Recent Advances in Metal–Organic Frameworks and Their Derivatives for Adsorption of Radioactive Iodine
Abstract
1. Introduction
2. Performance and Mechanisms of Iodine Adsorption over MOFs and Their Derivatives
2.1. Van Der Waals Interactions
2.2. Complexing Interactions
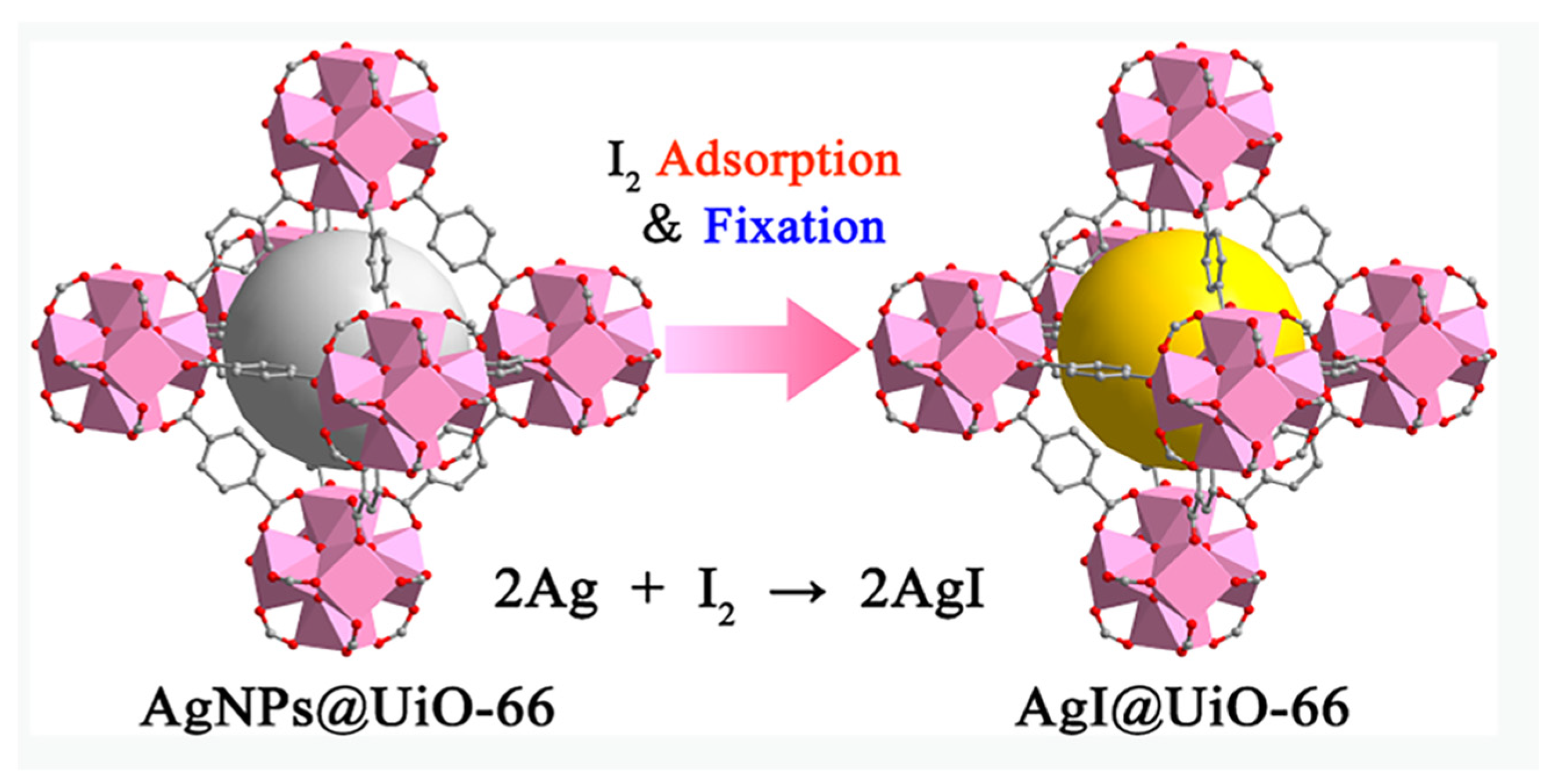
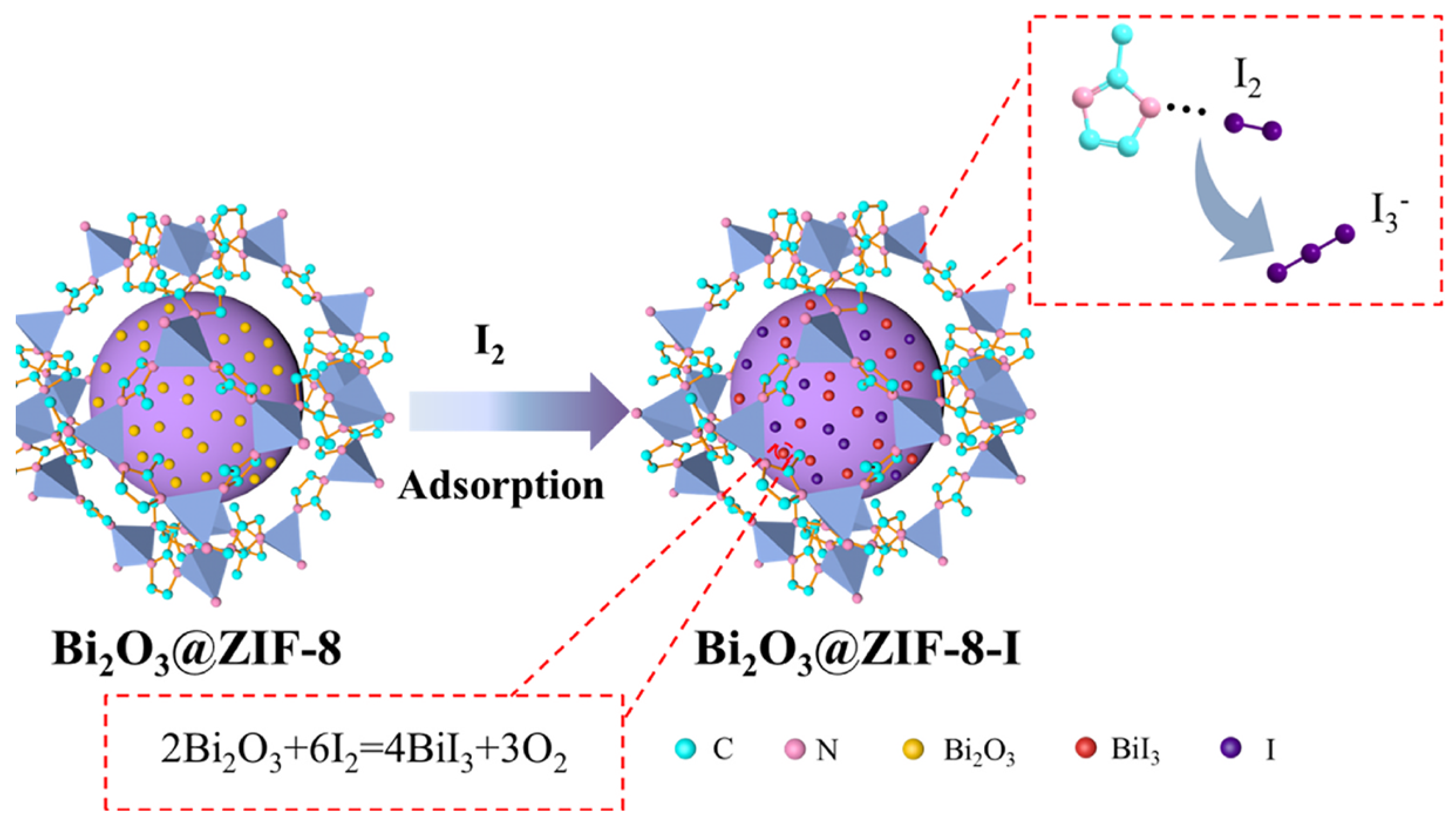
2.3. Chemical Precipitation
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rozhkova, A.; Logutenkova, E.; Dodonova, K.; Kuzmenkova, N.; Petrov, V. Sorption behavior of fission products (Tc, I) in marine and freshwater bottom sediments. J. Radioanal. Nucl. Chem. 2024, 333, 1963–1973. [Google Scholar] [CrossRef]
- Kurisingal, J.F.; Yun, H.; Hong, C.S. Porous organic materials for iodine adsorption. J. Hazard. Mater. 2023, 458, 131835. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Shahbazi-Gahrouei, D. A review on theranostic applications of iodine nanoparticles: Recent findings and perspectives. Nanomed. J. 2021, 8, 234–240. [Google Scholar]
- Zhang, X.; Maddock, J.; Nenoff, T.M.; Denecke, M.A.; Yang, S.; Schröder, M. Adsorption of iodine in metal–organic framework materials. Chem. Soc. Rev. 2022, 51, 3243–3262. [Google Scholar] [CrossRef] [PubMed]
- Shamim, M.A.; Zia, H.; Zeeshan, M.; Khan, M.Y.; Shahid, M. Metal organic frameworks (MOFs) as a cutting-edge tool for the selective detection and rapid removal of heavy metal ions from water: Recent progress. J. Environ. Chem. Eng. 2022, 10, 106991. [Google Scholar] [CrossRef]
- Jaswal, A.; Singh, A.; Sarkar, S.; Singh, M. Optimized Phytoremediation Process for the Sustainable Management of Radionuclides in the Food Chain. J. Food. Chem. Nanotechnol. 2023, 9, S291–S299. [Google Scholar]
- Fujiwara, H. Observation of radioactive iodine (131I, 129I) in cropland soil after the Fukushima nuclear accident. Sci. Total Environ. 2016, 566, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Zia, H.; Shamim, M.A.; Zeeshan, M.; Khan, M.Y.; Shahid, M. Metal organic frameworks as a versatile platform for the radioactive iodine capture: State of the art developments and future prospects. Inorg. Chim. Acta 2022, 539, 121026. [Google Scholar] [CrossRef]
- Hao, Y.; Tian, Z.; Liu, C.; Xiao, C. Recent advances in the removal of radioactive iodine by bismuth-based materials. Front. Chem. 2023, 11, 1122484. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, X.; Wang, H.; Ma, D.; Tan, K.; Jensen, S.; Deibert, B.J.; Butler, J.; Cure, J.; Shi, Z. Capture of organic iodines from nuclear waste by metal-organic framework-based molecular traps. Nat. Commun. 2017, 8, 485. [Google Scholar] [CrossRef]
- Haymart, M.R.; Banerjee, M.; Stewart, A.K.; Koenig, R.J.; Birkmeyer, J.D.; Griggs, J.J. Use of radioactive iodine for thyroid cancer. JAMA 2011, 306, 721–728. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, C.D.; Jimenez, J.L.; Bahreini, R.; Flagan, R.C.; Seinfeld, J.H.; Hämeri, K.; Pirjola, L.; Kulmala, M.; Jennings, S.G.; Hoffmann, T. Marine aerosol formation from biogenic iodine emissions. Nature 2002, 417, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, K.; Predeep, P. Thermal Stability, Crystallization, and Melting Behaviours of Iodine Doped Semiconducting Polyethylene Terephthalate (PET) Thin Films. J. Macromol. Sci. Part B Phys. 2024. [Google Scholar] [CrossRef]
- Sava, D.F.; Garino, T.J.; Nenoff, T.M. Iodine confinement into metal–organic frameworks (MOFs): Low-temperature sintering glasses to form novel glass composite material (GCM) alternative waste forms. Ind. Eng. Chem. Res. 2012, 51, 614–620. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Dong, X.; Li, M.; He, Q.; Zhao, S.; Xie, L. Fluorinated metal–organic frameworks for enhanced stability and iodine adsorption selectivity under humid conditions. Chem. Eng. J. 2023, 461, 142058. [Google Scholar] [CrossRef]
- Zheng, Z.; Lin, Q.; Gao, Y.; Ji, X.; Sessler, J.L.; Wang, H. Recent advances in iodine adsorption from water. Coord. Chem. Rev. 2024, 511, 215860. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, M.; Taghizadeh, A.; Ghaedi, M. Adsorbent. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 71–210. [Google Scholar]
- Ayadi, T.; Badawi, M.; Cantrel, L.; Lebègue, S. Rational approach for an optimized formulation of silver-exchanged zeolites for iodine capture from first-principles calculations. Mol. Syst. Des. Eng. 2022, 7, 422–433. [Google Scholar] [CrossRef]
- Riley, B.J.; Chong, S.; Schmid, J.; Marcial, J.; Nienhuis, E.T.; Bera, M.K.; Lee, S.; Canfield, N.L.; Kim, S.; Derewinski, M.A. Role of zeolite structural properties toward iodine capture: A head-to-head evaluation of framework type and chemical composition. ACS Appl. Mater. Interfaces 2022, 14, 18439–18452. [Google Scholar] [CrossRef] [PubMed]
- Balumuru, C.; Raja, K.; Sabharwall, P.; Utgikar, V. Adsorption of Radioactive Iodine Using Nanocarbon on ETS-10 as Adsorbent. Nucl. Technol. 2024. [Google Scholar] [CrossRef]
- Parker, K.E.; Golovich, E.C.; Wellman, D.M. Iodine Adsorption on Ion-Exchange Resins and Activated Carbons: Batch Testing; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2014.
- Nandanwar, S.U.; Coldsnow, K.; Green, M.; Utgikar, V.; Sabharwall, P.; Aston, D.E. Activity of nanostructured C@ETS-10 sorbent for capture of volatile radioactive iodine from gas stream. Chem. Eng. J. 2016, 287, 593–601. [Google Scholar] [CrossRef]
- Qian, X.; Zhu, Z.-Q.; Sun, H.-X.; Ren, F.; Mu, P.; Liang, W.; Chen, L.; Li, A. Capture and reversible storage of volatile iodine by novel conjugated microporous polymers containing thiophene units. ACS Appl. Mater. Interfaces 2016, 8, 21063–21069. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Cho, Y.-J.; Shin, J.M.; Yim, M.-S. Bismuth-embedded SBA-15 mesoporous silica for radioactive iodine capture and stable storage. J. Nucl. Mater. 2015, 465, 556–564. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Sarma, D.; Malliakas, C.D.; Polychronopoulou, K.; Riley, B.J.; Pierce, D.A.; Chun, J.; Kanatzidis, M.G. Chalcogenide aerogels as sorbents for radioactive iodine. Chem. Mater. 2015, 27, 2619–2626. [Google Scholar] [CrossRef]
- Mantasha, I.; Hussain, S.; Ahmad, M.; Shahid, M. Two dimensional (2D) molecular frameworks for rapid and selective adsorption of hazardous aromatic dyes from aqueous phase. Sep. Purif. Technol. 2020, 238, 116413. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, T.Y.; Chen, J.L.; Liu, Y.; Yuan, X.; Yan, J.; Sun, Q.; Xu, Z.; Zhang, D.; Wang, X. Heterostructured bimetallic MOF-on-MOF architectures for efficient oxygen evolution reaction. Adv. Mater. 2024, 36, 2306910. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Liu, Q.; Bai, W.; Li, H.; Wang, M.; Qian, Q.; Yang, Q.; Xiao, C.; Xie, Y. Directional shunting of photogenerated carriers in POM@MOF for promoting nitrogen adsorption and oxidation. Adv. Mater. 2023, 35, 2304532. [Google Scholar] [CrossRef] [PubMed]
- Sheta, S.M.; Hamouda, M.A.; Ali, O.I.; Kandil, A.; Sheha, R.R.; El-Sheikh, S.M. Recent progress in high-performance environmental impacts of the removal of radionuclides from wastewater based on metal–organic frameworks: A review. RSC Adv. 2023, 13, 25182–25208. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Liu, L.; Yan, B. Adsorption of uranium (VI) by metal-organic frameworks and covalent-organic frameworks from water. Coord. Chem. Rev. 2023, 475, 214917. [Google Scholar] [CrossRef]
- Drout, R.J.; Otake, K.; Howarth, A.J.; Islamoglu, T.; Zhu, L.; Xiao, C.; Wang, S.; Farha, O.K. Efficient capture of perrhenate and pertechnetate by a mesoporous Zr metal–organic framework and examination of anion binding motifs. Chem. Mater. 2018, 30, 1277–1284. [Google Scholar] [CrossRef]
- Ma, C.; Vasileiadis, A.; Wolterbeek, H.T.; Denkova, A.G.; Crespo, P.S. Adsorption of molybdenum on Zr-based MOFs for potential application in the 99Mo/99mTc generator. Appl. Surf. Sci. 2022, 572, 151340. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, Z.; Li, X.; Ma, K.; Jin, T.; Feng, Z.; Lan, T.; Zhao, J.; Xiao, S. Highly radiation-resistant Al-MOF selected based on the radiation stability rules of metal–organic frameworks with ultra-high thorium ion adsorption capacity. Environ. Sci. Nano 2024, 11, 2103–2111. [Google Scholar] [CrossRef]
- Chen, P.; He, X.; Pang, M.; Dong, X.; Zhao, S.; Zhang, W. Iodine capture using Zr-based metal–organic frameworks (Zr-MOFs): Adsorption performance and mechanism. ACS Appl. Mater. Interfaces 2020, 12, 20429–20439. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Cui, D.; Zhang, S.-R.; Xu, Y.-H.; Jiang, D.-L. Iodine capture in porous organic polymers and metal–organic frameworks materials. Mater. Horiz. 2019, 6, 1571–1595. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, W.; Chen, Y.; Xu, C.; Hu, G.; Han, Z. Highly efficient adsorption of iodine under ultrahigh pressure from aqueous solution. Sep. Purif. Technol. 2020, 233, 115999. [Google Scholar] [CrossRef]
- Li, Z.J.; Ju, Y.; Lu, H.; Wu, X.; Yu, X.; Li, Y.; Wu, X.; Zhang, Z.H.; Lin, J.; Qian, Y. Boosting the iodine adsorption and radioresistance of Th-UiO-66 MOFs via aromatic substitution. Chem.-Eur. J. 2021, 27, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Yang, K.; Dong, X.; Han, Y. Adsorption-based capture of iodine and organic iodines: Status and challenges. J. Mater. Chem. A 2023, 11, 5460–5475. [Google Scholar] [CrossRef]
- He, Y.-C.; Kan, W.-Q.; Guo, J.; Yang, Y.; Du, P.; Liu, Y.-Y.; Ma, J.-F. Iodine-templated assembly of an In(III) complex with a single-crystal-to-single-crystal transition. CrystEngComm 2013, 15, 7406–7409. [Google Scholar] [CrossRef]
- Yao, R.-X.; Cui, X.; Jia, X.-X.; Zhang, F.-Q.; Zhang, X.-M. A luminescent zinc(II) metal–organic framework (MOF) with conjugated π-electron ligand for high iodine capture and nitro-explosive detection. Inorg. Chem. 2016, 55, 9270–9275. [Google Scholar] [CrossRef]
- Salles, F.; Zajac, J. Impact of Structural Functionalization, Pore Size, and Presence of Extra-Framework Ions on the Capture of Gaseous I2 by MOF Materials. Nanomaterials 2021, 11, 2245. [Google Scholar] [CrossRef]
- Liu, B.; Ren, X.; Chen, L.; Ma, X.; Chen, Q.; Sun, Q.; Zhang, L.; Si, P.; Ci, L. High efficient adsorption and storage of iodine on S, N co-doped graphene aerogel. J. Hazard. Mater. 2019, 373, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-J.; Ju, Y.; Yu, B.; Wu, X.; Lu, H.; Li, Y.; Zhou, J.; Guo, X.; Zhang, Z.-H.; Lin, J.; et al. Modulated synthesis and isoreticular expansion of Th-MOFs with record high pore volume and surface area for iodine adsorption. Chem. Commun. 2020, 56, 6715–6718. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, Y.; Hong, S.; Yoon, K. Iodine as a visible probe for the evaluation of zeolite donor strength. J. Am. Chem. Soc. 1996, 118, 9377–9386. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Lu, G.; Wang, Y.; Li, S.; Cui, C.; Wu, J.; Xu, Z.; Tian, D.; Huang, W. Mesoporous metal–organic frameworks with size-, shape-, and space-distribution-controlled pore structure. Adv. Mater. 2015, 18, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, T.; Dong, X.; Pang, M.; Xiao, S.; Zhang, W. Thiophene-based MOFs for iodine capture: Effect of pore structures and interaction mechanism. Chem. Eng. J. 2021, 425, 130578. [Google Scholar] [CrossRef]
- Huang, J.-F.; Hu, H.-C.; Deng, S.-Q.; Cai, S.-L.; Fan, J.; Zhang, W.-G.; Zheng, S.-R. A Ni(II) metal–organic framework with helical channels for the capture of iodine via guest exchange induced amorphization. New J. Chem. 2022, 46, 7144–7152. [Google Scholar] [CrossRef]
- Sarkar, A.; Adhikary, A.; Mandal, A.; Chakraborty, T.; Das, D. Zn-BTC MOF as an Adsorbent for Iodine Uptake and Organic Dye Degradation. Cryst. Growth Des. 2020, 20, 7833–7839. [Google Scholar] [CrossRef]
- Hu, X.-L.; Sun, C.-Y.; Qin, C.; Wang, X.-L.; Wang, H.-N.; Zhou, E.-L.; Li, W.-E.; Su, Z.-M. Iodine-templated assembly of unprecedented 3d–4f metal–organic frameworks as photocatalysts for hydrogen generation. Chem. Commun. 2013, 49, 3564–3566. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Xie, X.-X.; Huang, Z.-Y.; Cai, X.-T.; Zhang, W.-G.; Cai, S.-L.; Fan, J.; Zheng, S.-R. Ethylenediamine grafted MIL-101 for iodine vapor capture with high capacity. J. Solid State Chem. 2022, 315, 123453. [Google Scholar] [CrossRef]
- Askari, S.; Khodaei, M.M.; Benassi, E.; Jafarzadeh, M. MIL-101-NH2-TFR and MIL-101-NH2-TFR/Cu2+ as novel hybrid materials for efficient adsorption of iodine and reduction of Cr(VI). Mater. Today Commun. 2023, 35, 105990. [Google Scholar] [CrossRef]
- Aa, S.; Zhang, Y.; Li, Z.; Xia, H.; Xue, M.; Liu, X.; Mu, Y. Highly efficient and reversible iodine capture using a metalloporphyrin-based conjugated microporous polymer. Chem. Commun. 2014, 50, 8495–8498. [Google Scholar] [CrossRef] [PubMed]
- Kamal, S.; Khalid, M.; Khan, M.S.; Shahid, M.; Ahmad, M. Amine- and Imine-Functionalized Mn-Based MOF as an Unusual Turn-On and Turn-Off Sensor for d10 Heavy Metal Ions and an Efficient Adsorbent to Capture Iodine. Cryst. Growth Des. 2022, 22, 3277–3294. [Google Scholar] [CrossRef]
- Kamal, S.; Khalid, M.; Khan, M.S.; Shahid, M.; Ahmad, M. A bifunctionalised Pb-based MOF for iodine capture and dye removal. Dalton Trans. 2023, 52, 4501–4516. [Google Scholar] [CrossRef] [PubMed]
- Vakil, F.; Ahmad, M.S.; Kumar, M.; Ansari, A.; Shahid, M.; Ahmad, M. Fabrication of a Cd(II) metal–organic framework as a dual functional material: Efficient iodine capture and selective adsorption of a cationic dye. CrystEngComm 2023, 25, 2280–2297. [Google Scholar] [CrossRef]
- Liu, L.; Chen, L.; Thummavichai, K.; Ye, Z.; Wang, Y.; Fujita, T.; Wang, X. Amino-functionalized MOF-on-MOF architectural nanocomplexes composed for radioactive-iodine efficient adsorption. Chem. Eng. J. 2023, 474, 145858. [Google Scholar] [CrossRef]
- Guo, B.; Li, F.; Wang, C.; Zhang, L.; Sun, D. A rare (3,12)-connected zirconium metal–organic framework with efficient iodine adsorption capacity and pH sensing. J. Mater. Chem. A 2019, 7, 13173–13179. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Sun, Y.; Chen, Z.; Zhang, Z.-Y.; Liang, F.; Liu, D.; Hu, H.-C. Post-synthetic modification of zeolitic imidazolate framework-90 via Schiff base reaction for ultrahigh iodine capture. J. Mater. Chem. A 2023, 11, 23922–23931. [Google Scholar] [CrossRef]
- Chapman, K.W.; Sava, D.F.; Halder, G.J.; Chupas, P.J.; Nenoff, T.M. Trapping guests within a nanoporous metal–organic framework through pressure-induced amorphization. J. Am. Chem. Soc. 2011, 133, 18583–18585. [Google Scholar] [CrossRef] [PubMed]
- Sava, D.F.; Rodriguez, M.A.; Chapman, K.W.; Chupas, P.J.; Greathouse, J.A.; Crozier, P.S.; Nenoff, T.M. Capture of volatile iodine, a gaseous fission product, by zeolitic imidazolate framework-8. J. Am. Chem. Soc. 2011, 133, 12398–12401. [Google Scholar] [CrossRef]
- Bhunia, M.K.; Hughes, J.T.; Fettinger, J.C.; Navrotsky, A. Thermochemistry of paddle wheel MOFs: Cu-HKUST-1 and Zn-HKUST-1. Langmuir 2013, 29, 8140–8145. [Google Scholar] [CrossRef]
- Hughes, J.T.; Sava, D.F.; Nenoff, T.M.; Navrotsky, A. Thermochemical evidence for strong iodine chemisorption by ZIF-8. J. Am. Chem. Soc. 2013, 135, 16256–16259. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Do, X.H.; Cho, K.Y.; Jeong, K.; Baek, K.-Y. Amine-Functionalized Zeolitic Imidazolate Framework-8 (ZIF-8) Nanocrystals for Adsorption of Radioactive Iodine. ACS Appl. Nano Mater. 2020, 3, 9852–9861. [Google Scholar] [CrossRef]
- Tong, D.; Zhao, Y.; Chen, Z.; Bo, T.; Nie, S.; Xiao, S. A systematic first-principles study of volatile iodine adsorption onto zeolitic imidazolate frameworks. Microporous Mesoporous Mater. 2021, 317, 111017. [Google Scholar] [CrossRef]
- Zhang, X.; da Silva, I.; Fazzi, R.; Sheveleva, A.M.; Han, X.; Spencer, B.F.; Sapchenko, S.A.; Tuna, F.; McInnes, E.J.L.; Li, M.; et al. Iodine Adsorption in a Redox-Active Metal–Organic Framework: Electrical Conductivity Induced by Host−Guest Charge-Transfer. Inorg. Chem. 2019, 58, 14145–14150. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically Conductive Porous Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef] [PubMed]
- Huve, J.; Ryzhikov, A.; Nouali, H.; Lalia, V.; Augé, G.; Daou, T.J. Porous sorbents for the capture of radioactive iodine compounds: A review. RSC Adv. 2018, 8, 29248–29273. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.-H.; Li, Z.-Y.; Chen, K.-W.; Gu, A.-T.; Wang, P.; Yang, Y. Synthesis and characterization of Ag@Cu-based MOFs as efficient adsorbents for iodine anions removal from aqueous solutions. J. Environ. Radioact. 2023, 265, 107211. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Zhang, J.; He, X.; Crittenden, J.C.; Zhang, W. Silver Ion-Exchanged Anionic Metal–Organic Frameworks for Iodine Adsorption: Silver Species Evolution from Ions to Nanoparticles. ACS Appl. Nano Mater. 2023, 6, 7206–7217. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, P.; Li, Y.; Gu, J. AgNPs-Containing Metal–Organic Frameworks for the Effective Adsorption and Immobilization of Radioactive Iodine. J. Chem. Eng. Data 2020, 65, 1986–1992. [Google Scholar] [CrossRef]
- Katsoulidis, A.P.; He, J.; Kanatzidis, M.G. Functional monolithic polymeric organic framework aerogel as reducing and hosting media for Ag nanoparticles and application in capturing of iodine vapors. Chem. Mater. 2012, 24, 1937–1943. [Google Scholar] [CrossRef]
- Liu, J.; Strachan, D.M.; Thallapally, P.K. Enhanced noble gas adsorption in Ag@MOF-74Ni. Chem. Commun. 2014, 50, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, H.; Wang, R.; Duan, M. Fabricating Ag@MOF-5 nanoplates by the template of MOF-5 and evaluating its antibacterial activity. Colloids Surf. A 2021, 626, 127093. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, H.; Zhao, X.; Liu, D. One-step preparation of Ag0-MOF composites for effective removal of iodine from water. J. Solid State Chem. 2022, 305, 122680. [Google Scholar] [CrossRef]
- Qi, B.; Liu, Y.; Zheng, T.; Gao, Q.; Yan, X.; Jiao, Y.; Yang, Y. Highly efficient capture of iodine by Cu/MIL-101. J. Solid State Chem. 2018, 258, 49–55. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Zhang, J.; Gao, Y.; Zhang, W. Cu-loaded MOF-303 for iodine adsorption: The roles of Cu species and pyrazole ligands. Appl. Surf. Sci. 2023, 619, 156819. [Google Scholar] [CrossRef]
- Tian, Z.; Chee, T.-S.; Zhang, X.; Lei, L.; Xiao, C. Novel bismuth-based electrospinning materials for highly efficient capture of radioiodine. Chem. Eng. J. 2021, 412, 128687. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, W.; Kang, J.; Li, B. Facile synthesis of mesoporous Fe-based MOFs loading bismuth with high speed adsorption of iodine from solution. J. Solid State Chem. 2019, 269, 558–565. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Chen, K.-W.; Gu, A.-T.; Zhou, X.-Y.; Wang, P.; Gong, C.-H.; Mao, P.; Jiao, Y.; Chen, K.; Lu, J.-G.; et al. Adsorption performance study of bismuth-doped ZIF-8 composites on radioactive iodine in the vapor and liquid phases. J. Solid State Chem. 2023, 325, 124186. [Google Scholar] [CrossRef]
- Xin, B.; Zeng, G.; Gao, L.; Li, Y.; Xing, S.; Hua, J.; Li, G.; Shi, Z.; Feng, S. An unusual copper(I) halide-based metal–organic framework with a cationic framework exhibiting the release/adsorption of iodine, ion-exchange and luminescent properties. Dalton Trans. 2013, 42, 7562–7568. [Google Scholar] [CrossRef]
- Lan, Y.; Tong, M.; Yang, Q.; Zhong, C. Computational screening of covalent organic frameworks for the capture of radioactive iodine and methyl iodine. CrystEngComm 2017, 19, 4920–4926. [Google Scholar] [CrossRef]
- Falaise, C.; Volkringer, C.; Facqueur, J.; Bousquet, T.; Gasnot, L.; Loiseau, T. Capture of iodine in highly stable metal–organic frameworks: A systematic study. Chem. Commun. 2013, 49, 10320–10322. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Liu, Y.; Wang, X.-F.; Chen, Z.; Wang, X. Optimizing iodine capture performance by metal-organic framework containing with bipyridine units. Front. Chem. Sci. Eng. 2022, 17, 395–403. [Google Scholar] [CrossRef]
- Yu, R.-L.; Sun, M.-Q.; Wang, X.-Y.; Li, D.-T.; Li, Z.-L.; Xia, L.-Z. C@MOF composite material for rapid and efficient capture of gaseous iodine. Chem. Eng. J. 2024, 489, 151423. [Google Scholar] [CrossRef]
- Xian, J.-Y.; Xie, X.-X.; Huang, Z.-Y.; Liu, Y.-L.; Song, H.-Y.; Chen, Z.-Q.; Ou, Y.-C.; Zheng, S.-R. Structure and Properties of a Mixed-Ligand Co-MOF That Was Synthesized in Situ from a Single Imidazole–Pyridyl–Tetrazole Trifunctional Ligand. Cryst. Growth Des. 2023, 23, 1448–1454. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, P.; Wang, D.; Li, T.T.; Wu, L.H.; Zheng, S.R. A mixed-ligand Co(II) MOF synthesized from a single organic ligand to capture iodine and methyl iodine vapour. Dalton Trans. 2023, 52, 7709–7717. [Google Scholar] [CrossRef]
- Wang, L.; Chen, P.; Dong, X.; Zhang, W.; Zhao, S.; Xiao, S.; Ouyang, Y. Porous MOF-808@PVDF beads for removal of iodine from gas streams. RSC Adv. 2020, 10, 44679–44687. [Google Scholar] [CrossRef]
- Andrade, P.H.M.; Dhainaut, J.; Volkringer, C.; Loiseau, T.; Moncomble, A.; Hureau, M.; Moissette, A. Stability of Iodine Species Trapped in Titanium-Based MOFs: MIL-125 and MIL-125_NH2. Small 2024, 20, e2400265. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Zhou, B.-x.; Lin, X.; Mo, Y.-H.; Cao, J.; Cai, S.-L.; Fan, J.; Zhang, W.-G.; Zheng, S.-R. Iodine Adsorption–Desorption-Induced Structural Transformation and Improved Ag+ Turn-On Luminescent Sensing Performance of a Nonporous Eu(III) Metal–Organic Framework. Inorg. Chem. 2024, 6, 4185–4195. [Google Scholar] [CrossRef]
- Suo, M.-M.; Yang, G.-P.; Wang, Y.-Y. Functionalized Metal–Organic Framework with Azo Double and Amide Groups for Highly Efficient Iodine Capture. Cryst. Growth Des. 2024, 24, 3933–3940. [Google Scholar] [CrossRef]
- Bhambri, H.; Mandal, S.K. Impact of Conformational Isomerism in Two Zn-MOFs on the Multimedia Incarceration of Iodine: In-Depth Experimental and Computational Assessments. ACS Appl. Energy Mater. 2023, 6, 12307–12317. [Google Scholar] [CrossRef]
- Andrade, P.H.M.; Ahouari, H.; Volkringer, C.; Loiseau, T.; Vezin, H.; Hureau, M.; Moissette, A. Electron-Donor Functional Groups, Band Gap Tailoring, and Efficient Charge Separation: Three Keys to Improve the Gaseous Iodine Uptake in MOF Materials. ACS Appl. Mater. Interfaces 2023, 15, 31032–31048. [Google Scholar] [CrossRef]
- Yu, R.L.; Li, Q.F.; Li, Z.L.; Wang, X.Y.; Xia, L.Z. Analysis of Radioactive Iodine Trapping Mechanism by Zinc-Based Metal-Organic Frameworks with Various N-Containing Carboxylate Ligands. ACS Appl. Mater. Interfaces 2023, 15, 35082–35091. [Google Scholar] [CrossRef] [PubMed]
- Sava, D.F.; Chapman, K.W.; Rodriguez, M.A.; Greathouse, J.A.; Crozier, P.S.; Zhao, H.; Chupas, P.J.; Nenoff, T.M. Competitive I2 Sorption by Cu-BTC from Humid Gas Streams. Chem. Mater. 2013, 25, 2591–2596. [Google Scholar] [CrossRef]
- Maddock, J.; Kang, X.; Liu, L.; Han, B.; Yang, S.; Schröder, M. The impact of structural defects on iodine adsorption in UiO-66. Chemistry 2021, 3, 525–531. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, H.; Li, J.; Xue, W.; Zhong, C. IL-induced formation of dynamic complex iodine anions in IL@MOF composites for efficient iodine capture. J. Mater. Chem. A 2019, 7, 18324–18329. [Google Scholar] [CrossRef]
- Zahid, M.; Zhang, D.; Xu, X.; Pan, M.; Ul Haq, M.H.; Reda, A.T.; Xu, W. Barbituric and thiobarbituric acid-based UiO-66-NH2 adsorbents for iodine gas capture: Characterization, efficiency and mechanisms. J. Hazard. Mater. 2021, 416, 125835. [Google Scholar] [CrossRef]
- Brunet, G.; Safin, D.A.; Aghaji, M.Z.; Robeyns, K.; Korobkov, I.; Woo, T.K.; Murugesu, M. Stepwise crystallographic visualization of dynamic guest binding in a nanoporous framework. Chem. Sci. 2017, 8, 3171–3177. [Google Scholar] [CrossRef]
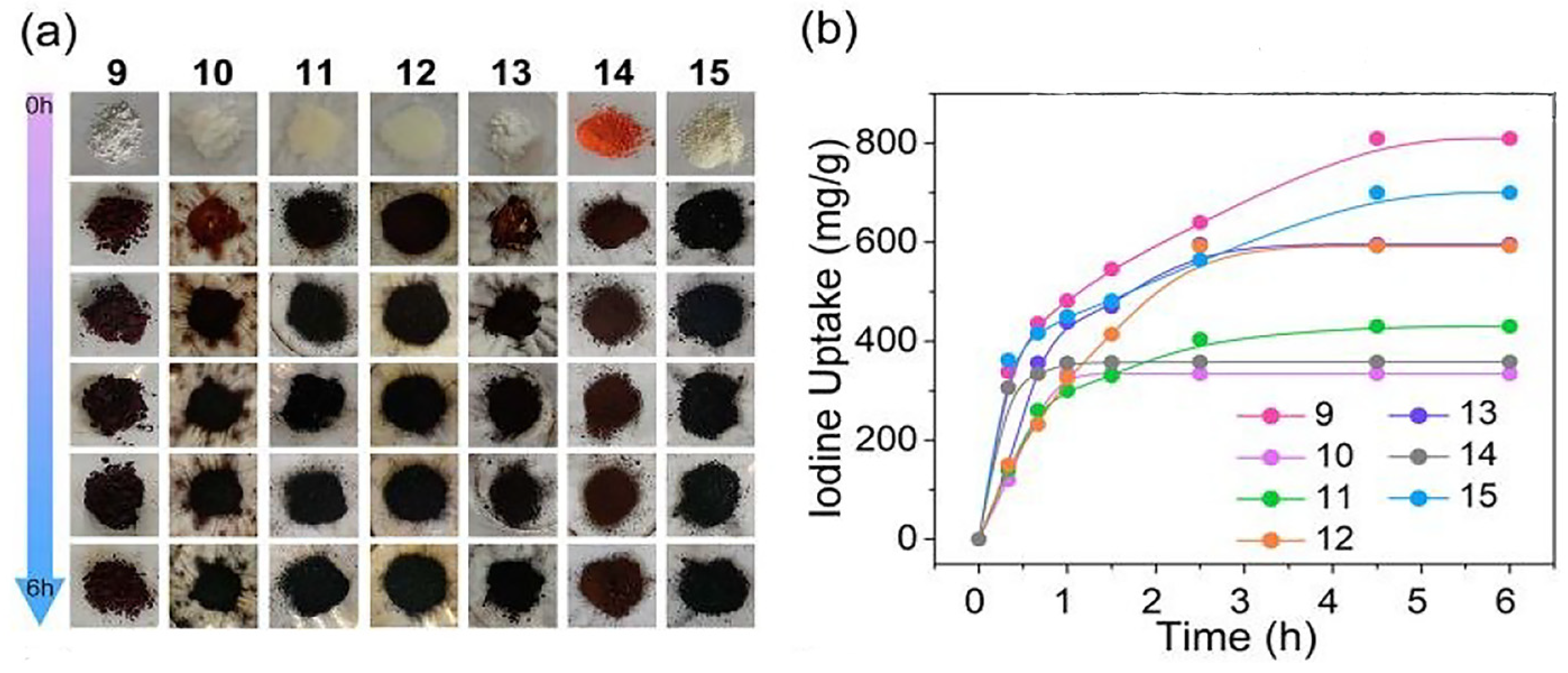

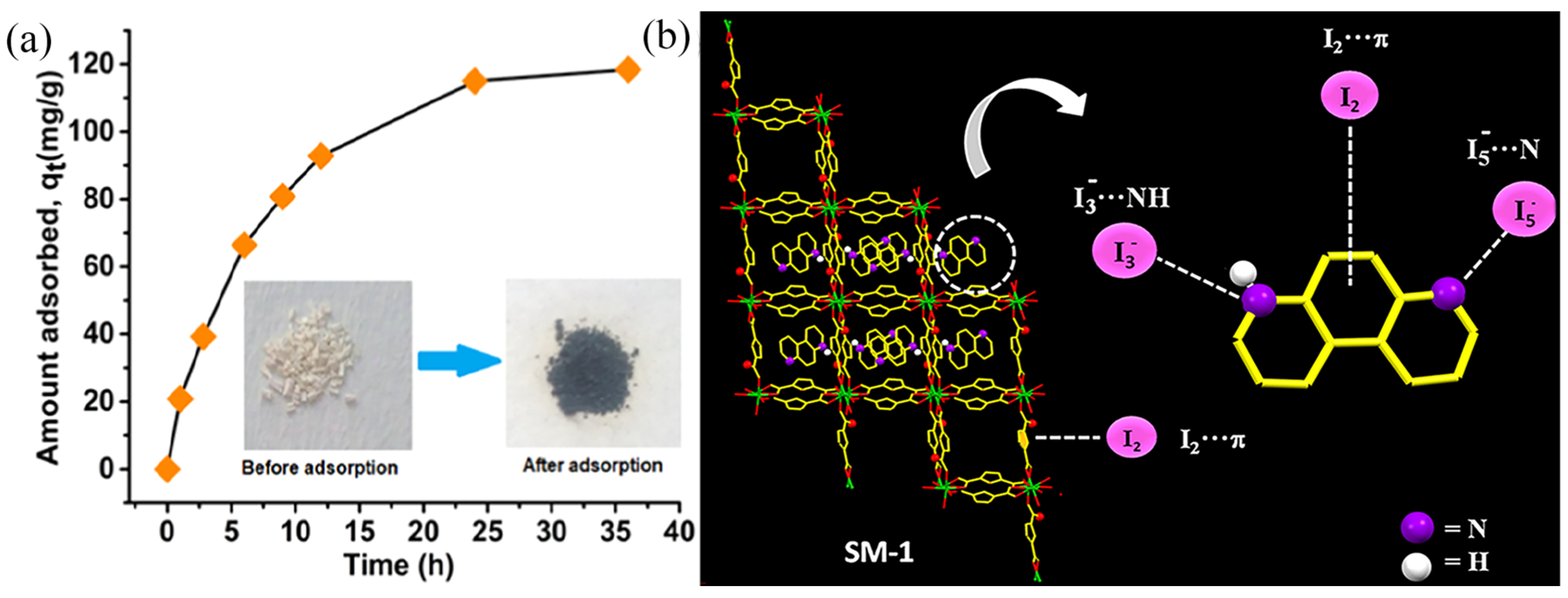

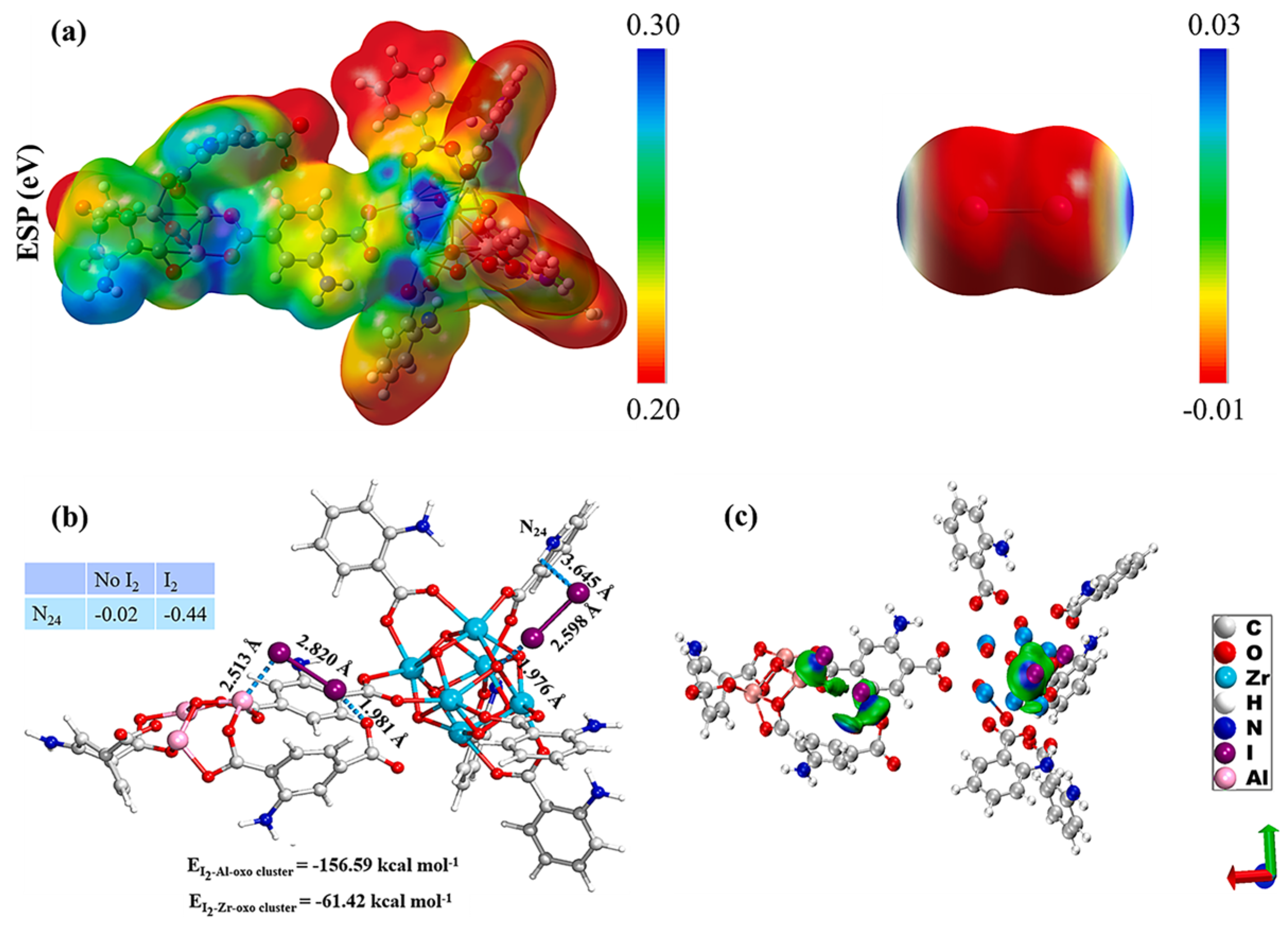

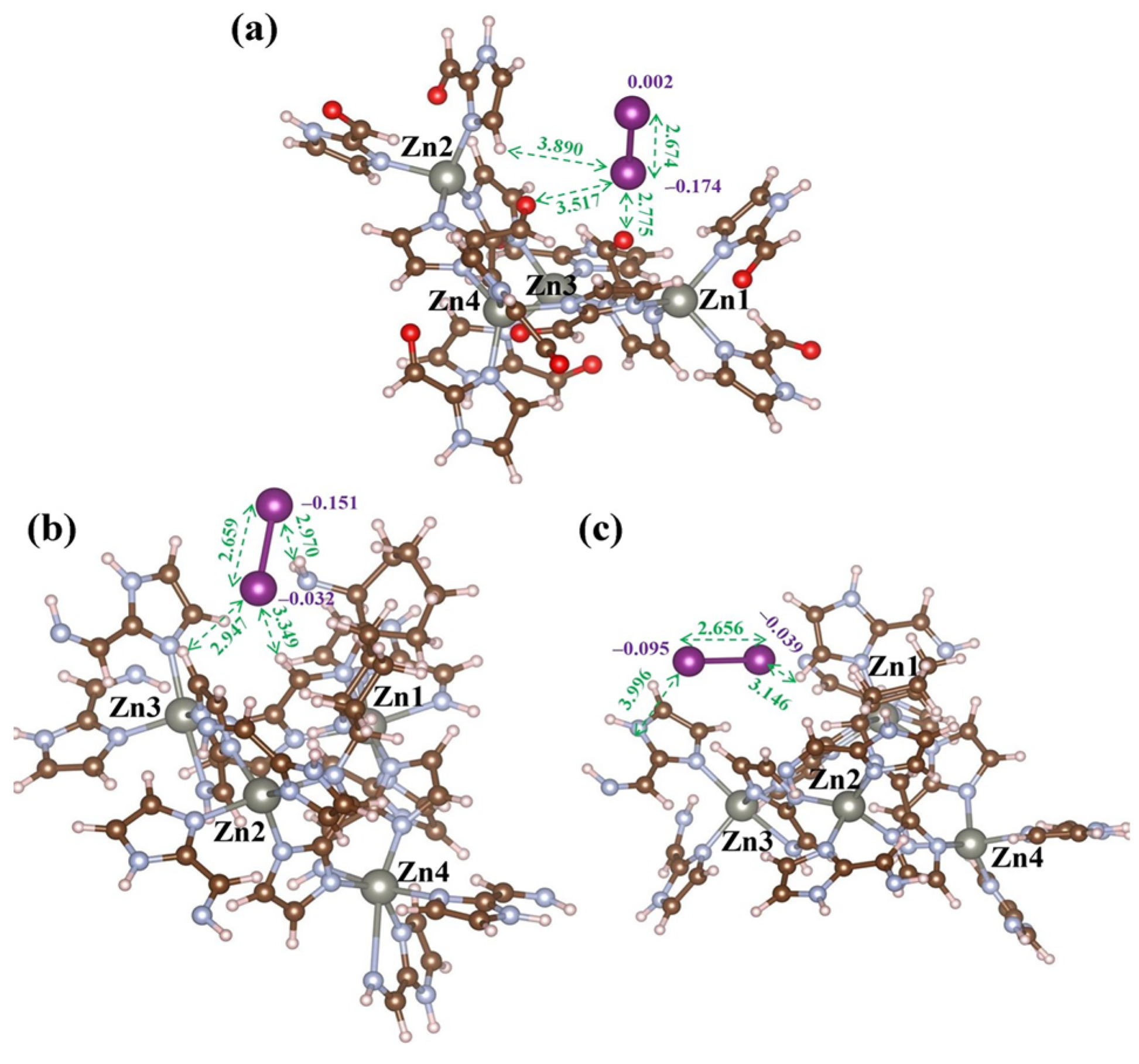


| Sample | Test Temperature (°C) | Adsorption Capacity (g g−1) | Ref. |
|---|---|---|---|
| Th-BPYDC | 75 | 2.23 | [84] |
| MOF-808 | 80 | 2.18 | [35] |
| NH2-MIL-101-on-NH2-UIO-66 | 80 | 1.93 | [57] |
| DUT-68 | 80 | 1.08 | [47] |
| CZ-3 | 75 | 1.15 | [85] |
| SCNU-Z7 | 80 | 2.7 | [86] |
| Co-IPT-IBA | 75 | 2.88 | [87] |
| MOF-808@PVDF | 80 | 1.42 | [88] |
| MIL-125 | 100 | 1.9 | [89] |
| MIL-125_NH2 | 100 | 1.6 | [89] |
| Eu-IPDA | 85 | 1.9 | [90] |
| Zn-ABTC | 75 | 2.02 | [91] |
| Co-IPT-IBA | 75 | 2.88 | [87] |
| {[Zn(bpaipa)]·DMF·2H2O}n | 75 | 2.95 | [92] |
| {[Zn(bpaipa)]·5H2O}n | 75 | 4.51 | [92] |
| MIL-125(Ti)_NH2 | 100 | 1.7 | [93] |
| CAU-1(Al)_NH2 | 100 | 1.3 | [93] |
| MIL-125(Ti) | 100 | 1.4 | [93] |
| Zn-MOF-1 | 75 | 1.25 | [94] |
| Zn-MOF-2 | 75 | 1.96 | [94] |
| Cu-BTC | 75 | 1.75 | [95] |
| ZIF-90-III | 75 | 6.6 | [59] |
| UPC-158-HF | 70 | 2.19 | [58] |
| UPC-158-HCl | 70 | 2.92 | [58] |
| UPC-158-HBr | 70 | 2.75 | [58] |
| UiO-66-FA | 80 | 2.25 | [96] |
| UiO-66 | 80 | 1.17 | [96] |
| PCN-333(Al) | 75 | 4.42 | [97] |
| IL@PCN-333(Al) | 75 | 7.35 | [97] |
| UiO-66-NH-B.D | 75 | 1.17 | [98] |
| UiO-66-NH-T.D | 75 | 1.33 | [98] |
| (ZnI2)3(TPT)2 | Room temperature | 1.73 | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Duan, J.; Liang, Y.; Zhang, H.; Duan, C.; Liu, S. Recent Advances in Metal–Organic Frameworks and Their Derivatives for Adsorption of Radioactive Iodine. Molecules 2024, 29, 4170. https://doi.org/10.3390/molecules29174170
Peng L, Duan J, Liang Y, Zhang H, Duan C, Liu S. Recent Advances in Metal–Organic Frameworks and Their Derivatives for Adsorption of Radioactive Iodine. Molecules. 2024; 29(17):4170. https://doi.org/10.3390/molecules29174170
Chicago/Turabian StylePeng, Li, Jiali Duan, Yu Liang, Haiqi Zhang, Chongxiong Duan, and Sibin Liu. 2024. "Recent Advances in Metal–Organic Frameworks and Their Derivatives for Adsorption of Radioactive Iodine" Molecules 29, no. 17: 4170. https://doi.org/10.3390/molecules29174170
APA StylePeng, L., Duan, J., Liang, Y., Zhang, H., Duan, C., & Liu, S. (2024). Recent Advances in Metal–Organic Frameworks and Their Derivatives for Adsorption of Radioactive Iodine. Molecules, 29(17), 4170. https://doi.org/10.3390/molecules29174170





