Self-Assembled Nanostructure of Ionic Sn(IV)porphyrin Complex Based on Multivalent Interactions for Photocatalytic Degradation of Water Contaminants
Abstract
1. Introduction
2. Results and Discussion
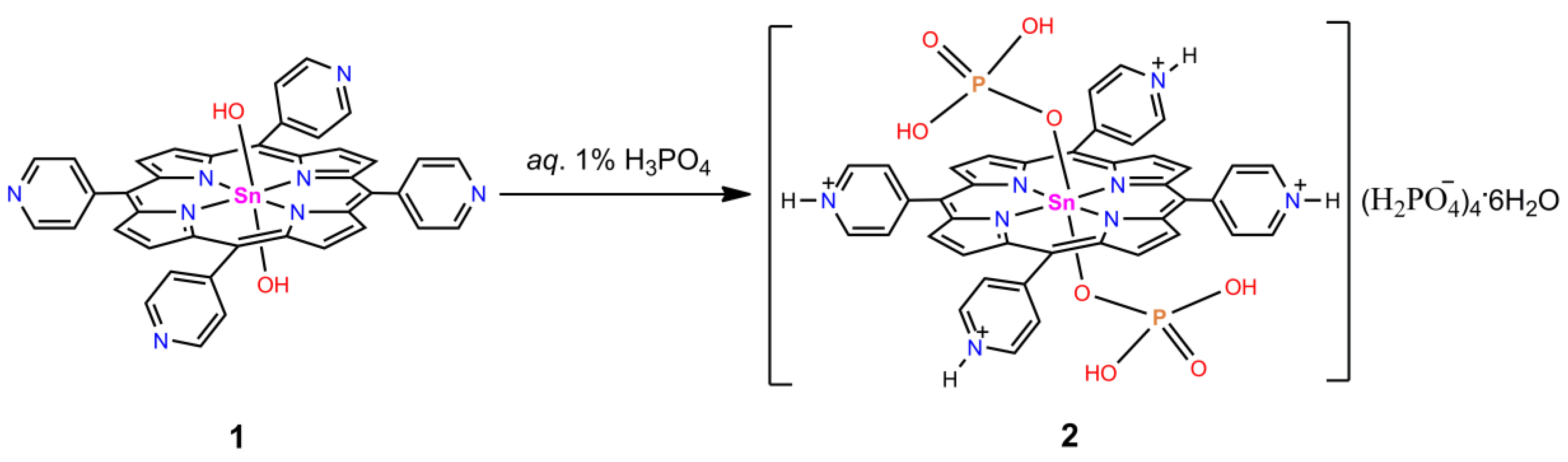
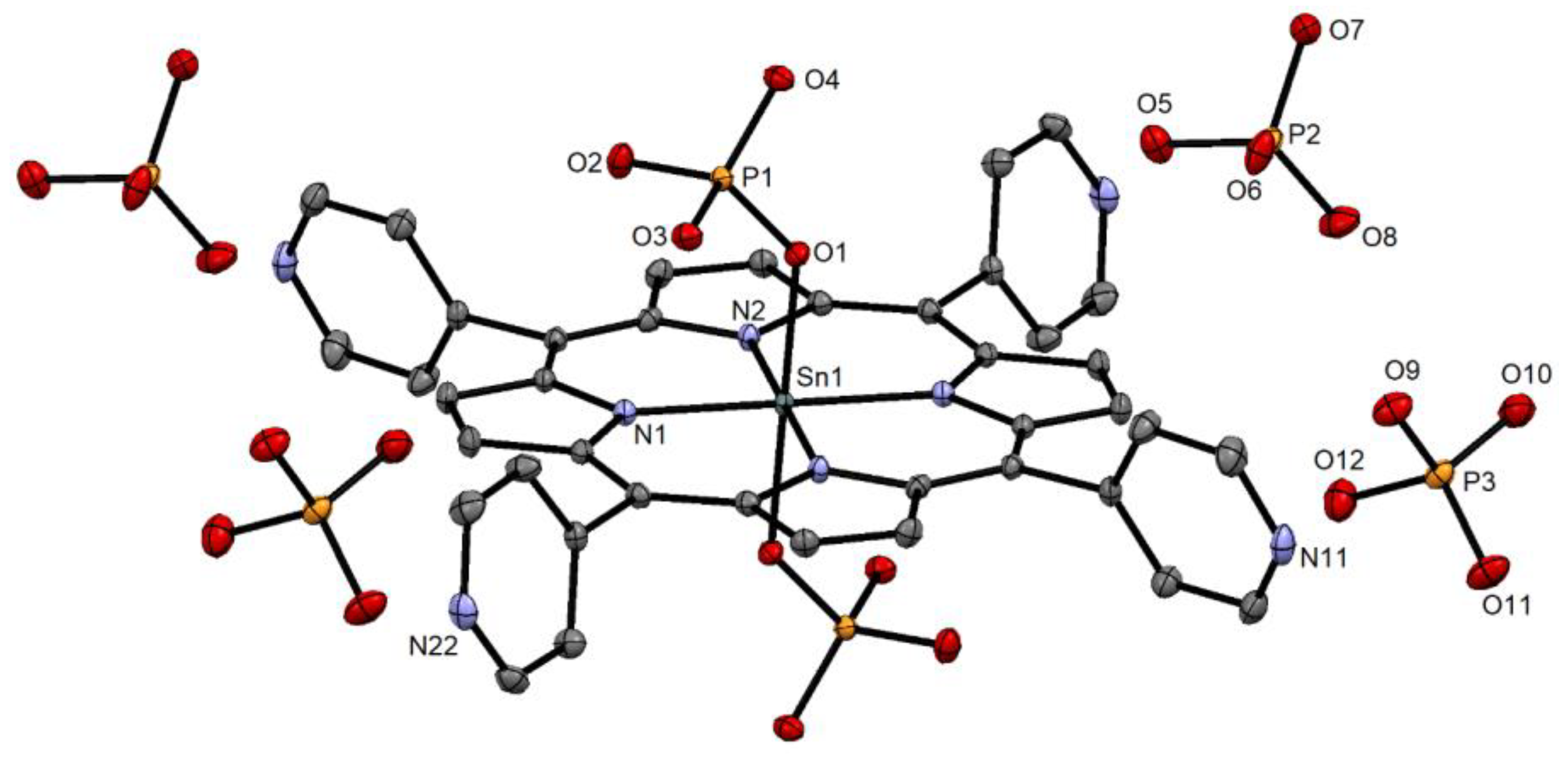
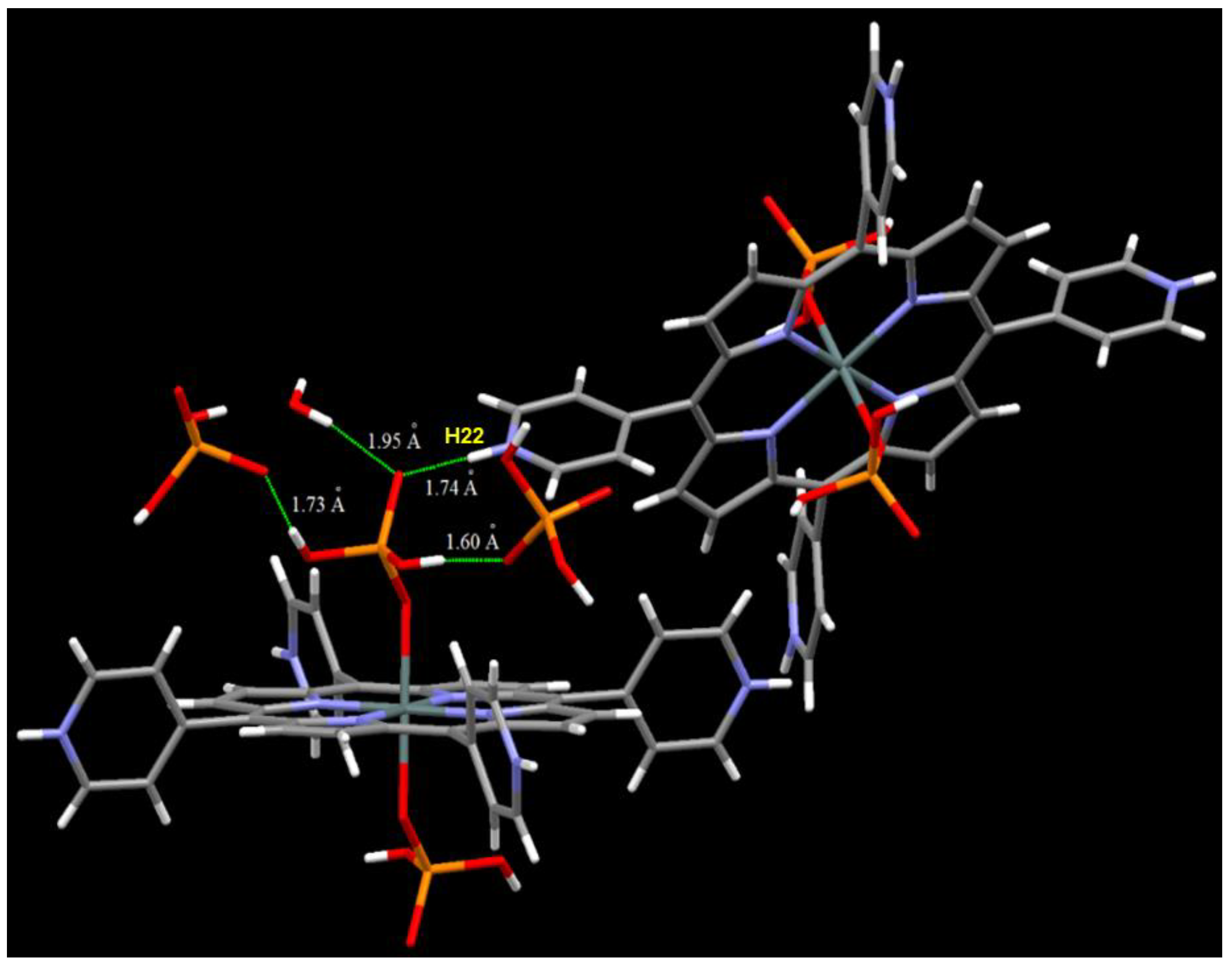
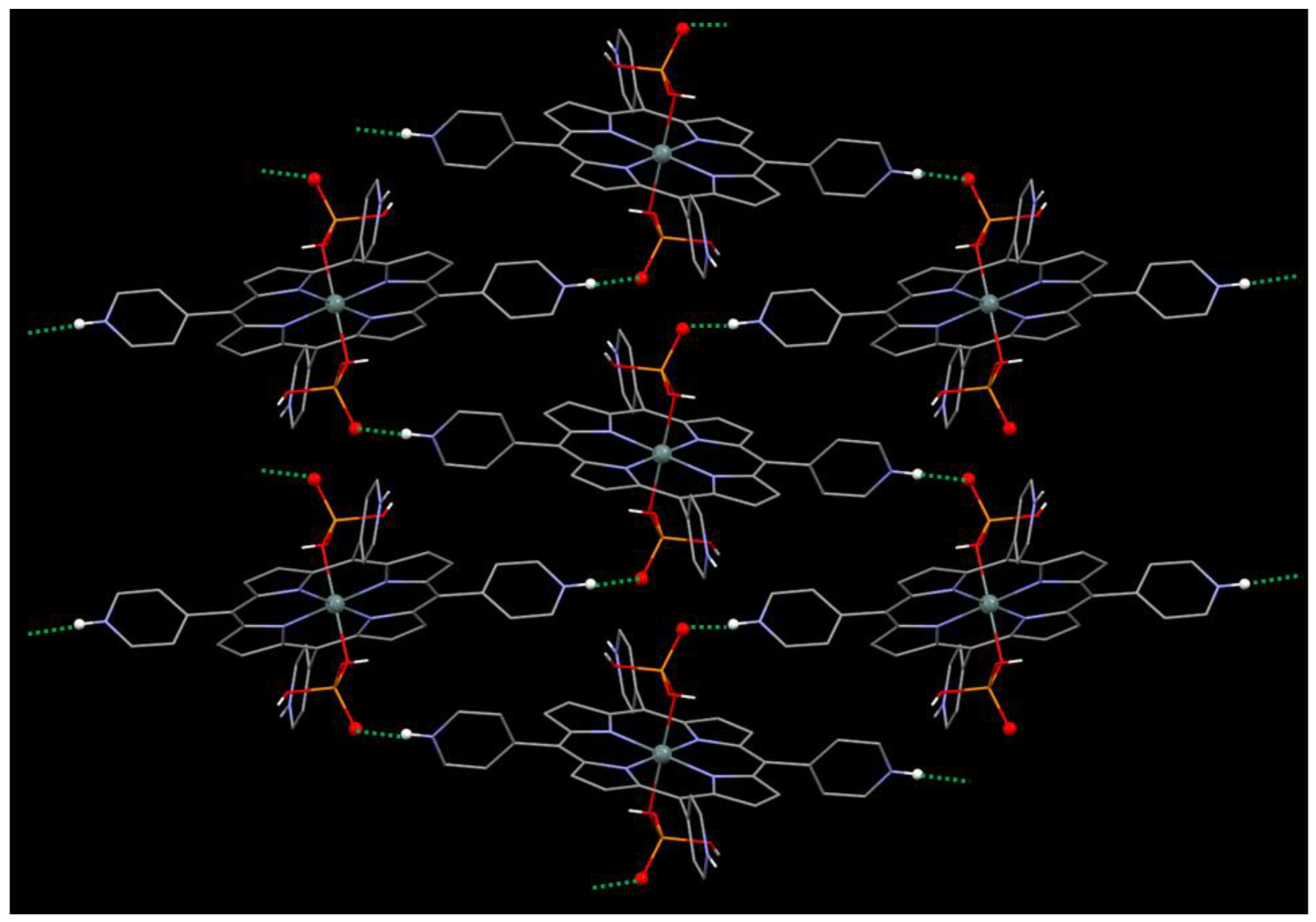
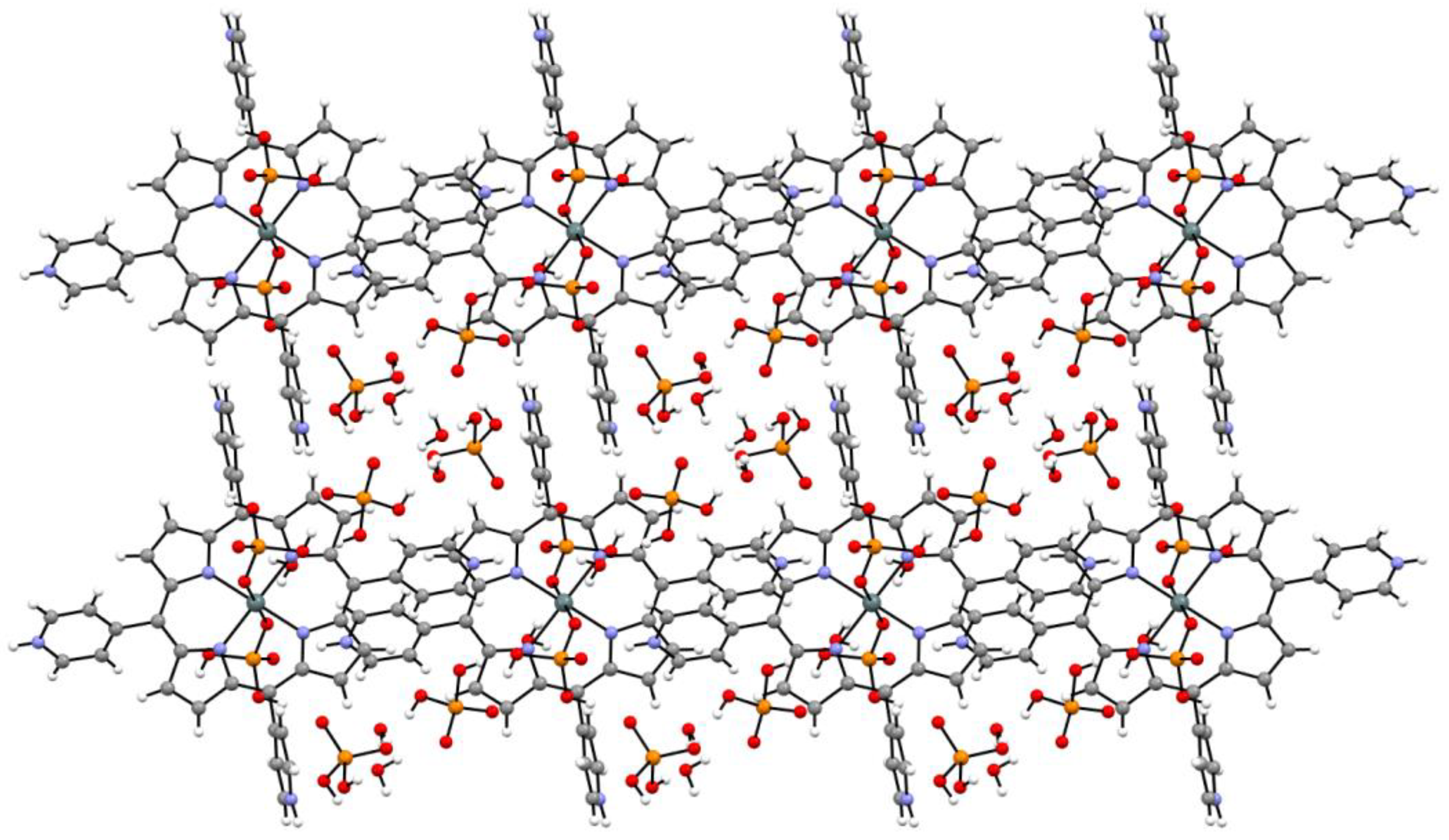
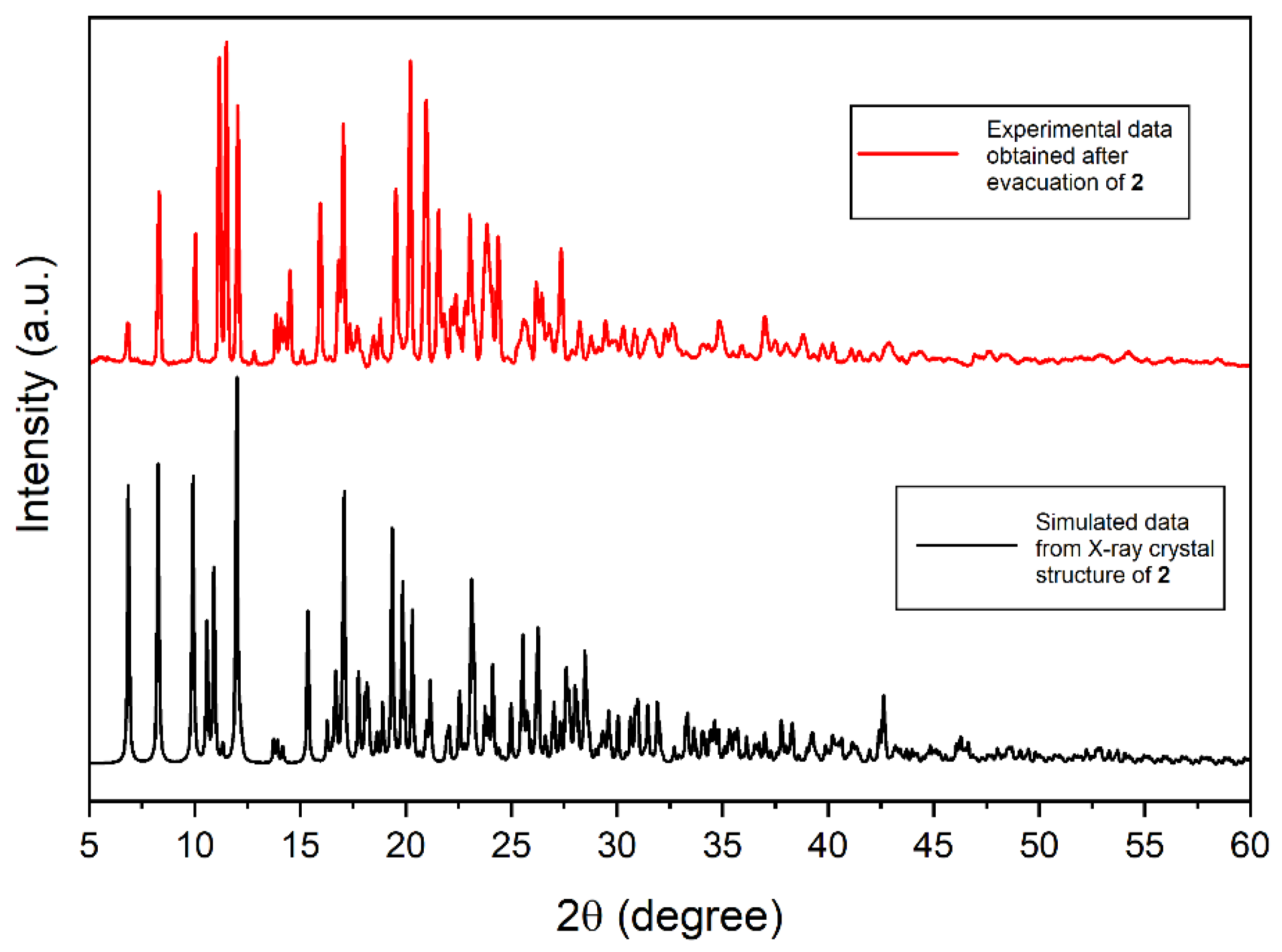
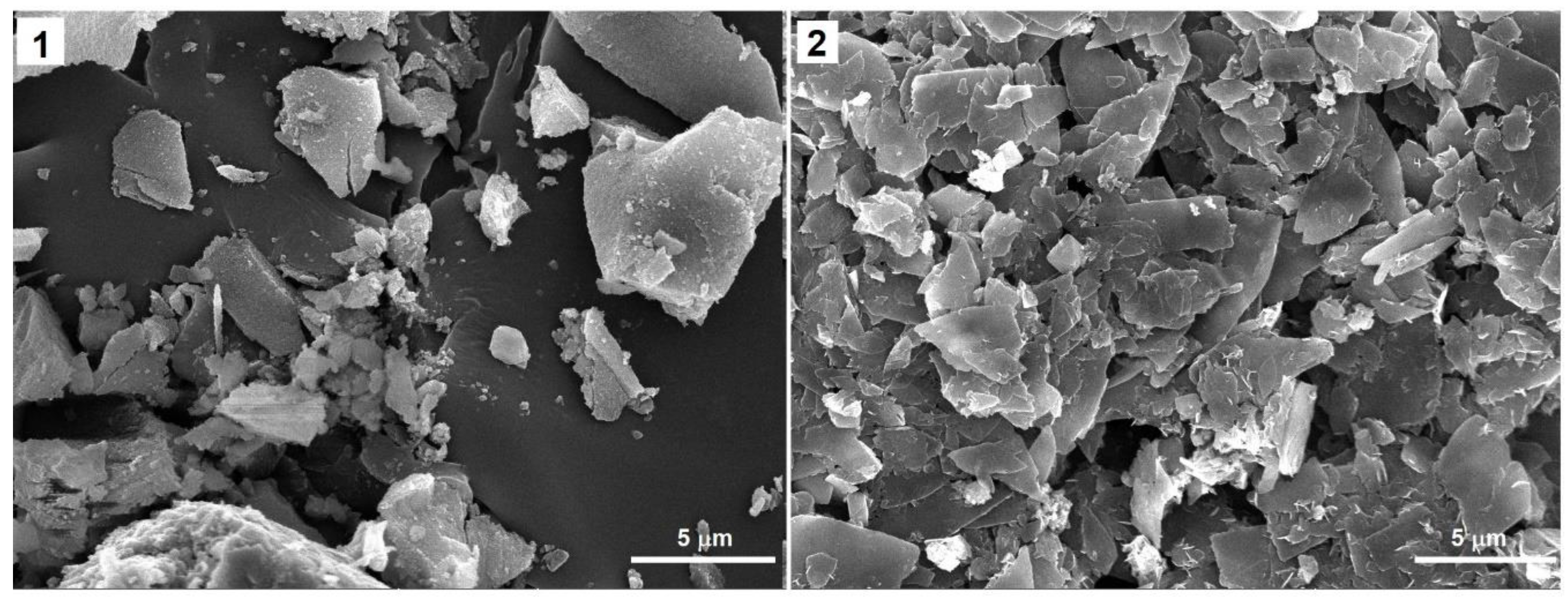
2.1. Synthesis and X-ray Single-Crystal Structural Analysis
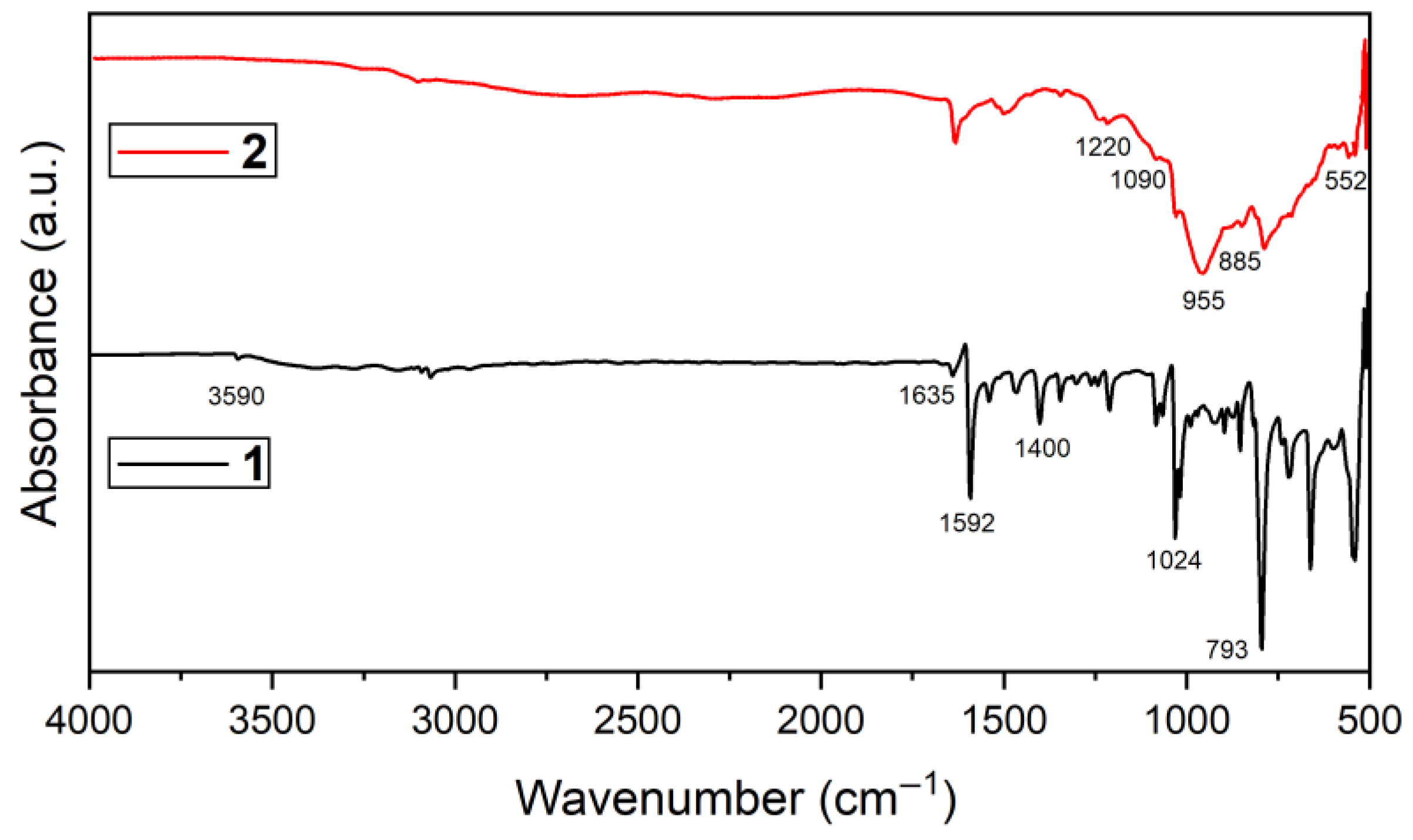
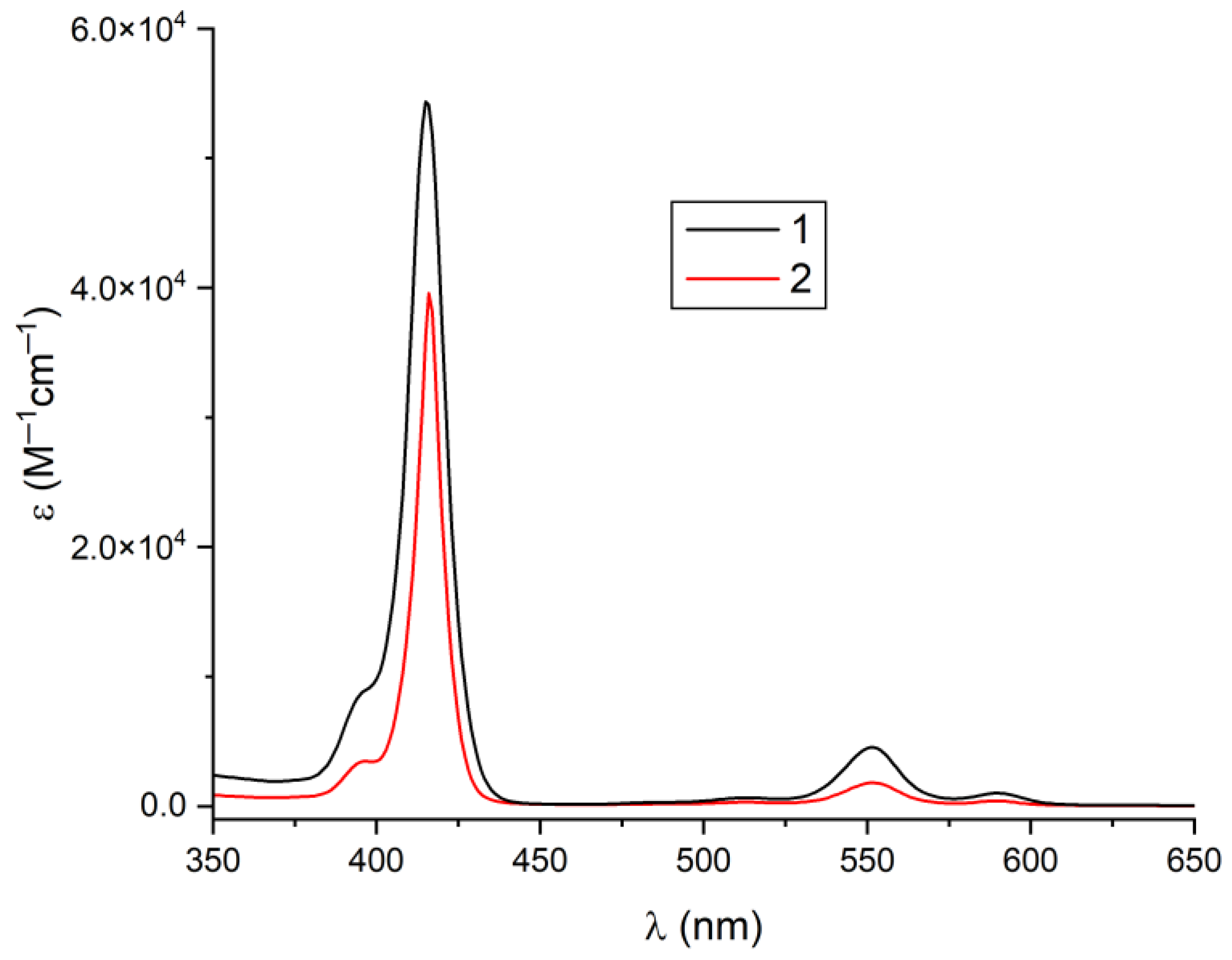
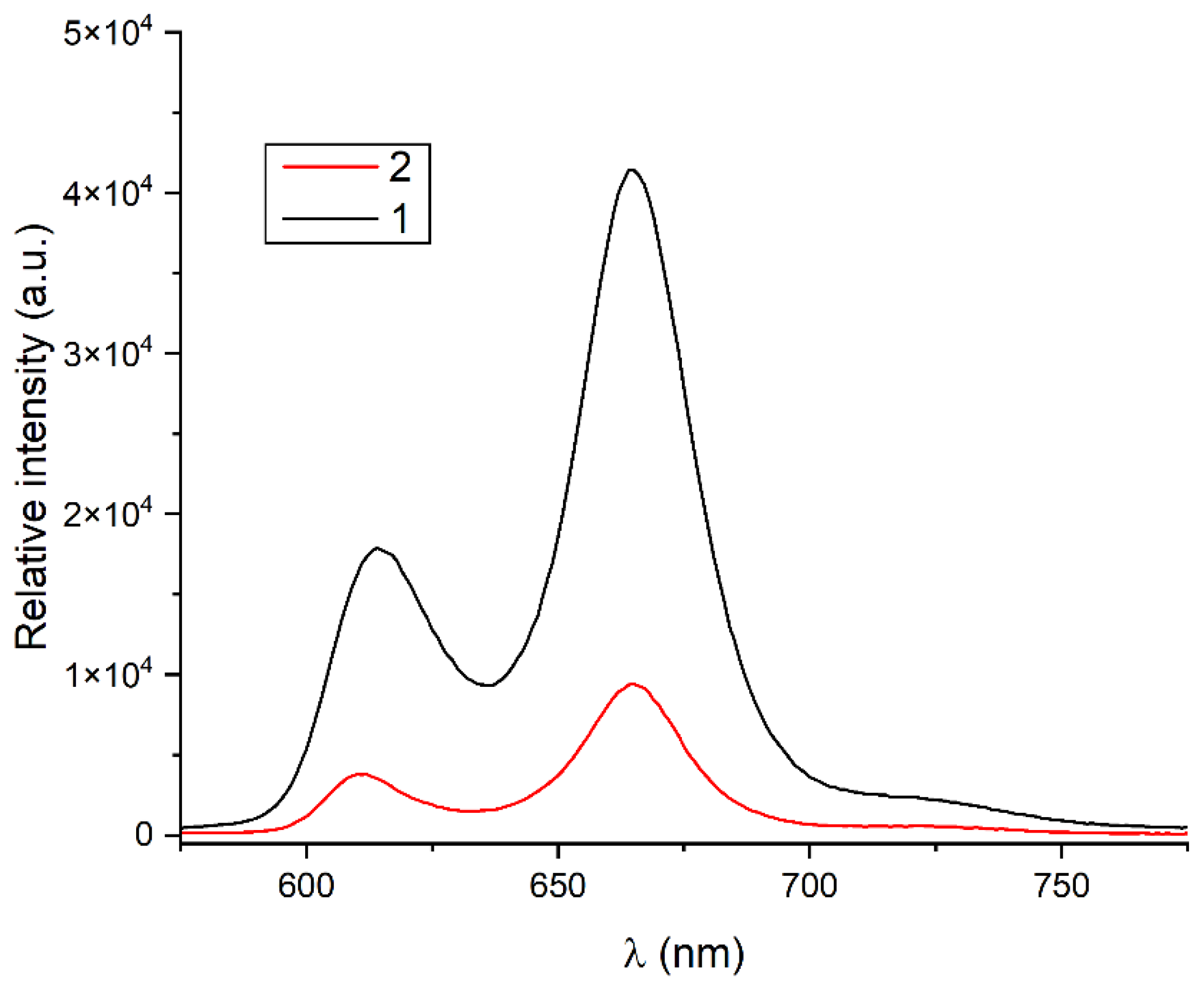
2.2. Spectroscopic Analysis
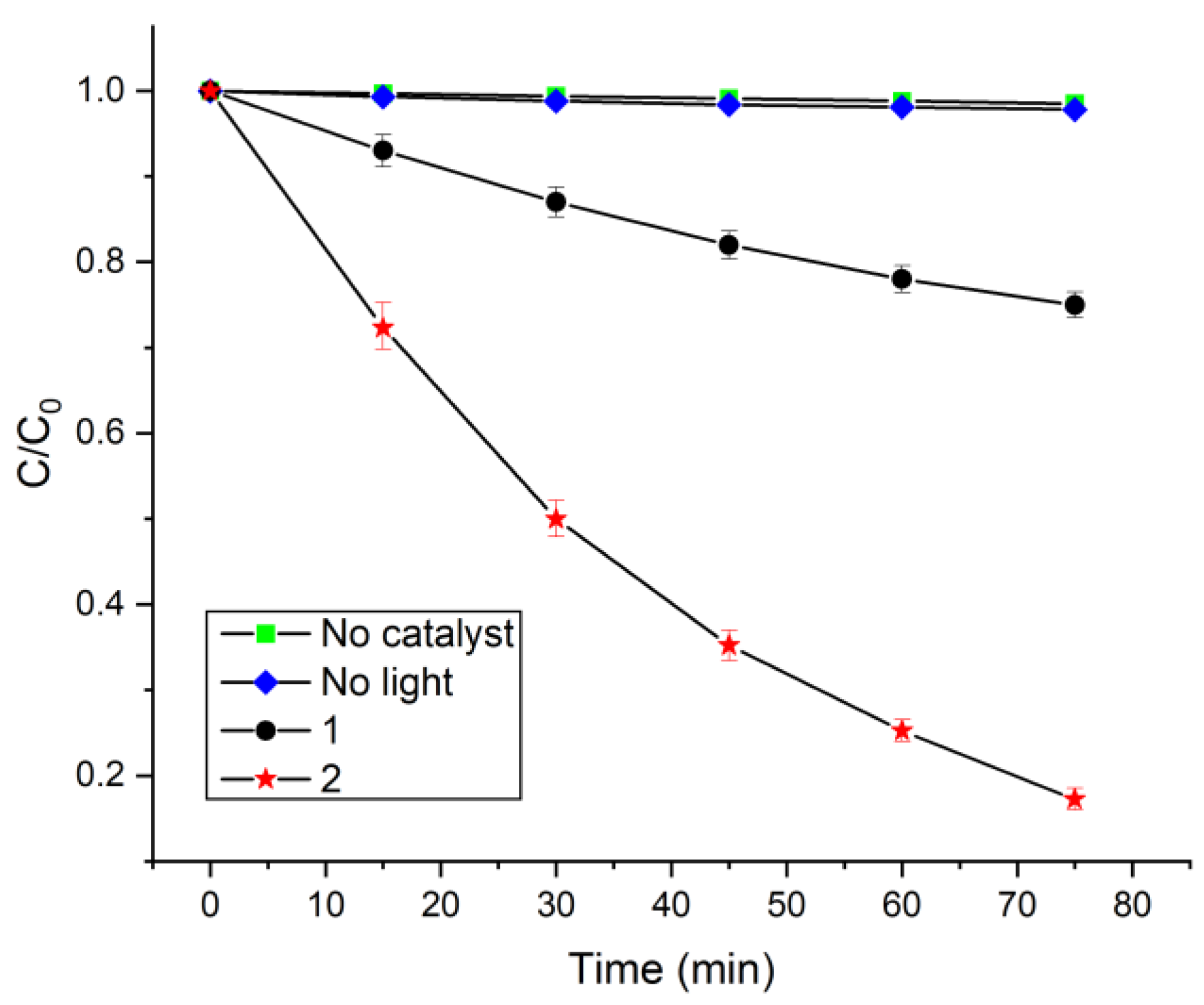
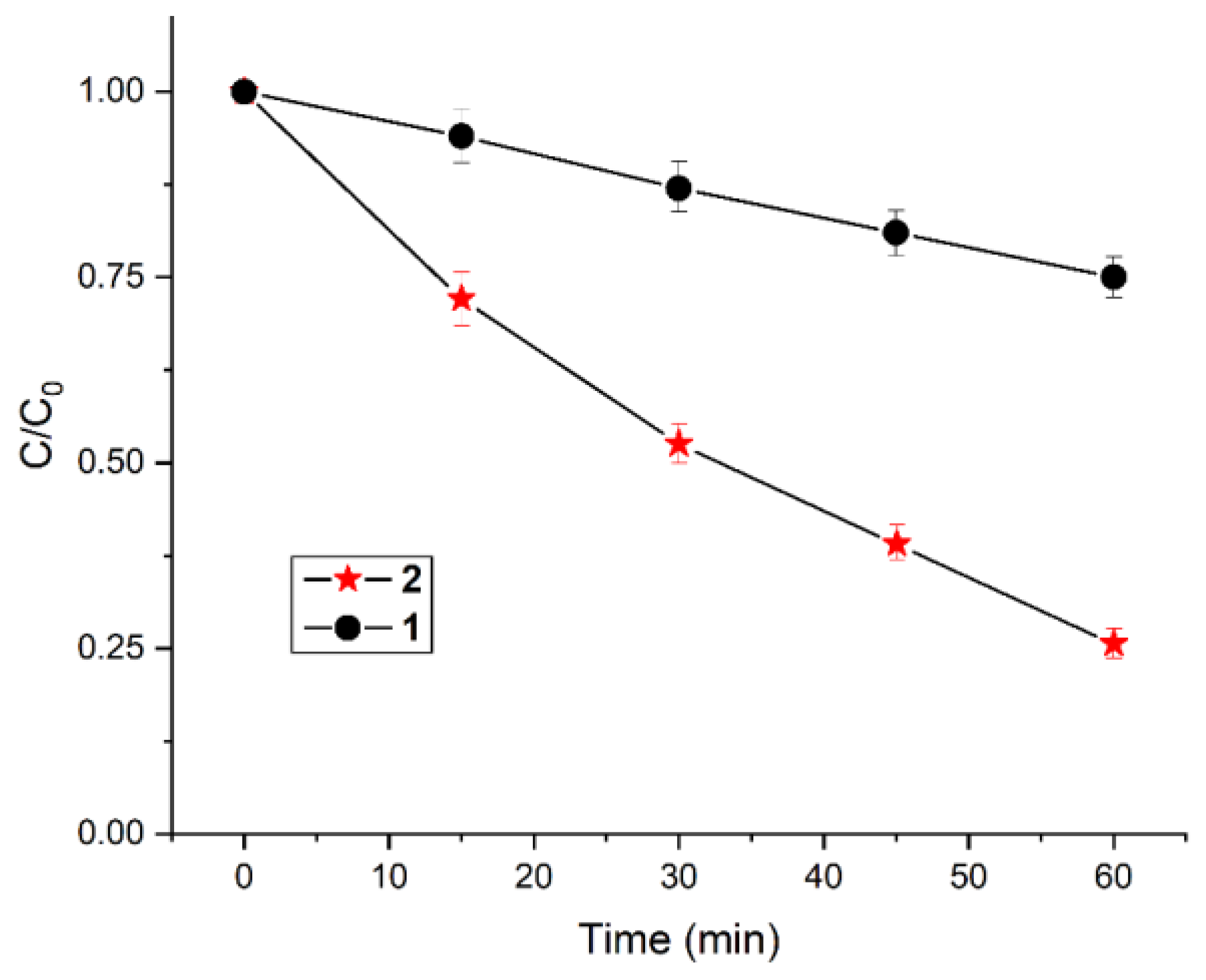
2.3. Catalytic Photodegradation of Pollutants
2.4. Possible Mechanism of the Catalytic Photodegradation of Pollutants
3. Materials and Methods
3.1. Synthesis of [Sn(H2PO4)2(TPyHP)](H2PO4)4∙6H2O (2)
3.2. X-ray Single-Crystal Structure Analysis
3.3. Photoelectrochemical Experiment
3.4. Catalytic Photodegradation Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Hasobe, T. Porphyrin-Based Supramolecular Nanoarchitectures for Solar Energy Conversion. J. Phys. Chem. Lett. 2013, 4, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, L.; Wang, H.; Cao, R.; Wang, J.; Bai, F.; Fan, H. Self-Assembled One-Dimensional Porphyrin Nanostructures with Enhanced Photocatalytic Hydrogen Generation. Nano Lett. 2018, 18, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Marianov, A.N.; Lu, T.; Zhong, J. A review of the development of porphyrin-based catalysts for electrochemical CO2 reduction. Chem. Eng. J. 2023, 470, 144249. [Google Scholar] [CrossRef]
- Magna, G.; Mandoj, F.; Stefanelli, M.; Pomarico, G.; Monti, D.; Di Natale, C.; Paolesse, R.; Nardis, S. Recent Advances in Chemical Sensors Using Porphyrin-Carbon Nanostructure Hybrid Materials. Nanomaterials 2021, 11, 997. [Google Scholar] [CrossRef]
- Pan, D.; Liang, P.; Zhong, X.; Wang, D.; Cao, H.; Wang, W.; He, W.; Yang, Z.; Dong, X. Self-Assembled Porphyrin-Based Nanoparticles with Enhanced Near-Infrared Absorbance for Fluorescence Imaging and Cancer Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 2, 999–1005. [Google Scholar] [CrossRef]
- Yao, B.; He, Y.; Wang, S.; Sun, H.; Liu, X. Recent Advances in Porphyrin-Based Systems for Electrochemical Oxygen Evolution Reaction. Int. J. Mol. Sci. 2022, 23, 6036. [Google Scholar] [CrossRef]
- Xie, M.-H.; Yang, X.-L.; Zou, C.; Wu, C.-D. A SnIV–Porphyrin-Based Metal-Organic Framework for the Selective Photo-Oxygenation of Phenol and Sulfides. Inorg. Chem. 2011, 50, 5318–5320. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Porphyrin-Based Nanomaterials for the Photocatalytic Remediation of Wastewater: Recent Advances and Perspectives. Molecules 2024, 29, 611. [Google Scholar] [CrossRef]
- Drain, C.M.; Varotto, A.; Radivojevic, I. Self-Organized Porphyrinic Materials. Chem. Rev. 2009, 109, 1630–1658. [Google Scholar] [CrossRef]
- Hasobe, T. Photo- and electro-functional self-assembled architectures of porphyrins. Phys. Chem. Chem. Phys. 2012, 14, 15975–15987. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Integration of Sn (IV) porphyrin on Mesoporous Alumina Support and Visible Light Catalytic Photodegradation of Methylene Blue. Mater. Today Commun. 2024, 39, 109033. [Google Scholar] [CrossRef]
- Shee, N.K.; Lee, G.-S.; Kim, H.-J. Sn(IV)porphyrin-Incorporated TiO2 Nanotubes for Visible Light-Active Photocatalysis. Molecules 2024, 29, 1612. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Complementary metalloporphyrin-based nanostructure decorated with silver nanoparticles for photocatalytic degradation of organic dyes. Inorg. Chem. Commun. 2024, 163, 112252. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Recent Developments in Porphyrin-Based Metal–Organic Framework Materials for Water Remediation under Visible-Light Irradiation. Int. J. Mol. Sci. 2024, 25, 4183. [Google Scholar] [CrossRef]
- Lehn, J.-M. Perspectives in Supramolecular Chemistry—From Molecular Recognition towards Molecular Information Processing and Self-Organization. Angew. Chem. Int. Ed. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular chemistry of metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Y.; Wang, L.; Zhang, N.; Cao, R.; Bian, K.; Alarid, L.; Haddad, R.E.; Bai, F.; Fan, H. Morphology-Controlled Synthesis and Metalation of Porphyrin Nanoparticles with Enhanced Photocatalytic Performance. Nano Lett. 2016, 16, 6523–6528. [Google Scholar] [CrossRef]
- Lu, J.; Li, Z.; An, W.; Liu, L.; Cui, W. Tuning the Supramolecular Structures of Metal-Free Porphyrin via Surfactant Assisted Self-Assembly to Enhance Photocatalytic Performance. Nanomaterials 2019, 9, 1321. [Google Scholar] [CrossRef]
- Wang, S.-P.; Lin, W.; Wang, X.; Cen, T.-Y.; Xie, H.; Huang, J.; Zhu, B.-Y.; Zhang, Z.; Song, A.; Hao, J.; et al. Controllable hierarchical self-assembly of porphyrin-derived supra-amphiphiles. Nat. Commun. 2019, 10, 1399–1411. [Google Scholar] [CrossRef]
- Shee, N.K.; Seo, J.-W.; Kim, H.-J. Spectrophotometric Study of Bridging N-Donor Ligand-Induced Supramolecular Assembly of Conjugated Zn-Trisporphyrin with a Triphenylamine Core. Molecules 2021, 26, 4771. [Google Scholar] [CrossRef]
- Gong, X.; Milic, T.; Xu, C.; Batteas, J.D.; Drain, C.M. Preparation and Characterization of Porphyrin Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14290–14291. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Nayak, S.K.; Mallampalli, S.; Patra, A. Surfactant-assisted porphyrin based hierarchical nano/micro assemblies and their efficient photocatalytic behavior. ACS Appl. Mater. Inter. 2013, 6, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lin, C.; Zhong, Z.; Li, X.; Xu, X.; Liu, J.; Kang, L.-T.; Chai, G.; Yao, J. Effect of axial coordination of iron porphyrin on their nanostructures and photocatalytic performance. Cryst. Growth Des. 2019, 19, 3279–3287. [Google Scholar] [CrossRef]
- Rebelo, S.L.; Neves, C.M.; de Almeida, M.P.; Pereira, E.; Simões, M.M.; Neves, M.G.P.; de Castro, B.; Medforth, C.J. Binary ionic iron(III) porphyrin nanostructured materials with catalase-like activity. Appl. Mater. Today 2020, 21, 100830. [Google Scholar] [CrossRef]
- Bera, K.; Mondal, A.; Pal, U.; Maiti, N.C. Porphyrin-Armored Gold Nanospheres Modulate the Secondary Structure of α-Synuclein and Arrest Its Fibrillation. J. Phys. Chem. C 2020, 124, 6418–6434. [Google Scholar] [CrossRef]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin Nanotubes by Ionic Self-Assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef]
- Wang, Z.; Ho, K.J.; Medforth, C.J.; Shelnutt, J.A. Porphyrin Nanofiber Bundles from Phase-Transfer Ionic Self-Assembly and Their Photocatalytic Self-Metallization. Adv. Mater. 2006, 18, 2557–2560. [Google Scholar] [CrossRef]
- Tian, Y.; Beavers, C.M.; Busani, T.; Martin, K.E.; Jacobsen, J.L.; Mercado, B.Q.; Swartzentruber, B.S.; van Swol, F.; Medfortheg, C.J.; Shelnutt, J.A. Binary ionic porphyrin nanosheets: Electronic and light-harvesting properties regulated by crystal structure. Nanoscale 2012, 4, 1695–1700. [Google Scholar] [CrossRef]
- Tian, Y.; Busani, T.; Uyeda, G.H.; Martin, K.E.; van Swol, F.; Medforth, C.J.; Montan, G.A.; Shelnutt, J.A. Hierarchical cooperative binary ionic porphyrin nanocomposites. Chem. Commun. 2012, 48, 4863–4865. [Google Scholar] [CrossRef]
- Schwab, A.D.; Smith, D.E.; Rich, C.S.; Young, E.R.; Smith, W.F.; de Paula, J.C. Porphyrin Nanorods. J. Phys. Chem. B 2003, 107, 11339–11345. [Google Scholar] [CrossRef]
- Shee, N.K.; Lee, C.-J.; Kim, H.-J. Crystal structure of bis (benzoato-κO)[5,15-di-phenyl-10,20-bis(pyridin-4-yl)porphyrinato-κ4N,N′,N″,N‴]tin(IV). IUCrData 2019, 4, x190787. [Google Scholar] [CrossRef]
- Lee, C.-J.; Shee, N.K.; Kim, H.-J. Fabrication and Properties of Sn(IV)Porphyrin-Linked Porous Organic Polymer for Environmental Applications. RSC Adv. 2023, 13, 24077–24085. [Google Scholar] [CrossRef] [PubMed]
- Shetti, V.S.; Pareek, Y.; Ravikanth, M. Sn(IV) Porphyrin Scaffold for Multiporphyrin Arrays. Coord. Chem. Rev. 2012, 256, 2816–2842. [Google Scholar] [CrossRef]
- Amati, A.; Cavigli, P.; Demitri, N.; Natali, M.; Indelli, M.T.; Iengo, E. Sn(IV) Multiporphyrin Arrays as Tunable Photoactive Systems. Inorg. Chem. 2019, 58, 4399–4411. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Sn(IV)-Porphyrin-Based Nanostructures Featuring Pd(II)-Mediated Supramolecular Arrays and Their Photocatalytic Degradation of Acid Orange 7 Dye. Int. J. Mol. Sci. 2022, 23, 13702. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Supramolecular squares of Sn(IV)porphyrins with Re(I)-corners for the fabrication of self-assembled nanostructures performing photocatalytic degradation of Eriochrome Black T dye. Inorg. Chem. Front. 2022, 10, 174–183. [Google Scholar] [CrossRef]
- Dvivedi, A.; Pareek, Y.; Ravikanth, M. SnIV Porphyrin Scaffolds for Axially Bonded Multiporphyrin Arrays: Synthesis and Structure Elucidation by NMR Studies. Chem. Eur. J. 2014, 20, 4481–4490. [Google Scholar] [CrossRef]
- Fasting, C.; Schalley, C.A.; Weber, M.; Seitz, O.; Hecht, S.; Koksch, B.; Dernedde, J.; Graf, C.; Knapp, E.-W.; Haag, R. Multivalency as a Chemical Organization and Action Principle. Angew. Chem. Int. Ed. 2012, 51, 2–29. [Google Scholar] [CrossRef]
- Jo, H.J.; Kim, S.H.; Kim, H.-J. Supramolecular Assembly of Tin(IV) Porphyrin Cations Stabilized by Ionic Hydrogen-Bonding Interactions. Bull. Korean Chem. Soc. 2015, 36, 2348–2351. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Supramolecular Self-Assembly of the Zwitterionic Sn(IV)-Porphyrin Complex. Molbank 2023, 2023, M1723. [Google Scholar] [CrossRef]
- Cao, J.; Xu, B.; Luo, B.; Lin, H.; Chen, S. Novel BiOI/BiOBr Heterojunction Photocatalysts with Enhanced Visible Light Photocatalytic Properties. Catal. Commun. 2011, 13, 63–68. [Google Scholar] [CrossRef]
- Wang, X.-j.; Yang, W.-y.; Li, F.-t.; Zhao, J.; Liu, R.-h.; Liu, S.-j.; Li, B. Construction of Amorphous TiO2/BiOBr Heterojunctions via Facets Coupling for Enhanced Photocatalytic Activity. J. Hazard. Mater. 2015, 292, 126–136. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Hangarge, R.V.; Bhosale, S.V.; Ninh, H.D.; Jones, L.A.; Bhosale, S.V. Arginine-Mediated Self-Assembly of Porphyrin on Graphene: A Photocatalyst for Degradation of Dyes. Appl. Sci. 2017, 7, 643. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, Z.; Long, Y.; Li, W. Single-Source-Precursor-Assisted Synthesis of Porous WO3/g-C3N4 with Enhanced Photocatalytic Property. Colloids Surf., A 2019, 582, 123857. [Google Scholar] [CrossRef]
- Mota, H.P.; Quadrado, R.N.; Iglesias, B.A.; Fajardo, A.R. Enhanced photocatalytic degradation of organic pollutants mediated by Zn (II)-porphyrin/poly(acrylic acid) hybrid microparticles. Appl. Catal. B 2020, 277, 119208. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhang, H.; Wu, Y.; Jia, Y.; Jin, R.; Gao, S. CTAB-Assisted Solvothermal Construction of Hierarchical Bi2MoO6/Bi5O7Br with Improved Photocatalytic Performances. Sep. Purif. Technol. 2020, 242, 116775. [Google Scholar] [CrossRef]
- Andrade Neto, N.F.; Lima, A.B.; Bomio, M.R.D.; Motta, F.V. Microwave-Assisted Hydrothermal Synthesis of Ag2Mo1-XWxO4 (x = 0, 0.25, 0.50, 0.75 and 1 Mol%) Heterostructures for Enhanced Photocatalytic Degradation of Organic Dyes. J. Alloys Compd. 2020, 844, 156077. [Google Scholar] [CrossRef]
- Dhir, R. Photocatalytic degradation of methyl orange dye under UV irradiation in the presence of synthesized PVP capped pure and gadolinium doped ZnO nanoparticles. Chem. Phys. Lett. 2020, 746, 137302. [Google Scholar] [CrossRef]
- Shubha, J.P.; Savitha, H.S.; Adil, S.F.; Khan, M.; Hatshan, M.R.; Kavalli, K.; Shaik, B. Straightforward Synthesis of Mn3O4/ZnO/Eu2O3-Based Ternary Heterostructure Nano-Photocatalyst and Its Application for the Photodegradation of Methyl Orange and Methylene Blue Dyes. Molecules 2021, 26, 4661. [Google Scholar] [CrossRef]
- Kite, S.V.; Kadam, A.N.; Sathe, D.J.; Patil, S.; Mali, S.S.; Hong, C.K.; Lee, S.W.; Garadkar, K.M. Nanostructured TiO2 sensitized with MoS2 nanoflowers for enhanced photodegradation efficiency toward methyl orange. ACS Omega 2021, 6, 17071–17085. [Google Scholar] [CrossRef]
- Adeel, M.; Saeed, M.; Khan, I.; Muneer, M.; Akram, N. Synthesis and characterization of Co–ZnO and evaluation of its photocatalytic activity for photodegradation of methyl orange. ACS Omega 2021, 6, 1426–1435. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Yuan, X.; Jiang, L.; Xia, Q.; Chen, H. Photocatalytic removal of antibiotics from natural water matrices and swine wastewater via Cu(I) coordinately polymeric carbon nitride framework. Chem. Eng. J. 2020, 392, 123638. [Google Scholar] [CrossRef]
- Nasri, S.; Guergueb, M.; Brahmi, J.; Al-Ghamdi, Y.O.; Loiseau, F.; Nasri, H. Synthesis of a Novel Zinc(II) Porphyrin Complex, Halide Ion Reception, Catalytic Degradation of Dyes, and Optoelectronic Application. Crystals 2023, 13, 238. [Google Scholar] [CrossRef]
- Bouich, M.A.; Hirchi, S.; Banaoues, R.C.; Ghalla, H.; Guergueb, M.; Babba, H.; Roisnel, T.; Nasri, H. Spectroscopic, X-ray structure, radical scavenging and in vitro antifungal activities and catalytic degradation of methyl orange dye investigation of the Meso-tetrakis(p-tolyl) diprotonated porphyrin. J. Mol. Struct. 2024, 1304, 137650. [Google Scholar] [CrossRef]
- Bouicha, M.A.; Moulahi, N.; Guergueb, M.; Chaabane, R.B.; Nasri, H. A new Ni(II) metalloporphyrin: Characterization, theoretical sensing calculations and catalytic degradation of methylene blue and methyl orange dyes. J. Iran. Chem. Soc. 2024, 21, 1611–1633. [Google Scholar] [CrossRef]
- Yan, X.; Wang, X.; Gu, W.; Wu, M.; Yan, Y.; Hu, B.; Che, G.; Han, D.; Yang, J.; Fan, W. Single-crystalline AgIn (MoO4)2 nanosheets grafted Ag/AgBr composites with enhanced plasmonic photocatalytic activity for degradation of tetracycline under visible light. Appl. Catal. B Environ. 2015, 164, 297–304. [Google Scholar] [CrossRef]
- Yuan, X.; Jiang, L.; Chen, X.; Leng, L.; Wang, H.; Wu, Z.; Xiong, T.; Liang, J.; Zeng, G. Highly Efficient Visible-Light-Induced Photoactivity of Z-Scheme Ag2CO3/Ag/WO3 Photocatalysts for Organic Pollutant Degradation. Environ. Sci. Nano 2017, 4, 2175–2185. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Cheng, R.; Ali, J.; Wang, Z.; Mailhot, G.; Pan, G. Microcystis aeruginosa Synergistically Facilitate the Photocatalytic Degradation of Tetracycline Hydrochloride and Cr(VI) on PAN/TiO2/Ag Nanofiber Mats. Catalysts 2018, 8, 628. [Google Scholar] [CrossRef]
- Xiao, P.; Jiang, D.; Ju, L.; Jing, J.; Chen, M. Construction of RGO/CdIn2S4/g-C3N4 ternary hybrid with enhanced photocatalytic activity for the degradation of tetracycline hydrochloride. Appl. Surf. Sci. 2018, 433, 388–397. [Google Scholar] [CrossRef]
- Li, M.Y.; Tang, Y.-B.; Shi, W.-L.; Chen, F.-Y.; Shi, Y.; Gu, H.C. Design of visible-light-response core–shell Fe2O3/CuBi2O4 heterojunctions with enhanced photocatalytic activity towards the degradation of tetracycline: Z-scheme photocatalytic mechanism insight. Inorg. Chem. Front. 2018, 5, 3148–3154. [Google Scholar] [CrossRef]
- Guo, F.; Li, M.; Ren, H.; Huang, X.; Hou, W.; Wang, C.; Shi, W.; Lu, C. Fabrication of p-n CuBi2O4/MoS2 heterojunction with nanosheets-on-microrods structure for enhanced photocatalytic activity towards tetracycline degradation. Appl. Surf. Sci. 2019, 491, 88–94. [Google Scholar] [CrossRef]
- Yu, X.; He, J.; Zhang, Y.; Hu, J.; Chen, F.; Wang, Y.; He, G.; Liu, J.; He, Q. Effective photodegradation of tetracycline by narrow-energy band gap photocatalysts La2–xSrxNiMnO6 (x = 0, 0.05, 0.10, and 0.125). J. Alloys Compd. 2019, 806, 451–463. [Google Scholar] [CrossRef]
- Nagamine, M.; Osial, M.; Jackowska, K.; Krysinski, P.; Widera-Kalinowska, J. Tetracycline Photocatalytic Degradation under CdS Treatment. J. Mar. Sci. Eng. 2020, 8, 483. [Google Scholar] [CrossRef]
- Wei, X.; Feng, H.; Li, L.; Gong, J.; Jiang, K.; Xue, S.; Chu, P.K. Synthesis of tetragonal prismatic γ-In2Se3 nanostructures with predominantly {110} facets and photocatalytic degradation of tetracycline. Appl. Catal. B-Environ. 2020, 260, 118218. [Google Scholar] [CrossRef]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Gao, X.; Ma, J.; Chen, Z.; Kang, B.; Liu, J.; Li, H.; Feng, Z.; Huang, J. Novel BP/BiOBr S-scheme nano-heterojunction for enhanced visible-light photocatalytic tetracycline removal and oxygen evolution activity. J. Hazard. Mater. 2020, 387, 121690. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, L.; Liang, J.; Xu, W.; Yu, H.; Zhang, J.; Ye, S.; Xing, W.; Yuan, X. Photocatalytic degradation of tetracycline antibiotics using delafossite silver ferrite-based Z-scheme photocatalyst: Pathways and mechanism insight. Chemosphere 2021, 270, 128651. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Liu, X.; Li, Q.; Zheng, Y. A Novel PVDF-TiO2@g-C3N4 Composite Electrospun Fiber for Efficient Photocatalytic Degradation of Tetracycline under Visible Light Irradiation. Ecotoxicol. Environ. Saf. 2021, 210, 111866. [Google Scholar] [CrossRef]
- Hemavibool, K.; Sansenya, T.; Nanan, S. Enhanced Photocatalytic Degradation of Tetracycline and Oxytetracycline Antibiotics by BiVO4 Photocatalyst under Visible Light and Solar Light Irradiation. Antibiotics 2022, 11, 761. [Google Scholar] [CrossRef]
- Chen, L.; Xu, B.; Jin, M.; Chen, L.; Yi, G.; Xing, B.; Zhang, Y.; Wu, Y.; Li, Z. Excellent photocatalysis of Bi2WO6 structured with oxygen vacancies in degradation of tetracycline. J. Mol. Struct. 2023, 1278, 134911. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Supramolecular Self-Assembled Nanostructures Derived from Amplified Structural Isomerism of Zn(II)–Sn(IV)–Zn(II) Porphyrin Triads and Their Visible Light Photocatalytic Degradation of Pollutants. Nanomaterials 2024, 14, 1104. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.J.; Jung, S.H.; Kim, H.-J. Synthesis and Hydrogen-Bonded Supramolecular Assembly of trans-Dihydroxotin(IV) Tetrapyridylporphyrin Complexes. Bull. Korean Chem. Soc. 2004, 25, 1869–1873. [Google Scholar]
- Xie, S.; Huang, P.; Kruzic, J.J.; Zeng, X.; Qian, H. A highly efficient degradation mechanism of methyl orange using Fe-based metallic glass powders. Sci. Rep. 2016, 6, 21947. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Q.Z.; Wang, Z.P.; Zhu, Y. An all-organic 0D/2D supramolecular porphyrin/g-C3N4 heterojunction assembled via π-π interaction for efficient visible photocatalytic oxidation. Appl. Catal. B Environ. 2021, 291, 120059. [Google Scholar] [CrossRef]
- Ning, L.; Xu, J.; Lou, Y.; Pan, C.; Wang, Z.; Zhu, Y. A 3D/0D cobalt-embedded nitrogen-doped porous carbon/supramolecular porphyrin magnetic-separation photocatalyst with highly efficient pollutant degradation and water oxidation performance. J. Mater. Sci. Technol. 2022, 124, 53–64. [Google Scholar] [CrossRef]
- Mahalakshmi, K.; Ranjith, R.; Thangavelu, P.; Priyadharshini, M.; Palanivel, B.; Manthrammel, M.A.; Shkir, M.; Diravidamani, B. Augmenting the Photocatalytic Performance of Direct Z-Scheme Bi2O3/g-C3N4 Nanocomposite. Catalysts 2022, 12, 1544. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Bruker. SHELXTL (Ver. 6.10): Program for Solution and Refinement of Crystal Structures; Bruker AXS Inc.: Madison, WI, USA, 2000. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

| Photocatalysts | Irradiation Time (min) | Catalyst Dosage (mg/L) | Dye Concentration (mg/L) | Rate Constant (min−1) | Reference |
|---|---|---|---|---|---|
| BiOI–BiOBr | 300 | 2000 | 10 | 0.0031 | [41] |
| BiOBr | 80 | 1000 | 10 | 0.0072 | [42] |
| 15% TiO2/BiOBr | 80 | 1000 | 10 | 0.0243 | [42] |
| graphene@porphyrin (GNPs@TCPP) | 180 | 5 | 5 | 0.0065 | [43] |
| g-C3N4 | 120 | 1000 | 10 | 0.0038 | [44] |
| WO3 | 120 | 1000 | 10 | 0.0018 | [44] |
| WO3-g-C3N4 | 120 | 1000 | 10 | 0.0213 | [44] |
| Zn(II)-porphyrin/poly(acrylic acid) | 180 | 50 | 10 | 0.048 | [45] |
| Bi2MoO6–Bi5O7Br | 180 | 200 | 16 | 0.0035 | [46] |
| Ag2Mo1–xWxO4 (x = 0.50) | 140 | 1000 | 5 | 0.0054 | [47] |
| Zn0.999Gd0.001O | 90 | 400 | 15 | 0.0212 | [48] |
| Mn3O4/ZnO/Eu2O3 | 150 | 150 | 5 | 0.0107 | [49] |
| TiO2 | 80 | 500 | 20 | 0.015 | [50] |
| 5.0 wt% MoS2-TiO2 | 80 | 500 | 20 | 0.028 | [50] |
| ZnO | 120 | 1000 | 100 | 0.005 | [51] |
| 10% Co–ZnO | 120 | 1000 | 100 | 0.014 | [51] |
| Zn(II)–Sn(IV)–Zn(II) Porphyrin-Triad | 100 | 67 | 20 | 0.0251 | [52] |
| 4α-[Zn(TAzPP)] | 70 | 200 | 20 | 0.016 | [53] |
| [H4TTP]Cl2∙3CHCl3 + H2O2 | 180 | 500 | 30 | 0.0042 | [54] |
| Ni(TAMPP) + H2O2 | 90 | 500 | 30 | 0.013 | [55] |
| 1 | 75 | 30 | 30 | 0.005 | This work |
| 2 | 75 | 30 | 30 | 0.023 | This work |
| Photocatalyst | Irradiation Time (min) | Catalyst Dose (mg/L) | TC Concentration (mg/L) | Rate Constant (min−1) | Reference |
|---|---|---|---|---|---|
| AgIn(MoO4)2 | 60 | 1000 | 10 | 0.00698 | [56] |
| 6%-Ag/AgIn(MoO4)2 | 60 | 1000 | 10 | 0.00985 | [56] |
| Ag2CO3/Ag/WO3 | 90 | 1000 | 10 | 0.0179 | [57] |
| polyacrylonitrile (PAN)-TiO2/Ag | 240 | 1000 | 20 | 0.013 | [58] |
| g-C3N4 | 180 | 1000 | 10 | 0.0032 | [59] |
| CdIn2S4 | 180 | 1000 | 10 | 0.00422 | [59] |
| rGO/g-C3N4 | 180 | 1000 | 10 | 0.00251 | [59] |
| rGO/CdIn2S4 | 180 | 1000 | 10 | 0.0042 | [59] |
| CdIn2S4/g-C3N4 | 180 | 1000 | 10 | 0.0043 | [59] |
| rGO/30%- CdIn2S4/g-C3N4 | 180 | 1000 | 10 | 0.00766 | [59] |
| CuBi2O4 | 120 | 500 | 10 | 0.00341 | [60] |
| Fe2O3 | 120 | 500 | 10 | 0.00485 | [60] |
| 30%- Fe2O3/CuBi2O4 | 120 | 500 | 10 | 0.01246 | [60] |
| CuBi2O4 | 120 | 500 | 10 | 0.0032 | [61] |
| MoS2 | 120 | 500 | 10 | 0.0034 | [61] |
| CuBi2O4/MoS2 (5%) | 120 | 500 | 10 | 0.0095 | [61] |
| La2-xSrxNiMnO6 (x = 0.10) | 240 | 1000 | 10 | 0.0101 | [62] |
| CdS | 90 | 250 | 40 | 0.0056 | [63] |
| γ-In2Se3 (0.04 M EDTA) | 120 | 1000 | 20 | 0.0175 | [64] |
| TiO2 (P-25) | 150 | 200 | 10 | 0.0069 | [65] |
| BP (black phosphorus) | 90 | 1000 | 50 | 0.0007 | [66] |
| BiOBr | 90 | 1000 | 50 | 0.0027 | [66] |
| 10BP/BiOBr | 90 | 1000 | 50 | 0.0110 | [66] |
| AgFeO2 | 60 | 500 | 40 | 0.0060 | [67] |
| Ag/AgFeO2 | 60 | 500 | 40 | 0.0104 | [67] |
| poly vinylidene fluoride-TiO2@g-C3N4-0.2g | 300 | 1000 | 50 | 0.0120 | [68] |
| BiVO4 | 240 | 250 | 10 | 0.0045 | [69] |
| Bi2WO6 | 180 | 300 | 20 | 0.0044 | [70] |
| Oxygen deficient Bi2WO6 | 180 | 300 | 20 | 0.0078 | [70] |
| Zn(II)-Sn(IV)-Zn(II) porphyrin triad | 45 | 50 | 100 | 0.0260 | [71] |
| 1 | 60 | 30 | 125 | 0.0040 | This work |
| 2 | 60 | 30 | 125 | 0.0180 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shee, N.K.; Kim, H.-J. Self-Assembled Nanostructure of Ionic Sn(IV)porphyrin Complex Based on Multivalent Interactions for Photocatalytic Degradation of Water Contaminants. Molecules 2024, 29, 4200. https://doi.org/10.3390/molecules29174200
Shee NK, Kim H-J. Self-Assembled Nanostructure of Ionic Sn(IV)porphyrin Complex Based on Multivalent Interactions for Photocatalytic Degradation of Water Contaminants. Molecules. 2024; 29(17):4200. https://doi.org/10.3390/molecules29174200
Chicago/Turabian StyleShee, Nirmal Kumar, and Hee-Joon Kim. 2024. "Self-Assembled Nanostructure of Ionic Sn(IV)porphyrin Complex Based on Multivalent Interactions for Photocatalytic Degradation of Water Contaminants" Molecules 29, no. 17: 4200. https://doi.org/10.3390/molecules29174200
APA StyleShee, N. K., & Kim, H.-J. (2024). Self-Assembled Nanostructure of Ionic Sn(IV)porphyrin Complex Based on Multivalent Interactions for Photocatalytic Degradation of Water Contaminants. Molecules, 29(17), 4200. https://doi.org/10.3390/molecules29174200







