The Influence of the Comonomer Ratio and Reaction Temperature on the Mechanical, Thermal, and Morphological Properties of Lignin Oil–Sulfur Composites
Abstract
:1. Introduction
2. Results and Discussion
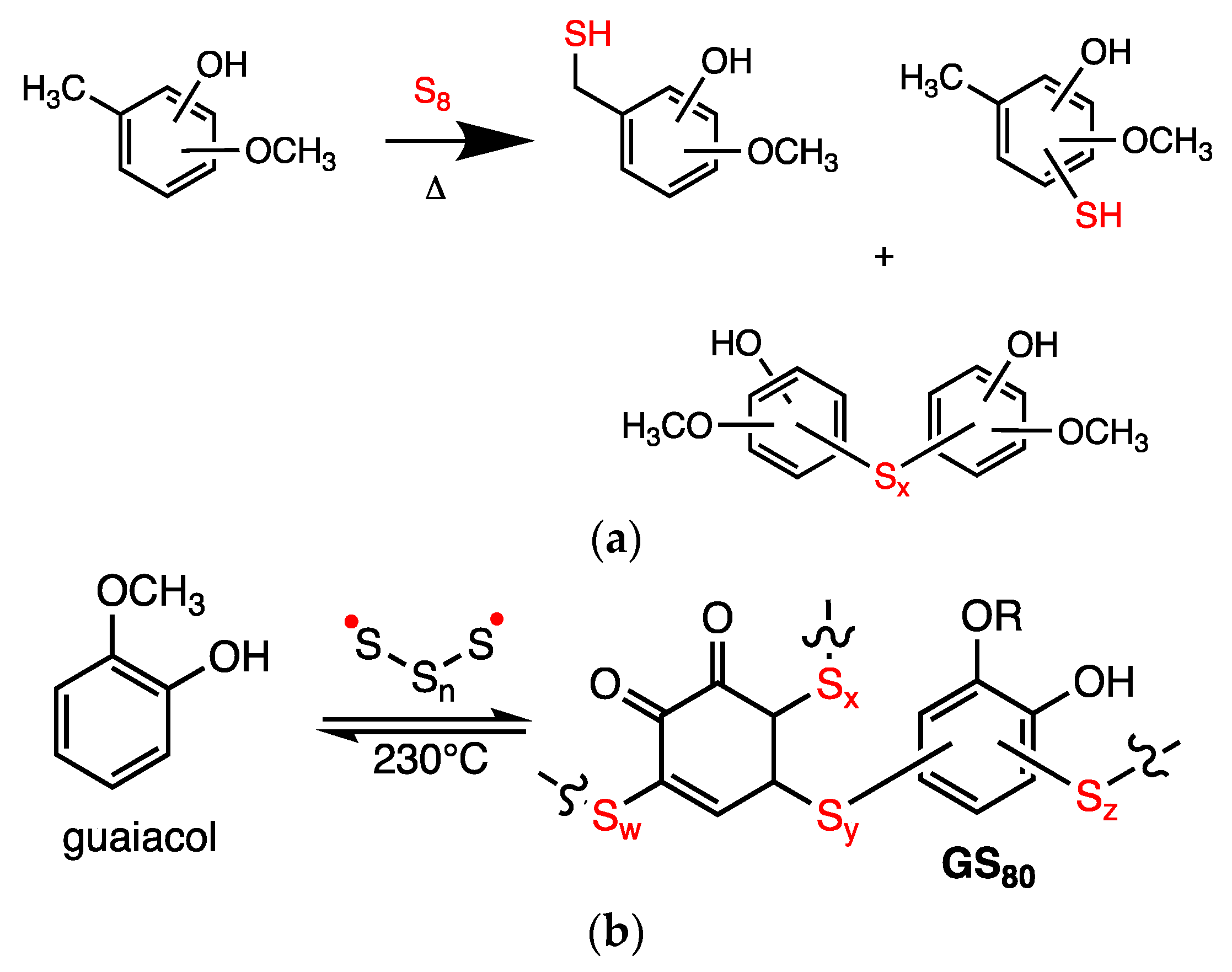
2.1. Synthesis and Chemical Characterization of Composites
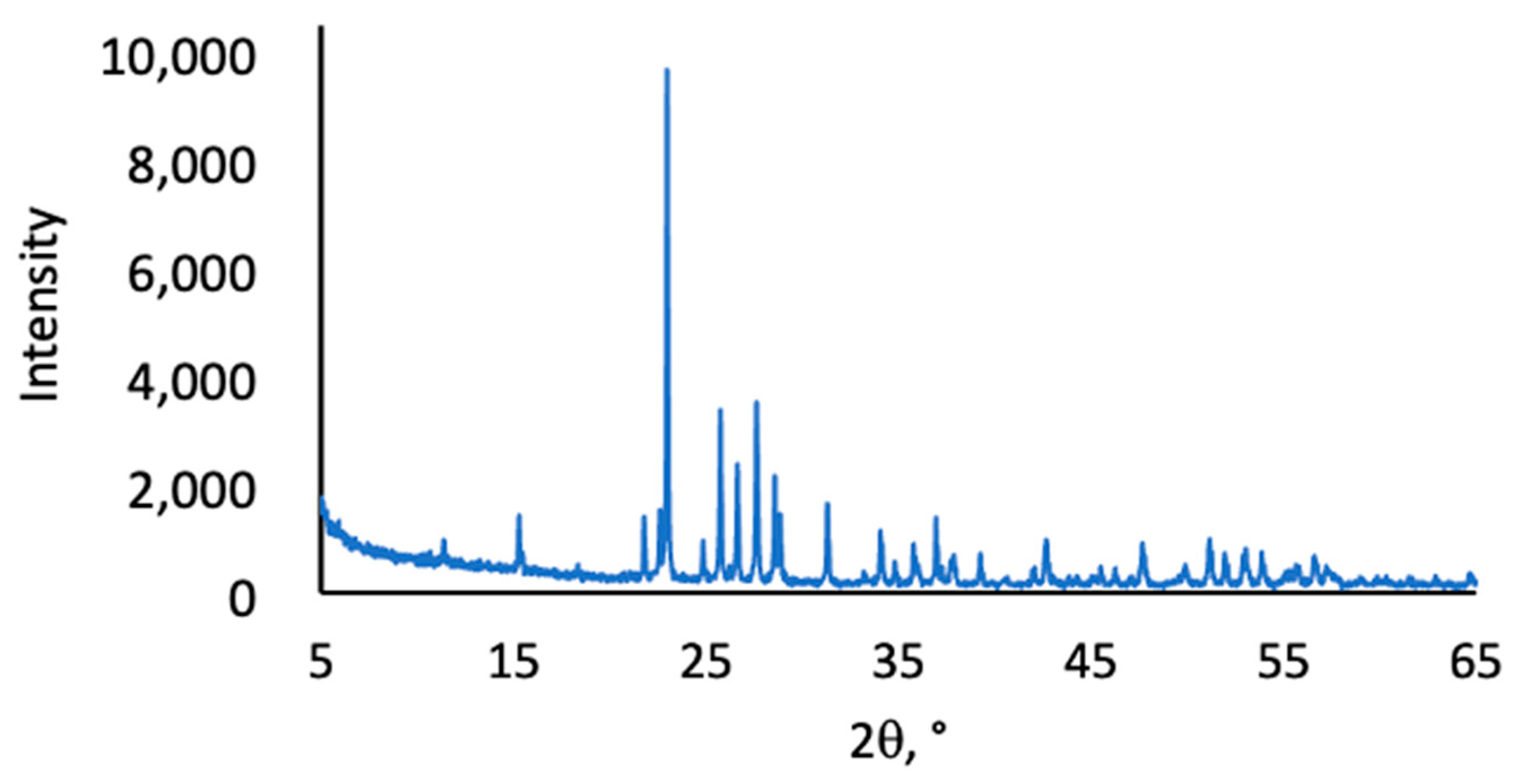
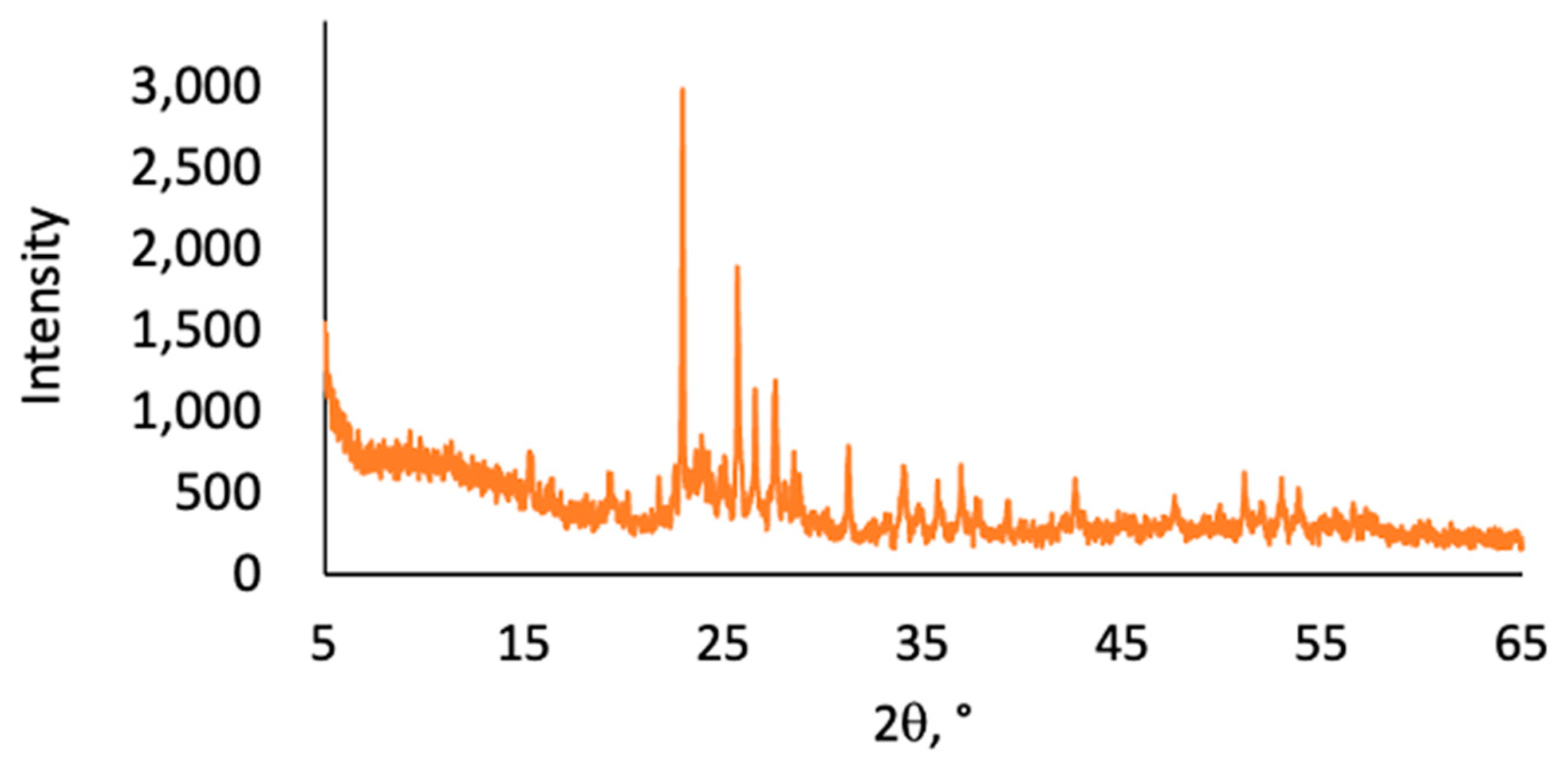
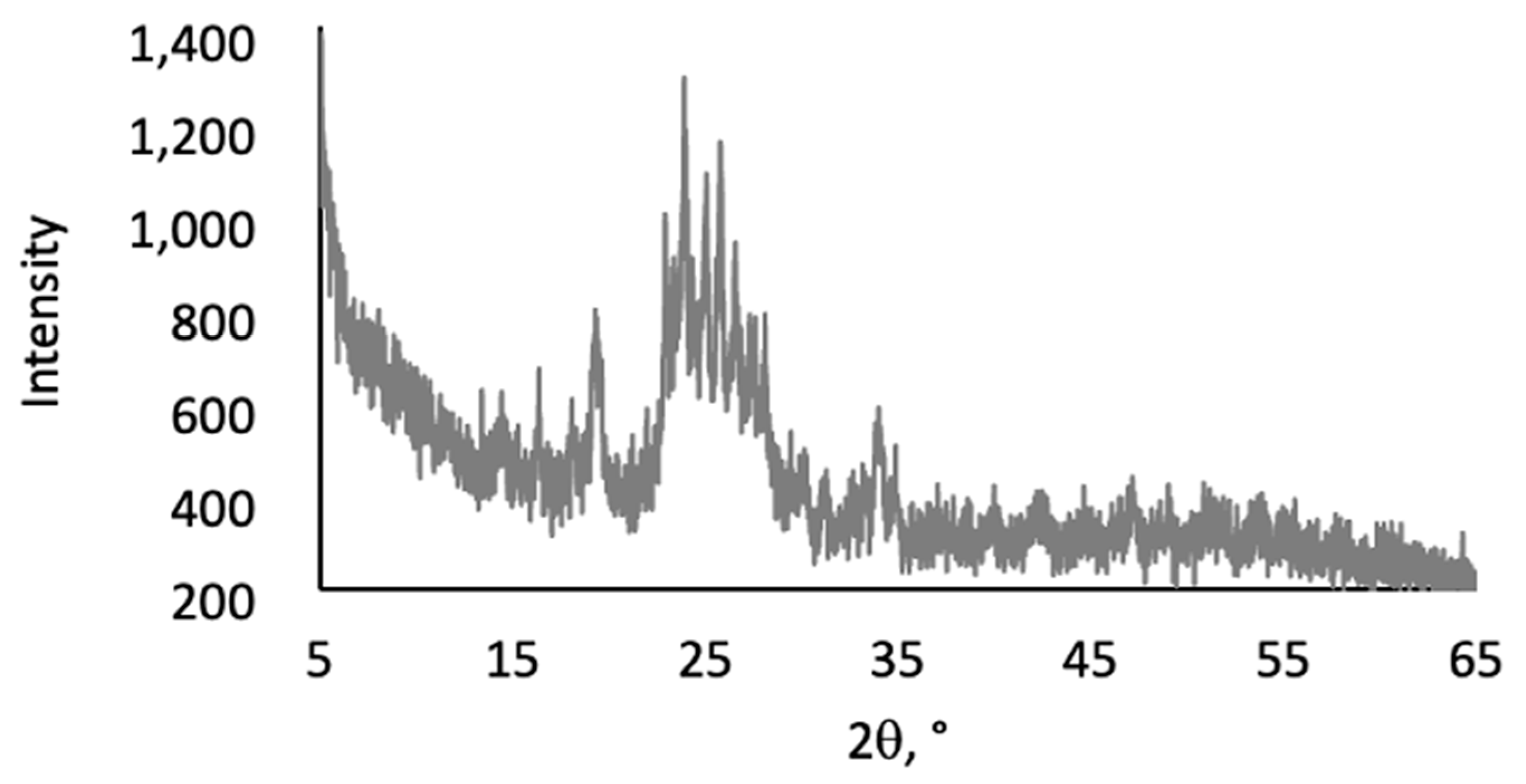
2.2. Thermal and Morphological Properties of LOSx@T
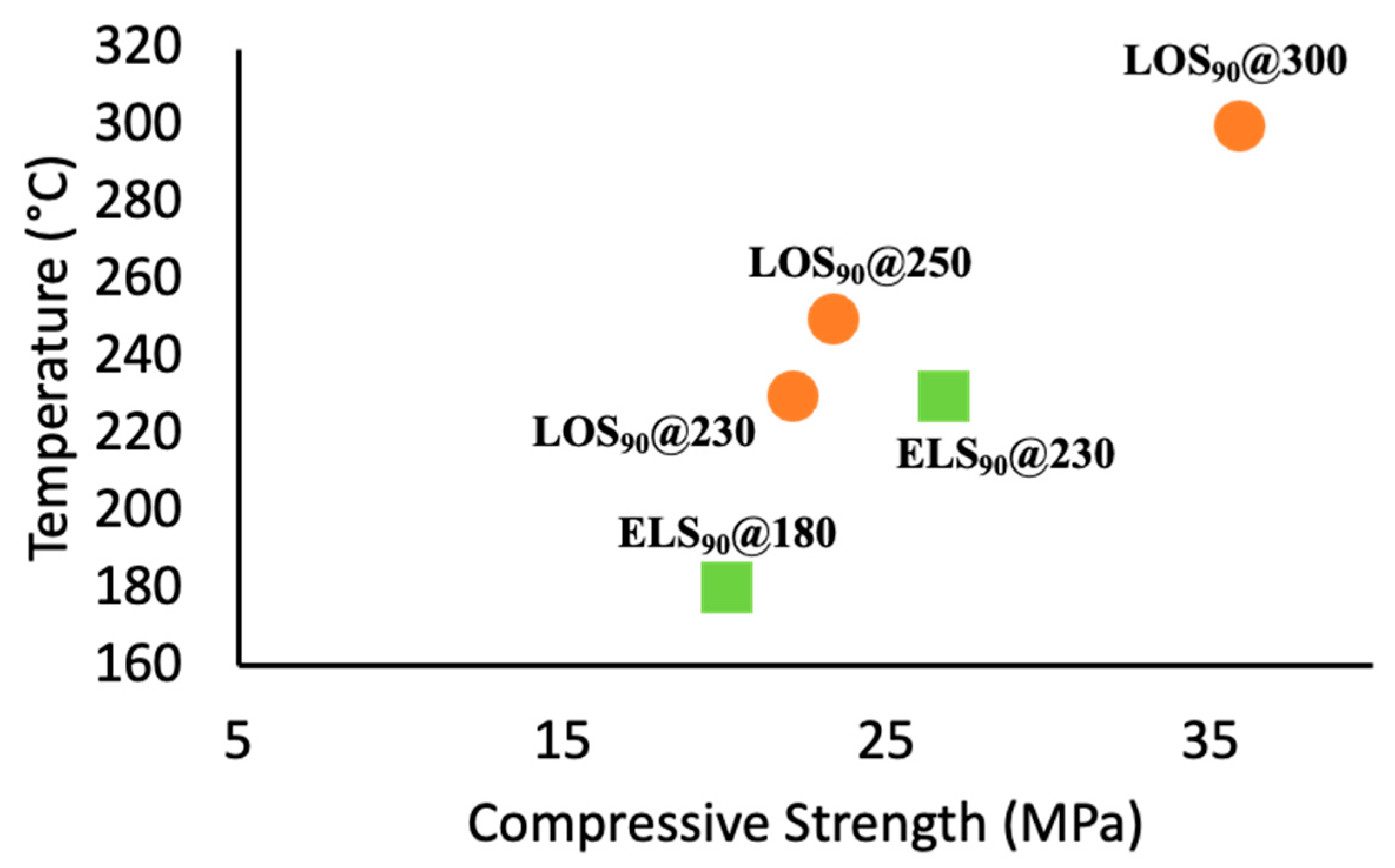
2.3. Mechanical Properties and Environmental Impact Considerations
3. Materials and Methods
3.1. Materials
3.2. General Considerations and Instrumentation
3.3. Synthesis of LOSx@T Composites
3.4. General Method for Dark Sulfur Quantification
3.5. General Method for Ethyl Acetate Extractions for Dark Sulfur Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Kelley, S.S.; Venditti, R.A. Lignin-Based Thermoplastic Materials. ChemSusChem 2016, 9, 770–783. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, D.S.; Jurasek, L.; Krištofová, L.; Xia, Z.; Sun, Y.; Paluš, E. Abundance and Reactivity of Dibenzodioxocins in Softwood Lignin. J. Agric. Food Chem. 2002, 50, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Ragauskas, A.J.; Yuan, J.S. Lignin Conversion: Opportunities and Challenges for the Integrated Biorefinery. Ind. Biotechnol. 2016, 12, 161–167. [Google Scholar] [CrossRef]
- Zoia, L.; Salanti, A.; Frigerio, P.; Orlandi, M. Exploring allylation and Claisen rearrangement as a novel chemical modification of lignin. BioResources 2014, 9, 6540–6561. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Katahira, R.; Elder, T.J.; Beckham, G.T. A Brief Introduction to Lignin Structure. In Lignin Valorization: Emerging Approaches; Beckham, G.T., Ed.; The Royal Society of Chemistry: Piccadilly, London, 2018; pp. 1–20. [Google Scholar]
- Hatakeyama, H.; Hatakeyama, T. Lignin Structure, Properties, and Applications. In Biopolymers: Lignin, Proteins, Bioactive Nanocomposites; Abe, A., Dusek, K., Kobayashi, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–63. [Google Scholar]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- Petridis, L.; Schulz, R.; Smith, J.C. Simulation Analysis of the Temperature Dependence of Lignin Structure and Dynamics. J. Am. Chem. Soc. 2011, 133, 20277–20287. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Sethupathy, S.; Murillo Morales, G.; Gao, L.; Wang, H.; Yang, B.; Jiang, J.; Sun, J.; Zhu, D. Lignin valorization: Status, challenges and opportunities. Bioresour. Technol. 2022, 347, 126696. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.-C. Lignin Source and Structural Characterization. ChemSusChem 2020, 13, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, R.; Athiyaman Balakumaran, P.; Divakar, K.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sirohi, R.; Binod, P.; Kumar Awasthi, M.; Sindhu, R. Microbial valorization of lignin: Prospects and challenges. Bioresour. Technol. 2022, 344, 126240. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.; Kim, B.S. Lignin valorization using biological approach. Biotechnol. Appl. Biochem. 2021, 68, 459–468. [Google Scholar] [CrossRef]
- Crestini, C.; Lange, H.; Sette, M.; Argyropoulos, D.S. On the structure of softwood kraft lignin. Green Chem. 2017, 19, 4104–4121. [Google Scholar] [CrossRef]
- Suota, M.J.; da Silva, T.A.; Zawadzki, S.F.; Sassaki, G.L.; Hansel, F.A.; Paleologou, M.; Ramos, L.P. Chemical and structural characterization of hardwood and softwood LignoForce™ lignins. Ind. Crops Prod. 2021, 173, 114138. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Rohella, R.S.; Sahoo, N.; Chakravortty, V. Lignin macromolecule. Resonance 1997, 2, 60–66. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Adler, E. Structural elements of lignin. Ind. Eng. Chem. 1957, 49, 1377–1383. [Google Scholar] [CrossRef]
- Xia, M.; Valverde-Barrantes, O.J.; Suseela, V.; Blackwood, C.B.; Tharayil, N. Characterizing natural variability of lignin abundance and composition in fine roots across temperate trees: A comparison of analytical methods. New Phytol. 2022, 236, 2358–2373. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef]
- Sette, M.; Wechselberger, R.; Crestini, C. Elucidation of Lignin Structure by Quantitative 2D NMR. Chem.—A Eur. J. 2011, 17, 9529–9535. [Google Scholar] [CrossRef]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Á.T.; Rencoret, J.; Marques, G.; Gutiérrez, A.; Ibarra, D.; Jiménez-Barbero, J.; del Río, J.C. Monolignol acylation and lignin structure in some nonwoody plants: A 2D NMR study. Phytochemistry 2008, 69, 2831–2843. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current Understanding of the Correlation of Lignin Structure with Biomass Recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Chapple, C. The Genetics of Lignin Biosynthesis: Connecting Genotype to Phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef]
- Novaes, E.; Kirst, M.; Chiang, V.; Winter-Sederoff, H.; Sederoff, R. Lignin and Biomass: A Negative Correlation for Wood Formation and Lignin Content in Trees. Plant Physiol. 2010, 154, 555–561. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. (Int. Ed.) 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Wong, S.S.; Shu, R.; Zhang, J.; Liu, H.; Yan, N. Downstream processing of lignin derived feedstock into end products. Chem. Soc. Rev. 2020, 49, 5510–5560. [Google Scholar] [CrossRef]
- Sun, Z.; Cheng, J.; Wang, D.; Yuan, T.-Q.; Song, G.; Barta, K. Downstream Processing Strategies for Lignin-First Biorefinery. ChemSusChem 2020, 13, 5199–5212. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Farac, N.F.; Azimi, G. Supercritical Fluid Extraction of Rare Earth Elements from Nickel Metal Hydride Battery. ACS Sustain. Chem. Eng. 2018, 6, 1417–1426. [Google Scholar] [CrossRef]
- Janicka, P.; Płotka-Wasylka, J.; Jatkowska, N.; Chabowska, A.; Fares, M.Y.; Andruch, V.; Kaykhaii, M.; Gębicki, J. Trends in the new generation of green solvents in extraction processes. Curr. Opin. Green Sustain. Chem. 2022, 37, 100670. [Google Scholar] [CrossRef]
- Cañadas, R.; González-Miquel, M.; González, E.J.; Núñez de Prado, A.; Díaz, I.; Rodríguez, M. Sustainable Recovery of High Added-Value Vanilla Compounds from Wastewater Using Green Solvents. ACS Sustain. Chem. Eng. 2021, 9, 4850–4862. [Google Scholar] [CrossRef]
- Cañadas, R.; González-Miquel, M.; González, E.J.; Díaz, I.; Rodríguez, M. Evaluation of bio-based solvents for phenolic acids extraction from aqueous matrices. J. Mol. Liq. 2021, 338, 116930. [Google Scholar] [CrossRef]
- Rapinel, V.; Rombaut, N.; Rakotomanomana, N.; Vallageas, A.; Cravotto, G.; Chemat, F. An original approach for lipophilic natural products extraction: Use of liquefied n-butane as alternative solvent to n-hexane. LWT-Food Sci. Technol. 2017, 85, 524–533. [Google Scholar] [CrossRef]
- Rapinel, V.; Breil, C.; Makerri, C.; Jacotet-Navarro, M.; Rakotomanomana, N.; Vallageas, A.; Chemat, F. Feasibility of using liquefied gas HFO-1234ze (trans-1,3,3,3-tetrafluoroprop-1-ene) as an alternative to conventional solvents for solid–liquid extraction of food ingredients and natural products. LWT-Food Sci. Technol. 2017, 83, 225–234. [Google Scholar] [CrossRef]
- Kouris, P.D.; van Osch, D.J.G.P.; Cremers, G.J.W.; Boot, M.D.; Hensen, E.J.M. Mild thermolytic solvolysis of technical lignins in polar organic solvents to a crude lignin oil. Sustain. Energy Fuels 2020, 4, 6212–6226. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Tekin, K.; Hao, N.; Karagoz, S.; Ragauskas, A.J. Ethanol: A Promising Green Solvent for the Deconstruction of Lignocellulose. ChemSusChem 2018, 11, 3559–3575. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Mabee, W.E.; McFarlane, P.N.; Saddler, J.N. Biomass availability for lignocellulosic ethanol production. Biomass Bioenergy 2011, 35, 4519–4529. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Qu, S.; Da, J.; Hao, Z. H2S-Selective Catalytic Oxidation: Catalysts and Processes. ACS Catal. 2015, 5, 1053–1067. [Google Scholar] [CrossRef]
- Lim, J.; Pyun, J.; Char, K. Recent approaches for the direct use of elemental sulfur in the synthesis and processing of advanced materials. Angew. Chem. Int. Ed. 2015, 54, 3249–3258. [Google Scholar] [CrossRef]
- Crockett, M.P.; Evans, A.M.; Worthington, M.J.; Albuquerque, I.S.; Slattery, A.D.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Bernardes, G.J.; Chalker, J.M. Sulfur-Limonene Polysulfide: A Material Synthesized Entirely from Industrial By-Products and Its Use in Removing Toxic Metals from Water and Soil. Angew. Chem. Int. Ed. 2016, 55, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Worthington, M.J.H.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; Tonkin, S.J.; Chalker, J.M.; Schiller, T.L.; Hasell, T. Stretchable and Durable Inverse Vulcanized Polymers with Chemical and Thermal Recycling. Chem. Mater. 2022, 34, 1167–1178. [Google Scholar] [CrossRef]
- Davis, A.E.; Sayer, K.B.; Jenkins, C.L. A comparison of adhesive polysulfides initiated by garlic essential oil and elemental sulfur to create recyclable adhesives. Polym. Chem. 2022, 13, 4634–4640. [Google Scholar] [CrossRef]
- Eder, M.L.; Call, C.B.; Jenkins, C.L. Utilizing Reclaimed Petroleum Waste to Synthesize Water-Soluble Polysulfides for Selective Heavy Metal Binding and Detection. ACS Appl. Polym. Mater. 2022, 4, 1110–1116. [Google Scholar] [CrossRef]
- Herrera, C.; Ysinga, K.J.; Jenkins, C.L. Polysulfides Synthesized from Renewable Garlic Components and Repurposed Sulfur Form Environmentally Friendly Adhesives. ACS Appl. Mater. Interfaces 2019, 11, 35312–35318. [Google Scholar] [CrossRef]
- Orme, K.; Fistrovich, A.H.; Jenkins, C.L. Tailoring Polysulfide Properties through Variations of Inverse Vulcanization. Macromolecules 2020, 53, 9353–9361. [Google Scholar] [CrossRef]
- Westerman, C.R.; Jenkins, C.L. Dynamic Sulfur Bonds Initiate Polymerization of Vinyl and Allyl Ethers at Mild Temperatures. Macromolecules 2018, 51, 7233–7238. [Google Scholar] [CrossRef]
- Grimm, A.P.; Scheiger, J.M.; Roesky, P.W.; Théato, P. Inverse vulcanization of trimethoxyvinylsilane particles. Polym. Chem. 2022, 13, 5852–5860. [Google Scholar] [CrossRef]
- Gomez, I.; Leonet, O.; Blazquez, J.A.; Mecerreyes, D. Inverse Vulcanization of Sulfur using Natural Dienes as Sustainable Materials for Lithium-Sulfur Batteries. ChemSusChem 2016, 9, 3419–3425. [Google Scholar] [CrossRef]
- Wagenfeld, J.-G.; Al-Ali, K.; Almheiri, S.; Slavens, A.F.; Calvet, N. Sustainable applications utilizing sulfur, a by-product from oil and gas industry: A state-of-the-art review. Waste Manag. 2019, 95, 78–89. [Google Scholar] [CrossRef]
- Wujcik, K.H.; Wang, D.R.; Raghunathan, A.; Drake, M.; Pascal, T.A.; Prendergast, D.; Balsara, N.P. Lithium Polysulfide Radical Anions in Ether-Based Solvents. J. Phys. Chem. C 2016, 120, 18403–18410. [Google Scholar] [CrossRef]
- Diez, S.; Hoefling, A.; Theato, P.; Pauer, W. Mechanical and Electrical Properties of Sulfur-Containing Polymeric Materials Prepared via Inverse Vulcanization. Polymers 2017, 9, 59. [Google Scholar] [CrossRef]
- Hoefling, A.; Lee, Y.J.; Theato, P. Sulfur-Based Polymer Composites from Vegetable Oils and Elemental Sulfur: A Sustainable Active Material for Li–S Batteries. Macromol. Chem. Phys. 2017, 218, 1600303. [Google Scholar] [CrossRef]
- Westerman, C.R.; Walker, P.M.; Jenkins, C.L.; Westerman, C.R.; Walker, P.M. Synthesis of Terpolymers at Mild Temperatures Using Dynamic Sulfur Bonds in Poly(S-Divinylbenzene). J. Vis. Exp. 2019, 147, e59620. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Thiounn, T.; Smith, R.C.; Tennyson, A.G. Valorization of waste to yield recyclable composites of elemental sulfur and lignin. J. Mater. Chem. A 2019, 7, 15683–15690. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Smith, R.C. Facile route to an organosulfur composite from biomass-derived guaiacol and waste sulfur. J. Mater. Chem. A 2020, 8, 20318–20322. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Maladeniya, C.P.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Durable composites by vulcanization of oleyl-esterified lignin. RSC Adv. 2023, 13, 3234–3240. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.-M.O.; El Gamal, M. Sulfur based hazardous waste solidification. Environ. Geol. 2007, 53, 159–175. [Google Scholar] [CrossRef]
- Rauchfuss, T. Under sulfur’s spell. Nat. Chem. 2011, 3, 648. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Namnabat, S.; Anderson, L.E.; Glass, R.S.; Norwood, R.A.; Mackay, M.E.; Char, K.; Pyun, J. Dynamic Covalent Polymers via Inverse Vulcanization of Elemental Sulfur for Healable Infrared Optical Materials. ACS Macro Lett. 2015, 4, 862–866. [Google Scholar] [CrossRef]
- Zhang, Y.; Konopka, K.M.; Glass, R.S.; Char, K.; Pyun, J. Chalcogenide hybrid inorganic/organic polymers (CHIPs) via inverse vulcanization and dynamic covalent polymerizations. Polym. Chem. 2017, 8, 5167–5173. [Google Scholar] [CrossRef]
- Tonkin, S.J.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Karton, A.; Hasell, T.; Chalker, J.M. Chemically induced repair, adhesion, and recycling of polymers made by inverse vulcanization. Chem. Sci. 2020, 11, 5537–5546. [Google Scholar] [CrossRef]
- Smith, J.A.; Wu, X.; Berry, N.G.; Hasell, T. High sulfur content polymers: The effect of crosslinker structure on inverse vulcanization. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1777–1781. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; Zhang, B.; Jiang, L.; Petcher, S.; Smith, J.A.; Parker, D.J.; Cooper, A.I.; Lei, J.; Hasell, T. Inverse Vulcanized Polymers with Shape Memory, Enhanced Mechanical Properties, and Vitrimer Behavior. Angew. Chem. Int. Ed. 2020, 59, 13371–13378. [Google Scholar] [CrossRef]
- Parker, D.J.; Chong, S.T.; Hasell, T. Sustainable inverse-vulcanised sulfur polymers. RSC Adv. 2018, 8, 27892–27899. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Wang, H.; Dodd, L.J.; Hasell, T. Processable crosslinked terpolymers made from elemental sulfur with wide range of thermal and mechanical properties. Commun. Mater. 2023, 4, 89. [Google Scholar] [CrossRef]
- Zhang, Y.; Pavlopoulos, N.G.; Kleine, T.S.; Karayilan, M.; Glass, R.S.; Char, K.; Pyun, J. Nucleophilic Activation of Elemental Sulfur for Inverse Vulcanization and Dynamic Covalent Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 7–12. [Google Scholar] [CrossRef]
- Wu, X.; Smith, J.A.; Petcher, S.; Zhang, B.; Parker, D.J.; Griffin, J.M.; Hasell, T. Catalytic inverse vulcanization. Nat. Commun. 2019, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, N.A.; Tikoalu, A.D.; Worthington, M.J.H.; Shapter, R.; Tonkin, S.J.; Stojcevski, F.; Mann, M.; Gibson, C.T.; Gascooke, J.R.; Karton, A.; et al. Reactive Compression Molding Post-Inverse Vulcanization: A Method to Assemble, Recycle, and Repurpose Sulfur Polymers and Composites. Chem.—A Eur. J. 2020, 26, 10035–10044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gao, H.; Yan, P.; Petcher, S.; Hasell, T. Inverse vulcanization below the melting point of sulfur. Mater. Chem. Front. 2020, 4, 669–675. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; McBride, F.; Cai, D.; Dale, J.; Hanna, V.; Hasell, T. Mechanochemical synthesis of inverse vulcanized polymers. Nat. Commun. 2022, 13, 4824. [Google Scholar] [CrossRef]
- Jia, J.; Liu, J.; Wang, Z.-Q.; Liu, T.; Yan, P.; Gong, X.-Q.; Zhao, C.; Chen, L.; Miao, C.; Zhao, W.; et al. Photoinduced inverse vulcanization. Nat. Chem. 2022, 14, 1249–1257. [Google Scholar] [CrossRef]
- Alex, A.; Singha, N.K.; Choudhury, S. Exploring inverse vulcanization in lithium–sulfur batteries. Curr. Opin. Electrochem. 2023, 39, 101271. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Copolymerization of an aryl halide and elemental sulfur as a route to high sulfur content materials. Polym. Chem. 2020, 11, 1621–1628. [Google Scholar] [CrossRef]
- Lopez, C.V.; Maladeniya, C.P.; Smith, R.C. Lithium-Sulfur Batteries: Advances and Trends. Electrochem 2020, 1, 226–259. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Green Synthesis of Thermoplastic Composites from a Terpenoid-Cellulose Ester. ACS Appl. Polym. Mater. 2020, 2, 3761–3765. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Recyclable, sustainable, and stronger than portland cement: A composite from unseparated biomass and fossil fuel waste. Mater. Adv. 2020, 1, 590–594. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Robust, remeltable and remarkably simple to prepare biomass–sulfur composites. Mater. Adv. 2020, 1, 2271–2278. [Google Scholar] [CrossRef]
- Maladeniya, C.P.; Karunarathna, M.S.; Lauer, M.K.; Lopez, C.V.; Thiounn, T.; Smith, R.C. A role for terpenoid cyclization in the atom economical polymerization of terpenoids with sulfur to yield durable composites. Mater. Adv. 2020, 1, 1665–1674. [Google Scholar] [CrossRef]
- Smith, A.D.; Tennyson, A.G.; Smith, R.C. Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sustain. Chem. 2020, 1, 209–237. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Inverse vulcanization of octenyl succinate-modified corn starch as a route to biopolymer–sulfur composites. Mater. Adv. 2021, 2, 2391–2397. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, A.D.; Smith, R.C. High strength composites from low-value animal coproducts and industrial waste sulfur. RSC Adv. 2022, 12, 1535–1542. [Google Scholar] [CrossRef]
- Thiounn, T.; Karunarathna, M.S.; Slann, L.M.; Lauer, M.K.; Smith, R.C. Sequential crosslinking for mechanical property development in high sulfur content composites. J. Polym. Sci. 2020, 58, 2943–2950. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C.; Knuutinen, J.S.; Ahkola, H.S.J.; Herve, S.H. Refractory organic pollutants and toxicity in pulp and paper mill wastewaters. Environ. Sci. Pollut. Res. 2015, 22, 6473–6499. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Noh, G.Y.; Kim, B.G.; Yoo, Y.; Choi, W.J.; Kim, D.-G.; Yoon, H.G.; Kim, Y.S. Synthesis of Poly(phenylene polysulfide) Networks from Elemental Sulfur and p-Diiodobenzene for Stretchable, Healable, and Reprocessable Infrared Optical Applications. ACS Macro Lett. 2019, 8, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Facile new approach to high sulfur-content materials and preparation of sulfur-lignin copolymers. J. Mater. Chem. A 2020, 8, 548–553. [Google Scholar] [CrossRef]
- Melro, E.; Duarte, H.; Eivazi, A.; Costa, C.; Faleiro, M.L.; da Costa, A.M.R.; Antunes, F.E.; Valente, A.J.M.; Romano, A.; Norgren, M.; et al. Poly(butylene succinate)-Based Composites with Technical and Extracted Lignins from Wood Residues. ACS Appl. Polym. Mater. 2024, 6, 1169–1181. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Shen, X.; Cao, M.; Wang, Y.; Zhang, W.; Xu, Y.; Ling, Z.; Chen, S.; Xu, F. Transparent Cellulose/Lignin Composite Films with Adjustable Haze and UV-Blocking Performance for Light Management. ACS Sust. Chem. Eng. 2024, 12, 5427–5435. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Sun, Y.; Qiu, X.; Li, X.; Sun, C.; Liu, Y.; Yu, Q.; Yu, B.; Cai, M.; et al. BTA-P4444-Lig-Functionalized MXene to Prepare Anticorrosion and Wear-Resistant Integrated Waterborne Epoxy Composite Coating. ACS Sust. Chem. Eng. 2024, 12, 8247–8260. [Google Scholar] [CrossRef]
- Singh, K.; Yuvrajsinh, G.M.; Mehra, S.; Mishra, A.; Kumar, A. Bioionic Liquid-Assisted Transparent Sodium Alginate-κ-Carrageenan-Lignin Composite Films with Excellent Ultraviolet Shielding, Antioxidant, and Antibacterial Properties. ACS Sustain. Chem. Eng. 2024, 12, 4314–4327. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Zhang, J.; Wang, F.; Liu, Z.; Jiang, J. Investigation on self-healing polyurethane coating doped with lignin composites for protecting cementitious materials. Constr. Build. Mater. 2024, 411, 134368. [Google Scholar] [CrossRef]
- Fazeli, M.; Mukherjee, S.; Baniasadi, H.; Abidnejad, R.; Mujtaba, M.; Lipponen, J.; Seppala, J.; Rojas, O.J. Lignin beyond the status quo: Recent and emerging composite applications. Green Chem. 2024, 26, 593–630. [Google Scholar] [CrossRef]
- Yang, W.; Wang, D.; Feng, S.; He, S.; Xiao, H.; Dai, H.; Han, J. Heteroatom-doped hierarchically porous thick bulk carbon derived from a Pleurotus eryngii/lignin composite: A free-standing and high mass loading electrode for high-energy-density storage. Green Chem. 2024, 26, 4633–4644. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Q.; Wang, K.; Cho, M.; Jiang, L. Thermoplastic starch nanocomposites derived directly from cornmeal. J. Compos. Mater. 2024, 58, 1077–1088. [Google Scholar] [CrossRef]
- Campos, G.N.; da Rocha, E.B.D.; Furtado, C.R.G.; de Figueiredo, M.A.G.; de Sousa, A.M.F. Using carboxyl groups to improve the compatibility of XNBR/lignin composites. Polym. Compos. 2024, 45, 4124–4137. [Google Scholar] [CrossRef]
- Montazeri, M.; Norouzbeigi, R. Investigation of synergistic effects incorporating esterified lignin and guar gum composite aerogel for sustained oil spill cleanup. Sci. Rep. 2024, 14, 13892. [Google Scholar] [CrossRef]
- Thiounn, T.; Karunarathna, M.S.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Detoxification of bisphenol A via sulfur-mediated carbon–carbon σ-bond scission. RSC Sustain. 2023, 1, 535–542. [Google Scholar] [CrossRef]
- Kapuge Dona, N.L.; Maladeniya, C.P.; Smith, R.C. Reactivity of Biomass-Derived Olefins with Elemental Sulfur: Mechanistic Insight. Eur. J. Org. Chem. 2024, 27, e202301269. [Google Scholar] [CrossRef]
- Tisdale, K.A.; Dona, N.L.K.; Maladeniya, C.P.; Smith, R.C. Green and Atom Economical Route to High Compressive Strength Lignin Oil-Sulfur Composites. J. Polym. Environ. 2024, 1–13. [Google Scholar] [CrossRef]
- Dale, J.J.; Petcher, S.; Hasell, T. Dark Sulfur: Quantifying Unpolymerized Sulfur in Inverse Vulcanized Polymers. ACS Appl. Polym. Mater. 2022, 4, 3169–3173. [Google Scholar] [CrossRef]
- Dale, J.J.; Stanley, J.; Dop, R.A.; Chronowska-Bojczuk, G.; Fielding, A.J.; Neill, D.R.; Hasell, T. Exploring Inverse Vulcanisation Mechanisms from the Perspective of Dark Sulfur. Eur. Polym. J. 2023, 195, 112198. [Google Scholar] [CrossRef]
- Shankarayya Wadi, V.K.; Jena, K.K.; Khawaja, S.Z.; Yannakopoulou, K.; Fardis, M.; Mitrikas, G.; Karagianni, M.; Papavassiliou, G.; Alhassan, S.M. NMR and EPR Structural Analysis and Stability Study of Inverse Vulcanized Sulfur Copolymers. ACS Omega 2018, 3, 3330–3339. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Venkataraghavan, R.; Kasturi, T.R. Contribution to the Infrared Spectra of Organosulphur Compounds. Can. J. Chem. 1964, 42, 36–42. [Google Scholar] [CrossRef]
- Bastian, E.J., Jr.; Martin, R.B. Disulfide vibrational spectra in the sulfur-sulfur and carbon-sulfur stretching region. J. Phys. Chem. 1973, 77, 1129–1133. [Google Scholar] [CrossRef]
- Stark, N.M.; Yelle, D.J.; Agarwal, U.P. Techniques for characterizing lignin. Lignin Polym. Compos. 2016, 2016, 49–66. [Google Scholar]
- Collier, W.E.; Schultz, T.P.; Kalasinsky, V.F. Infrared Study of Lignin: Reexamination of Aryl-Alkyl Ether C—O Stretching Peak Assignments. Holzforschung 1992, 46, 523–528. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Griebel, J.J.; Glass, R.S.; Char, K.; Pyun, J. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability, energy and defense. Prog. Polym. Sci. 2016, 58, 90–125. [Google Scholar] [CrossRef]
- Tobolsky, A.V.; MacKnight, W.; Beevers, R.B.; Gupta, V.D. The glass transition temperature of polymeric sulphur. Polymer 1963, 4, 423–427. [Google Scholar] [CrossRef]
- Bandzierz, K.; Reuvekamp, L.; Dryzek, J.; Dierkes, W.; Blume, A.; Bielinski, D. Influence of Network Structure on Glass Transition Temperature of Elastomers. Materials 2016, 9, 607. [Google Scholar] [CrossRef]
- Smith, J.A.; Green, S.J.; Petcher, S.; Parker, D.J.; Zhang, B.; Worthington, M.J.H.; Wu, X.; Kelly, C.A.; Baker, T.; Gibson, C.T.; et al. Crosslinker Copolymerization for Property Control in Inverse Vulcanization. Chem.—A Eur. J. 2019, 25, 10433–10440. [Google Scholar] [CrossRef]



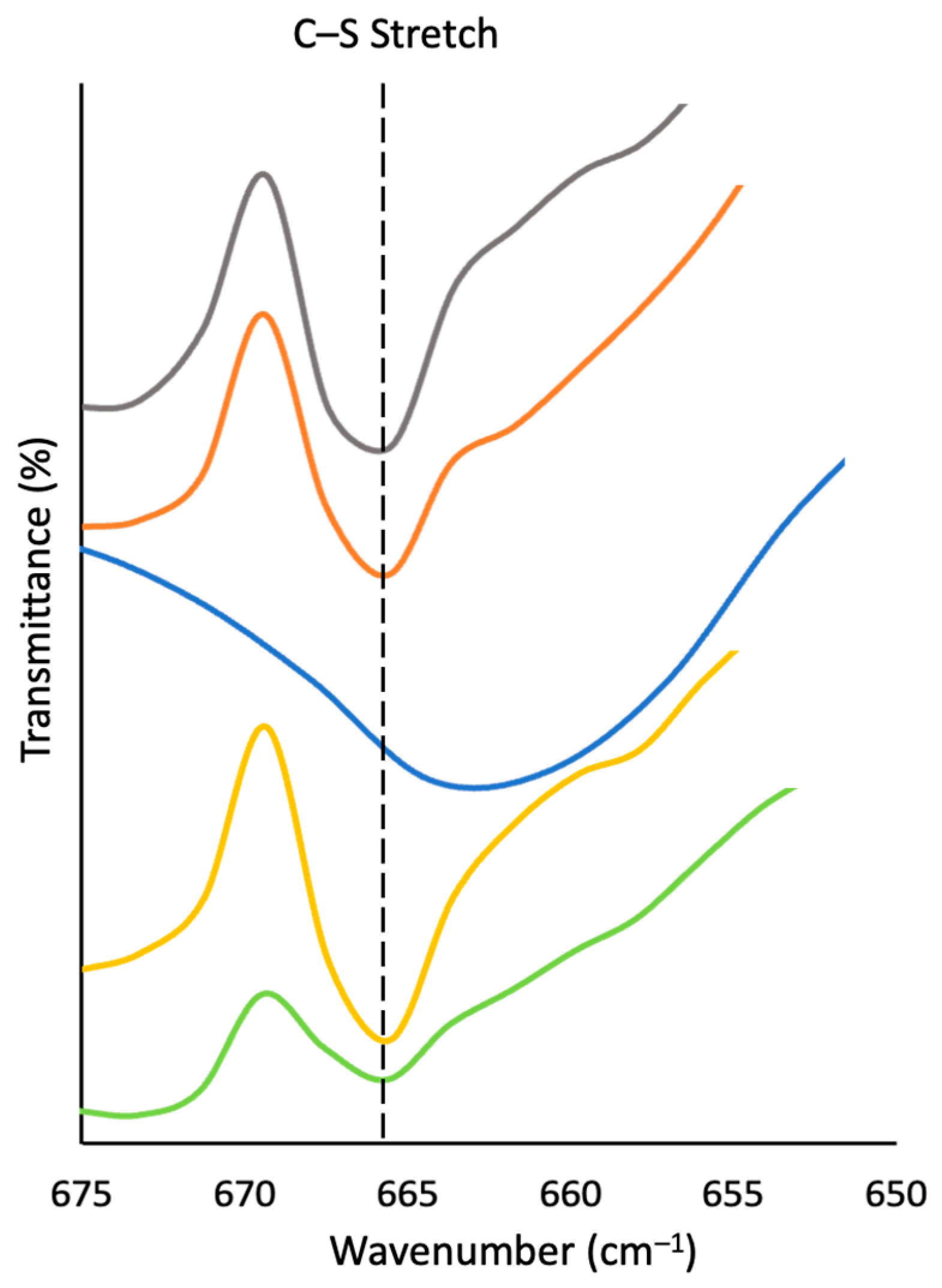

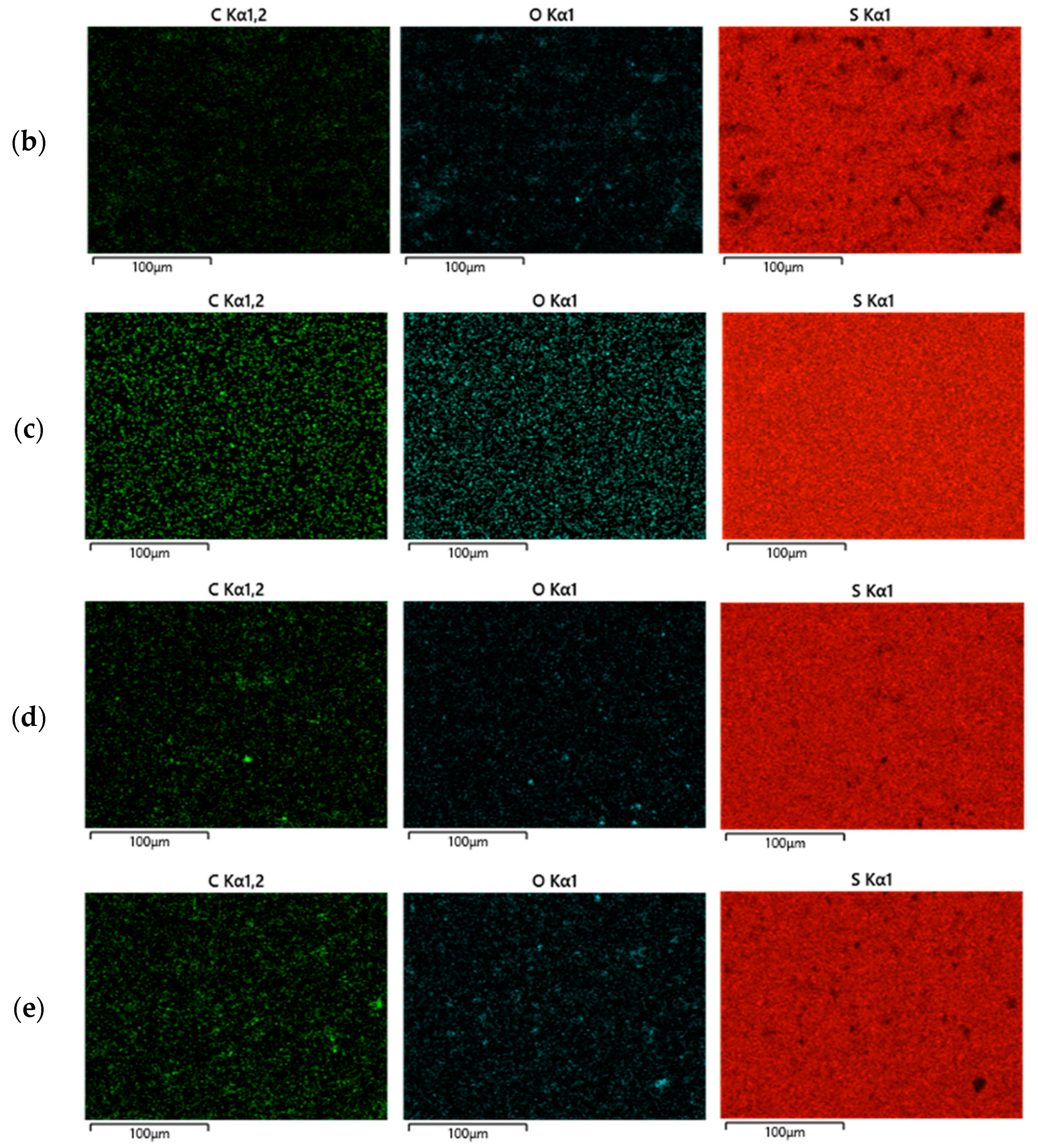

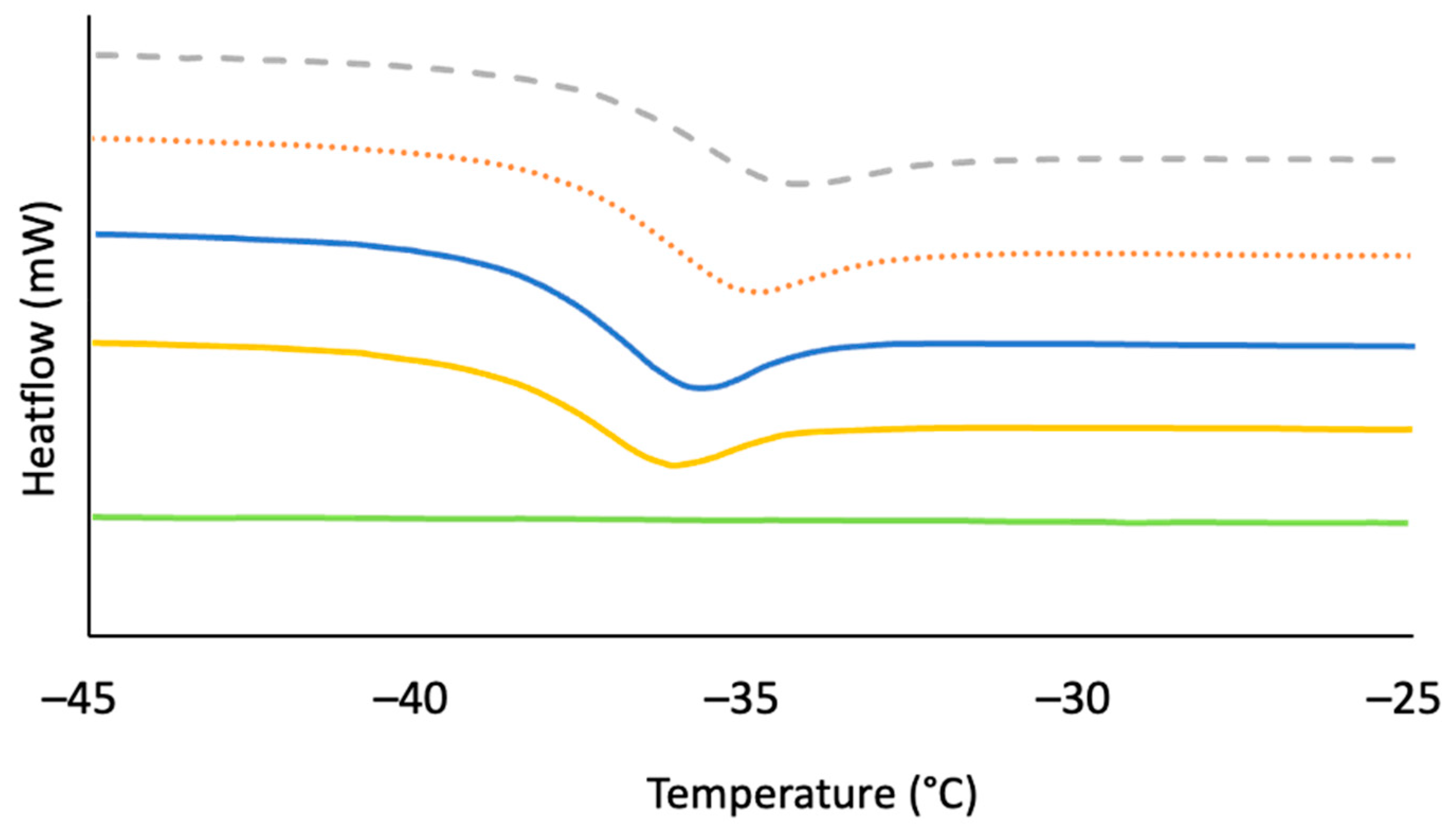

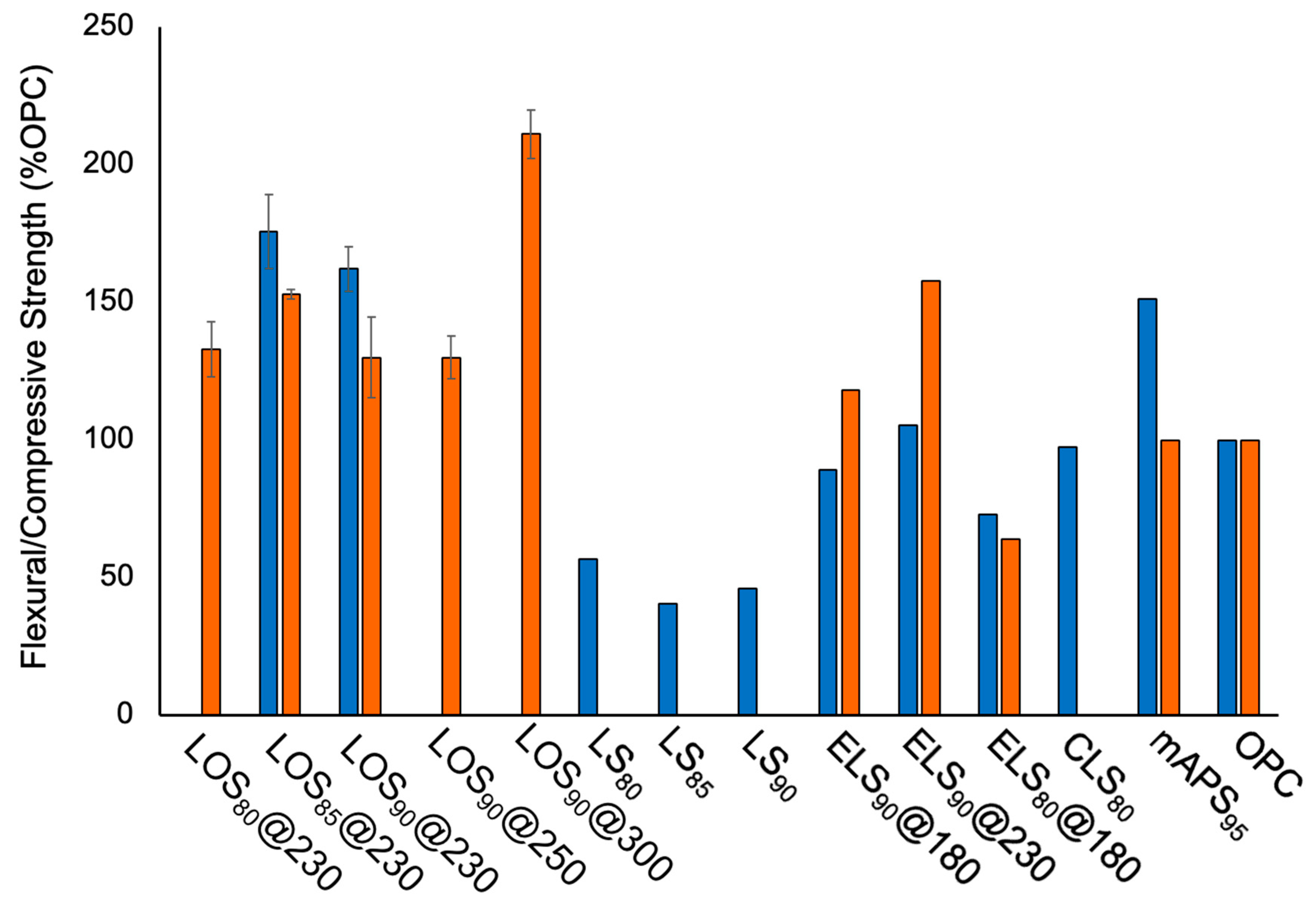
| Materials | Dark Sulfur [a] (wt. %) | Crosslinked Material [a] (wt. %) |
|---|---|---|
| LOS80@230 | 22 | 82 |
| LOS85@230 | 31 | 77 |
| LOS90@230 | 21 | 82 |
| LOS90@250 | 24 | 78 |
| LOS90@300 | 22 | 81 |
| Materials | /°C | /°C | /°C | ∆Hm J/g | ∆Hcc J/g | Percentage Crystallinity [d] |
|---|---|---|---|---|---|---|
| LOS90@230 [e] | 229 | 114 | −36 | 26 | ND [j] | 57 |
| LOS85@230 [e] | 230 | 106 | −35 | 35 | 26 | 19 |
| LOS80@230 [e] | 230 | 105 | −35 | 32 | 32 | 1 |
| LOS90@250 [e] | 231 | 117 | −36 | 30 | 16 | 30 |
| LOS90@300 [e] | 230 | 119 | ND [j] | ND [j] | ND [j] | ND [j] |
| LS80 [f] | 236 | 118 | −34 | 30 | 25 | 8 |
| LS85 [f] | 235 | 118 | −35 | 34 | 13 | 40 |
| LS90 [f] | 237 | 117 | ND [j] | 34 | ND [j] | 67 |
| LS95 [f] | 235 | 107 | ND [j] | 37 | ND [j] | 63 |
| LS99 [f] | 233 | 113 | ND [j] | 47 | ND [j] | 91 |
| GS80 [g] | 264 | ND [j] | −30 | ND [j] | ND [j] | ND [j] |
| ELS90@180 [h] | 230 | 117 | −37 | ND [j] | ND [j] | ND [j] |
| ELS90@230 [h] | 234 | 117 | −37 | ND [j] | ND [j] | ND [j] |
| ELS80@180 [h] | 231 | 117 | −37 | ND [j] | ND [j] | ND [j] |
| S8 [i] | 228/229 | 119 | ND [j] | 45 | ND [j] | 100 |
| Materials | Compressive Strength (MPa) | Flexural Strength/Modulus (MPa) | Compressive Strength (% of OPC) |
|---|---|---|---|
| LOS90@230 [a] | 22.1 ± 2.5 | 5.7/186 | 130 |
| LOS85@230 [a] | 26.0 ± 0.3 | 6.5/236 | 153 |
| LOS80@230 [a] | 22.6 ± 1.7 | ND [k] | 133 |
| LOS90@250 [a] | 22.1 ± 1.3 | ND [k] | 130 |
| LOS90@300 [a] | 35.9 ± 1.5 | ND [k] | 211 |
| LS80 [b] | ND [k] | 2.1/87 | ND [k] |
| LS85 [b] | ND [k] | 1.5/76 | ND |
| LS90 [b] | ND [k] | 1.7/57 | ND |
| LS95 [b] | ND [k] | ND [k] | ND |
| LS99 [b] | ND [k] | ND [k] | ND |
| GS80 [c] | ND [k] | ND [k] | ND |
| ELS90@180 [d] | 20.1 ± 2.3 | 3.3/ND [k] | 118 |
| ELS90@230 [d] | 26.8 ± 0.5 | 3.9/ND [k] | 158 |
| ELS80@180 [d] | 10.9 ± 0.85 | 2.7/ND [k] | 64 |
| CLS80 [e] | ND [k] | 3.6/ND [k] | ND [k] |
| mAPS95 [f] | 17.0 | 5.6/ND [k] | 100 |
| OPC [g] | 17.0 | 3.7/580 | 100 |
| S-DCPD (1:1) [h] | ND | 6.0/3700 | ND |
| S-DCPD–linseed oil (2:1:1) [i] | ND | 4.7/1250 | ND |
| S-DCPD–limonene (2:1:1) [j] | ND | 1.9/1750 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tisdale, K.A.; Kapuge Dona, N.L.; Smith, R.C. The Influence of the Comonomer Ratio and Reaction Temperature on the Mechanical, Thermal, and Morphological Properties of Lignin Oil–Sulfur Composites. Molecules 2024, 29, 4209. https://doi.org/10.3390/molecules29174209
Tisdale KA, Kapuge Dona NL, Smith RC. The Influence of the Comonomer Ratio and Reaction Temperature on the Mechanical, Thermal, and Morphological Properties of Lignin Oil–Sulfur Composites. Molecules. 2024; 29(17):4209. https://doi.org/10.3390/molecules29174209
Chicago/Turabian StyleTisdale, Katelyn A., Nawoda L. Kapuge Dona, and Rhett C. Smith. 2024. "The Influence of the Comonomer Ratio and Reaction Temperature on the Mechanical, Thermal, and Morphological Properties of Lignin Oil–Sulfur Composites" Molecules 29, no. 17: 4209. https://doi.org/10.3390/molecules29174209







