Naphthalimide-Based Amphiphiles: Synthesis and DFT Studies of the Aggregation and Interaction of a Simplified Model System with Water Molecules
Abstract
:1. Introduction
2. Results
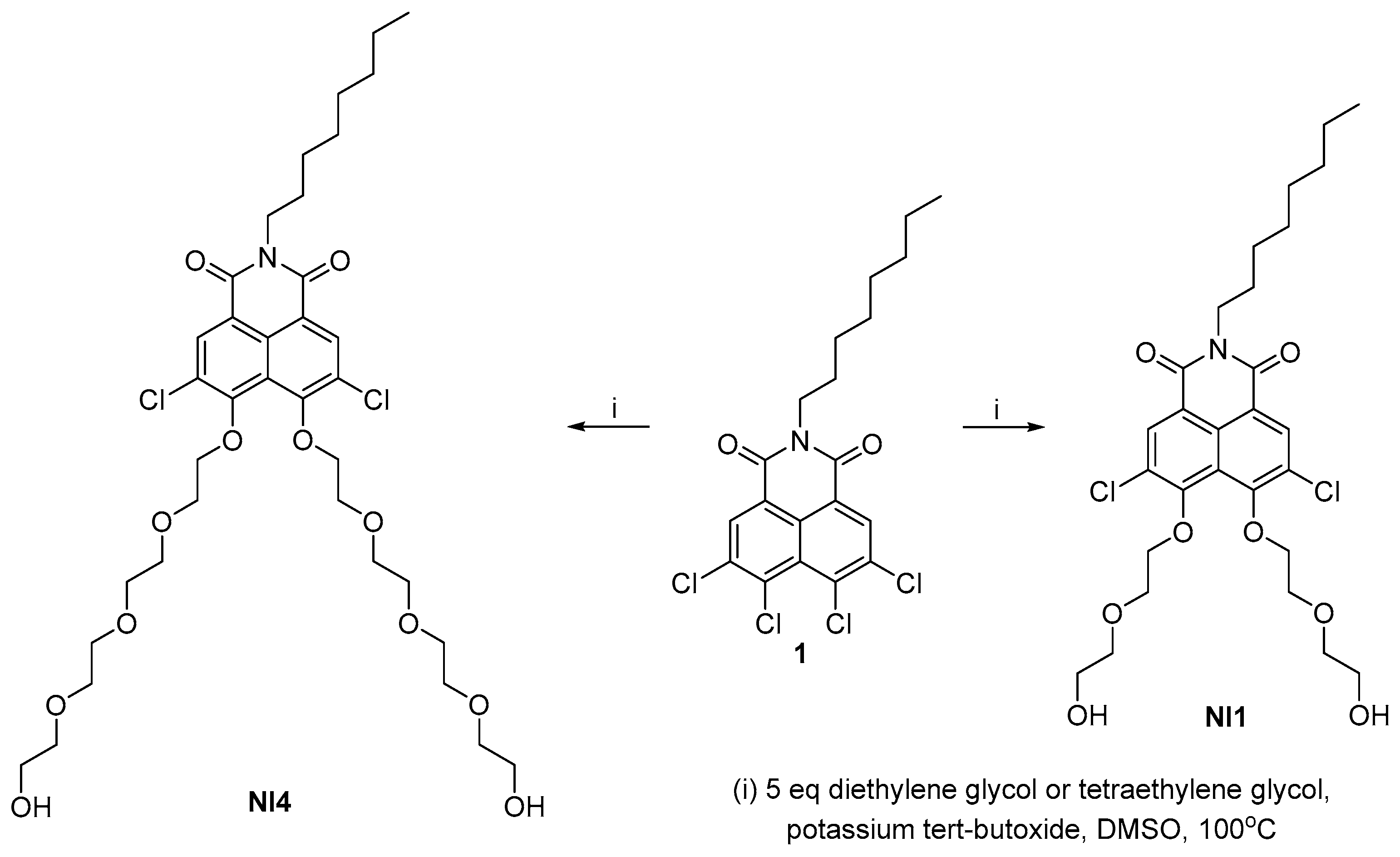
2.1. Synthesis of PEG-Substituted 1,8-Naphthalimides
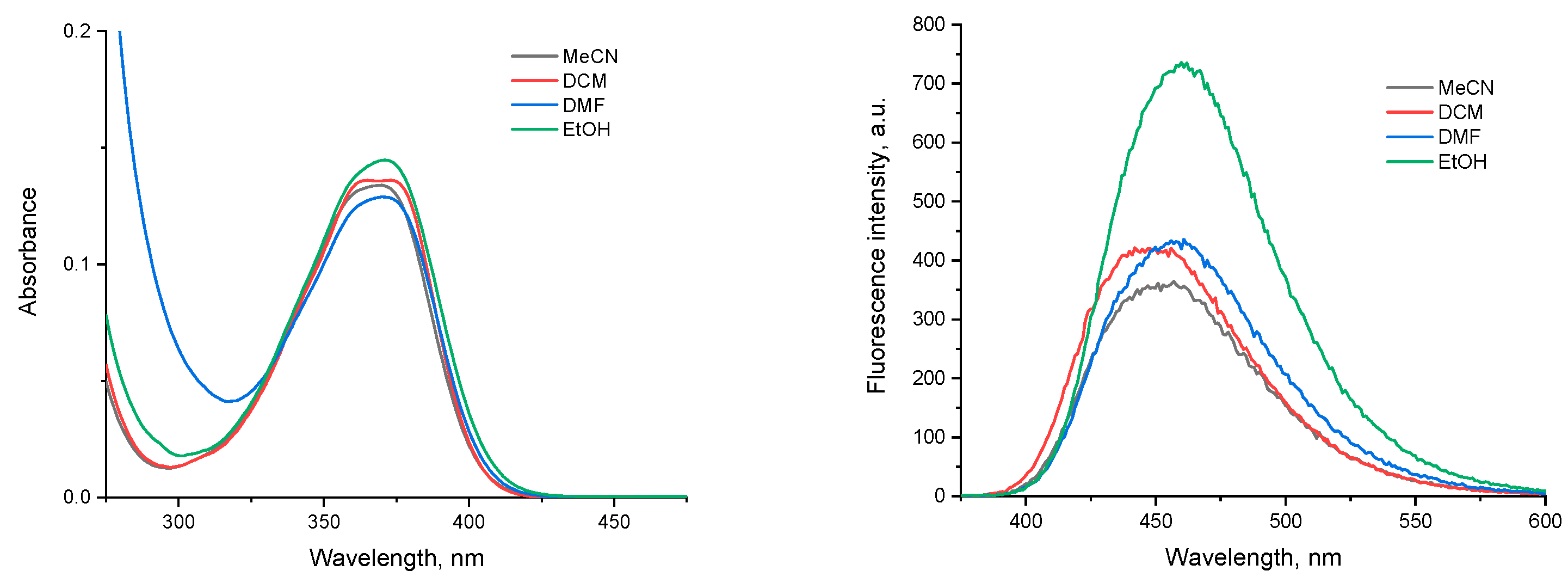
2.2. DFT Studies of the Aggregation and Interaction of PEG-Substituted 1,8-Naphthalimides with Water Molecules
2.2.1. Water Dimer
2.2.2. (P)EG/Water
2.2.3. Naphthalimide-PEG Compounds
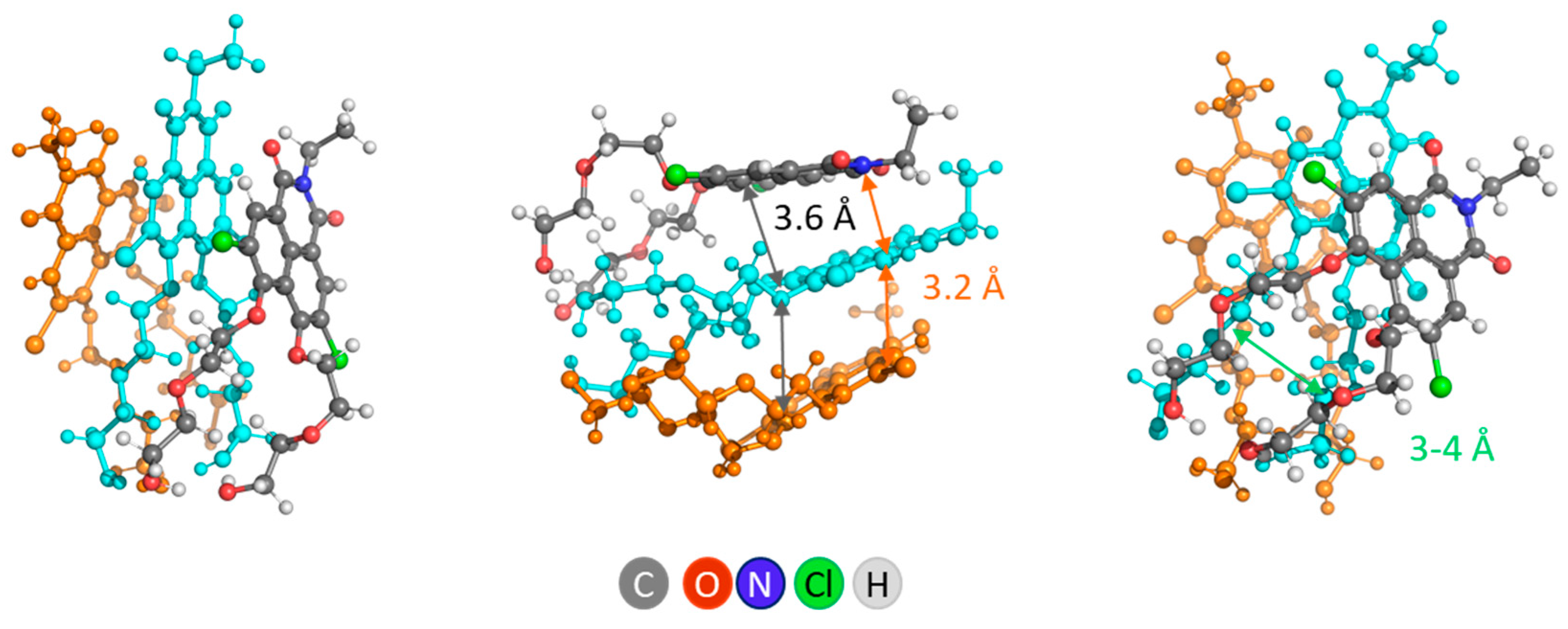
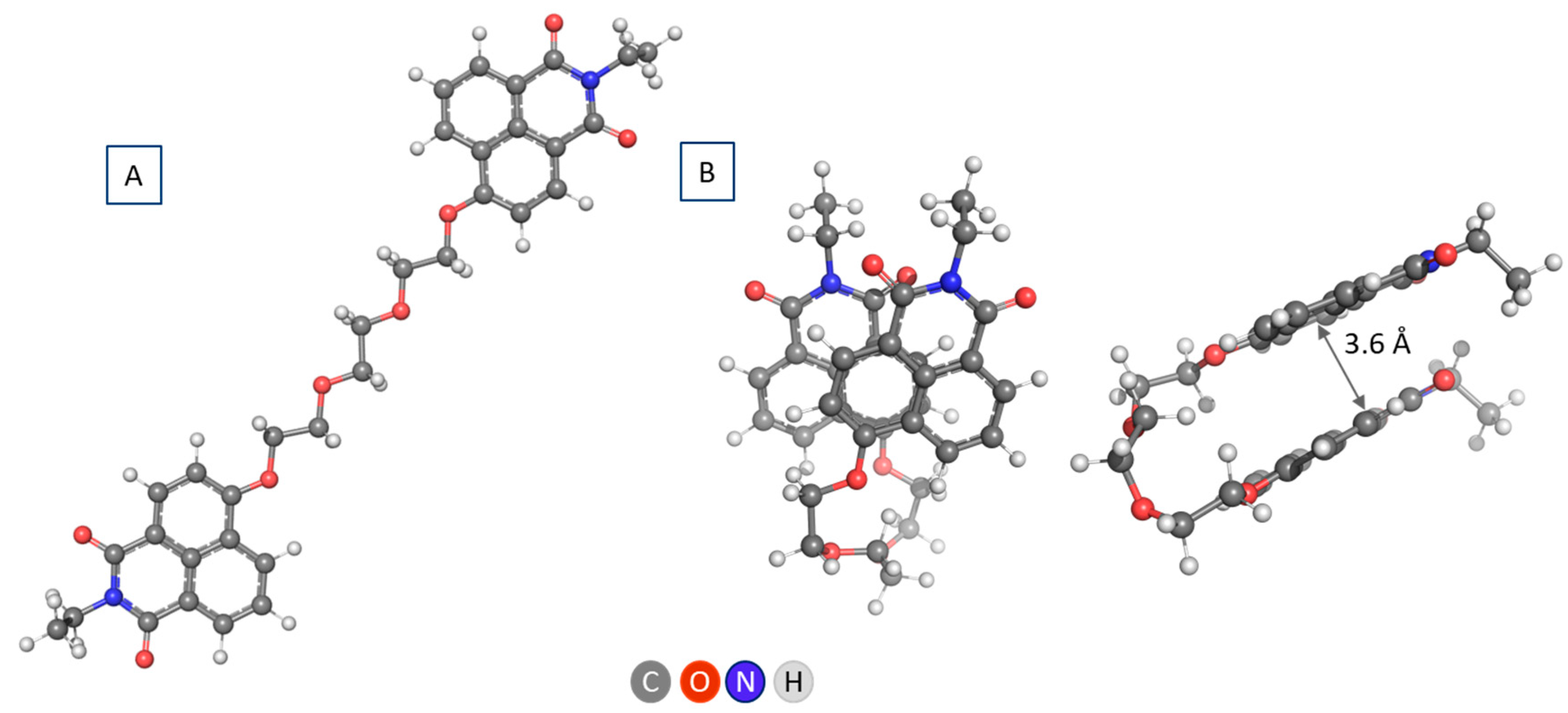
Aggregation
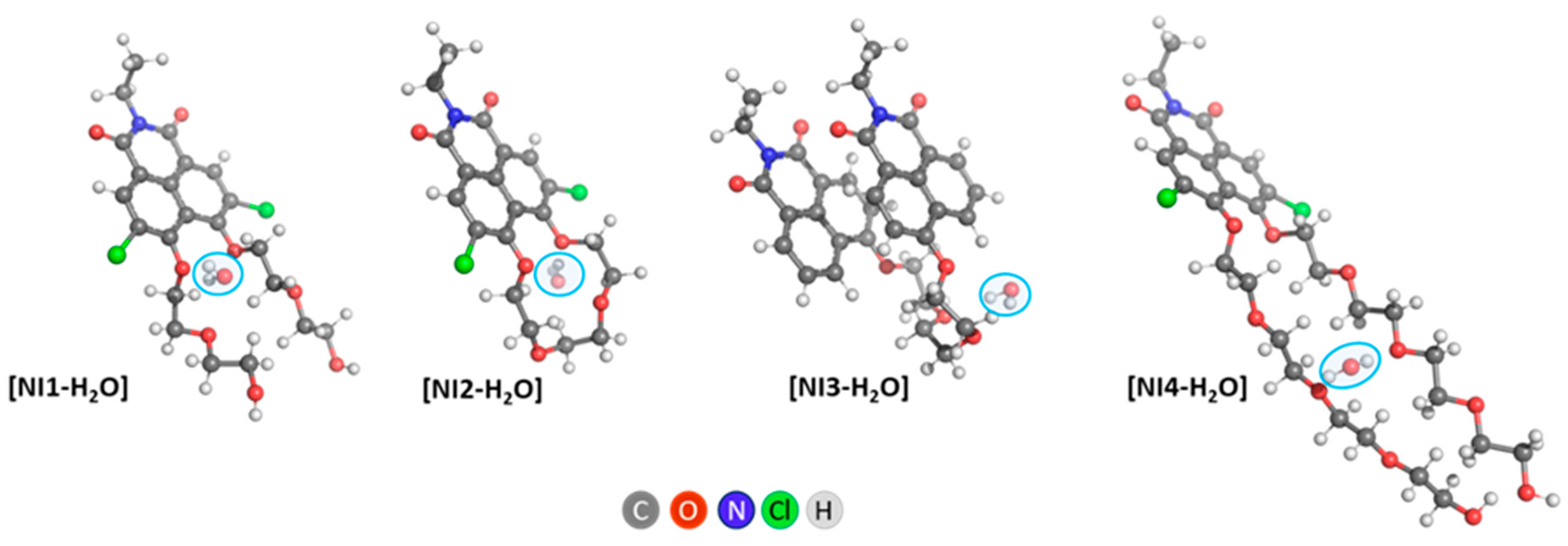
Interaction with Water Molecules
2.3. A Look at Experimental Evidence on Aggregation of NI4 in Water Environment
3. Materials and Methods
3.1. Synthesis of NI1 and NI4
3.2. Synthesis of NI2
3.3. Synthesis of NI3
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J. Amphiphilic Molecules BT—Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 72–75. ISBN 978-3-662-44324-8. [Google Scholar]
- Lindman, B.; Alexandridis, P. (Eds.) Amphiphilic Molecules: Small and Large; Elsevier Science B.V.: Amsterdam, The Netherlands, 2000; pp. 1–12. ISBN 978-0-444-82441-7. [Google Scholar]
- Ghosh, S.; Ray, A.; Pramanik, N. Self-Assembly of Surfactants: An Overview on General Aspects of Amphiphiles. Biophys. Chem. 2020, 265, 106429. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W. Role of Amphiphilic Compounds in the Evolution of Membrane Structure on the Early Earth. Orig. Life Evol. Biosph. 1986, 17, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Milshteyn, D.; Damer, B.; Havig, J.; Deamer, D. Amphiphilic Compounds Assemble into Membranous Vesicles in Hydrothermal Hot Spring Water but Not in Seawater. Life 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jiang, S. Super-Hydrophilic Zwitterionic Poly(Carboxybetaine) and Amphiphilic Non-Ionic Poly(Ethylene Glycol) for Stealth Nanoparticles. Nano Today 2012, 7, 404–413. [Google Scholar] [CrossRef]
- Inada, Y.; Furukawa, M.; Sasaki, H.; Kodera, Y.; Hiroto, M.; Nishimura, H.; Matsushima, A. Biomedical and Biotechnological Applications of PEG- and PM-Modified Proteins. Trends Biotechnol. 1995, 13, 86–91. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Parray, Z.A.; Hassan, M.I.; Ahmad, F.; Islam, A. Amphiphilic Nature of Polyethylene Glycols and Their Role in Medical Research. Polym. Test. 2020, 82, 106316. [Google Scholar] [CrossRef]
- Deng, J.; Dai, Z.; Yan, J.; Sandru, M.; Sandru, E.; Spontak, R.J.; Deng, L. Facile and Solvent-Free Fabrication of PEG-Based Membranes with Interpenetrating Networks for CO2 Separation. J. Memb. Sci. 2019, 570–571, 455–463. [Google Scholar] [CrossRef]
- Feng, Y.; Han, G.; Chung, T.-S.; Weber, M.; Widjojo, N.; Maletzko, C. Effects of Polyethylene Glycol on Membrane Formation and Properties of Hydrophilic Sulfonated Polyphenylenesulfone (SPPSU) Membranes. J. Memb. Sci. 2017, 531, 27–35. [Google Scholar] [CrossRef]
- Tunuguntla, R.H.; Hu, A.Y.; Zhang, Y.; Noy, A. Impact of PEG Additives and Pore Rim Functionalization on Water Transport through Sub-1 Nm Carbon Nanotube Porins. Faraday Discuss. 2018, 209, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Winterhalter, M.; Bürner, H.; Marzinka, S.; Benz, R.; Kasianowicz, J.J. Interaction of Poly(Ethylene-Glycols) with Air-Water Interfaces and Lipid Monolayers: Investigations on Surface Pressure and Surface Potential. Biophys. J. 1995, 69, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Mohammed-Sadhakathullah, A.H.M.; Paulo-Mirasol, S.; Molina, B.G.; Torras, J.; Armelin, E. PLA-PEG-Cholesterol Biomimetic Membrane for Electrochemical Sensing of Antioxidants. Electrochim. Acta 2024, 476, 143716. [Google Scholar] [CrossRef]
- Lentz, B.R. PEG as a Tool to Gain Insight into Membrane Fusion. Eur. Biophys. J. 2007, 36, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Yang, G.; Liu, Y.; Liu, T. Research Progress in Membrane Fusion-Based Hybrid Exosomes for Drug Delivery Systems. Front. Bioeng. Biotechnol. 2022, 10, 939441. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Goh, K.; Wang, R. The Coming of Age of Water Channels for Separation Membranes: From Biological to Biomimetic to Synthetic. Chem. Soc. Rev. 2022, 51, 4537–4582. [Google Scholar] [CrossRef]
- Song, W.; Kumar, M. Artificial Water Channels: Toward and beyond Desalination. Curr. Opin. Chem. Eng. 2019, 25, 9–17. [Google Scholar] [CrossRef]
- Song, W.; Kumar, M. Beyond Aquaporins: Recent Developments in Artificial Water Channels. Langmuir 2022, 38, 9085–9091. [Google Scholar] [CrossRef]
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.M.; Gunnlaugsson, T. Recent Advances in the Development of 1,8-Naphthalimide Based DNA Targeting Binders, Anticancer and Fluorescent Cellular Imaging Agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef]
- Misra, S.; Singh, P.; Das, A.; Brandão, P.; Sahoo, P.; Sepay, N.; Bhattacharjee, G.; Datta, P.; Mahapatra, A.K.; Satpati, B.; et al. Supramolecular Assemblies of a 1,8-Naphthalimide Conjugate and Its Aggregation-Induced Emission Property. Mater. Adv. 2020, 1, 3532–3538. [Google Scholar] [CrossRef]
- Liu, H.; Wei, S.; Qiu, H.; Zhan, B.; Liu, Q.; Lu, W.; Zhang, J.; Ngai, T.; Chen, T. Naphthalimide-Based Aggregation-Induced Emissive Polymeric Hydrogels for Fluorescent Pattern Switch and Biomimetic Actuators. Macromol. Rapid Commun. 2020, 41, 2000123. [Google Scholar] [CrossRef] [PubMed]
- Kavita, N.; Krishnan, I.P. Molecular Engineering of Naphthalimide Methylcyclohexane Luminogen: Unraveling J*-Aggregation Pattern and Sensing Melamine in Aqueous Media. CCS Chem. 2023, 6, 923–931. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Bojinov, V.B. Design, Synthesis and Sensor Activity of a Highly Photostable Blue Emitting 1,8-Naphthalimide. J. Lumin. 2012, 132, 2235–2241. [Google Scholar] [CrossRef]
- Mutovska, M.; Simeonova, N.; Stoyanov, S.; Zagranyarski, Y.; Stanchovska, S.; Marinova, D. Naphthalene Monoimides with Peri-Annulated Disulfide Bridge—Synthesis and Electrochemical Redox Activity. Materials 2023, 16, 7471. [Google Scholar] [CrossRef] [PubMed]
- Mutovska, M.; Skabeev, A.; Konstantinov, K.; Cabanetos, C.; Stoyanov, S.; Zagranyarski, Y. One-Pot Synthesis of Fused-Rings Heterocyclic Systems Based on Symmetrically Benzofuran Annulated 1,8-Naphthalimides. Dye. Pigment. 2023, 220, 111701. [Google Scholar] [CrossRef]
- Morokuma, K.; Pedersen, L. Molecular-Orbital Studies of Hydrogen Bonds. An Ab Initio Calculation for Dimeric H2O. J. Chem. Phys. 1968, 48, 3275–3282. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Topics in Physical Chemistry—Oxford University Press; Oxford University Press: Oxford, UK, 1997; ISBN 9780195095494. [Google Scholar]
- Ghosh, S.R.; Debnath, B.; Jana, A.D. Water Dimer Isomers: Interaction Energies and Electronic Structure. J. Mol. Model. 2020, 26, 20. [Google Scholar] [CrossRef]
- Leforestier, C.; Szalewicz, K.; van der Avoird, A. Spectra of Water Dimer from a New Ab Initio Potential with Flexible Monomers. J. Chem. Phys. 2012, 137, 14305. [Google Scholar] [CrossRef]
- Ruscic, B. Active Thermochemical Tables: Water and Water Dimer. J. Phys. Chem. A 2013, 117, 11940–11953. [Google Scholar] [CrossRef]
- Rozza, A.M.; Vanpoucke, D.E.P.; Krammer, E.-M.; Bouckaert, J.; Blossey, R.; Lensink, M.F.; Ondrechen, M.J.; Bakó, I.; Oláh, J.; Roos, G. Hydration Sphere Structure of Architectural Molecules: Polyethylene Glycol and Polyoxymethylene Oligomers. J. Mol. Liq. 2023, 384, 122172. [Google Scholar] [CrossRef]
- Tasaki, K. Poly(Oxyethylene)–Water Interactions: A Molecular Dynamics Study. J. Am. Chem. Soc. 1996, 118, 8459–8469. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tadokoro, H. Structural Studies of Polyethers, (-(CH2)m-O-)n. X. Crystal Structure of Poly(Ethylene Oxide). Macromolecules 1973, 6, 672–675. [Google Scholar] [CrossRef]
- Arnold, K.; Herrmann, A.; Pratsch, L.; Gawrisch, K. The Dielectric Properties of Aqueous Solutions of Poly(Ethylene Glycol) and Their Influence on Membrane Structure. Biochim. Biophys. Acta-Biomembr. 1985, 815, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Koizuim, N.; Hanai, T. Dielectric Properties of Lower-Membered Polyethylene Glycols at Low Frequencies. J. Phys. Chem. 1956, 60, 1496–1500. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Walshe, J.; Kennedy, M.; Confrey, T.; Doran, J.; McCormack, S.J. Enhancement in Solar Cell Efficiency by Luminescent Down-Shifting Layers. Adv. Energy Res. 2013, 1, 117–126. [Google Scholar] [CrossRef]
- Felip-León, C.; Galindo, F.; Miravet, J.F. Insights into the Aggregation-Induced Emission of 1,8-Naphthalimide-Based Supramolecular Hydrogels. Nanoscale 2018, 10, 17060–17069. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, Z.; Fan, C.; Liu, G.; Pu, S. 1,8-Naphthalimide-Based Highly Emissive Luminophors with Various Mechanofluorochromism and Aggregation-Induced Characteristics. ACS Omega 2019, 4, 14324–14332. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Bryaskova, R.G.; Ismail, S.R.; Philipova, N.D.; Uzunova, V.P.; Bakov, V.V.; Tzoneva, R.D.; Bojinov, V.B. Aggregation Induced Emission in 1,8-Naphthalimide Embedded Nanomicellar Architecture as a Platform for Fluorescent Ratiometric PH-Probe with Biomedical Applications. J. Photochem. Photobiol. A Chem. 2021, 418, 113380. [Google Scholar] [CrossRef]
- Said, A.I.; Staneva, D.; Angelova, S.; Grabchev, I. Self-Associated 1,8-Naphthalimide as a Selective Fluorescent Chemosensor for Detection of High PH in Aqueous Solutions and Their Hg2+ Contamination. Sensors 2023, 23, 399. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Bakov, V.V.; Bojinov, V.B. Photoinduced Electron Transfer and Aggregation-Induced Emission in 1,8-Naphthalimide Probes as a Platform for Detection of Acid/Base Vapors. Photonics 2022, 9, 994. [Google Scholar] [CrossRef]
- Bursch, M.; Mewes, J.M.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202205735. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom-Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F. Atomic Orbital Basis Sets. WIREs Comput. Mol. Sci. 2013, 3, 273–295. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. Thirty Years of Density Functional Theory in Computational Chemistry: An Overview and Extensive Assessment of 200 Density Functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Goerigk, L.; Hansen, A.; Bauer, C.; Ehrlich, S.; Najibi, A.; Grimme, S. A Look at the Density Functional Theory Zoo with the Advanced GMTKN55 Database for General Main Group Thermochemistry, Kinetics and Noncovalent Interactions. Phys. Chem. Chem. Phys. 2017, 19, 32184–32215. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Runge, E.; Gross, E.K.U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- PyMOL; Schrödinger, LLC.: New York, NY, USA, 2010.




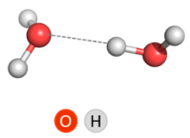 | O∙∙∙O Distance, Å | O–H∙∙∙O, ° | ΔE | ΔH | ΔE (+BSSE) | ΔH (+BSSE) |
|---|---|---|---|---|---|---|
| B3LYP/6-311++G(d,p) [30] | 2.901 | 175.55 | −5.85 | −5.05 | ||
| B3LYP/6-311++G(d,p) | 2.901 | 174.92 | −5.83 | −4.08 | −5.02 | −3.28 |
| B3LYP-D3/6-311++G(d,p) | 2.892 | 174.51 | −6.55 | −4.78 | −5.74 | −3.96 |
| B3LYP/6-31G(d,p) | 2.878 | 164.51 | ||||
| ωb97xd/6-31G(d,p) | 2.870 | 164.17 | −7.64 | −5.87 | −5.65 | −3.87 |
| ωb97xd/6-31+G(d,p) | 2.871 | 173.21 | −6.45 | −4.67 | −5.63 | −3.85 |
| ωb97xd/6-311+G(d,p) | 2.879 | 174.51 | −6.31 | −4.54 | −5.49 | −3.72 |
| ωb97xd/6-311++G(d,p) | 2.880 | 174.76 | −6.29 | −4.53 | −5.45 | −3.73 |
| M062X/6-31G(d,p) | 2.792 | 141.54 | −7.61 | −6.43 | −5.20 | −4.03 |
| M062X/6-31+G(d,p) | 2.880 | 172.53 | −6.64 | −4.88 | −5.77 | −4.01 |
| ε | ΔE (+BSSE) | ΔH (+BSSE) | O(H2O)∙∙∙O(dmEG) |
|---|---|---|---|
| 1 | −8.18 | −6.30 | 2.923 |
| 2 | −7.86 | −5.70 | 2.898 |
| 5 | −7.52 | −5.20 | 2.869 |
| 10 | −6.49 | −4.31 | 2.852 |
| 78 | −4.25 | −2.87 | 2.824 |
| ε | ΔE (+BSSE) | ΔH (+BSSE) | O(H2O)∙∙∙O(NI) |
|---|---|---|---|
| NI1 + H2O → [NI1-H2O] | |||
| 1 | −4.62 | −3.68 | 2.905 |
| 2 | −4.30 | −3.07 | 2.891 |
| 5 | −4.32 | −3.25 | 2.888 |
| 10 | −4.37 | −3.25 | 2.876 |
| 78 | −5.67 | −4.60 | 2.871 |
| NI2 + H2O → [NI2-H2O] | |||
| 1 | −5.82 | −4.22 | 2.846 |
| 2 | −4.25 | −2.70 | 2.856 |
| 5 | −3.45 | −1.85 | 2.869 |
| 10 | −2.90 | −1.41 | 2.876 |
| 78 | −3.44 | −2.18 | 2.895 |
| NI3 + H2O → [NI3-H2O] | |||
| 1 | −9.93 | −8.27 | 2.875; 2.933 |
| 2 | −9.00 | −6.66 | 2.867; 2.886 |
| 5 | −8.25 | −6.34 | 2.854; 2.849 |
| 10 | −7.38 | −5.45 | 2.841; 2.839 |
| 78 | −4.96 | −3.49 | 2.828; 2.809 |
| NI4 + H2O → [NI4-H2O] | |||
| 1 | −7.19 | −5.16 | 2.892; 2.871 |
| 2 | −8.00 | −4.46 | 2.887; 2.866 |
| 5 | −7.55 | −3.97 | 2.882; 2.857 |
| 10 | −6.58 | −2.92 | 2.875; 2.848 |
| 78 | −5.01 | −2.09 | 2.828; 2.873 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkova, V.; Anastasova, D.; Dobrev, S.; Mutovska, M.; Kircheva, N.; Nikolova, V.; Kolev, S.D.; Stoyanov, S.; Zagranyarski, Y.; Dudev, T.; et al. Naphthalimide-Based Amphiphiles: Synthesis and DFT Studies of the Aggregation and Interaction of a Simplified Model System with Water Molecules. Molecules 2024, 29, 4204. https://doi.org/10.3390/molecules29174204
Petkova V, Anastasova D, Dobrev S, Mutovska M, Kircheva N, Nikolova V, Kolev SD, Stoyanov S, Zagranyarski Y, Dudev T, et al. Naphthalimide-Based Amphiphiles: Synthesis and DFT Studies of the Aggregation and Interaction of a Simplified Model System with Water Molecules. Molecules. 2024; 29(17):4204. https://doi.org/10.3390/molecules29174204
Chicago/Turabian StylePetkova, Vladislava, Denitsa Anastasova, Stefan Dobrev, Monika Mutovska, Nikoleta Kircheva, Valya Nikolova, Spas D. Kolev, Stanimir Stoyanov, Yulian Zagranyarski, Todor Dudev, and et al. 2024. "Naphthalimide-Based Amphiphiles: Synthesis and DFT Studies of the Aggregation and Interaction of a Simplified Model System with Water Molecules" Molecules 29, no. 17: 4204. https://doi.org/10.3390/molecules29174204
APA StylePetkova, V., Anastasova, D., Dobrev, S., Mutovska, M., Kircheva, N., Nikolova, V., Kolev, S. D., Stoyanov, S., Zagranyarski, Y., Dudev, T., & Angelova, S. (2024). Naphthalimide-Based Amphiphiles: Synthesis and DFT Studies of the Aggregation and Interaction of a Simplified Model System with Water Molecules. Molecules, 29(17), 4204. https://doi.org/10.3390/molecules29174204










