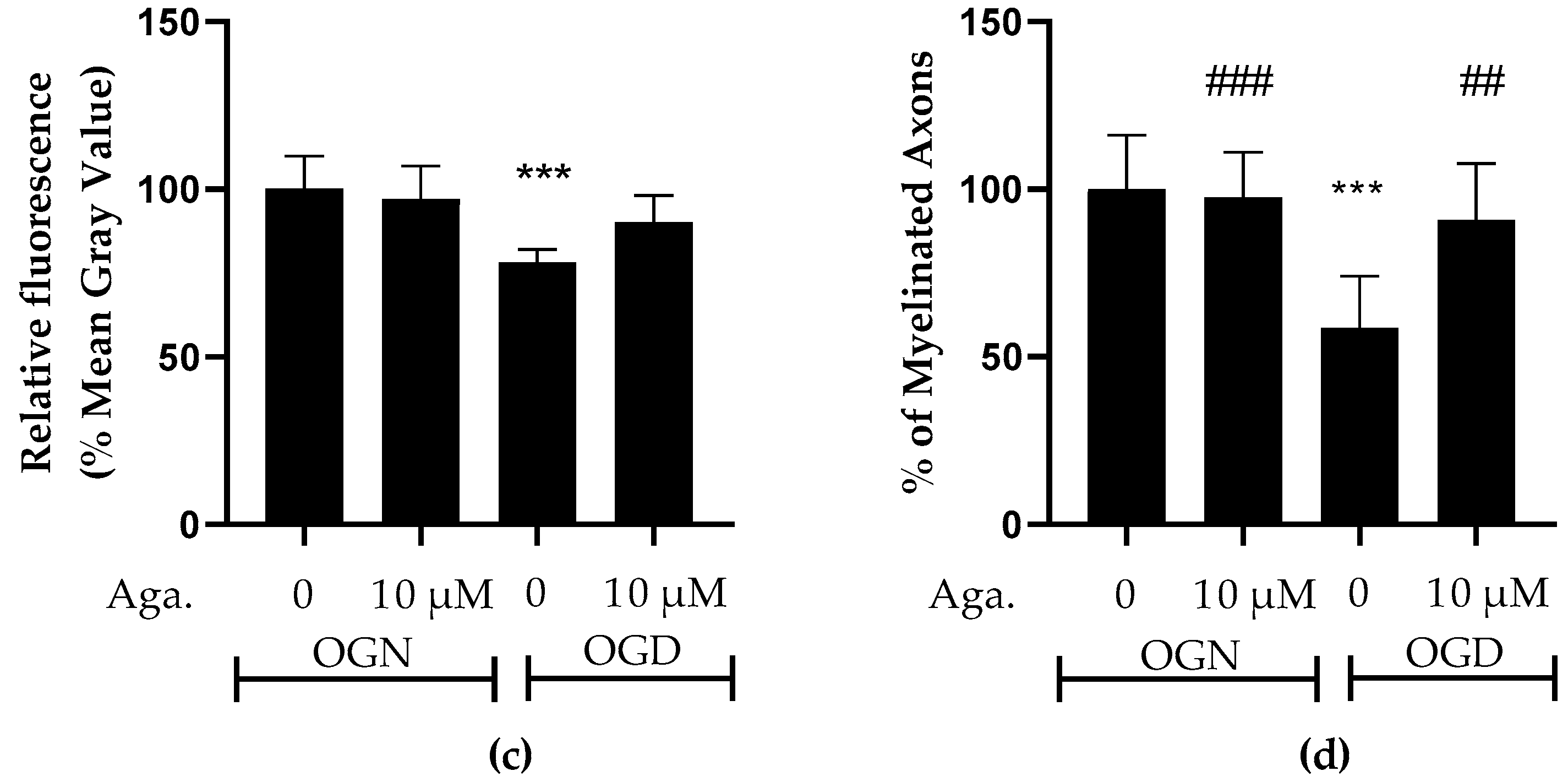
2.2. Agathisflavone Prevented White Matter Loss in Cerebellar Slices Subjected to Ischemic Damage
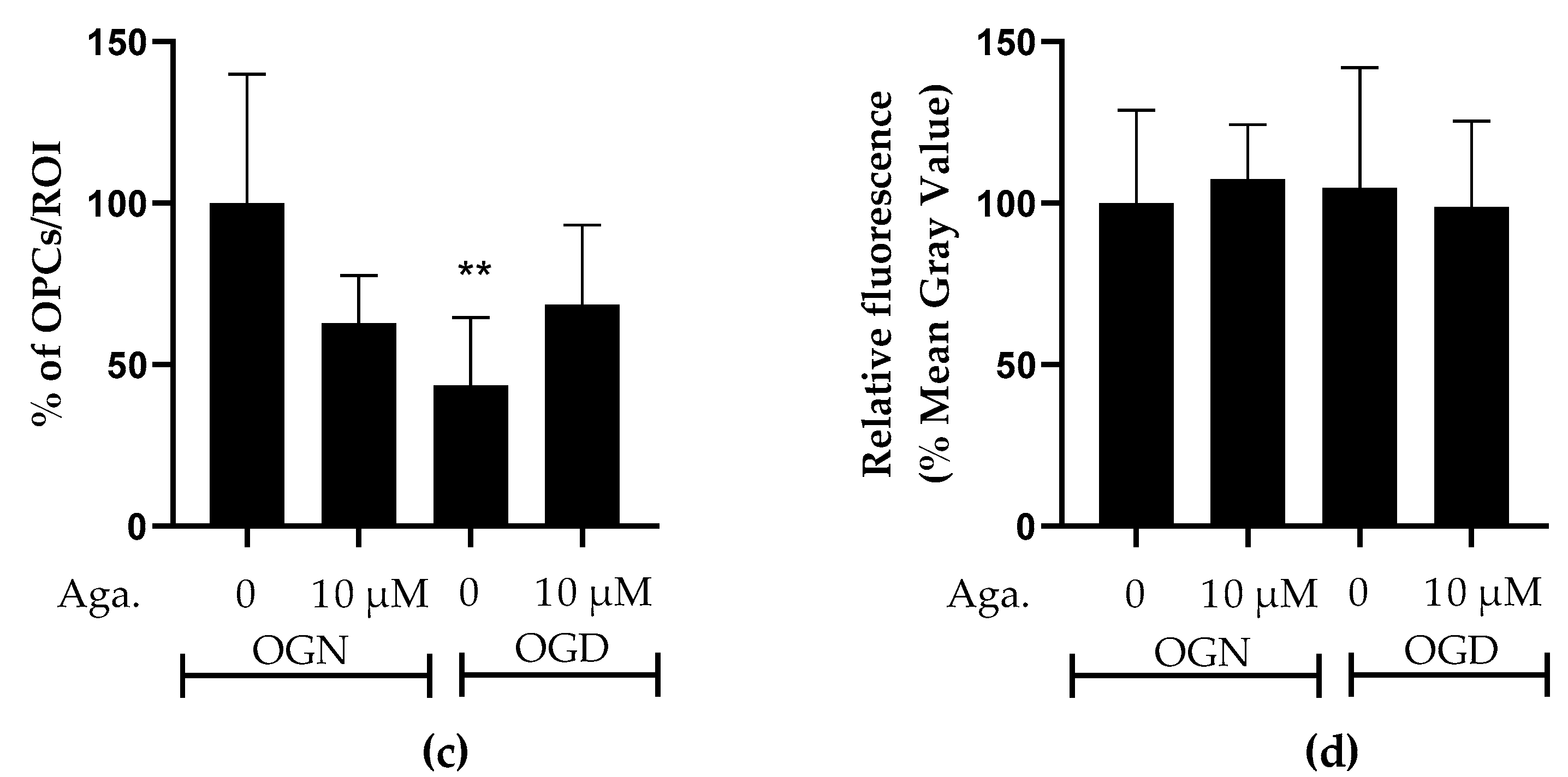
2.3. Agathisflavone Prevents the Loss of OPCs in the Molecular Layer in Cerebellar Slices Subjected to Ischemic Damage
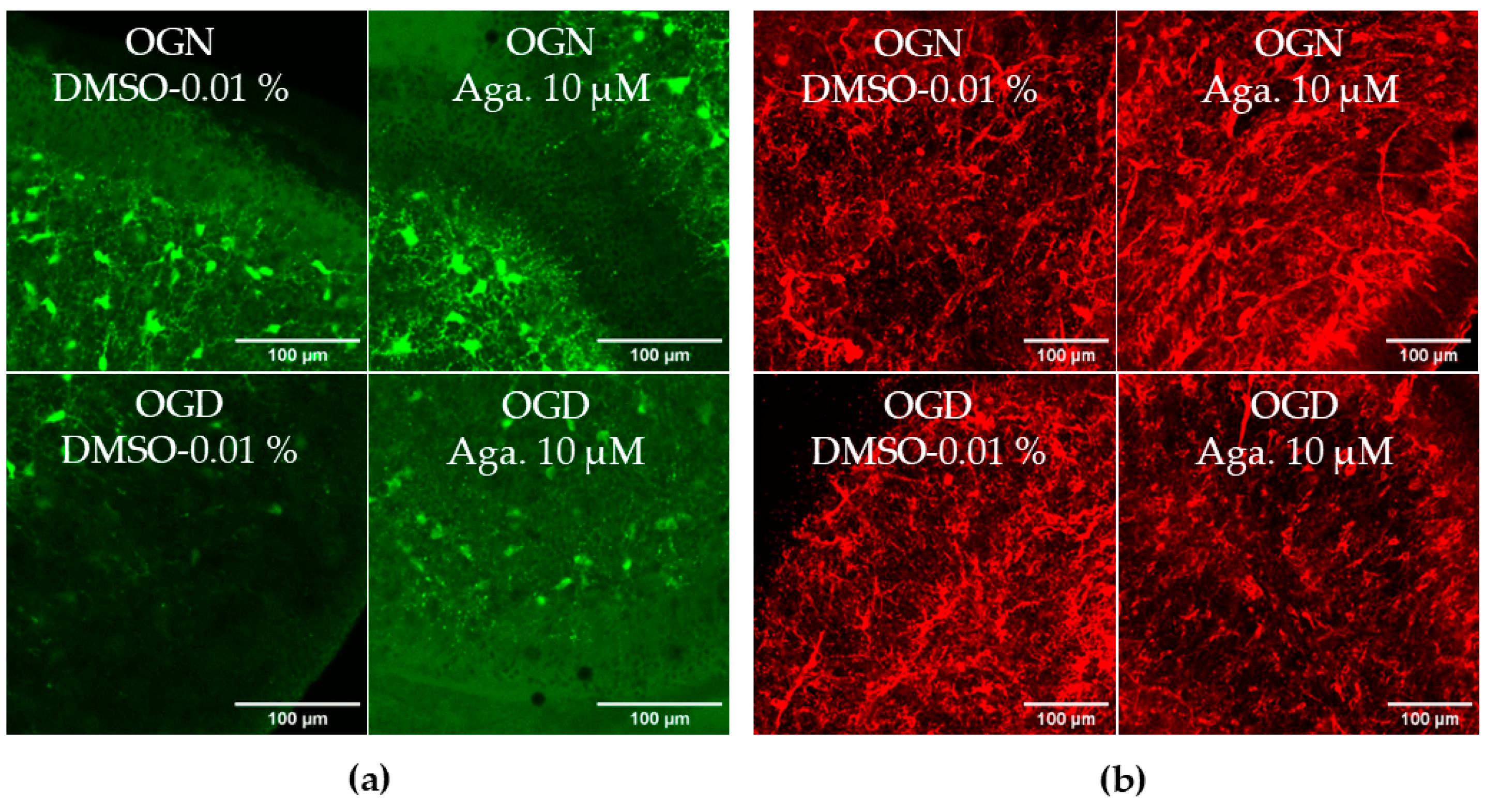
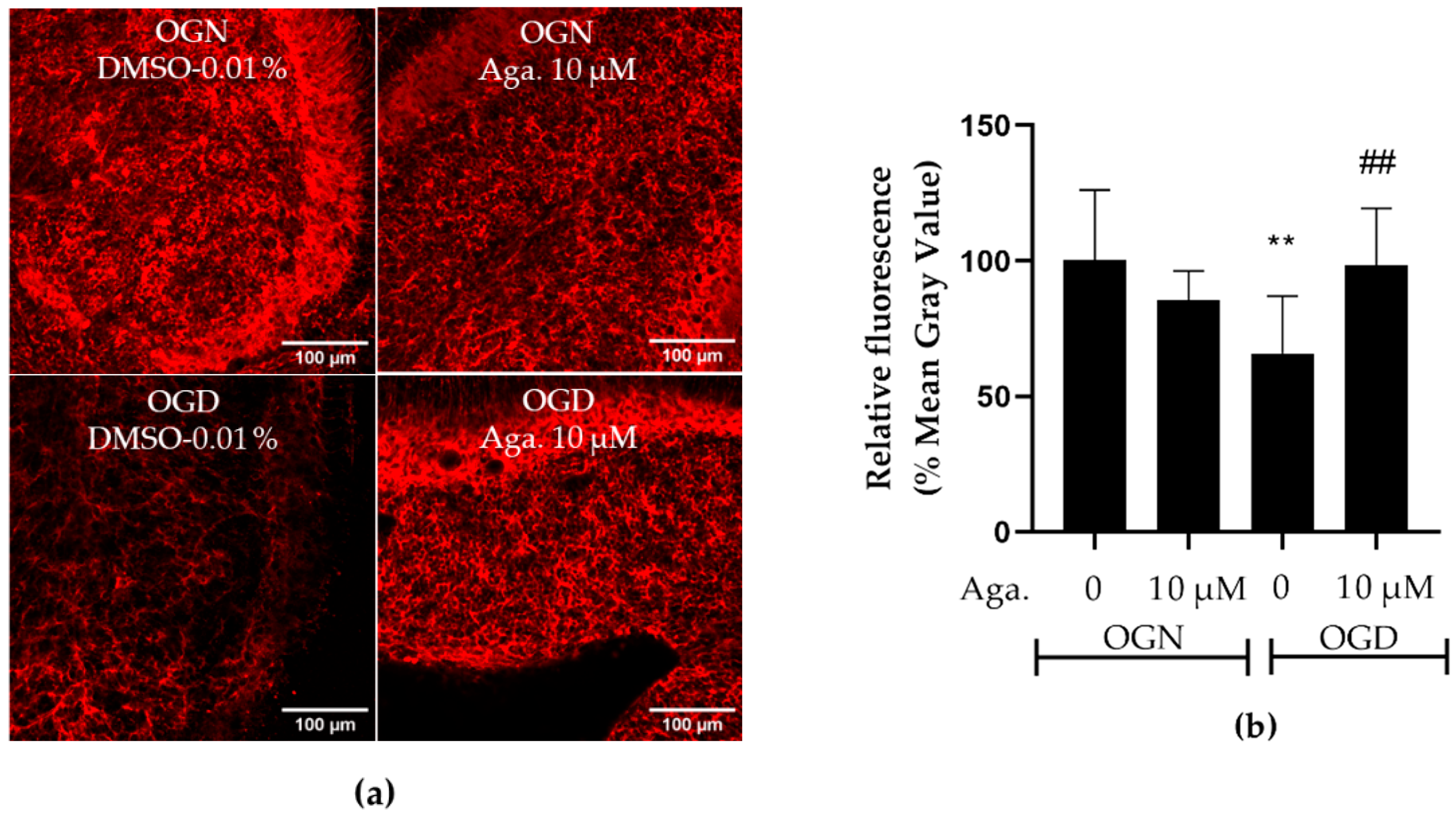
2.4. Flavonoid Agathisflavone Prevented Astrocyte Reactivity in Acute Ischemic Damage in Cerebellar Slices
2.5. Agathisflavone Prevented the Loss of Glutamine Synthetase Expression in Ischemic Damage in Cerebellar Slices
2.6. Agathisflavone Protects Purkinje Cells from Acute Ischemic Damage
2.7. The Expression Profile of Cytokines Related to the Inflammatory Response
3. Discussion
4. Materials and Methods
4.1. Agathisflavone
4.2. Animals and Tissue
4.3. Ex Vivo Cerebellar Slice Preparation and Oxygen–Glucose Deprivation (OGD)
4.4. Immunofluorescence Labeling
4.5. Image Acquisition and Analysis
4.6. Quantitative RT-PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, X.; De Silva, T.M.; Chen, J.; Faraci, F.M. Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke. Circ. Res. 2017, 120, 449–471. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Butt, A.M. Chapter 10—Inflammatory diseases of the CNS. In Neuroglia; Verkhratsky, A., Butt, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 533–561. [Google Scholar] [CrossRef]
- Shin, T.H.; Lee, D.Y.; Basith, S.; Manavalan, B.; Paik, M.J.; Rybinnik, I.; Mouradian, M.M.; Ahn, J.H.; Lee, G. Metabolome Changes in Cerebral Ischemia. Cells 2020, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chopp, M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016, 144, 103–120. [Google Scholar] [CrossRef]
- Choudhury, G.R.; Ding, S. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol. Dis. 2016, 85, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Lambertsen, K.L.; Biber, K.; Finsen, B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 2012, 32, 1677–1698. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.S.; Ribeiro, P.R.; Silva, J.; Hoppe, J.B.; de Almeida, M.M.A.; de Lima Ferreira, B.C.; Andrade, G.B.; de Souza, S.B.; Ferdandez, L.G.; de Fátima Dias Costa, M.; et al. Amburana cearensis seed extract stimulates astrocyte glutamate homeostatic mechanisms in hippocampal brain slices and protects oligodendrocytes against ischemia. BMC Complement. Med. Ther. 2023, 23, 154. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.G.; Fern, R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature 2005, 438, 1167–1171. [Google Scholar] [CrossRef]
- Micu, I.; Jiang, Q.; Coderre, E.; Ridsdale, A.; Zhang, L.; Woulfe, J.; Yin, X.; Trapp, B.D.; McRory, J.E.; Rehak, R.; et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 2006, 439, 988–992. [Google Scholar] [CrossRef]
- Caprariello, A.V.; Mangla, S.; Miller, R.H.; Selkirk, S.M. Apoptosis of oligodendrocytes in the central nervous system results in rapid focal demyelination. Ann. Neurol. 2012, 72, 395–405. [Google Scholar] [CrossRef]
- Shi, H.; Hu, X.; Leak, R.K.; Shi, Y.; An, C.; Suenaga, J.; Chen, J.; Gao, Y. Demyelination as a rational therapeutic target for ischemic or traumatic brain injury. Exp. Neurol. 2015, 272, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Baldassarro, V.A.; Marchesini, A.; Giardino, L.; Calzà, L. Differential effects of glucose deprivation on the survival of fetal versus adult neural stem cells-derived oligodendrocyte precursor cells. Glia 2020, 68, 898–917. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Rosenberg, P.A.; Volpe, J.J.; Jensen, F.E. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc. Natl. Acad. Sci. USA 2003, 100, 6801–6806. [Google Scholar] [CrossRef]
- Dennis, S.H.; Jaafari, N.; Cimarosti, H.; Hanley, J.G.; Henley, J.M.; Mellor, J.R. Oxygen/glucose deprivation induces a reduction in synaptic AMPA receptors on hippocampal CA3 neurons mediated by mGluR1 and adenosine A3 receptors. J. Neurosci. 2011, 31, 11941–11952. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Diplock, A.T.; Charleux, J.L.; Crozier-Willi, G.; Kok, F.J.; Rice-Evans, C.; Roberfroid, M.; Stahl, W.; Viña-Ribes, J. Functional food science and defence against reactive oxidative species. Br. J. Nutr. 1998, 80 (Suppl. 1), S77–S112. [Google Scholar] [CrossRef]
- Costa, S.L.; Silva, V.D.A.; dos Santos Souza, C.; Santos, C.C.; Paris, I.; Muñoz, P.; Segura-Aguilar, J. Impact of Plant-Derived Flavonoids on Neurodegenerative Diseases. Neurotox. Res. 2016, 30, 41–52. [Google Scholar] [CrossRef]
- de Sousa, L.M.S.; Santos, B.N.G.; Medeiros, M.; Lima, I.B.C.; Santos-Filho, F.S.; Santana, A.; Moreno, L.; Nunes, L.C.C. Poincianella pyramidalis (Tul) L.P. Queiroz: A review on traditional uses, phytochemistry and biological-pharmacological activities. J. Ethnopharmacol. 2021, 264, 113181. [Google Scholar] [CrossRef]
- do Nascimento, R.P.; Dos Santos, B.L.; Amparo, J.A.O.; Soares, J.R.P.; da Silva, K.C.; Santana, M.R.; Almeida, Á.M.A.N.; da Silva, V.D.A.; Costa, M.F.D.; Ulrich, H.; et al. Neuroimmunomodulatory Properties of Flavonoids and Derivates: A Potential Action as Adjuvants for the Treatment of Glioblastoma. Pharmaceutics 2022, 14, 116. [Google Scholar] [CrossRef]
- Dos Santos, B.L.; Dos Santos, C.C.; Soares, J.R.P.; da Silva, K.C.; de Oliveira, J.V.R.; Pereira, G.S.; de Araújo, F.M.; Costa, M.F.D.; David, J.M.; da Silva, V.D.A.; et al. The Flavonoid Agathisflavone Directs Brain Microglia/Macrophages to a Neuroprotective Anti-Inflammatory and Antioxidant State via Regulation of NLRP3 Inflammasome. Pharmaceutics 2023, 15, 1410. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.M.A.; Pieropan, F.; de Mattos Oliveira, L.; Dos Santos Junior, M.C.; David, J.M.; David, J.P.; da Silva, V.D.A.; Dos Santos Souza, C.; Costa, S.L.; Butt, A.M. The flavonoid agathisflavone modulates the microglial neuroinflammatory response and enhances remyelination. Pharmacol. Res. 2020, 159, 104997. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.M.A.; Pieropan, F.; Footz, T.; David, J.M.; David, J.P.; da Silva, V.D.A.; Dos Santos Souza, C.; Voronova, A.; Butt, A.M.; Costa, S.L. Agathisflavone Modifies Microglial Activation State and Myelination in Organotypic Cerebellar Slices Culture. J. Neuroimmune Pharmacol. 2022, 17, 206–217. [Google Scholar] [CrossRef]
- de Almeida, M.M.A.; Souza, C.D.S.; Dourado, N.S.; da Silva, A.B.; Ferreira, R.S.; David, J.M.; David, J.P.; Costa, M.F.D.; da Silva, V.D.A.; Butt, A.M.; et al. Phytoestrogen Agathisflavone Ameliorates Neuroinflammation-Induced by LPS and IL-1β and Protects Neurons in Cocultures of Glia/Neurons. Biomolecules 2020, 10, 562. [Google Scholar] [CrossRef]
- Küspert, M.; Hammer, A.; Bösl, M.R.; Wegner, M. Olig2 regulates Sox10 expression in oligodendrocyte precursors through an evolutionary conserved distal enhancer. Nucleic Acids Res. 2011, 39, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Sekimoto, K.; Hayashi, C.; Mabuchi, Y.; Nakamura, T.; Akazawa, C. Differentiation of Oligodendrocyte Precursor Cells from Sox10-Venus Mice to Oligodendrocytes and Astrocytes. Sci. Rep. 2017, 7, 14133. [Google Scholar] [CrossRef]
- Buffo, A.; Rossi, F. Origin, lineage and function of cerebellar glia. Prog. Neurobiol. 2013, 109, 42–63. [Google Scholar] [CrossRef]
- Nishiyama, A.; Komitova, M.; Suzuki, R.; Zhu, X. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009, 10, 9–22. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity from the Perspective of Glial Cells. Front. Cell Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Souza, C.; Grangeiro, M.S.; Lima Pereira, E.P.; dos Santos, C.C.; da Silva, A.B.; Sampaio, G.P.; Ribeiro Figueiredo, D.D.; David, J.M.; David, J.P.; da Silva, V.D.A.; et al. Agathisflavone, a flavonoid derived from Poincianella pyramidalis (Tul.), enhances neuronal population and protects against glutamate excitotoxicity. NeuroToxicology 2018, 65, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Chen, X.; Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment. Int. J. Mol. Med. 2022, 49, 1–9. [Google Scholar] [CrossRef]
- Kook, S.Y.; Jeong, H.; Kang, M.J.; Park, R.; Shin, H.J.; Han, S.H.; Son, S.M.; Song, H.; Baik, S.H.; Moon, M.; et al. Crucial role of calbindin-D28k in the pathogenesis of Alzheimer’s disease mouse model. Cell Death Differ. 2014, 21, 1575–1587. [Google Scholar] [CrossRef]
- Bastianelli, E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum 2003, 2, 242–262. [Google Scholar] [CrossRef] [PubMed]
- Vila, N.; Castillo, J.; Dávalos, A.; Esteve, A.; Planas, A.M.; Chamorro, A. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke 2003, 34, 671–675. [Google Scholar] [CrossRef]
- Dewar, D.; Underhill, S.M.; Goldberg, M.P. Oligodendrocytes and ischemic brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 263–274. [Google Scholar] [CrossRef]
- Molina-Gonzalez, I.; Miron, V.E.; Antel, J.P. Chronic oligodendrocyte injury in central nervous system pathologies. Commun. Biol. 2022, 5, 1274. [Google Scholar] [CrossRef]
- Inoue, M.; Tanida, T.; Kondo, T.; Takenaka, S.; Nakajima, T. Oxygen-glucose deprivation-induced glial cell reactivity in the rat primary neuron-glia co-culture. J. Vet. Med. Sci. 2023, 85, 799–808. [Google Scholar] [CrossRef]
- Gallo, V.; Ghiani, C.A. Glutamate receptors in glia: New cells, new inputs and new functions. Trends Pharmacol. Sci. 2000, 21, 252–258. [Google Scholar] [CrossRef]
- Itoh, T.; Beesley, J.; Itoh, A.; Cohen, A.S.; Kavanaugh, B.; Coulter, D.A.; Grinspan, J.B.; Pleasure, D. AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J. Neurochem. 2002, 81, 390–402. [Google Scholar] [CrossRef]
- Kukley, M. Recent Insights into the Functional Role of AMPA Receptors in the Oligodendrocyte Lineage Cells In Vivo. Int. J. Mol. Sci. 2023, 24, 4138. [Google Scholar] [CrossRef]
- Hardt, S.; Tascio, D.; Passlick, S.; Timmermann, A.; Jabs, R.; Steinhäuser, C.; Seifert, G. Auxiliary Subunits Control Function and Subcellular Distribution of AMPA Receptor Complexes in NG2 Glia of the Developing Hippocampus. Front. Cell Neurosci. 2021, 15, 669717. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, A.; Vidos, C.; Monteverde Busso, M.; Cieri, M.B.; Ramos, A.J. Pathological Neuroinflammatory Conversion of Reactive Astrocytes Is Induced by Microglia and Involves Chromatin Remodeling. Front. Pharmacol. 2021, 12, 689346. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2015, 7, a020420. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, J.; Shao, A.; Zhang, J.H.; Zhang, J. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front. Immunol. 2020, 11, 294. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Reactive astrocytes in neural repair and protection. Neuroscientist 2005, 11, 400–407. [Google Scholar] [CrossRef]
- Rakers, C.; Schleif, M.; Blank, N.; Matušková, H.; Ulas, T.; Händler, K.; Torres, S.V.; Schumacher, T.; Tai, K.; Schultze, J.L.; et al. Stroke target identification guided by astrocyte transcriptome analysis. Glia 2019, 67, 619–633. [Google Scholar] [CrossRef]
- Vanzulli, I.; Butt, A.M. mGluR5 protect astrocytes from ischemic damage in postnatal CNS white matter. Cell Calcium 2015, 58, 423–430. [Google Scholar] [CrossRef]
- Dourado, N.S.; Souza, C.D.S.; De Almeida, M.M.A.; Bispo Da Silva, A.; Dos Santos, B.L.; Silva, V.D.A.; De Assis, A.M.; Da Silva, J.S.; Souza, D.O.; Costa, M.D.F.D.; et al. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated with Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 119. [Google Scholar] [CrossRef]
- de Amorim, V.C.M.; Júnior, M.S.O.; da Silva, A.B.; David, J.M.; David, J.P.L.; de Fátima Dias Costa, M.; Butt, A.M.; da Silva, V.D.A.; Costa, S.L. Agathisflavone modulates astrocytic responses and increases the population of neurons in an in vitro model of traumatic brain injury. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Kostandy, B.B. The role of glutamate in neuronal ischemic injury: The role of spark in fire. Neurol. Sci. 2012, 33, 223–237. [Google Scholar] [CrossRef]
- Dao, D.N.; Ahdab-Barmada, M.; Schor, N.F. Cerebellar glutamine synthetase in children after hypoxia or ischemia. Stroke 1991, 22, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Petito, C.K.; Chung, M.C.; Verkhovsky, L.M.; Cooper, A.J. Brain glutamine synthetase increases following cerebral ischemia in the rat. Brain Res. 1992, 569, 275–280. [Google Scholar] [CrossRef]
- Jeitner, T.M.; Battaile, K.; Cooper, A.J. Critical Evaluation of the Changes in Glutamine Synthetase Activity in Models of Cerebral Stroke. Neurochem. Res. 2015, 40, 2544–2556. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.N.; Starke-Reed, P.E.; Stadtman, E.R.; Liu, G.J.; Carney, J.M.; Floyd, R.A. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc. Natl. Acad. Sci. USA 1990, 87, 5144–5147. [Google Scholar] [CrossRef]
- D’Amelio, F.; Eng, L.F.; Gibbs, M.A. Glutamine synthetase immunoreactivity is present in oligodendroglia of various regions of the central nervous system. Glia 1990, 3, 335–341. [Google Scholar] [CrossRef]
- Ben Haim, L.; Schirmer, L.; Zulji, A.; Sabeur, K.; Tiret, B.; Ribon, M.; Chang, S.; Lamers, W.H.; Boillée, S.; Chaumeil, M.M.; et al. Evidence for glutamine synthetase function in mouse spinal cord oligodendrocytes. Glia 2021, 69, 2812–2827. [Google Scholar] [CrossRef]
- Butts, T.; Rook, V.; Varela, T.; Wilson, L.; Wingate, R.J.T. Specification of granule cells and purkinje cells. In Handbook of the Cerebellum and Cerebellar Disorders; Springer: Cham, Switzerland, 2021; pp. 99–119. [Google Scholar] [CrossRef]
- Fujishima, K.; Kawabata Galbraith, K.; Kengaku, M. Dendritic Self-Avoidance and Morphological Development of Cerebellar Purkinje Cells. Cerebellum 2018, 17, 701–708. [Google Scholar] [CrossRef]
- Sotelo, C.; Rossi, F. Purkinje cell migration and differentiation. In Handbook of the Cerebellum and Cerebellar Disorders; Springer: Cham, Switzerland, 2021; pp. 173–205. [Google Scholar] [CrossRef]
- Hirano, T. Purkinje Neurons: Development, Morphology, and Function. Cerebellum 2018, 17, 699–700. [Google Scholar] [CrossRef]
- Hausmann, R.; Seidl, S.; Betz, P. Hypoxic changes in Purkinje cells of the human cerebellum. Int. J. Legal Med. 2007, 121, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Helleringer, R.; Chever, O.; Daniel, H.; Galante, M. Oxygen and Glucose Deprivation Induces Bergmann Glia Membrane Depolarization and Ca(2+) Rises Mainly Mediated by K(+) and ATP Increases in the Extracellular Space. Front. Cell Neurosci. 2017, 11, 349. [Google Scholar] [CrossRef]
- Kelley, M.H.; Ortiz, J.; Shimizu, K.; Grewal, H.; Quillinan, N.; Herson, P.S. Alterations in Purkinje cell GABAA receptor pharmacology following oxygen and glucose deprivation and cerebral ischemia reveal novel contribution of β1 -subunit-containing receptors. Eur. J. Neurosci. 2013, 37, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Ardeshiri, A.; Kelley, M.H.; Korner, I.P.; Hurn, P.D.; Herson, P.S. Mechanism of progesterone neuroprotection of rat cerebellar Purkinje cells following oxygen-glucose deprivation. Eur. J. Neurosci. 2006, 24, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, A.B.; Madsen, K.D.; Liljefors, T.; Stafford, G.I.; van Staden, J.; Jäger, A.K. Biflavones from Rhus species with affinity for the GABA(A)/benzodiazepine receptor. J. Ethnopharmacol. 2006, 103, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Piochon, C.; Irinopoulou, T.; Brusciano, D.; Bailly, Y.; Mariani, J.; Levenes, C. NMDA receptor contribution to the climbing fiber response in the adult mouse Purkinje cell. J. Neurosci. 2007, 27, 10797–10809. [Google Scholar] [CrossRef]
- Tirandi, A.; Sgura, C.; Carbone, F.; Montecucco, F.; Liberale, L. Inflammatory biomarkers of ischemic stroke. 779 Intern. Emerg. Med. 2023, 18, 723–732. [Google Scholar] [CrossRef]
- Planas, A.M.; Gorina, R.; Chamorro, A. Signalling pathways mediating inflammatory responses in brain ischaemia. Biochem. Soc. Trans. 2006, 34, 1267–1270. [Google Scholar] [CrossRef]
- Bonetti, N.R.; Diaz-Cañestro, C.; Liberale, L.; Crucet, M.; Akhmedov, A.; Merlini, M.; Reiner, M.F.; Gobbato, S.; Stivala, S.; Kollias, G.; et al. Tumour Necrosis Factor-α Inhibition Improves Stroke Outcome in a Mouse Model of Rheumatoid Arthritis. Sci. Rep. 2019, 9, 2173. [Google Scholar] [CrossRef]
- Pickering, M.; Cumiskey, D.; O’Connor, J.J. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp. Physiol. 2005, 90, 663–670. [Google Scholar] [CrossRef]
- Liberale, L.; Bonetti, N.R.; Puspitasari, Y.M.; Vukolic, A.; Akhmedov, A.; Diaz-Cañestro, C.; Keller, S.; Montecucco, F.; Merlini, M.; Semerano, A.; et al. TNF-α antagonism rescues the effect of ageing on stroke: Perspectives for targeting inflamm-ageing. Eur. J. Clin. Investig. 2021, 51, e13600. [Google Scholar] [CrossRef] [PubMed]
- Boutin, H.; LeFeuvre, R.A.; Horai, R.; Asano, M.; Iwakura, Y.; Rothwell, N.J. Role of IL-1alpha and IL-1beta in ischemic brain damage. J. Neurosci. 2001, 21, 5528–5534. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Diaz-Cañestro, C.; Bonetti, N.R.; Paneni, F.; Akhmedov, A.; Beer, J.H.; Montecucco, F.; Lüscher, T.F.; Camici, G.G. Post-ischaemic administration of the murine Canakinumab-surrogate antibody improves outcome in experimental stroke. Eur. Heart J. 2018, 39, 3511–3517. [Google Scholar] [CrossRef] [PubMed]
- Liesz, A.; Bauer, A.; Hoheisel, J.D.; Veltkamp, R. Intracerebral interleukin-10 injection modulates post-ischemic neuroinflammation: An experimental microarray study. Neurosci. Lett. 2014, 579, 18–23. [Google Scholar] [CrossRef]
- Mendes, C.C.; Bahia, M.V.; David, J.M.; David, J.P. Constituents of Caesalpinia pyramidalis. Fitoterapia 2000, 71, 205–207. [Google Scholar] [CrossRef]
- Bahia, M.V.; Santos, J.B.d.; David, J.P.; David, J.M. Biflavonoids and other phenolics from Caesalpinia pyramidalis (Fabaceae). J. Braz. Chem. Soc. 2005, 16, 1402–1405. [Google Scholar] [CrossRef]
- Bahia, M.V.; David, J.P.; David, J.M. Occurrence of biflavones in leaves of Caesalpinia pyramidalis specimens. Química Nova 2010, 33, 1297–1300. [Google Scholar] [CrossRef]
- De Simoni, A.; Yu, L.M. Preparation of organotypic hippocampal slice cultures: Interface method. Nat. Protoc. 2006, 1, 1439–1445. [Google Scholar] [CrossRef]
- Stoppini, L.; Buchs, P.A.; Muller, D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 1991, 37, 173–182. [Google Scholar] [CrossRef]
- Butt, A.M.; Vanzulli, I.; Papanikolaou, M.; De La Rocha, I.C.; Hawkins, V.E. Metabotropic Glutamate Receptors Protect Oligodendrocytes from Acute Ischemia in the Mouse Optic Nerve. Neurochem. Res. 2017, 42, 2468–2478. [Google Scholar] [CrossRef]
- Azim, K.; Rivera, A.; Raineteau, O.; Butt, A.M. GSK3β regulates oligodendrogenesis in the dorsal microdomain of the subventricular zone via Wnt-β-catenin signaling. Glia 2014, 62, 778–779. [Google Scholar] [CrossRef]




| Gene | Gene ID | Direct Sequence | Reverse Sequence |
|---|---|---|---|
| TNF-α | 21926 | GGTGCCTATGTCTCAGCCTCTT | GCCATAGAACTGATGAGAGGGAG |
| IL-1β | 16176 | TGGACCTTCCAGGATGAGGACA | GTTCATCTCGGAGCCTGTAGTG |
| IL-10 | 16153 | CGGGAAGACAATAACTGCACCC | CGGTTAGCAGTATGTTGTCCAGC |
| GAPDH | 14433 | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
| ACTB | 11461 | CATTGCTGACAGGATGCAGAAGG | TGCTGGAAGGTGGACAGTGAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreira, R.B.; dos Santos, C.C.; de Oliveira, J.V.R.; da Silva, V.D.A.; David, J.M.; Butt, A.M.; Costa, S.L. Neuroprotective Effect of Flavonoid Agathisflavone in the Ex Vivo Cerebellar Slice Neonatal Ischemia. Molecules 2024, 29, 4159. https://doi.org/10.3390/molecules29174159
Carreira RB, dos Santos CC, de Oliveira JVR, da Silva VDA, David JM, Butt AM, Costa SL. Neuroprotective Effect of Flavonoid Agathisflavone in the Ex Vivo Cerebellar Slice Neonatal Ischemia. Molecules. 2024; 29(17):4159. https://doi.org/10.3390/molecules29174159
Chicago/Turabian StyleCarreira, Rodrigo Barreto, Cleonice Creusa dos Santos, Juciele Valeria Ribeiro de Oliveira, Victor Diogenes Amaral da Silva, Jorge Maurício David, Arthur Morgan Butt, and Silvia Lima Costa. 2024. "Neuroprotective Effect of Flavonoid Agathisflavone in the Ex Vivo Cerebellar Slice Neonatal Ischemia" Molecules 29, no. 17: 4159. https://doi.org/10.3390/molecules29174159
APA StyleCarreira, R. B., dos Santos, C. C., de Oliveira, J. V. R., da Silva, V. D. A., David, J. M., Butt, A. M., & Costa, S. L. (2024). Neuroprotective Effect of Flavonoid Agathisflavone in the Ex Vivo Cerebellar Slice Neonatal Ischemia. Molecules, 29(17), 4159. https://doi.org/10.3390/molecules29174159








