Abstract
Inspired by the potent tyrosinase inhibitory activity of phenolic compounds with a 2-phenylbenzo[d]thiazole scaffold, we explored phenolic compounds 1–15 with 2-phenylbenzo[d]oxazole, which is isosterically related to 2-phenylbenzo[d]thiazole, as novel tyrosinase inhibitors. Among these, compounds 3, 8, and 13, featuring a resorcinol structure, exhibited significantly stronger mushroom tyrosinase inhibition than kojic acid, with compound 3 showing a nanomolar IC50 value of 0.51 μM. These results suggest that resorcinol plays an important role in tyrosinase inhibition. Kinetic studies using Lineweaver–Burk plots demonstrated the inhibition mechanisms of compounds 3, 8, and 13, while docking simulation results indicated that the resorcinol structure contributed to tyrosinase binding through hydrophobic and hydrogen bonding interactions. Additionally, these compounds effectively inhibited tyrosinase activity and melanin production in B16F10 cells and inhibited B16F10 tyrosinase activity in situ in a concentration-dependent manner. As these compounds showed no cytotoxicity to epidermal cells, melanocytes, or keratinocytes, they are appropriate for skin applications. Compounds 8 and 13 demonstrated substantially higher depigmentation effects on zebrafish larvae than kojic acid, even at 800- and 400-times lower concentrations than kojic acid, respectively. These findings suggest that 2-phenylbenzo[d]oxazole is a promising candidate for tyrosinase inhibition.
1. Introduction
Melanin is a pigment that varies in color from a red-yellow to black-brown pigment and is found in nearly all living organisms, including bacteria, plants, animals, and humans [1,2]. Melanin is an oligomer or polymer. Eumelanin has a 5,6-dihydroxyindole structure, whereas pheomelanin contains benzothiazine and benzothiazole structures [3]. Melanin determines the color of the skin, eyes, and hair [4] and plays a protective role by absorbing ultraviolet rays [5]. However, excessive melanin pigmentation in certain areas can lead to cosmetic variations or medical problems such as freckles, melasma, senile spots, and hyperpigmentation [6,7]. Meanwhile, the production of melanin in fruits and vegetables acts as a representative factor that reduces quality via causing browning and unpleasant odors [8].
Melanin is synthesized through several chemical and enzymatic reactions [9]. Tyrosinase, tyrosinase-related protein-1 (TRP-1), and TRP-2 are involved in the melanin biosynthesis process, or melanogenesis [10]. Tyrosinase participates in the initial two oxidation processes using l-tyrosine and l-dopa as substrates during melanogenesis. TRP-1 and -2 are involved only in the synthesis of eumelanin. Among these enzymes, tyrosinase is reported to be a rate-determining enzyme in melanogenesis [11,12]. Thus, tyrosinase is an important target in the regulation of melanogenesis. Arbutin [13,14], kojic acid [15], hydroquinone [15], 4-butylresorcinol [13], tranexamic acid [13,16], and ascorbic acids [13,17] are representative tyrosinase inhibitors (Figure 1). Despite their use, the unmet demand for new whitening agents continues to rise because of their low clinical efficacy, instability during product manufacturing, and side effects [18,19,20,21].
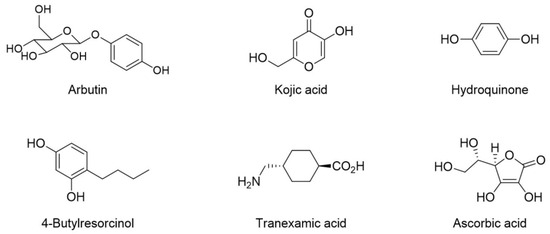
Figure 1.
The structures of representative tyrosinase inhibitors.
Phenolic compounds with 2-phenylbenzo[d]thiazole scaffolds have been reported to display potent tyrosinase inhibitory activities against murine and mushroom tyrosinases (Figure 2) [22]. 2-Phenylbenzo[d]oxazole scaffolds are isosterically related to 2-phenylbenzo[d]thiazole. Additionally, compounds containing 2-phenylbenzo[d]oxazole exhibit various biological activities, including antimicrobial [23,24], antifungal [25], and anticancer [26] activities and the inhibition of transthyretin amyloid formation [27]. Therefore, phenolic compounds bearing a 2-phenylbenzo[d]oxazole scaffold were synthesized, and their abilities to inhibit tyrosinase activity, melanin production, and antioxidant activity were evaluated. In addition, their depigmentation effects in zebrafish larvae were examined. Furthermore, their inhibitory modes of action on tyrosinase and plausible chemical interactions with tyrosinase were examined.
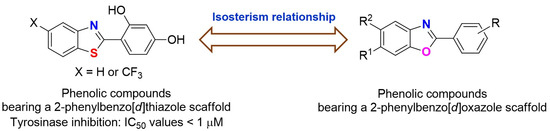
Figure 2.
Structures of phenolic compounds bearing 2-phenylbenzo[d]thiazole and 2-phenylbenzo[d]oxazole scaffolds.
2. Results and Discussion
2.1. Preparation of Target Compounds, 2-Phenylbenzo[d]oxazoles 1–15
Through more than a decade of research on tyrosinase inhibitors, it has been established that 4-hydroxyphenyl, 2,4-dihydroxyphenyl, 3,4-dihydroxyphenyl, and 3-hydroxy-4-methoxyphenyl moieties play significant roles in inhibiting tyrosinase activity [28,29,30,31]. Consequently, for the synthesis of target compounds bearing the 2-phenylbenzoxazole scaffold, we introduced five 2-phenyl groups, including these phenyl moieties, into the target compounds. Three benzoxazoles, 6-methylbenzoxazole (series A), 6-chlorobenzoxazole (series B), and 5-methylbenzoxazole (series C), were introduced into the benzo[d]oxazole portion of the 2-phenylbenzoxazoles (Scheme 1). Fifteen 2-phenylbenzoxazole compounds, 1–15, were synthesized as previously reported [32]. Except for the target compounds with two hydroxyl groups on the 2-phenyl ring, the remaining target compounds were synthesized via two-step reactions. The condensation of 2-hydroxyanilines (2-hydroxy-4-methylaniline, 4-chloro-2-hydroxyaniline, or 2-hydroxy-5-methylaniline) with the appropriate benzaldehyde in ethanol produced the resulting imine intermediates. These intermediates were then equilibrated with 2,3-dihydrobenzo[d]oxazoles. Treatment with 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) oxidized the 2,3-dihydrobenzo[d]oxazoles to their corresponding benzo[d]oxazoles to afford the target compounds (1, 2, 4, 6, 7, 9, 11, 12, and 14). Further, the O-demethylation of the target compounds 2, 7, and 12 using BBr3 generated the target compounds 3, 8, and 13, respectively, with a resorcinol group. The O-Demethylation of the target compounds 4, 9, and 14 using BBr3 afforded the target compounds 5, 10, and 15, respectively, with a catechol group. The structures of the target compounds were confirmed by 1H and 13C NMR spectroscopy.
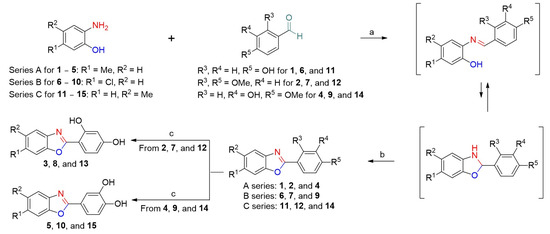
Scheme 1.
Synthetic outline of target compounds 1–15 using three series starting materials (series A: 2-hydroxy-4-methylaniline, series B: 4-chloro-2-hydroxyaniline, and series C: 2-hydroxy-5-methylaniline). Reagents and conditions: (a) anhydrous ethanol, reflux, 14–40 h; (b) 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ), CH2Cl2, 19 °C, 12–16 h, 45–60% (2-step yield); (c) BBr3, CH2Cl2, 19 °C, 2–15 h, 74–95%.
2.2. Mushroom Tyrosinase Inhibition of 2-Phenylbenzo[d]oxazole Compounds 1–15
The inhibitory potency of compounds 1–15 against mushroom tyrosinase was examined using l-dopa and l-tyrosine as substrates. Overall, the IC50 value was lower when l-tyrosine was used as the substrate compared to when l-dopa was used as the substrate. The numbering of the phenyl ring at position 2 of benzoxazole followed the numbering of the common phenyl ring.
When l-tyrosine was used as a substrate, kojic acid exhibited an IC50 value of 14.33 ± 1.63 μM, indicating a strong inhibitory potency (Table 1). Among the 6-methyl-2-phenylbenzoxazole series of compounds (1–5), compound 1, which had a hydroxyl group substituted at position 4 of the phenyl ring, showed a somewhat weak mushroom tyrosinase inhibition effect (IC50 value = 152.51 ± 14.33 μM). Introducing an additional hydroxyl group into the phenyl ring of 1 resulted in different effects, depending on the position of the substitution: compound 3 with a 2,4-dihydroxyphenyl ring showed a very high tyrosinase inhibition potency with a nanomolar IC50 value of 0.51 ± 0.00 μM, while compound 5 bearing a 3,4-dihydroxyphenyl ring exhibited a tyrosinase inhibition potency with an IC50 value of 144.06 ± 3.10 μM, similar to 1. Interestingly, compound 2, in which the 2,4-dihydroxyl group of the phenyl ring of 3 was substituted with a 2,4-dimethoxyl group, showed a very weak inhibitory potency (IC50 value > 200 μM). Replacing the 4-hydroxyl group on the phenyl ring of 5 with a 4-methoxyl group significantly reduced tyrosinase inhibition (IC50 value of 4 > 200 μM). The 6-Chloro-2-phenylbenzoxazole series compounds (6–10) and the 2-phenyl-5-methylbenzoxazole series compounds (11–15) showed tyrosinase inhibition trends similar to the 6-methyl-2-phenylbenzoxazole series compounds (1–5). Notably, compounds 3, 8, and 13, each bearing a 2,4-dihydroxyphenyl ring, demonstrated the most potent mushroom tyrosinase inhibitory activities, with IC50 values of 0.51 ± 0.00, 2.22 ± 0.16, and 3.50 ± 0.07 μM, respectively. Compound 3 had a 28-times higher tyrosinase potency than kojic acid.

Table 1.
Half-maximal inhibitory concentration (IC50) values of 2-phenylbenzo[d]oxazole compounds 1–15 and kojic acid in the presence of l-dopa and l-tyrosine and substitution patterns.
When l-dopa was used as a substrate, kojic acid exhibited an IC50 value of 40.42 ± 3.70 μM, which was higher compared to when l-tyrosine was used. Similar to the observation with l-tyrosine, compounds 3, 8, and 13, each bearing a 2,4-dihydroxyphenyl ring, exhibited stronger tyrosinase inhibitions than kojic acid. The IC50 values of 3, 8, and 13 were 16.78 ± 0.57, 20.38 ± 1.99, and 20.76 ± 1.02 μM, respectively. The remaining compounds had IC50 values of >200 μΜ, except for compound 5 (IC50 value = 187.13 ± 30.28 μM). Among the 2-phenylbenzoxazole compounds bearing a 2,4-dihydroxyphenyl ring (3, 8, and 13), compound 3, which belonged to series A, exerted the strongest tyrosinase inhibitory activity, regardless of the substrate type.
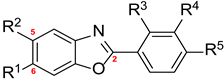
The structure–activity relationship is outlined in Figure 3. The presence of R5 played a crucial role in providing the basic tyrosinase inhibitory activity, with the hydroxyl group contributing more to tyrosinase inhibition than the methoxy group. Adding a hydroxyl group at R4 reduced the tyrosinase inhibitory activity. The insertion of a substituent into R3 induced dramatic changes in the tyrosinase inhibitory activity; the insertion of a hydroxyl group significantly increased the inhibitory potency, whereas the insertion of a methoxyl group significantly decreased the inhibitory potency. Additionally, the introduction of a methyl group at R1 slightly increased the inhibitory activity, more than when a methyl group was added to R2.
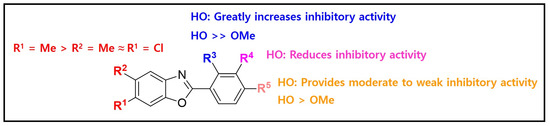
Figure 3.
The structure–mushroom tyrosinase inhibitory activity relationships of 2-phenylbenzoxazole compounds.
2.3. Inhibition Mode of Action of 2-Phenylbenzoxazole Compounds
For the inhibition mechanism study of the 2-phenylbenzoxazole compounds, kinetic studies of mushroom tyrosinase were conducted. As compounds 3, 8, and 13 exhibited potent inhibition activity against mushroom tyrosinase, their initial rate of dopachrome production was measured in the presence of four different compound concentrations (0, 5, 10, and 20 µM for 3 and 13 and 0, 10, 20, and 40 µM for 8). Kinetic experiments were performed using various concentrations of l-dopa. Lineweaver–Burk plots were obtained for each compound by plotting the inverse of the l-dopa concentration against the inverse of the initial rate of dopachrome production (Figure 4). In the Lineweaver–Burk plots (Figure 4B,C), the lines for compounds 8 and 13 merged at one point on the y-axis, whereas the lines for 3 (Figure 4A) merged at one point in the second quadrant. These results suggest that 8 and 13 are competitive inhibitors whose maximum reaction rates do not change, regardless of the inhibitor concentration, and that 3 is a mixed-type inhibitor in which the maximum reaction rate decreases and the Michaelis constant increases as the inhibitor concentration increases.
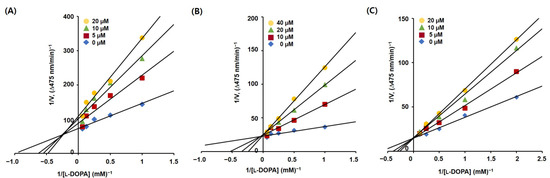
Figure 4.
Lineweaver–Burk plots of 2-phenylbenzoxazole inhibitors 3 (A), 8 (B), and 13 (C) obtained from kinetic studies. The plots were generated using several concentrations (0.5–16 mM) of l-dopa and four different concentrations (20, 10, 5, and 0 µM for 3 and 13 and 40, 20, 10, and 0 µM for 8) of the inhibitors were used.
For the inhibition constants (Ki) of these compounds, each Lineweaver–Burk plot for the competitive inhibitors 8 and 13 was transformed into a corresponding Dixon plot. These Dixon plots were obtained by plotting the inhibitor concentration against the inverse of the initial dopachrome production rate (Figure 5). The four lines for each Dixon plot were merged at one point in the second quadrant. The absolute x-coordinate of each merged point represents the Ki value of each compound. The Ki values of 8 and 13 were 12.50 and 17.22 µM, respectively.
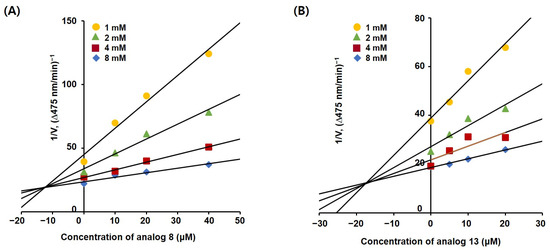
Figure 5.
Dixon plots of 2-phenylbenzoxazole competitive inhibitors 8 (A) and 13 (B) obtained from kinetic studies. Four different concentrations (1, 2, 4, and 8 mM) of l-dopa and four different concentrations (40, 20, 10, and 0 µM for 8 and 20, 10, 5, and 0 µM for 13) were used.
2.4. In Silico Docking Simulation of 2-Phenylbenoxazole Compounds 3, 8, and 13 and Mushroom Tyrosinase Using AutoDock Vina
Compounds 3, 8, and 13 showed the strongest mushroom tyrosinase inhibition. To investigate the chemical interactions and binding affinities between these 2-phenylbenoxazole compounds and mushroom tyrosinase amino acid residues, in silico docking simulations were carried out using AutoDock Vina 1.2.0.
According to the docking simulation results shown in Figure 6, compound 3 interacted hydrophobically with the amino acid residues at the tyrosinase active site. The fused benzene ring in 3 interacted with two amino acids, Ala286 and Val283, while the 6-methyl substituent interacted with three amino acids, Ala286, Val283, and Phe292. Additionally, the 2-phenyl moiety of 2-phenylbenoxazole compound 3 interacted with two amino acids, Phe264 and Val248. These hydrophobic interactions resulted in a binding affinity of −6.9 kcal/mol to compound 3. Notably, compounds 8 and 13 also exhibited hydrophobic interactions with the same amino acid residues at the mushroom tyrosinase active site, analogous to compound 3. The binding affinities of these compounds were −6.8 and −6.7 kcal/mol, respectively. On the other hand, kojic acid, used for comparing the binding affinity, formed hydrogen bonds with three histidine residues (His296, His61, and His263) and Met280 and interacted with His263 via pi-pi stacking, resulting in a binding affinity of −5.4 kcal/mol. The in silico docking simulation results suggest that the 2-phenylbenzoxazole compounds 3, 8, and 13 bound more tightly to the tyrosinase active site compared to kojic acid.
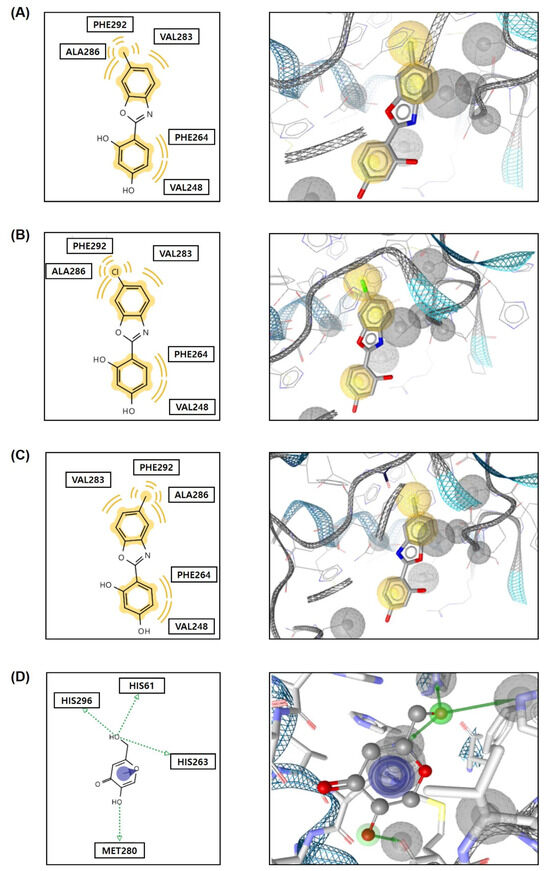
Figure 6.
Plausible chemical interactions between 2-phenylbenzoxazole compounds (3 (A), 8 (B), and 13 (C)) or kojic acid (D) and the amino acid residues in the active site of tyrosinase, as determined using AutoDock Vina. Yellow wave indicates hydrophobic interaction; green dotted arrows denote hydrogen bond; and blue arrow represents pi-pi stacking.
The kinetic results showed that compound 3 was a mixed-type inhibitor that could bind to both the tyrosinase active site and the allosteric site. Thus, the potential of compound 3 to bind to the tyrosinase allosteric site was assessed using a docking simulation.
Figure 7 shows compound 3’s binding to the allosteric (red) and active (turquoise) sites. Compound 3 interacted with three amino acids (Glu67, Lys5, and Gln72) via four hydrogen bonds, and the 4-hydroxyl group of the 2-phenyl ring formed three hydrogen bonds. Notably, the 4-hydroxyl group interacted with Gln72 as a hydrogen bond acceptor and donor. These interactions provided compound 3 with a binding affinity of −7.1 kcal/mol, indicating that compound 3 could bind tightly to the tyrosinase allosteric site.
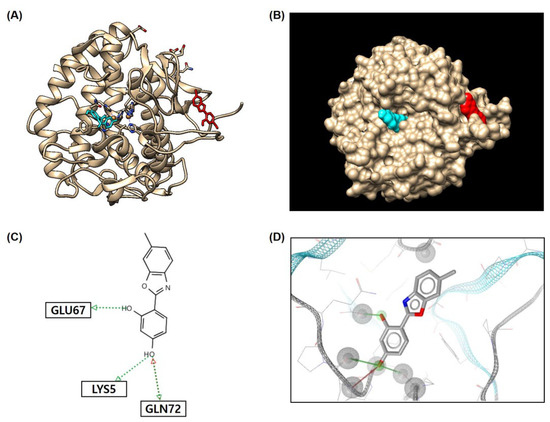
Figure 7.
In silico docking simulation results between compound 3 and tyrosinase amino acid residues using AutoDock Vina. Rounded ribbon representation (A) and surface representation (B) showing compound 3 bound to both the active site and allosteric site of tyrosinase. Two-dimensional (C) and three-dimensional (D) plausible chemical interactions between compound 3 and the amino acid residues of tyrosinase present in the allosteric site. Red and green dotted arrows, hydrogen bond acceptor and donor, respectively.
2.5. Cytotoxicity in B16F10 Cells
Since the 2-phenylbenzoxazole compounds 3, 8, and 13 were identified as being potent mushroom tyrosinase inhibitors, they were used in B16F10-cell-based experiments. Before assessing the biological impact of compounds 3, 8, and 13 in the B16F10 cells, the effect of these compounds on B16F10 cell viability was evaluated. The cells were treated with the compounds at concentrations of 0, 1, 2, and 5 μM for 48 and 72 h, respectively.
Compounds 3, 8, and 13 were not cytotoxic to the B16F10 cells after 48 h at any of the concentrations tested (Figure 8). After 72 h of treatment, compounds 8 and 13 did not show cytotoxicity at any of the concentrations tested; however, compound 3 showed weak cytotoxicity at the highest concentration tested. Despite this, the level of cytotoxicity of compound 3 at 5 μM, was minimal. Therefore, the melanin inhibition ability and cellular tyrosinase inhibitory activity of compounds 3, 8, and 13 were evaluated at a concentration of 5 μM.
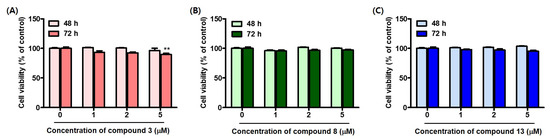
Figure 8.
B16F10 cell viability at 48 and 72 h, following treatment with compounds 3 (A), 8 (B), and 13 (C). Each compound was tested at concentrations of 0, 1, 2, and 5 μM. ** p < 0.01.
2.6. Effect of 2-Phenylbenzoxazole Compounds 3, 8, and 13 on Melanogenesis in B16F10 Cells
We investigated whether compounds 3, 8, and 13, which exhibited a strong inhibition of mushroom tyrosinase, could inhibit melanogenesis in B16F10 cells. The B16F10 cells were exposed to each compound (1, 2, and 5 μM) for 1 h. Following this, stimulators (1 μM α-MSH [α-melanocyte-stimulating hormone] and 200 μM IBMX [3-isobutyl-1-methylxanthine]) were treated for 72 h. Kojic acid (5 μM) was utilized as a comparative control.
Exposure to the stimulators greatly increased melanogenesis, but the treatment with 3, 8, or 13 decreased the melanin content in a concentration-dependent manner (Figure 9). At 2 μM, all compounds exerted strong anti-melanogenic effects with lower melanin content levels than kojic acid. Additionally, compounds 3 and 8 at 5 μM decreased the melanin content levels much more strongly than kojic acid at the same concentration. These results suggest that compounds 3, 8, and 13, which potently inhibit mushroom tyrosinase, have the potential to inhibit melanogenesis by inhibiting cellular tyrosinase. Thus, the inhibitory effects of 3, 8, and 13 on B16F10 cellular tyrosinase were assessed.
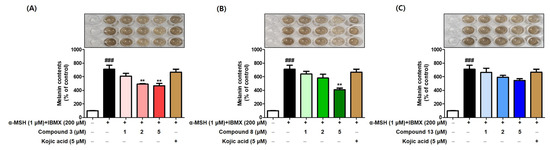
Figure 9.
The anti-melanogenic effect of compounds 3 (A), 8 (B), and 13 (C) in B16F10 cells. Compounds (3, 8, and 13) were treated at 1, 2, and 5 μM, and kojic acid (5 μM) was used as positive material. α-MSH (1 μM) and IBMX (200 μM) were used as stimulators. ** p < 0.01 vs. the stimulators-treated group and ### p < 0.001 vs. the non-treated group.
2.7. Effect of 2-Phenylbenzoxazole Compounds 3, 8, and 13 on B16F10 Cellular Tyrosinase Inhibition
To investigate the origins of the anti-melanogenic effects of compounds 3, 8, and 13, their impacts on B16F10 cellular tyrosinase inhibition were evaluated. The experiment utilized a similar experimental method and the same concentrations as those used in the melanin content experiment. Compounds 3, 8, and 13 (1, 2, and 5 μM), kojic acid (5 μM: positive control), and stimulators (1 μM α-MSH and 200 μM IBMX) were used in this experiment. Prior to treatment with the stimulators for 72 h, the test samples (3, 8, 13, and kojic acid) were pre-treated for 1 h.
The exposure of the B16F10 cells to stimulators led to a significant increase in their cellular tyrosinase activity (Figure 10). However, the addition of compounds 3, 8, and 13 reduced the stimulator-induced tyrosinase activity in a concentration-dependent manner. The potency of these compounds in inhibiting cellular tyrosinase activity was similar to or stronger than that of kojic acid at the same concentration. In addition, the cellular tyrosinase activity results were similar to the anti-melanogenic effects of the compounds, suggesting that the anti-melanogenic properties of these compounds are primarily related to their ability to inhibit cellular tyrosinase activity.
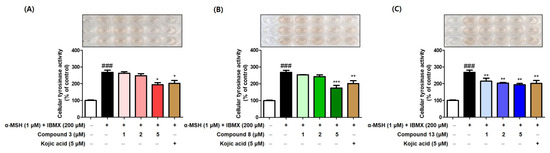
Figure 10.
The tyrosinase activity inhibitory effects of compounds 3 (A), 8 (B), and 13 (C) in B16F10 cells. Compounds (3, 8, and 13) were treated at 1, 2, and 5 μM, and kojic acid (5 μM) acted as positive control. α-MSH (1 μM) and IBMX (200 μM) were used as stimulators. *** p, ** p, and * p represent <0.001, <0.01, and <0.05, respectively, vs. the stimulators-treated group and ### p < 0.001 vs. the non-treated group.
2.8. In Situ Tyrosinase Activity in B16F10 Cells
To evaluate the effect of the compounds on in situ tyrosinase activity, an experiment using B16F10 cells was performed as previously described [34,35]. Since compounds 3, 8, and 13 exhibited potent anti-melanogenic effects, these compounds were used in this experiment, and excess l-dopa was used as a substrate to produce melanin. B16F10 cells were treated with kojic acid, a comparative control, or compounds 3, 8, and 13 at 5 μM and 1, 2, and 5 μM, respectively, for 1 h. Afterward, the cells were treated with stimulators (α-MSH [1 μM] and IBMX [200 μM]) for 72 h to increase their tyrosinase activity. Excess l-dopa was added to increase their melanin production prior to the acquisition of cell images.
The stimulators led to a significant increase in melanin production (Figure 11). However, treatment with 5 μM kojic acid markedly decreased melanin formation. Compounds 3, 8, and 13 reduced melanin formation in a concentration-dependent manner. All compounds at 2 μM showed a stronger melanin reduction than kojic acid at 5 μM. Compared to the concentration of 5 μM, all compounds exerted a much stronger melanin production inhibitory effect than kojic acid. These results suggest that the melanin reduction effects were derived from the in situ reduction in tyrosinase activity caused by these compounds.
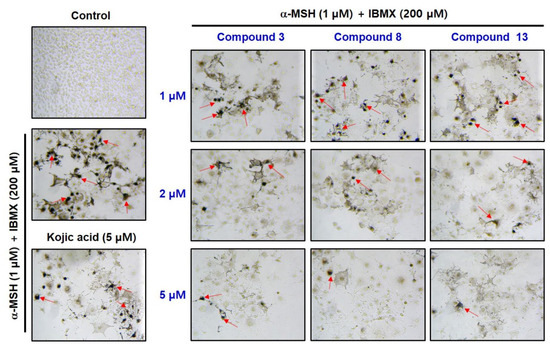
Figure 11.
Cell photos obtained from an in situ B16F10 cell tyrosinase activity experiment. B16F10 cells were treated with compounds 3, 8, and 13 for 1 h at 1, 2, and 5 μM, or kojic acid (5 μM) as a positive material. Then, the cells were treated with 1 μM α-MSH and 200 μM IBMX for 72 h. The cells were treated with excess l-dopa for 2 h, before acquiring their photos. Arrows indicate melanin pigments in melanoma cells. Arrows indicate excessive melanin formation.
2.9. Cell Viability on HaCaT
HaCaT cells, human keratinocytes, are the primary cells comprising the human epidermis. Dermal medicines and cosmetic ingredients should not be cytotoxic to epidermal cells, including HaCaT cells. Therefore, the effects of compounds 3, 8, and 13 on HaCaT cell viability were investigated. HaCaT cells were exposed to each compound at concentrations of 2, 5, and 10 μM for 24 h.
None of the compounds exhibited cytotoxicity against the HaCaT cells after 24 h (Figure 12). These results suggest that these compounds are suitable for dermal applications.
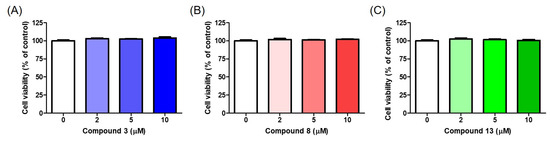
Figure 12.
Cell viability on HaCaT cells in the presence of compounds 3 (A), 8 (B), and 13 (C). All compounds were treated at 2, 5, and 10 μM, and cell viability was analyzed after 24 h.
2.10. In Vivo Depigmentation Effect of 2-Phenylbenzoxazole Compounds on Zebrafish Larvae
The 2-Phenylbenzoxazole compounds exhibited inhibitory effects on both mushroom tyrosinase enzymes and B16F10 cellular tyrosinase, leading to anti-melanogenic potency in the cells. Therefore, we investigated whether these compounds induce anti-melanogenic activity in vivo. Zebrafish embryos were used in this study. Zebrafish are very similar to humans in terms of their genetic sequence and function [36,37,38]. The zebrafish embryos were obtained through natural mating in our laboratory. The chorion of the zebrafish embryos was removed 24 h post-fertilization (hpf). After 4 h, the 2-phenylbenzoxazole compounds 8 and 13 and kojic acid, a positive control, were used. Kojic acid was used at a concentration of 20 mM and compound 13 was used at concentrations of 0.025 and 0.05 mM. In contrast, compound 8 was used at 0.025 mM, because it was judged to have a weak toxicity at 0.05 mM. Before obtaining photographs of the zebrafish larvae, the zebrafish embryos were placed in an incubator for zebrafish embryos for 48 h. Photographs were acquired from dorsal and lateral views of the zebrafish larvae.
At 76 hpf, the control zebrafish larvae displayed dark pigmentation throughout their bodies, including their eyes (Figure 13B). Exposure to kojic acid resulted in reduced pigmentation in all regions except the eyes, compared with the pigmentation in the control zebrafish larvae. Compound 13 demonstrated a concentration-dependent depigmentation effect and exhibited a significantly stronger depigmentation efficacy than kojic acid, even at a concentration (0.05 mM) 400-times lower than that of kojic acid. In particular, the pigmentation of the eyes significantly decreased. Compound 8 exhibited a strong potency at 0.025 mM, similar to 13 at 0.05 mM. According to a pigmentation area analysis using CS analyzer software version 3.0, kojic acid at 20 mM decreased the pigmentation area compared to the control (Figure 13C). Compound 13 demonstrated a similar or stronger efficacy than kojic acid at 20 mM in reducing the pigmentation area at 0.025 and 0.05 mM, respectively. Compound 8 also exhibited the smallest pigmentation area, even at a concentration of 0.025 mM, which was 800-times lower than that of kojic acid. These in vivo results suggest that the 2-phenylbenzoxazole compounds 8 and 13 are promising agents for reducing melanogenesis.
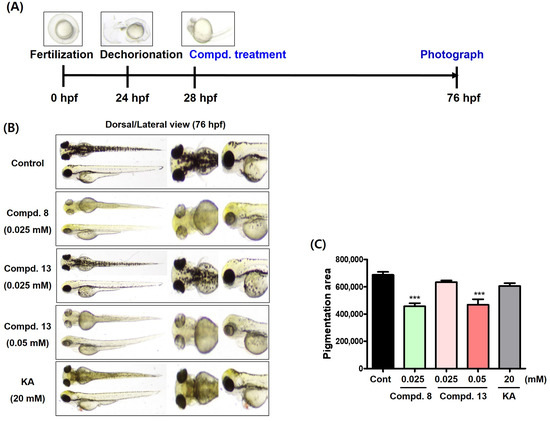
Figure 13.
Depigmentation results of compounds 8 and 13 in zebrafish larvae. (A) Experimental manipulation according to timeline. (B) Zebrafish photographs (dorsal and lateral views) obtained 48 h after treatment of test samples (compounds 8 [0.025 mM] and 13 [0.025 and 0.05 mM] and kojic acid [KA, 20 mM; positive control]). (C) Pigmentation areas of zebrafish larvae obtained using CS analyzer software. *** p < 0.001 vs. the untreated control.
Because compound 8 showed the most potent depigmentation effect on the zebrafish larvae, the melanin quantification and tyrosinase inhibitory activity of 8 were evaluated using kojic acid as a positive control for comparison. Zebrafish embryos obtained by natural mating were dechorionated at 24 hpf and treated with compound 8 (0.025 mM) or kojic acid (20 mM) at 28 hpf. After 46 h, the melanin content and tyrosinase activity were determined.
Kojic acid significantly reduced the melanin content of the zebrafish larvae (53%) compared to that of the untreated control (100%), and compound 8 exhibited a stronger reduction in melanin content (35%) than kojic acid, even at concentrations 800-times lower than kojic acid (Figure 14B). The inhibitory effect of 8 on the tyrosinase activity in the zebrafish larvae was similar to that of melanin (Figure 14C). After treatment with kojic acid or 8, their % tyrosinase activity were 57 and 29%, respectively, compared with the untreated control (100%), and compound 8 showed a better tyrosinase inhibitory activity than kojic acid, even at a much lower concentration. These results suggest that the depigmentation effect of 8 in zebrafish larvae can be attributed to its tyrosinase inhibitory ability.
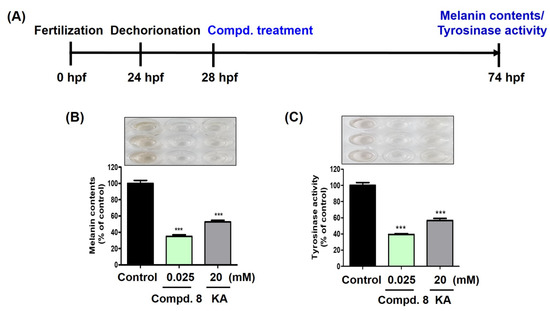
Figure 14.
Effects of compound 8 on melanin contents and tyrosinase activity in zebrafish larvae. Compound 8 was used at 0.025 mM and kojic acid (KA, 20 mM) was used as positive control. (A) Experimental manipulation according to timeline. (B) Melanin content results. (C) Tyrosinase activity results. *** p < 0.001 vs. the untreated control.
2.11. Antioxidant Ability of 2-Phenylbenzoxazole Compounds 1–15 Using 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Radical Cation, and Reactive Oxygen Species (ROS)
Since a positive relationship between their anti-melanogenic effect and antioxidant ability has been reported [39], the antioxidant efficacy of 2-phenylbenzoxazole compounds 1–15 was investigated.
The DPPH radical scavenging activity was assessed by mixing DPPH solution with compounds 1–15 and incubating them in the dark for 30 min. All test samples were evaluated at 500 μM. Except for compounds 10 and 15, which contain a catechol group, the remaining compounds showed no or weak DPPH radical scavenging activity (Figure 15A). Compounds 10 and 15 both strongly scavenged DPPH radicals, with a scavenging activity of 86%, comparable to that of l-ascorbic acid (96%), which was used as a positive control. In contrast, despite having a catechol structure, compound 5 was found to have a weak DPPH radical scavenging effect, unlike compounds 10 and 15.
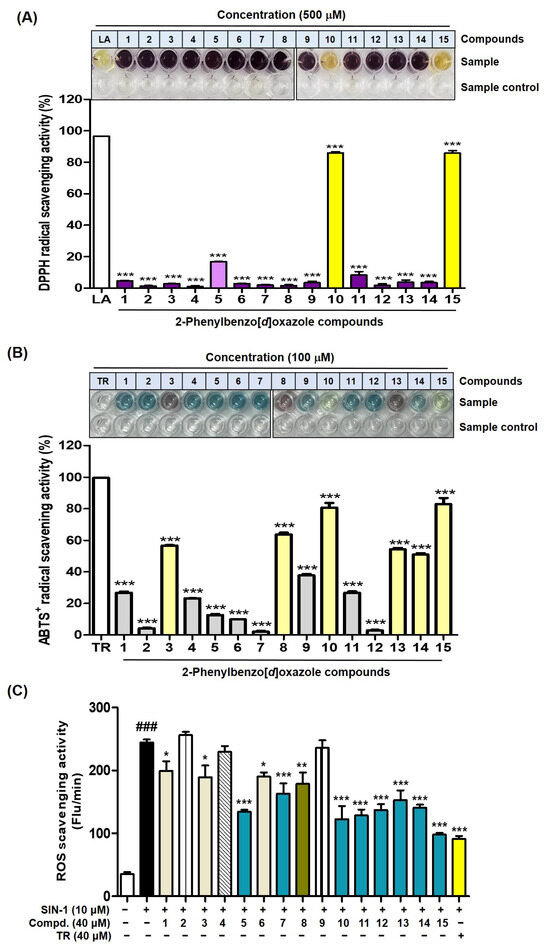
Figure 15.
Effects of 2-phenylbenzoxazole compounds 1–15 on DPPH (A) and ABTS+• (B) extinction and ROS scavenging (C). All test samples, including l-ascorbic acid [LA; positive control], for DPPH radical scavenging (A), all test samples, including Trolox [TR; positive control], for ABTS radical scavenging (B), and all test samples, including Trolox [TR], for ROS scavenging (C) were treated at concentrations of 500, 100, and 40 μM, respectively. (C) SIN-1 was used to generate ROS and treated at 10 μM, and Trolox was used as a positive control. *** p < 0.001 vs. LA-treated group for (A) or TR-treated group for (B). ### p < 0.001 vs. the untreated control, *** p < 0.001, ** p < 0.01, and * p < 0.05 vs. SIN-1-treated group for (C).
For ABTS+• scavenging experiments, ABTS+• was generated by oxidizing ABTS using potassium persulfate. All test samples (Trolox [TR; positive material] and 1–15) were used at a final concentration of 100 μM. Among the test samples, Trolox exhibited the highest ABTS+•-scavenging activity (99% inhibition) (Figure 15B). The 2-Phenylbenzoxazole compounds showed a wide range of scavenging efficacies ranging from weak to strong. Compounds 10 and 15 were the most potent ABTS+• scavengers (81 and 83% inhibition, respectively). Both compounds share a common catechol structure. Interestingly, compound 5, which has a catechol structure, was found to have a very weak ABTS+• scavenging ability (13% inhibition). Similar results were obtained in the DPPH radical scavenging experiment. Compounds 3, 8, and 13, which showed potent inhibitions of both mushroom and murine tyrosinases, exhibited a moderate antioxidant ability to scavenge ABTS+• (57, 64, and 54% inhibition, respectively). In contrast, compounds 2, 7, and 12, with no hydroxyl substituents, exhibited less than 5% inhibition. Compounds 4, 9, and 14, bearing a 4-hydroxy-3-methoxyphenyl, scavenged ABTS+• moderately (23–51%).
For ROS scavenging experiments, test samples (1–15 and Trolox [positive control]; 40 μM) containing 3-morpholinosydnonimine (SIN-1; 10 μM) were mixed with a 2′,7′-dichlorodihydrofluorescein (DCFH) solution. After 30 min, the ROS scavenging activity of the test samples was assessed. Trolox exhibited the most potent ROS scavenging activity (Figure 15C). Among the 15 compounds tested, eight compounds, including compounds 5, 10, and 15, each containing a catechol structure, demonstrated a strong to moderate ROS-scavenging activity. Notably, compound 15 showed a strong ROS scavenging efficacy comparable to that of Trolox. Interestingly, compound 5 displayed antioxidant potency with a strong ROS scavenging efficacy, in contrast to the DPPH and ABTS radical scavenging experiments.
3. Materials and Methods
3.1. Synthesis
3.1.1. General Methods
Solvents and chemicals were purchased from Thermo Fisher Scientific (Waltham, MA, USA), Daejung (Siheung-si, Gyeonggi-do, Republic of Korea), and SEJIN CI Co. (Seoul, Republic of Korea). Thin-layer chromatography (TLC; Silica gel 60 F254) plates were purchased from Merck Millipore (Darmstadt, Germany). The NMR data of the compounds were obtained using a JEOL ECZ400S instrument (JEOL Ltd., Tokyo, Japan). The NMR peak-splitting patterns are represented as s (singlet), m (multiplet), brs (broad singlet), d (doublet), and dd (doublet of doublets).
3.1.2. Preparation of 2-Phenylbenzo[d]oxazole Compounds 1–15
General Synthetic Method for the Synthesis of 1 [40], 2 [41], 4, 6, 7, 9, 11 [25,42,43,44], 12 [41], and 14
Solutions of 2-hydroxyaniline (series A: 2-hydroxy-4-methylaniline, series B: 4-chloro-2-hydroxyaniline, and series C: 2-hydroxy-5-methylaniline) and 1.0 equivalents of the appropriate benzaldehydes (4-hydroxybenzaldehyde, 2,4-dimethoxybenzaldehyde, and 3-hydroxy-4-methoxybenzaldehyde) were heated under reflux in ethyl alcohol for 14–40 h and then evaporated to yield the corresponding crude imine intermediates. Without further purification, the crude imine was treated with 1.0 equivalent 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) in dichloromethane and stirred at 19 °C for 12–16 h. After the reaction mixture was filtered through a pad of Celite®, the filtrate was partitioned between dichloromethane and saturated NaHCO3 solution, and the organic layer was evaporated. The resulting residue was purified by silica gel column chromatography using hexane/ethyl acetate (1.5:1 to 5:1) or dichloromethane/methanol (50:1 to 70:1) as the eluent to obtain 1, 2, 4, 6, 7, 9, 11, 12, and 14 in yields of 45–60%.
General Synthetic Method for the Synthesis of 3, 5, 8, 10, 13 [45], and 15 (Supplementary Materials)
A 1.0 M BBr3 in dichloromethane solution (4.0 equivalent for 2, 7, and 12 and 3.0 equivalent for 4, 9, and 14) was added dropwise to a dichloromethane solution of 2, 7, and 12 bearing a 2,4-dimethoxyphenyl ring or 4, 9, and 14 bearing a 3-hydroxy-4-methoxyphenyl ring at 0 °C. The reaction mixture was then stirred at 19 °C for 2–15 h. After, the reaction mixture was partitioned between a saturated NaHCO3 solution and ethyl acetate. The organic layer was evaporated, and the resultant residue was purified by silica gel column chromatography using dichloromethane/methanol (50:1 to 100:1) or hexane/ethyl acetate (1:1) as the eluent to obtain 3, 5, 8, 10, 13, and 15 in yields of 74–95%.
4-(6-Methylbenzo[d]oxazol-2-yl)phenol (compound 1)
1H NMR (400 MHz, DMSO-d6) δ 10.23 (s, 1H, OH), 7.97 (d, 2H, J = 8.8 Hz, 2′-H, 6′-H), 7.55 (d, 1H, J = 8.4 Hz, 4-H), 7.49 (d, 1H, J = 1.6 Hz, 7-H), 7.14 (dd, 1H, J = 8.4, 1.6 Hz, 5-H), 6.92 (d, 2H, J = 8.8 Hz, 3′-H, 5′-H), 2.41 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 162.8, 161.3, 150.8, 140.1, 135.2, 129.6, 126.2, 119.2, 117.9, 116.6, 111.2, 21.8.
2-(2,4-Dimethoxyphenyl)-6-methylbenzo[d]oxazole (compound 2)
1H NMR (400 MHz, CDCl3) δ 8.07 (d, 1H, J = 8.8 Hz, 6′-H), 7.63 (d, 1H, J = 8.0 Hz, 4-H), 7.34 (d, 1H, J = 1.6 Hz, 7-H), 7.10 (dd, 1H, J = 8.0, 1.6 Hz, 5-H), 6.61 (dd, 1H, J = 8.8, 2.4 Hz, 5′-H), 6.58 (d, 1H, J = 2.4 Hz, 3′-H), 4.00 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 2.47 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ 163.4, 161.2, 159.9, 150.5, 140.2, 134.9, 132.4, 125.4, 119.3, 110.5, 109.4, 105.3, 99.2, 56.3, 55.6, 21.9.
4-(6-Methylbenzo[d]oxazol-2-yl)benzene-1,3-diol (compound 3)
1H NMR (400 MHz, DMSO-d6) δ 11.21 (s, 1H, OH), 10.32 (s, 1H, OH), 7.77 (d, 1H, J = 8.4 Hz, 6′-H), 7.59 (d, 1H, J = 8.0 Hz, 4-H), 7.54 (d, 1H, J = 1.6 Hz, 7-H), 7.18 (dd, 1H, J = 8.0, 1.6 Hz, 5-H), 6.47 (dd, 1H, J = 8.4, 2.4 Hz, 5′-H), 6.42 (d, 1H, J = 2.4 Hz, 3′-H), 2.42 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 163.2, 162.9, 160.2, 149.2, 137.8, 135.7, 129.1, 126.6, 118.4, 111.2, 109.2, 103.3, 102.5, 21.7.
2-Methoxy-5-(6-methylbenzo[d]oxazol-2-yl)phenol (compound 4)
1H NMR (400 MHz, CDCl3) δ 7.79–7.76 (m, 2H, 2′-H, 6′-H), 7.59 (d, 1H, J = 8.0 Hz, 4-H), 7.34 (d, 1H, J = 1.6 Hz, 7-H), 7.13 (dd, 1H, J = 8.0, 1.6 Hz, 5-H), 6.95 (d, 1H, J = 8.8 Hz, 5′-H), 5.79 (s, 1H, OH), 3.95 (s, 3H, OCH3), 2.48 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ 162.8, 151.0, 149.4, 146.0, 139.8, 135.2, 125.7, 120.6, 120.5, 119.1, 113.7, 110.8, 110.7, 56.1, 21.9.
4-(6-Methylbenzo[d]oxazol-2-yl)benzene-1,2-diol (compound 5)
1H NMR (400 MHz, DMSO-d6) δ 9.68 (brs, 2H, 2 × OH), 7.53 (d, 1H, J = 8.0 Hz, 4-H), 7.52 (d, 1H, J = 2.0 Hz, 2′-H), 7.48 (d, 1H, J = 1.0 Hz, 7-H), 7.45 (dd, 1H, J = 8.4, 2.0 Hz, 6′-H), 7.12 (dd, 1H, J = 8.0, 1.0 Hz, 5-H), 6.87 (d, 1H, J = 8.4 Hz, 5′-H), 2.40 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 162.9, 150.8, 149.8, 146.3, 140.1, 135.1, 126.1, 120.0, 119.1, 118.1, 116.6, 114.7, 111.1, 21.8.
4-(6-Chlorobenzo[d]oxazol-2-yl)phenol (compound 6)
1H NMR (400 MHz, DMSO-d6) δ 10.36 (s, 1H, OH), 7.97 (d, 2H, J = 8.8 Hz, 2′-H, 6′-H), 7.87 (d, 1H, J = 2.0 Hz, 7-H), 7.69 (d, 1H, J = 8.4 Hz, 4-H), 7.36 (dd, 1H, J = 8.4, 2.0 Hz, 5-H), 6.93 (d, 2H, J = 8.8 Hz, 3′-H, 5′-H); 13C NMR (100 MHz, DMSO-d6) δ 164.2, 161.8, 150.9, 141.4, 130.0, 129.3, 125.5, 120.7, 117.2, 116.7, 111.7.
6-Chloro-2-(2,4-dimethoxyphenyl)benzo[d]oxazole (compound 7)
1H NMR (400 MHz, CDCl3) δ 8.05 (d, 1H, J = 8.4 Hz, 6′-H), 7.65 (d, 1H, J = 8.4 Hz, 4-H), 7.54 (d, 1H, J = 2.0 Hz, 7-H), 7.27 (dd, 1H, J = 8.4, 2.0 Hz, 5-H), 6.61 (dd, 1H, J = 8.4, 2.4 Hz, 5′-H), 6.58 (d, 1H, J = 2.4 Hz, 3′-H), 3.98 (s, 3H, OCH3), 3.88 (s, 3H, OCH3); 13C NMR (100 MHz, CDCl3) δ 163.9, 162.4, 160.1, 150.3, 141.2, 132.6, 130.0, 124.9, 120.4, 110.9, 108.6, 105.5, 99.2, 56.3, 55.7.
4-(6-Chlorobenzo[d]oxazol-2-yl)benzene-1,3-diol (compound 8)
1H NMR (400 MHz, DMSO-d6) δ 10.99 (s, 1H, OH), 10.39 (s, 1H, OH), 7.91 (d, 1H, J = 2.0 Hz, 7-H), 7.76 (d, 1H, J = 8.4 Hz, 6′-H), 7.72 (d, 1H, J = 8.4 Hz, 4-H), 7.40 (dd, 1H, J = 8.4, 2.0 Hz, 5-H), 6.48 (dd, 1H, J = 8.4, 2.4 Hz, 5′-H), 6.43 (d, 1H, J = 2.4 Hz, 3′-H); 13C NMR (100 MHz, DMSO-d6) δ 164.1, 163.6, 160.3, 149.5, 139.4, 129.7, 129.6, 125.9, 120.0, 111.8, 109.4, 103.4, 102.3.
5-(6-Chlorobenzo[d]oxazol-2-yl)-2-methoxyphenol (compound 9)
1H NMR (400 MHz, DMSO-d6) δ 9.61 (s, 1H, OH), 7.86 (d, 1H, J = 2.0 Hz, 7-H), 7.69 (d, 1H, J = 8.8 Hz, 4-H), 7.57 (dd, 1H, J = 8.4, 2.4 Hz, 6′-H), 7.54 (d, 1H, J = 2.4 Hz, 2′-H), 7.36 (dd, 1H, J = 8.8, 2.0 Hz, 5-H), 7.07 (d, 1H, J = 8.4 Hz, 5′-H), 3.82 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d6) δ 163.9, 151.8, 151.0, 147.5, 141.3, 129.5, 125.6, 120.8, 120.1, 118.8, 114.4, 112.9, 111.8, 56.2.
4-(6-Chlorobenzo[d]oxazol-2-yl)benzene-1,2-diol (compound 10)
1H NMR (400 MHz, DMSO-d6) δ 7.84 (d, 1H, J = 2.0 Hz, 7-H), 7.65 (d, 1H, J = 8.4 Hz, 4-H), 7.46 (d, 1H, J = 2.4 Hz, 2′-H), 7.44 (dd, 1H, J = 8.0, 2.4 Hz, 6′-H), 7.34 (dd, 1H, J = 8.4, 2.0 Hz, 5-H), 6.80 (d, 1H, J = 8.0 Hz, 5′-H); 13C NMR (100 MHz, DMSO-d6) δ 164.7, 152.6, 150.9, 147.1, 141.6, 128.9, 125.3, 120.5, 120.4, 116.5, 115.7, 114.1, 111.6.
4-(5-Methylbenzo[d]oxazol-2-yl)phenol (compound 11)
1H NMR (400 MHz, DMSO-d6) δ 10.25 (brs, 1H, OH), 7.98 (d, 2H, J = 8.8 Hz, 2′-H, 6′-H), 7.54 (d, 1H, J = 8.4 Hz, 7-H), 7.48 (d, 1H, J = 1.2 Hz, 4-H), 7.13 (dd, 1H, J = 8.4, 1.2 Hz, 6-H), 6.92 (d, 2H, J = 8.8 Hz, 3′-H, 5′-H), 2.38 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 163.4, 161.4, 148.8, 142.5, 134.4, 129.7, 126.2, 119.7, 117.8, 116.6, 110.5, 21.5.
2-(2,4-Dimethoxyphenyl)-5-methylbenzo[d]oxazole (compound 12)
1H NMR (400 MHz, CDCl3) δ 8.07 (d, 1H, J = 8.4 Hz, 6′-H), 7.54 (d, 1H, J = 2.0 Hz, 4-H), 7.40 (d, 1H, J = 8.4 Hz, 7-H), 7.09 (dd, 1H, J = 8.4, 2.0 Hz, 6-H), 6.60 (dd, 1H, J = 8.4, 2.0 Hz, 5′-H), 6.58 (d, 1H, J = 2.0 Hz, 3′-H), 3.98 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 2.45 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ 163.5, 161.9, 160.0, 148.5, 142.5, 133.9, 132.5, 125.6, 119.9, 109.6, 109.4, 105.4, 99.2, 56.2, 55.6, 21.6.
4-(5-Methylbenzo[d]oxazol-2-yl)benzene-1,3-diol (compound 13)
1H NMR (400 MHz, DMSO-d6) δ 11.25 (s, 1H, OH), 10.37 (s, 1H, OH), 7.80 (d, 1H, J = 8.4 Hz, 6′-H), 7.59 (d, 1H, J = 8.4 Hz, 7-H), 7.52 (d, 1H, J = 1.6 Hz, 4-H), 7.17 (dd, 1H, J = 8.4, 1.6 Hz, 6-H), 6.47 (dd, 1H, J = 8.4, 2.4 Hz, 5′-H), 6.42 (d, 1H, J = 2.4 Hz, 3′-H), 2.39 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 163.5, 163.2, 160.3, 147.2, 140.2, 135.1, 129.3, 126.5, 118.9, 110.7, 109.3, 103.4, 102.6, 21.5.
2-Methoxy-5-(5-methylbenzo[d]oxazol-2-yl)phenol (compound 14)
1H NMR (400 MHz, DMSO-d6) δ 9.53 (s, 1H, OH), 7.58 (dd, 1H, J = 8.4, 2.0 Hz, 6′-H), 7.55 (d, 1H, J = 8.4 Hz, 7-H), 7.55 (d, 1H, J = 2.0 Hz, 4-H), 7.49 (d, 1H, J = 2.0 Hz, 2′-H), 7.14 (dd, 1H, J = 8.4, 2.0 Hz, 6-H), 7.07 (d, 1H, J = 8.4 Hz, 5′-H), 3.82 (s, 3H, OCH3), 2.38 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 163.1, 151.5, 148.9, 147.4, 142.5, 134.5, 126.3, 119.8, 119.8, 119.5, 114.3, 112.9, 110.6, 56.2, 21.5.
4-(5-Methylbenzo[d]oxazol-2-yl)benzene-1,2-diol (compound 15)
1H NMR (400 MHz, DMSO-d6) δ 9.72 (brs, 1H, OH), 9.45 (brs, 1H, OH), 7.53 (d, 1H, J = 8.4 Hz, 7-H), 7.52 (d, 1H, J = 2.0 Hz, 4-H), 7.48–7.45 (m, 2H, 2′-H, 6′-H), 7.11 (dd, 1H, J = 8.4, 2.0 Hz, 6-H), 6.87 (d, 1H, J = 8.0 Hz, 5′-H), 2.38 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ 163.5, 149.9, 148.8, 146.3, 142.6, 134.4, 126.1, 120.1, 119.6, 118.1, 116.7, 114.8, 110.5, 21.5.
3.2. Reagents for Biological Experiments
Potassium hydrogen phosphate, potassium dihydrogen phosphate, phenylmethylsulfonyl fluoride, 3-morpholinosydnomimine (SIN-1), 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), α-melanocyte-stimulating hormone (α-MSH), mushroom tyrosinase, dimethyl sulfoxide (DMSO), l-tyrosine, and l-dopa were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.3. Tyrosinase Activity Assay Using Mushroom Tyrosinase
The tyrosinase inhibitory activity of the 2-phenylbenzoxazoles was evaluated using a mushroom tyrosinase activity assay, following a previously described method [46]. An aqueous substrate mixture, consisting of phosphate buffer (pH 6.5; 17.2 mM) and l-tyrosine or l-dopa (345 μM), was prepared. A 96-well plate containing the test sample in dimethyl sulfoxide (DMSO) solution (10 μL), aqueous mushroom tyrosinase solution (20 μL; 200 units), and aqueous substrate mixture (170 μL) in each well was incubated at 37 °C for 15 min for l-dopa and 30 min for l-tyrosine, respectively. Using a VersaMax® ELISA reader (VersaMax Pro 6.5.1; Molecular Devices, Sunnyvale, CA, USA), the well absorbance at 475 nm was recorded to calculate the % tyrosinase activity inhibition: % tyrosinase activity inhibition = (1 − Abssam/Abscon) × 100, where Abssam and Abscon are the absorbances of the samples and control, respectively. Kojic acid was used to compare tyrosinase inhibitory activity. For IC50 values, test samples were used at three concentrations (4, 20, and 100 μM for 1, 2, 4, 5, 7, and 9–12, 2, 10, and 50 μM for 6, 14, 15, and kojic acid, 0.16, 0.8, and 4 μM for 8 and 13, and 0.032, 0.8, and 4 μM for 3 in the presence of l-tyrosine; 4, 20, and 100 μM for 1, 2, 4–7, 9–12, 14, 15, and kojic acid and 0.8, 4, and 20 μM for 3, 8, and 13 in the presence of l-dopa).
3.4. Kinetic Study Experiment Using Mushroom Tyrosinase
Mushroom tyrosinase was incubated with 2-phenylbenzoxazole inhibitors (3, 8, or 13) in the presence of various concentrations (0.5–16 mM) of l-dopa to measure the initial dopachrome production rate. An aqueous substrate mixture, consisting of phosphate buffer (pH 6.5; 17.2 mM) and l-dopa (345 μM), was prepared. A 96-well plate containing the inhibitor DMSO solution (10 μL), aqueous mushroom tyrosinase solution (20 μL; 20 units), and aqueous substrate mixture (170 μL) in each well was incubated for 20 min at 37 °C. During incubation, the absorbance at 475 nm was recorded at 5 min intervals using an ELISA reader to prepare Lineweaver–Burk plots for each inhibitor.
3.5. Docking Simulation Using AutoDock Vina
The 2-phenylbenzoxazole compounds 3, 8, and 13, which exhibited the most potent mushroom tyrosinase inhibitory activities, were selected for the docking simulation. The ligand 3D structure for the docking simulation was prepared using Chem3D Pro 12.0. The 3D X-ray crystal structure of mushroom tyrosinase (PDB ID: 2Y9X) was obtained from the RCSB Protein Data Bank (PDB). Docking simulations between the ligand (2-phenylbenzoxazoles 3, 8, and 13 and kojic acid [positive material]) and tyrosinase were conducted using AutoDock Vina 1.2.0 after the original ligand, tropolone, was removed from the active site. Chimera 1.13.1 and LigandScout 4.3 were utilized to obtain information on the plausible chemical interactions between the ligand and tyrosinase amino acid residues.
3.6. Cell Culture
B16F10 cells and HaCaT cells, obtained from the American Type Culture Collection (VA, USA), were cultured under an environment including 5% CO2 at 37 °C in Dulbecco’s modified Eagle’s medium, including 10% heat-inactivated FBS and penicillin–streptomycin (100X) solution (Welgene, Gyeongsangbuk-do, Republic of Korea).
3.7. B16F10 Cell Viability Assay
The influence of compounds 3, 8, and 13 on cytotoxicity was measured in B16F10 cells using a previously described method [47]. The B16F10 cells were inoculated at a density of 1 × 103 cells per well and cultured for 24 h in a 96-well plate under the same incubation conditions used for the cell culture. Test samples (3, 8, and 13) were added to each well at final concentrations of 0, 1, 2, and 5 μM and cultivated for 48 and 72 h, respectively. Each well was treated with 10 μL of EZ-Cytox solution (EZ-1000®, DoGenBio, Seoul, Republic of Korea) and cultivated for 2 h. The absorbance of each well was measured at 450 nm using an ELISA reader.
3.8. Cellular Melanin Content Level Assay in B16F10 Cells
The effects of compounds 3, 8, and 13 on B16F10 cellular melanin production were investigated using a previously reported method [48]. B16F10 cells (5 × 103 cells per well) were seeded in a 6-well plate and cultured under the same conditions as the cell culture for 24 h. The cells were pre-treated with test samples (kojic acid [positive control]: 5 μM or compounds 3, 8, and 13: 1, 2, and 5 μM) for 1 h. Stimulators, including 1 μM α-melanocyte-stimulating hormone (α-MSH) and 200 μM 3-isobutyl-1-methylxanthine (IBMX), were then added. After 72 h of cultivation, the cells were rinsed with PBS, detached from the bottom of each well using Trypsin-EDTA, and centrifuged for 10 min at 10,000× g and 4 °C. The obtained pellets were lysed with 100 μL of 1N-NaOH solution for 1 h at 60 °C and transferred to a 96-well plate. The optical density was measured at 405 nm using an ELISA reader. Normalization was conducted using a Pierce BCA Protein Assay Kit (Thermo Scientific, MA, USA).
Melanin content (%) = (ΔODsam/ΔODcon) × 100%, where ODsam and ODcon represent the optical densities of the sample and control, respectively.
3.9. Cellular Tyrosinase Activity Assay in B16F10 Cells
The effects of compounds 3, 8, and 13 on B16F10 cellular tyrosinase activity were assessed using a previously reported method [48]. B16F10 cells were seeded, treated with the samples and stimulators, and cultured for 72 h in the same manner as for the cellular melanin content assay. To lyse the cultured cells, a lysis buffer was prepared containing 1 mM phenylmethylsulfonyl fluoride, 50 mM phosphate buffer, and 1% Triton X-100 (5:90:5, v/v/v). The cultivated cells were lysed by adding the prepared lysis buffer (100 µL) after washing twice with PBS. The lysed cells were centrifuged for 30 min at 4 °C and 10,000 g after freezing for 1 h at −80 °C. The supernatants (80 µL) and 10 mM l-dopa (20 µL) were added to each well of a 96-well plate and cultivated for 10 min at 37 °C. The optical density was measured at 475 nm using an ELISA reader. Normalization was performed using a Pierce BCA Protein Assay Kit.
3.10. In Situ Tyrosinase Activity Assay Using B16F10 Cells and l-dopa
In situ tyrosinase activity was measured in B16F10 cells using l-dopa, as reported previously [34]. Compounds 3, 8, and 13, which exhibited potent melanin inhibition in the B16F10 cells, were tested at 1, 2, and 5 μM. The B16F10 cells were seeded at a density of 1 × 103 cells per well in a 24-well microplate at 37 °C with 5% CO2 for 24 h. Before exposure to stimulators (200 μM IBMX and 1 μM α-MSH), the cells were pre-treated with test samples (compounds 3, 8, and 13 or kojic acid [positive material; 5 μM]) for 1 h. The cells were processed as follows: (1) fixing with 4% paraformaldehyde for 40 min, (2) washing with PBS, and (3) permeabilizing with 0.1% Triton X-100 for 2 min. After washing with PBS, the cells were stained with l-dopa (2 mM; 500 μL) at 37 °C for 2 h. Stained images were collected using a camera connected to a microscope (Motic, Hong Kong).
3.11. HaCaT Cell Viability Assay
The cytotoxicity of compounds 3, 8, and 13 was assessed in HaCaT cells using a previously described method [38]. The HaCaT cells were inoculated at a density of 1 × 104 cells/well in a 96-well plate and cultured for 24 h under the standard incubation conditions. Test samples (3, 8, and 13) were added at the final concentrations of 0, 2, 5, and 10 μM and incubated for an additional 24 h. Each well was treated with 10 μL of EZ-Cytox solution and cultivated for 2 h. The absorbance of each well was measured at 450 nm using an ELISA reader.
3.12. In Vivo Depigmentation Experiment Using Zebrafish Embryos
A depigmentation experiment was performed using zebrafish embryos, as previously described [38,49,50]. Wild-type zebrafish (Danio rerio) were maintained in a fish tank at 28 °C and fed with dried brine shrimp (Artemia salina, San Francisco Bay Brand, San Francisco, CA, USA) three times daily. The zebrafish embryos were obtained through natural mating in mating cages. The obtained zebrafish embryos were transferred to a 90 mm culture dish containing 200 μL of E3-methylene blue (MB) solution, consisting of 0.001% MB, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, and 5 mM NaCl, and incubated in an incubator set at 28 °C. At 24 h post-fertilization (hpf), the zebrafish embryos were dechorionated using pronase (Sigma-Aldrich, St. Louis, MO, USA). Test samples (20 mM kojic acid, a positive control, 0.025 mM compound 8, and 0.025 and 0.05 mM compound 13) were added to each well of a 48-well plate containing four dechorionated zebrafish embryos and 200 μL of E3 solution per well. The 48-well plates were incubated in an incubator set at 28 °C for 48 h. At 76 hpf, the zebrafish larvae were mounted on a 1% methylcellulose block after anesthesia with tricaine methanesulfonate (Thermo Fisher Scientific, Waltham, MA, USA), and photographs of the zebrafish larvae were obtained using a SMZ745T stereoscopic microscope (Nikon, Tokyo, Japan). The pigmented areas of the zebrafish larvae were determined using the CS analyzer 3.0 image analysis software (ATTO, Tokyo, Japan).
3.13. Measurement of Melanin Content and Tyrosinase Activity in Zebrafish Larvae
The effects of the 2-phenylbenzo[d]oxazole compound 8 on the melanin content and tyrosinase activity in the zebrafish larvae were assessed using a previously defined procedure with minor modifications [49,51]. To quantify the melanin content in the zebrafish larvae, zebrafish embryos were obtained through natural mating and operated in a similar manner to the in vivo depigmentation experiment (25 zebrafish embryos per well in a 24-well plate; dechorionation at 24 hpf; test sample treatment (8 [0.025 mM] and kojic acid [20 mM; positive control]) at 28 hpf). Melanin quantification and tyrosinase activity were quantified at 74 hpf. For melanin quantification, the zebrafish larvae were lysed in RIPA buffer (Biosesang, Gyeonggi-do, Korea) and homogenized using a sonicator. After centrifuging the lysates (10,000× g and 10 min), the pellet was dissolved in 100 μL of 1N NaOH at 100 °C for 30 min and roughly vortexed to solubilize the melanin. The absorbance was measured at 405 nm using a microplate reader. For the tyrosinase activity measurement, the supernatants were transferred to new Eppendorf tubes and the protein was quantified using a Pierce BCA protein assay kit, with the absorbance being measured at 570 nm using a microplate reader. A total of 500 μg of protein in 100 μL of protein lysate was transferred into each well of a 96-well plate, followed by 100 μL of 1 mM l-dopa. The mixture was incubated for 1 h at 37 °C. The absorbance was measured at 475 nm to determine the tyrosinase activity. Kojic acid was used as a positive control.
3.14. 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
As previously reported [52,53], the ability of compounds 1–15 to remove DPPH radicals was evaluated. A 0.2 mM methanolic DPPH solution was mixed with a 5 mM l-ascorbic acid aqueous solution or 5 mM DMSO solution of 1–15 at a ratio of 10:1 (v/v, 180 μL:20 μL) in each well of a 96-well microplate. After incubating the microplate in the dark for 30 min, the optical density of each well was measured at 517 nm using an ELISA reader.
DPPH scavenging activity (%) = (OD[control] − OD[sample]) × 100/OD[control].
3.15. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Radical Cation Scavenging Assay
The effect of the 2-phenylbenzoxazole compounds 1–15 on radical scavenging was assessed using an ABTS assay, as previously reported [54]. An ABTS aqueous solution (7 mM) was mixed with a K2S2O8 aqueous solution (2.45 mM) in equal volumes (each 20 mL) and incubated in the dark for 20 h at 25 °C to generate ABTS+•. The ABTS+• solution was then diluted with pure EtOH to adjust the absorbance of the solution to 0.7 ± 0.02 at 730 nm. The test samples (1–15 and Trolox [positive control]) were dissolved in a co-solvent of EtOH and DMSO (9:1, v/v) to make a 1 mM solution. The diluted ABTS+• solution was mixed with the test sample solution at a volume ratio of 9:1 (90 μL:10 μL) and the mixture was stored for 2 min at 20 °C in the dark. The optical density was measured at 730 nm using an ELISA reader at 1 min intervals for 10 min. All test samples were used at a final concentration of 100 µM.
ABTS+• radical scavenging activity (%) = [(ODcon − ODsam)/ODcon] × 100, where ODsam and ODcon represent the absorbances of the test sample and the control, respectively.
3.16. Reactive Oxygen Species (ROS) Scavenging Assay
The ability of compounds 1–15 to remove ROS was assayed as previously reported [55]. 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA; 1.25 mM, 50 μL) was treated with esterase (0.6 units/μL, 50 μL) in phosphate pH 7.4 buffer (50 mM, 4.9 mL) and left at 25 °C for 30 min to generate DCFH. A 3-morpholinosydnonimine (SIN-1) solution (10 μL, 250 μM) and phosphate buffer (180 μL) were mixed with a test sample (1–15; 10 μL, 1 mM) in DMSO solution or Trolox (10 μL, 1 mM; positive control) in each well of a 96-well black plate and left in the dark 25 °C for 5 min. The SIN-1-test sample mixture was then combined with the generated DCFH solution (50 μL). Fluorescence was measured in 5 min intervals for 30 min at 530 nm using a microplate reader (Berthold Advances GmbH & Co., Bad Wildbad, Germany) with excitation at 485 nm.
3.17. Statistical Analysis
Results from three independent experiments are presented as the mean ± SEM. The statistical significance between groups was assessed using one-way ANOVA followed by the Newman–Keuls test using GraphPad Prism 5 (La Jolla, CA, USA). p < 0.05 was considered to be significant.
4. Conclusions
Compounds with a 2-phenylbenzo[d]thiazole scaffold exhibit potent tyrosinase inhibitory activity against murine and mushroom tyrosinases, and 2-phenylbenzo[d]thiazole is bioisosteric with 2-phenylbenzo[d]oxazole. Therefore, we designed and synthesized 15 compounds with a 2-phenylbenzo[d]oxazole scaffold as potential tyrosinase inhibitors. Among them, compounds 3, 8, and 13 demonstrated potent mushroom tyrosinase inhibition, with compound 3 having a nanomolar IC50 value of 0.51 ± 0.00 μM, which was significantly lower than that of kojic acid (IC50 value: 14.33 ± 1.63 μM). Kinetic studies confirmed that 3 is a mixed-type inhibitor, while 8 and 13 are competitive inhibitors. Docking simulations identified the chemical interactions occurring between the amino acid residues of tyrosinase and these compounds. In B16F10-cell-based experiments, compounds 3, 8, and 13 inhibited cellular tyrosinase activity and melanin production more effectively than kojic acid. Additionally, a staining method using a l-dopa to measure in situ tyrosinase activity also demonstrated that these compounds had stronger B16F10 cellular tyrosinase inhibitory activity than kojic acid. Compounds 3, 8, and 13 also exhibited superior depigmentation effects on zebrafish larvae, even at concentrations hundreds of times lower than those of kojic acid. Although the 2-phenylbenzo[d]oxazole derivatives were shown to be promising tyrosinase inhibitors, there are structural differences between the tetramer of mushroom tyrosinase and the glycosylated monomer of human tyrosinase. In addition, pharmacokinetic parameters cannot be predicted using simple animal models. Therefore, further studies are needed for clinical application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29174162/s1, Figures S1–S30: 1H and 13C NMR (nuclear magnetic resonance) spectroscopy data of compounds 1–15.
Author Contributions
Conceptualization, H.Y.C. and H.R.M.; formal analysis, H.J.J., H.S.P. (Hyeon Seo Park) and D.Y.; funding acquisition, H.J.J. and H.R.M.; resources, H.S.P. (Hyeon Seo Park), H.S.P. (Hye Soo Park) and H.J.K.; software, Y.P. and P.C.; supervision, H.R.M.; validation, H.J.J., H.S.P. (Hyeon Seo Park), H.S.P. (Hye Soo Park) and H.J.K.; writing—original draft, H.J.J. and H.R.M.; writing—review and editing, P.C., H.Y.C. and H.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the 2024 Post-Doc. Development Program of Pusan National University, and the Basic Science Research Program of the National Research Foundation (NRF) of Korea funded by the Ministry of Education (grant no. NRF-2021R1I1A1A01052284).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article and Supplementary Materials.
Acknowledgments
We thank the Zebrafish Center for Disease Modeling (ZCDM), Chungnam National University (Daejeon, Korea), for providing wild-type zebrafish (Danio rerio).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Choi, K.-Y. Bioprocess of Microbial Melanin Production and Isolation. Front. Bioeng. Biotechnol. 2021, 9, 765110. [Google Scholar] [CrossRef] [PubMed]
- Narang, U.; Kurian, N.K.; Bayram, S. Chapter 16—Enigmatic Secondary Metabolites: Microbial Melanins and Their Applications. In Fungal Secondary Metabolites; Abd-Elsalam, K.A., Mohamed, H.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 337–351. [Google Scholar]
- Pena, A.-M.; Ito, S.; Bornschlögl, T.; Brizion, S.; Wakamatsu, K.; Del Bino, S. Multiphoton FLIM Analyses of Native and UVA-Modified Synthetic Melanins. Int. J. Mol. Sci. 2023, 24, 4517. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.Z.; Zhou, A.E.; Hoegler, K.M.; Khachemoune, A. Oculocutaneous albinism: Epidemiology, genetics, skin manifestation, and psychosocial issues. Arch. Dermatol. Res. 2023, 315, 107–116. [Google Scholar] [CrossRef]
- Zamudio Díaz, D.F.; Busch, L.; Kröger, M.; Klein, A.L.; Lohan, S.B.; Mewes, K.R.; Vierkotten, L.; Witzel, C.; Rohn, S.; Meinke, M.C. Significance of melanin distribution in the epidermis for the protective effect against UV light. Sci. Rep. 2024, 14, 3488. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, Y.; Zhang, Y.; Huang, P.; Yan, J.; Song, Z.; Yuan, Q.; Huang, J. A Reactive Oxygen Species-Tyrosinase Cascade-Activated Prodrug for Selectively Suppressing Melanoma. CCS Chem. 2022, 4, 1654–1670. [Google Scholar] [CrossRef]
- Weerawardana, M.B.S.; Thiripuranathar, G.; Paranagama, P.A. Natural Antibrowning Agents against Polyphenol Oxidase Activity in Annona muricata and Musa acuminata. J. Chem. 2020, 2020, 1904798. [Google Scholar] [CrossRef]
- Marshall, M.R.; Kim, J.M.; Wei, C.-i. Enzymatic browning in fruits, vegetables and sea foods. Chem. Agric. Food Sci. 2000, 41, 259–312. [Google Scholar]
- Lv, J.; Jiang, S.; Yang, Y.; Zhang, X.; Gao, R.; Cao, Y.; Song, G. FGIN-1-27 Inhibits Melanogenesis by Regulating Protein Kinase A/cAMP-Responsive Element-Binding, Protein Kinase C-β, and Mitogen-Activated Protein Kinase Pathways. Front. Pharmacol. 2020, 11, 602889. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Ullah, S.; Son, S.; Yun, H.Y.; Kim, D.H.; Chun, P.; Moon, H.R. Tyrosinase inhibitors: A patent review (2011-2015). Expert Opin. Ther. Pat. 2016, 26, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Mota, S.; Rosa, G.P.; Barreto, M.C.; Garrido, J.; Sousa, E.; Cruz, M.T.; Almeida, I.F.; Quintas, C. Comparative Studies on the Photoreactivity, Efficacy, and Safety of Depigmenting Agents. Pharmaceuticals 2024, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Zhang, P.-X.; Shen, R.; Xu, M.; Han, L.; Shi, X.; Zhou, Z.-R.; Yang, J.-Y.; Liu, J.-Q. Determination of arbutin in vitro and in vivo by LC-MS/MS: Pre-clinical evaluation of natural product arbutin for its early medicinal properties. J. Ethnopharmacol. 2024, 330, 118232. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, J.O.; Fabiyi, O.S.; Adelakin, L.A.; Ekwerike, M.C. Effects of Skin Lightening Cream Agents—Hydroquinone and Kojic Acid, on the Skin of Adult Female Experimental Rats. Clin. Cosmet. Investig. Dermatol. 2020, 13, 283–289. [Google Scholar] [CrossRef]
- Galache, T.R.; Galache, M.; Sena, M.M.; Pavani, C. Amber photobiomodulation versus tranexamic acid for the treatment of melasma: Protocol for a double-blind, randomised controlled trial. BMJ Open 2023, 13, e073568. [Google Scholar] [CrossRef]
- Li, J.; Duan, N.; Song, S.; Nie, D.; Yu, M.; Wang, J.; Xi, Z.; Li, J.; Sheng, Y.; Xu, C.; et al. Transfersomes improved delivery of ascorbic palmitate into the viable epidermis for enhanced treatment of melasma. Int. J. Pharm. 2021, 608, 121059. [Google Scholar] [CrossRef]
- Westerhof, W.; Kooyers, T.J. Hydroquinone and its analogues in dermatology—A potential health risk. J. Cosmet. Dermatol. 2005, 4, 55–59. [Google Scholar] [CrossRef]
- Gaskell, M.; McLuckie, K.I.; Farmer, P.B. Genotoxicity of the benzene metabolites parabenzoquinone and hydroquinone. Chem. Biol. Interact. 2005, 153, 267–270. [Google Scholar] [CrossRef]
- Ogiwara, Y.; Sugiura, M.; Watanabe, K.; Tawara, J.; Endo, E.; Maruyama, H.; Tsuji, S.; Matsue, K.; Yamada, H.; Wako, Y.; et al. Evaluation of the repeated-dose liver, bone marrow and peripheral blood micronucleus and comet assays using kojic acid. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 780, 111–116. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Sang-Hun Jung, S.H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 17, 7395–7418. [Google Scholar] [CrossRef]
- Ha, Y.M.; Kim, J.-A.; Park, Y.J.; Park, D.; Choi, Y.J.; Kim, J.M.; Chung, K.W.; Han, Y.K.; Park, J.Y.; Lee, J.Y.; et al. Synthesis and biological activity of hydroxybenzylidenyl pyrrolidine-2,5-dione derivatives as new potent inhibitors of tyrosinase. MedChemComm 2011, 2, 542–549. [Google Scholar] [CrossRef]
- Nitulescu, G.; Margina, D.; Zanfirescu, A.; Olaru, O.T.; Nitulescu, G.M. Targeting Bacterial Sortases in Search of Anti-virulence Therapies with Low Risk of Resistance Development. Pharmaceuticals 2021, 14, 415. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, G.S.; Bhargava, A.; Pal Singh, G.; Joshi, S.D.; Singh Chundawat, N. Synthesis, molecular simulation studies, in vitro biological assessment of 2-substituted benzoxazole derivatives as promising antimicrobial agents. Turk. J. Chem. 2023, 47, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Sirgamalla, R.; Kommakula, A.; Konduru, S.; Ponakanti, R.; Devaram, J.; Boda, S. Cupper-catalyzed an efficient synthesis, characterization of 2-substituted benzoxazoles, 2-substituted benzothiazoles derivatives and their anti-fungal activity. Chem. Data Collect. 2020, 27, 100362. [Google Scholar] [CrossRef]
- Desai, S.; Desai, V.; Shingade, S. In-vitro Anti-cancer assay and apoptotic cell pathway of newly synthesized benzoxazole-N-heterocyclic hybrids as potent tyrosine kinase inhibitors. Bioorg. Chem. 2020, 94, 103382. [Google Scholar] [CrossRef]
- Johnson, S.M.; Connelly, S.; Wilson, I.A.; Kelly, J.W. Biochemical and Structural Evaluation of Highly Selective 2-Arylbenzoxazole-Based Transthyretin Amyloidogenesis Inhibitors. J. Med. Chem. 2008, 51, 260–270. [Google Scholar] [CrossRef]
- Jung, H.J.; Noh, S.G.; Park, Y.; Kang, D.; Chun, P.; Chung, H.Y.; Moon, H.R. In vitro and in silico insights into tyrosinase inhibitors with (E)-benzylidene-1-indanone derivatives. Comput. Struct. Biotechnol. J. 2019, 17, 1255–1264. [Google Scholar] [CrossRef]
- Ullah, S.; Kang, D.; Lee, S.; Ikram, M.; Park, C.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Synthesis of cinnamic amide derivatives and their anti-melanogenic effect in α-MSH-stimulated B16F10 melanoma cells. Eur. J. Med. Chem. 2019, 161, 78–92. [Google Scholar] [CrossRef]
- Ko, J.; Lee, J.; Jung, H.J.; Ullah, S.; Jeong, Y.; Hong, S.; Kang, M.K.; Park, Y.J.; Hwang, Y.; Kang, D.; et al. Design and Synthesis of (Z)-5-(Substituted benzylidene)-3-cyclohexyl-2-thioxothiazolidin-4-one Analogues as Anti-Tyrosinase and Antioxidant Compounds: In Vitro and In Silico Insights. Antioxidants 2022, 11, 1918. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, Y.; Jin Jung, H.; Ullah, S.; Ko, J.; Young Kim, G.; Yoon, D.; Hong, S.; Kang, D.; Park, Y.; et al. Anti-tyrosinase flavone derivatives and their anti-melanogenic activities: Importance of the β-phenyl-α,β-unsaturated carbonyl scaffold. Bioorg. Chem. 2023, 135, 106504. [Google Scholar] [CrossRef]
- Qi, S.-L.; Liu, Y.-P.; Li, Y.; Luan, Y.-X.; Ye, M. Ni-catalyzed hydroarylation of alkynes with unactivated β-C(sp2)−H bonds. Nat. Commun. 2022, 13, 2938. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.G.; Saura-Sanmartin, A.; Berna, J.; Teruel, J.A.; Muñoz, J.L.M.; López, J.N.R.; Cánovas, F.G.; García Molina, F.G. Considerations about the inhibition of monophenolase and diphenolase activities of tyrosinase. Characterization of the inhibitor concentration which generates 50% of inhibition, type and inhibition constants. A review. Int. J. Biol. Macromol. 2024, 267, 131513. [Google Scholar]
- Park, J.; Jung, H.; Jang, B.; Song, H.-K.; Han, I.-O.; Oh, E.-S. D-tyrosine adds an anti-melanogenic effect to cosmetic peptides. Sci. Rep. 2020, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Jin Jung, H.; Jin Kim, H.; Soo Park, H.; Young Kim, G.; Jung Park, Y.; Lee, J.; Kyung Kang, M.; Yoon, D.; Kang, D.; Park, Y.; et al. Highly potent anti-melanogenic effect of 2-thiobenzothiazole derivatives through nanomolar tyrosinase activity inhibition. Bioorg. Chem. 2024, 150, 107586. [Google Scholar] [CrossRef]
- Lamason, R.L.; Mohideen, M.A.; Mest, J.R.; Wong, A.C.; Norton, H.L.; Aros, M.C.; Jurynec, M.J.; Mao, X.; Humphreville, V.R.; Humbert, J.E.; et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 2005, 310, 1782–1786. [Google Scholar] [CrossRef]
- Santoriello, C.; Zon, L.I. Hooked! Modeling human disease in zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef]
- Yoon, D.; Jung, H.J.; Lee, J.; Kim, H.J.; Park, H.S.; Park, Y.J.; Kang, M.K.; Kim, G.Y.; Kang, D.; Park, Y.; et al. In vitro and in vivo anti-pigmentation effects of 2-mercaptobenzimidazoles as nanomolar tyrosinase inhibitors on mammalian cells and zebrafish embryos: Preparation of pigment-free zebrafish embryos. Eur. J. Med. Chem. 2024, 266, 116136. [Google Scholar] [CrossRef]
- Han, H.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Heo, H.J. Anti-Melanogenic Effect of Ethanolic Extract of Sorghum bicolor on IBMX-Induced Melanogenesis in B16/F10 Melanoma Cells. Nutrients 2020, 12, 832. [Google Scholar] [CrossRef]
- Shrivastava, D. Synthesis, evaluation and antimicrobial activity of benzoxazole derivatives. World J. Pharm. Pharm. Sci. 2012, 1, 754–774. [Google Scholar]
- Temiz, Ö.; Ören, İ.; Şener, E.; Yalçin, İ.; Uçartürk, N. Synthesis and microbiological activity of some novel 5- or 6-methyl-2-(2,4-disubstituted phenyl) benzoxazole derivatives. Il Farm. 1998, 53, 337–341. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, W.; Zhang, X.; Yin, L.; Chen, B.; Song, J. Synthesis and pharmacological evaluation of piperidine (piperazine)-substituted benzoxazole derivatives as multi-target antipsychotics. Bioorg. Med. Chem. Lett. 2015, 25, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Thi Hang, A.-H. Deep eutectic solvent-catalyzed arylation of benzoxazoles with aromatic aldehydes. RSC Adv. 2018, 8, 11127–11133. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Thi Hang, A.-H.; Ho Nguyen, T.-L.; Nguyen Chau, D.-K.; Tran, P.H. Phosphonium acidic ionic liquid: An efficient and recyclable homogeneous catalyst for the synthesis of 2-arylbenzoxazoles, 2-arylbenzimidazoles, and 2-arylbenzothiazoles. RSC Adv. 2018, 8, 11834–11842. [Google Scholar] [CrossRef] [PubMed]
- Schrader, F.C.; Glinca, S.; Sattler, J.M.; Dahse, H.-M.; Afanador, G.A.; Prigge, S.T.; Lanzer, M.; Mueller, A.-K.; Klebe, G.; Schlitzer, M. Novel Type II Fatty Acid Biosynthesis (FAS II) Inhibitors as Multistage Antimalarial Agents. ChemMedChem 2013, 8, 442–461. [Google Scholar] [CrossRef]
- Hyun, S.K.; Lee, W.-H.; Jeong, D.M.; Kim, Y.; Choi, J.S. Inhibitory Effects of Kurarinol, Kuraridinol, and Trifolirhizin from <i>Sophora flavescens</i> on Tyrosinase and Melanin Synthesis. Biol. Pharm. Bull. 2008, 31, 154–158. [Google Scholar] [CrossRef]
- Jung, H.J.; Choi, D.C.; Noh, S.G.; Choi, H.; Choi, I.; Ryu, I.Y.; Chung, H.Y.; Moon, H.R. New Benzimidazothiazolone Derivatives as Tyrosinase Inhibitors with Potential Anti-Melanogenesis and Reactive Oxygen Species Scavenging Activities. Antioxidants 2021, 10, 1078. [Google Scholar] [CrossRef]
- Lim, H.Y.; Kim, E.; Park, S.H.; Hwang, K.H.; Kim, D.; Jung, Y.J.; Kopalli, S.R.; Hong, Y.D.; Sung, G.H.; Cho, J.Y. Antimelanogenesis Effects of Theasinensin A. Int. J. Mol. Sci. 2021, 22, 7453. [Google Scholar] [CrossRef]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment. Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef]
- Chen, W.C.; Tseng, T.S.; Hsiao, N.W.; Lin, Y.L.; Wen, Z.H.; Tsai, C.C.; Lee, Y.C.; Lin, H.H.; Tsai, K.C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef]
- Hwang, K.S.; Yang, J.Y.; Lee, J.; Lee, Y.R.; Kim, S.S.; Kim, G.R.; Chae, J.S.; Ahn, J.H.; Shin, D.S.; Choi, T.Y.; et al. A novel anti-melanogenic agent, KDZ-001, inhibits tyrosinase enzymatic activity. J. Dermatol. Sci. 2018, 89, 165–171. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Boyles, M.; Murphy, F.; Mueller, W.; Wohlleben, W.; Jacobsen, N.R.; Braakhuis, H.; Giusti, A.; Stone, V. Development of a standard operating procedure for the DCFH2-DA acellular assessment of reactive oxygen species poduced by nanomaterials. Toxicol. Mech. Methods 2022, 32, 439–452. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).