Self-Assembled Ferritin Nanoparticles for Delivery of Antigens and Development of Vaccines: From Structure and Property to Applications
Abstract
1. Introduction
2. The Function and Structure of Ferritin
2.1. Non-Heme-Binding Ferritins (Ftn)
2.2. Heme-Binding Bacterioferritins (Bfr)
2.3. DNA-Binding Proteins from Starved Cells (Dps)
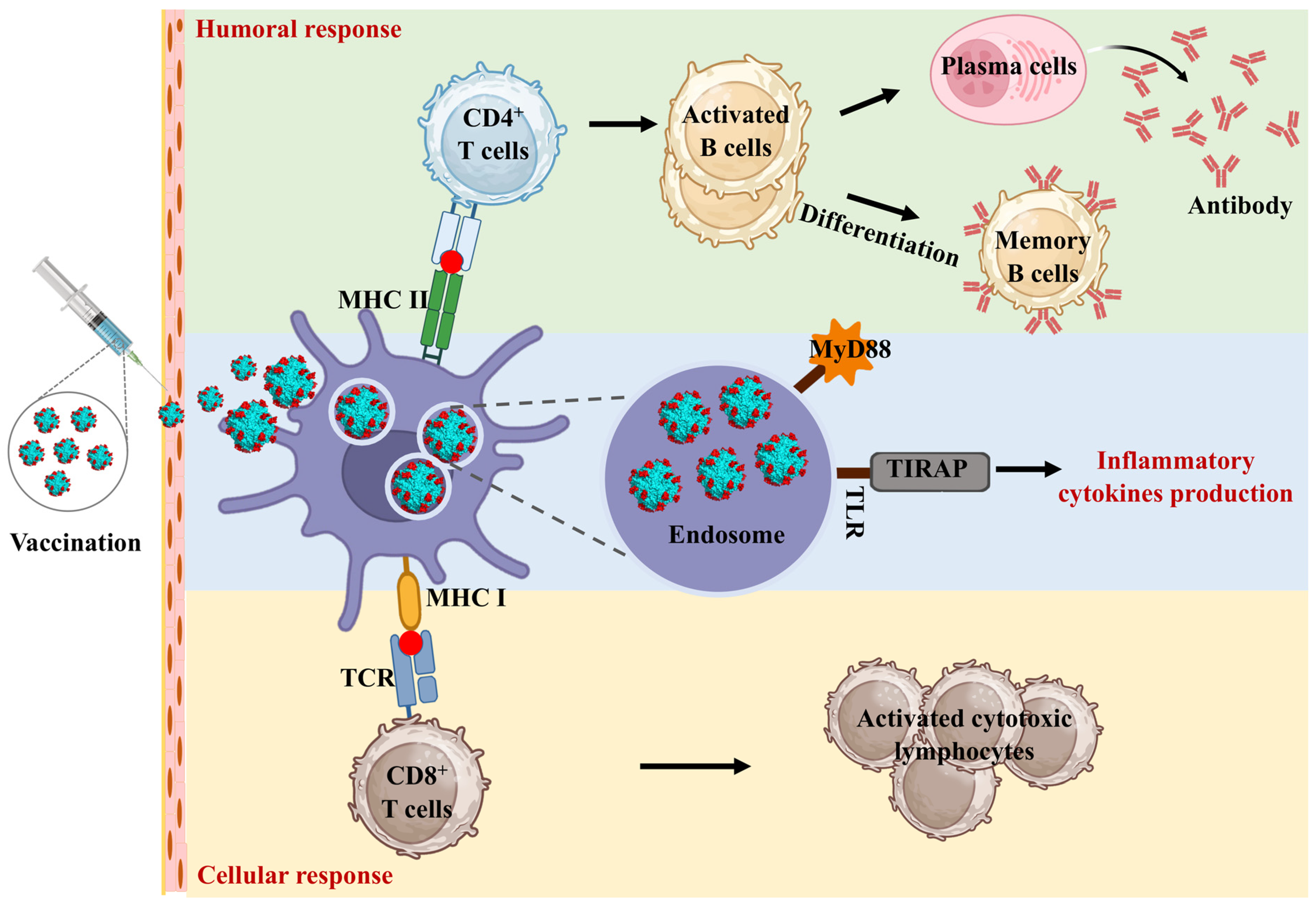
3. Self-Assembled Ferritin Nanoparticles for Vaccine Development
3.1. Self-Assembly and Hetero-Polymerization of Ferritin
3.2. Engineered Ferritin-Based Vaccine
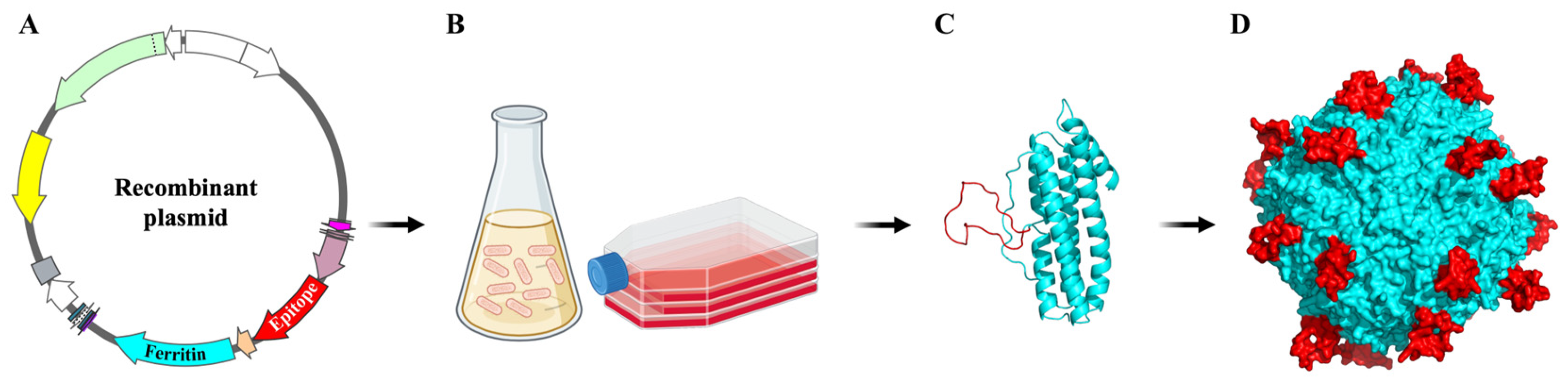
3.3. Production of Ferritin-Based Vaccine
4. Self-Assembled Ferritin Nanoparticles Display Different Types of Antigens
4.1. The Short Peptide Antigen Presented by Ferritin Nanoparticles
4.2. The Elongated Peptide Antigens Presented by Ferritin Nanoparticles
4.3. Enhancing Expression and Assembly of Ferritin Vaccines Harboring Extended Peptide Antigens in Mammalian Cells
4.4. The Bivalent Antigens Presented by Ferritin Nanoparticles
5. Molecular Mechanism of Self-Assembling Ferritin Nanoparticles to Enhance Immune Response
5.1. Ferritin Nanoparticles Activated the Inflammatory Response
5.2. Ferritin Nanoparticles Enhance Dendritic Cell Uptake
5.3. Ferritin Nanoparticles Increase the Duration of Antigen Presentation
5.4. Ferritin Nanoparticles Target Macrophages and Improve the Immune Response
6. The Advancement of Self-Assembled Ferritin Nanoparticles Vaccines Development
6.1. Self-Assembled Ferritin Nanoparticles Vaccines against Virus Infection
6.2. Self-Assembled Ferritin Nanoparticles Vaccines against Bacterial Infections
7. The Potential and Challenges of Self-Assembling Ferritin Nanoparticles in Vaccine Development
7.1. Advantage and Disadvantage of Self-Assembled Ferritin in Developing Vaccines
7.2. The Limitations and Challenges of Ferritin-Based Vaccines in Clinical Applications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munro, H.N.; Linder, M.C. Ferritin: Structure, biosynthesis, and role in iron metabolism. Physiol. Rev. 1978, 58, 317–396. [Google Scholar] [CrossRef]
- Theil, E.C.; Behera, R.K.; Tosha, T. Ferritins for Chemistry and for Life. Coord. Chem. Rev. 2013, 257, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Lim, S. Engineering protein nanocages as carriers for biomedical applications. NPG Asia Mater. 2017, 9, e371. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Arellano, A.; Vásquez-Procopio, J.; Gambis, A.; Blowes, L.M.; Steller, H.; Mollereau, B.; Missirlis, F. Ferritin Assembly in Enterocytes of Drosophila melanogaster. Int. J. Mol. Sci. 2016, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Torti, F.M.; Torti, S.V. Regulation of ferritin genes and protein. Blood 2002, 99, 3505–3516. [Google Scholar] [CrossRef]
- Lawson, D.M.; Artymiuk, P.J.; Yewdall, S.J.; Smith, J.M.; Livingstone, J.C.; Treffry, A.; Luzzago, A.; Levi, S.; Arosio, P.; Cesareni, G.; et al. Solving the structure of human H ferritin by genetically engineering intermolecular crystal contacts. Nature 1991, 349, 541–544. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin protein nanocages-the story. Nanotechnol. Percept. 2012, 8, 7–16. [Google Scholar] [CrossRef]
- Chakraborti, S.; Chakrabarti, P. Self-Assembly of Ferritin: Structure, Biological Function and Potential Applications in Nanotechnology. Adv. Exp. Med. Biol. 2019, 1174, 313–329. [Google Scholar]
- Han, J.A.; Kang, Y.J.; Shin, C.; Ra, J.S.; Shin, H.H.; Hong, S.Y.; Do, Y.; Kang, S. Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell (DC)-based vaccine development. Nanomedicine 2014, 10, 561–569. [Google Scholar] [CrossRef]
- Rodrigues, M.Q.; Alves, P.M.; Roldão, A. Functionalizing Ferritin Nanoparticles for Vaccine Development. Pharmaceutics 2021, 13, 1621. [Google Scholar] [CrossRef] [PubMed]
- Honarmand Ebrahimi, K.; Bill, E.; Hagedoorn, P.L.; Hagen, W.R. The catalytic center of ferritin regulates iron storage via Fe(II)-Fe(III) displacement. Nat. Chem. Biol. 2012, 8, 941–948. [Google Scholar] [CrossRef]
- Carmona, U.; Li, L.; Zhang, L.; Knez, M. Ferritin light-chain subunits: Key elements for the electron transfer across the protein cage. Chem. Commun. 2014, 50, 15358–15361. [Google Scholar] [CrossRef]
- Zang, J.; Chen, H.; Zhao, G.; Wang, F.; Ren, F. Ferritin cage for encapsulation and delivery of bioactive nutrients: From structure, property to applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 3673–3683. [Google Scholar] [CrossRef]
- He, D.; Marles-Wright, J. Ferritin family proteins and their use in bionanotechnology. N. Biotechnol. 2015, 32, 651–657. [Google Scholar] [CrossRef]
- Yang, R.; Chen, L.; Yang, S.; Lv, C.; Leng, X.; Zhao, G. 2D square arrays of protein nanocages through channel-directed electrostatic interactions with poly(α, l-lysine). Chem. Commun. 2014, 50, 2879–2882. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, B.; Bernacchioni, C.; Di Maio, D.; Turano, P.; Brancato, G. Electrostatic and Structural Bases of Fe2+ Translocation through Ferritin Channels. J. Biol. Chem. 2016, 291, 25617–25628. [Google Scholar] [CrossRef]
- Vicente, J.B.; Carrondo, M.A.; Teixeira, M.; Frazão, C. Structural studies on flavodiiron proteins. Meth. Enzymol. 2008, 437, 3–19. [Google Scholar]
- Vicente, J.B.; Justino, M.C.; Gonçalves, V.L.; Saraiva, L.M.; Teixeira, M. Biochemical, spectroscopic, and thermodynamic properties of flavodiiron proteins. Meth. Enzymol. 2008, 437, 21–45. [Google Scholar]
- Zhang, Y.; Orner, B.P. Self-assembly in the ferritin nano-cage protein superfamily. Int. J. Mol. Sci. 2011, 12, 5406–5421. [Google Scholar] [CrossRef]
- Bradley, J.M.; Le Brun, N.E.; Moore, G.R. Ferritins: Furnishing proteins with iron. J. Biol. Inorg. Chem. 2016, 21, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.A.; Filman, D.J.; Finkel, S.E.; Kolter, R.; Hogle, J.M. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat. Struct. Biol. 1998, 5, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Pesek, J.; Büchler, R.; Albrecht, R.; Boland, W.; Zeth, K. Structure and mechanism of iron translocation by a Dps protein from Microbacterium arborescens. J. Biol. Chem. 2011, 286, 34872–34882. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Kolter, R. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J. Bacteriol. 1997, 179, 5188–5194. [Google Scholar] [CrossRef] [PubMed]
- Hanna, E.S.; Roque-Barreira, M.C.; Mendes, G.M.; Soares, S.G.; Brocchi, M. Cloning, expression and purification of a glycosylated form of the DNA-binding protein Dps from Salmonella enterica Typhimurium. Protein Expr. Purif. 2008, 59, 197–202. [Google Scholar] [CrossRef]
- Zeth, K. Dps biomineralizing proteins: Multifunctional architects of nature. Biochem. J. 2012, 445, 297–311. [Google Scholar] [CrossRef]
- Chiancone, E.; Ceci, P. The multifaceted capacity of Dps proteins to combat bacterial stress conditions: Detoxification of iron and hydrogen peroxide and DNA binding. Biochim. Biophys. Acta 2010, 1800, 798–805. [Google Scholar] [CrossRef]
- Almirón, M.; Link, A.J.; Furlong, D.; Kolter, R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes. Dev. 1992, 6, 2646–2654. [Google Scholar] [CrossRef]
- Ceci, P.; Cellai, S.; Falvo, E.; Rivetti, C.; Rossi, G.L.; Chiancone, E. DNA condensation and self-aggregation of Escherichia coli Dps are coupled phenomena related to the properties of the N-terminus. Nucleic Acids Res. 2004, 32, 5935–5944. [Google Scholar] [CrossRef]
- Giorgi, A.; Mignogna, G.; Bellapadrona, G.; Gattoni, M.; Chiaraluce, R.; Consalvi, V.; Chiancone, E.; Stefanini, S. The unusual co-assembly of H- and M-chains in the ferritin molecule from the Antarctic teleosts Trematomus bernacchii and Trematomus newnesi. Arch. Biochem. Biophys. 2008, 478, 69–74. [Google Scholar] [CrossRef]
- Hamburger, A.E.; West, A.P., Jr.; Hamburger, Z.A.; Hamburger, P.; Bjorkman, P.J. Crystal structure of a secreted insect ferritin reveals a symmetrical arrangement of heavy and light chains. J. Mol. Biol. 2005, 349, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.F.; Duc, C.; Ravet, K.; Gaymard, F. Ferritins and iron storage in plants. Biochim. Biophys. Acta 2010, 1800, 806–814. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef]
- Singh, N.; Bhatla, S.C. Heme oxygenase-nitric oxide crosstalk-mediated iron homeostasis in plants under oxidative stress. Free Radic. Biol. Med. 2022, 182, 192–205. [Google Scholar] [CrossRef]
- Missirlis, F.; Kosmidis, S.; Brody, T.; Mavrakis, M.; Holmberg, S.; Odenwald, W.F.; Skoulakis, E.M.; Rouault, T.A. Homeostatic mechanisms for iron storage revealed by genetic manipulations and live imaging of Drosophila ferritin. Genetics 2007, 177, 89–100. [Google Scholar] [CrossRef]
- Corsi, B.; Perrone, F.; Bourgeois, M.; Beaumont, C.; Panzeri, M.C.; Cozzi, A.; Sangregorio, R.; Santambrogio, P.; Albertini, A.; Arosio, P.; et al. Transient overexpression of human H- and L-ferritin chains in COS cells. Biochem. J. 1998, 330 Pt 1, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, A.; Corsi, B.; Levi, S.; Santambrogio, P.; Albertini, A.; Arosio, P. Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: In vivo role of ferritin ferroxidase activity. J. Biol. Chem. 2000, 275, 25122–25129. [Google Scholar] [CrossRef]
- Santambrogio, P.; Levi, S.; Cozzi, A.; Rovida, E.; Albertini, A.; Arosio, P. Production and characterization of recombinant heteropolymers of human ferritin H and L chains. J. Biol. Chem. 1993, 268, 12744–12748. [Google Scholar] [CrossRef]
- Luscieti, S.; Santambrogio, P.; Langlois d’Estaintot, B.; Granier, T.; Cozzi, A.; Poli, M.; Gallois, B.; Finazzi, D.; Cattaneo, A.; Levi, S.; et al. Mutant ferritin L-chains that cause neurodegeneration act in a dominant-negative manner to reduce ferritin iron incorporation. J. Biol. Chem. 2010, 285, 11948–11957. [Google Scholar] [CrossRef]
- Stefanini, S.; Vecchini, P.; Chiancone, E. On the mechanism of horse spleen apoferritin assembly: A sedimentation velocity and circular dichroism study. Biochemistry 1987, 26, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Carmona, F.; Gozzelino, R.; Maccarinelli, F.; Poli, M. The importance of eukaryotic ferritins in iron handling and cytoprotection. Biochem. J. 2015, 472, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Ru, Y.; Hao, R.; Yang, Y.; Liu, H.; Li, Y.; Zhang, Y.; Mao, Y.; Yang, R.; Pan, Y.; et al. A ferritin-based nanoparticle displaying a neutralizing epitope for foot-and-mouth disease virus (FMDV) confers partial protection in guinea pigs. BMC Vet. Res. 2024, 20, 301. [Google Scholar] [CrossRef]
- Yin, C.; Yao, Y.F.; Yang, P.; Liu, H.; Gao, G.; Peng, Y.; Chen, M.; Lu, M.; Zhang, X.; Guo, W.; et al. A highly effective ferritin-based divalent nanoparticle vaccine shields Syrian hamsters against lethal Nipah virus. Front. Immunol. 2024, 15, 1387811. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Wei, C.J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Whittle, J.R.; Rao, S.S.; Kong, W.P.; Wang, L.; Nabel, G.J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499, 102–106. [Google Scholar] [CrossRef]
- Chen, Y.; Song, X.; Chen, W.; Zhao, X.; Yang, L.; Liu, D. Epitope screening and self-assembled nanovaccine molecule design of PDCoV-S protein based on immunoinformatics. Front. Microbiol. 2024, 15, 1402963. [Google Scholar] [CrossRef]
- He, L.; de Val, N.; Morris, C.D.; Vora, N.; Thinnes, T.C.; Kong, L.; Azadnia, P.; Sok, D.; Zhou, B.; Burton, D.R.; et al. Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat. Commun. 2016, 7, 12041. [Google Scholar] [CrossRef]
- He, L.; Kumar, S.; Allen, J.D.; Huang, D.; Lin, X.; Mann, C.J.; Saye-Francisco, K.L.; Copps, J.; Sarkar, A.; Blizard, S.S.; et al. HIV-1 vaccine design through minimizing envelope metastability. Sci. Adv. 2020, 6, eabd8600. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Tzarum, N.; Lin, X.; Shapero, B.; Sou, C.; Mann, C.J.; Stano, A.; Zhang, L.; Nagy, K.; Giang, E.; et al. Proof of concept for rational design of hepatitis C virus E2 core nanoparticle vaccines. Sci. Adv. 2020, 6, eaaz6225. [Google Scholar] [CrossRef]
- He, L.; Lin, X.; Wang, Y.; Abraham, C.; Sou, C.; Ngo, T.; Zhang, Y.; Wilson, I.A.; Zhu, J. Single-component, self-assembling, protein nanoparticles presenting the receptor binding domain and stabilized spike as SARS-CoV-2 vaccine candidates. Sci. Adv. 2021, 7, eabf1591. [Google Scholar] [CrossRef]
- Qu, Z.; Li, M.; Guo, Y.; Liu, Y.; Wang, J.; Gao, M. Expression, purification, and characterisation of recombinant ferritin in insect cells using the baculovirus expression system. Biotechnol. Lett. 2020, 42, 57–65. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Chen, H.; Li, X.; Qian, P. A ferritin nanoparticle vaccine for foot-and-mouth disease virus elicited partial protection in mice. Vaccine 2020, 38, 5647–5652. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Chen, Y.; Li, H.; Fang, K.; Chen, H.; Li, X.; Qian, P. A Self-Assembling Ferritin Nanoplatform for Designing Classical Swine Fever Vaccine: Elicitation of Potent Neutralizing Antibody. Vaccines 2021, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Kang, S.; Reichhardt, C.; Harlen, K.; Douglas, T. The ferritin superfamily: Supramolecular templates for materials synthesis. Biochim. Biophys. Acta 2010, 1800, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, L.; Yu, H.; Lv, P.; Lei, Z.; Zeng, Y.; Liu, G.; Cheng, T. Ferritin nanocage-based antigen delivery nanoplatforms: Epitope engineering for peptide vaccine design. Biomater. Sci. 2019, 7, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.; Piacentini, R.; Incocciati, A.; Bonamore, A.; Macone, A.; Rupert, J.; Zacco, E.; Miotto, M.; Milanetti, E.; Tartaglia, G.G.; et al. Design of protein-binding peptides with controlled binding affinity: The case of SARS-CoV-2 receptor binding domain and angiotensin-converting enzyme 2 derived peptides. Front. Mol. Biosci. 2023, 10, 1332359. [Google Scholar] [CrossRef]
- Liu, Z.; Hao, X.; Qian, J.; Zhang, H.; Bao, H.; Yang, Q.; Gu, W.; Huang, X.; Zhang, Y. Enzyme/pH Dual-Responsive Engineered Nanoparticles for Improved Tumor Immuno-Chemotherapy. ACS Appl. Mater. Interfaces 2024, 16, 12951–12964. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Wang, Q.; Li, C.; Zhou, Y.; Yao, X.; Xu, L.; Chang, Y.; Ding, F.; Sun, L.; Zhan, L.; et al. Self-Assembled Multiepitope Nanovaccine Provides Long-Lasting Cross-Protection against Influenza Virus. Adv. Heal. Healthc. Mater. 2024, 13, e2303531. [Google Scholar] [CrossRef]
- Georgiev, I.S.; Joyce, M.G.; Chen, R.E.; Leung, K.; McKee, K.; Druz, A.; Van Galen, J.G.; Kanekiyo, M.; Tsybovsky, Y.; Yang, E.S.; et al. Two-Component Ferritin Nanoparticles for Multimerization of Diverse Trimeric Antigens. ACS Infect. Dis. 2018, 4, 788–796. [Google Scholar] [CrossRef]
- Mehta, B.; Efthimiou, P. Ferritin in adult-onset still’s disease: Just a useful innocent bystander. Int. J. Inflam. 2012, 2012, 298405. [Google Scholar]
- Ruddell, R.G.; Hoang-Le, D.; Barwood, J.M.; Rutherford, P.S.; Piva, T.J.; Watters, D.J.; Santambrogio, P.; Arosio, P.; Ramm, G.A. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology 2009, 49, 887–900. [Google Scholar] [CrossRef]
- Zarjou, A.; Black, L.M.; McCullough, K.R.; Hull, T.D.; Esman, S.K.; Boddu, R.; Varambally, S.; Chandrashekar, D.S.; Feng, W.; Arosio, P.; et al. Ferritin Light Chain Confers Protection Against Sepsis-Induced Inflammation and Organ Injury. Front. Immunol. 2019, 10, 131. [Google Scholar] [CrossRef]
- Gasser, B.I. Determination of Serum Ferritin Glycosylation in Hyperferritinemia Associated to Iron Overload and Inflammation. EJIFCC 2009, 20, 136–140. [Google Scholar] [PubMed]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, E.M.; Ibrahim, K.S. Ameliorating effects of probiotics on alterations in iron homeostasis and inflammation in COVID-19. Mol. Biol. Rep. 2022, 49, 5153–5163. [Google Scholar] [CrossRef]
- Rosário, C.; Zandman-Goddard, G.; Meyron-Holtz, E.G.; D’Cruz, D.P.; Shoenfeld, Y. The hyperferritinemic syndrome: Macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Oltrogge, L.M.; Broomell, C.C.; Liepold, L.O.; Prevelige, P.E.; Young, M.; Douglas, T. Controlled assembly of bifunctional chimeric protein cages and composition analysis using noncovalent mass spectrometry. J. Am. Chem. Soc. 2008, 130, 16527–16529. [Google Scholar] [CrossRef]
- Santambrogio, P.; Pinto, P.; Levi, S.; Cozzi, A.; Rovida, E.; Albertini, A.; Artymiuk, P.; Harrison, P.M.; Arosio, P. Effects of modifications near the 2-, 3- and 4-fold symmetry axes on human ferritin renaturation. Biochem. J. 1997, 322 Pt 2, 461–468. [Google Scholar] [CrossRef]
- Berzofsky, J.A.; Ahlers, J.D.; Belyakov, I.M. Strategies for designing and optimizing new generation vaccines. Nat. Rev. Immunol. 2001, 1, 209–219. [Google Scholar] [CrossRef]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef]
- Roldão, A.; Mellado, M.C.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert. Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef]
- Elamanchili, P.; Lutsiak, C.M.; Hamdy, S.; Diwan, M.; Samuel, J. “Pathogen-mimicking” nanoparticles for vaccine delivery to dendritic cells. J. Immunother. 2007, 30, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Manayani, D.J.; Thomas, D.; Dryden, K.A.; Reddy, V.; Siladi, M.E.; Marlett, J.M.; Rainey, G.J.; Pique, M.E.; Scobie, H.M.; Yeager, M.; et al. A viral nanoparticle with dual function as an anthrax antitoxin and vaccine. PLoS Pathog. 2007, 3, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Li, Y.; Liang, N.; Hu, P.; Zhang, Y.; Qiao, L.; Zhang, Y.; Xie, J.; Luo, H.; Wang, F.; et al. Ferritin in cancer therapy: A pleiotropic tumoraffin nanocage-based transport. Cancer Med. 2023, 12, 11570–11588. [Google Scholar] [CrossRef]
- Lee, N.K.; Cho, S.; Kim, I.S. Ferritin—A multifaceted protein scaffold for biotherapeutics. Exp. Mol. Med. 2022, 54, 1652–1657. [Google Scholar] [CrossRef]
- Song, N.; Zhang, J.; Zhai, J.; Hong, J.; Yuan, C.; Liang, M. Ferritin: A Multifunctional Nanoplatform for Biological Detection, Imaging Diagnosis, and Drug Delivery. Acc. Chem. Res. 2021, 54, 3313–3325. [Google Scholar] [CrossRef]
- Incocciati, A.; Kubeš, J.; Piacentini, R.; Cappelletti, C.; Botta, S.; Bertuccini, L.; Šimůnek, T.; Boffi, A.; Macone, A.; Bonamore, A. Hydrophobicity-enhanced ferritin nanoparticles for efficient encapsulation and targeted delivery of hydrophobic drugs to tumor cells. Protein Sci. 2023, 32, e4819. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Jia, X.; Ji, T.; Zhou, M.; He, J.; Wang, K.; Tian, J.; Yan, X.; Fan, K. Ferritin nanocages for early theranostics of tumors via inflammation-enhanced active targeting. Sci. China Life Sci. 2022, 65, 328–340. [Google Scholar] [CrossRef]
- Liu, T.; Li, M.; Tian, Y.; Dong, Y.; Liu, N.; Wang, Z.; Zhang, H.; Zheng, A.; Cui, C. Immunogenicity and safety of a self-assembling ZIKV nanoparticle vaccine in mice. Int. J. Pharm. 2024, 660, 124320. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Jia, F.; Ge, C.; He, Y.; Tian, Y.; Wang, W.; Yang, G.; Huang, H.; Wang, J.; et al. Homologous Sequential Immunization Using Salmonella Oral Administration Followed by an Intranasal Boost with Ferritin-Based Nanoparticles Enhanced the Humoral Immune Response against H1N1 Influenza Virus. Microbiol. Spectr. 2023, 11, e0010223. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, X.; Guo, X.; Li, Z.; Yu, W.; Lv, S.; Zhang, W.; Jiao, F.; He, S.; Lu, S. Self-Assembled Nanoparticles with E Protein Domains I and II of Duck Tembusu Virus Can Induce a More Comprehensive Immune Response Against the Duck Tembusu Virus Challenge. Avian Dis. 2023, 67, 49–56. [Google Scholar]
- Li, D.; Song, H.; Li, J.; Liu, X.; Gao, X.; Wu, T.; Zhang, Z.; Li, Y. Expression and Evaluation of a Novel PPRV Nanoparticle Antigen Based on Ferritin Self-Assembling Technology. Pharmaceutics 2022, 14, 1902. [Google Scholar] [CrossRef]
- Qu, Z.; Li, M.; An, R.; Dai, H.; Yu, Y.; Li, C.; Cao, C.; Meng, Y.; Wang, J.; Gao, M. Self-assembled BPIV3 nanoparticles can induce comprehensive immune responses and protection against BPIV3 challenge by inducing dendritic cell maturation in mice. Vet. Microbiol. 2022, 268, 109415. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, S.; Qiao, Y.; Fu, Y.; Nie, J.; Jiang, S.; Yao, X.; Pan, Y.; Zhao, L.; Wu, C.; et al. Self-assembling ferritin nanoparticles coupled with linear sequences from canine distemper virus haemagglutinin protein elicit robust immune responses. J. Nanobiotechnol. 2022, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.; Lou, P.; Hu, Z.; Qu, P.; Li, D.; Li, Q.; Xu, Y.; Niu, J.; He, Y.; et al. A Nanoparticle-Based Hepatitis C Virus Vaccine With Enhanced Potency. J. Infect. Dis. 2020, 221, 1304–1314. [Google Scholar] [PubMed]
- Li, Z.; Cui, K.; Wang, H.; Liu, F.; Huang, K.; Duan, Z.; Wang, F.; Shi, D.; Liu, Q. A milk-based self-assemble rotavirus VP6-ferritin nanoparticle vaccine elicited protection against the viral infection. J. Nanobiotechnol. 2019, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Veggi, D.; Dello Iacono, L.; Malito, E.; Maruggi, G.; Giusti, F.; Goswami, P.; Pansegrau, W.; Marchi, S.; Tomei, S.; Luzzi, E.; et al. Effective Multivalent Oriented Presentation of Meningococcal NadA Antigen Trimers by Self-Assembling Ferritin Nanoparticles. Int. J. Mol. Sci. 2023, 24, 6183. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, X.; Liao, Y.; Zeng, S.; Li, Y.; Zhang, Y.; Gao, C.; Zhang, Y.; Wan, J.; Gu, J.; et al. Recombinant Pseudomonas aeruginosa flagellin delivered using ferritin nanoparticles provides enhanced cross-protection against lung infection in mice. Mol. Immunol. 2023, 163, 235–242. [Google Scholar] [CrossRef]
- Chang, X.; Ma, J.; Zhou, Y.; Xiao, S.; Xiao, X.; Fang, L. Development of a Ferritin Protein Nanoparticle Vaccine with PRRSV GP5 Protein. Viruses 2024, 16, 991. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.; Yang, X.; Qi, S.; Zhao, F.; Guo, D.; Li, C.; Zhu, Q.; Xing, X.; Cao, Y.; et al. Construction and immune effect evaluation of the S protein heptad repeat-based nanoparticle vaccine against porcine epidemic diarrhea virus. Virology 2024, 596, 110113. [Google Scholar] [CrossRef]
- Song, J.; Wang, M.; Zhou, L.; Tian, P.; Sun, Z.; Sun, J.; Wang, X.; Zhuang, G.; Jiang, D.; Wu, Y.; et al. A candidate nanoparticle vaccine comprised of multiple epitopes of the African swine fever virus elicits a robust immune response. J. Nanobiotechnol. 2023, 21, 424. [Google Scholar] [CrossRef]
- Zhong, D.; Lu, Z.; Xia, Y.; Wu, H.; Zhang, X.; Li, M.; Song, X.; Wang, Y.; Moon, A.; Qiu, H.J.; et al. Ferritin Nanoparticle Delivery of the E2 Protein of Classical Swine Fever Virus Completely Protects Pigs from Lethal Challenge. Vaccines 2024, 12, 629. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pu, R.; Zhang, Y.; Zhang, Y.; Wei, Y.; Zeng, S.; Gao, C.; Wang, Y.; Yin, D.; Zhang, Y.; et al. Self-assembled ferritin nanoparticles displaying PcrV and OprI as an adjuvant-free Pseudomonas aeruginosa vaccine. Front. Immunol. 2023, 14, 1184863. [Google Scholar] [CrossRef]
- Sudarev, V.V.; Dolotova, S.M.; Bukhalovich, S.M.; Bazhenov, S.V.; Ryzhykau, Y.L.; Uversky, V.N.; Bondarev, N.A.; Osipov, S.D.; Mikhailov, A.E.; Kuklina, D.D.; et al. Ferritin self-assembly, structure, function, and biotechnological applications. Int. J. Biol. Macromol. 2023, 224, 319–343. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Kikuchi, K.; Maity, B.; Ueno, T. The Versatile Manipulations of Self-Assembled Proteins in Vaccine Design. Int. J. Mol. Sci. 2021, 22, 1934. [Google Scholar] [CrossRef] [PubMed]

| Antigens | Target Pathogens | Attached Region of Ferritin | Expression System | Experiment Animal | Immunological Effects of the Ferritin Nanoparticles Vaccine | References |
|---|---|---|---|---|---|---|
| E protein of ZIKV | Zika virus (ZIKV) | N-terminus | E. coli | Mice | Enhanced high-affinity antigen-specific IgG antibody levels, increased secretion of the cytokines IL-4 and IFN-γ by splenocytes, significantly activated T/B lymphocytes, and improved the generation of memory T/B cells. | [78] |
| E2 protein | Classical swine fever virus (CSFV) | N-terminus | SF9 cells | Rabbits | Elicited higher neutralizing antibody titers and significantly induced CSFV-specific IFN-γ-secreting cells compared to monomeric E2 protein. | [52] |
| BA.5 RBD | Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) | N-terminus | Through Fc-Protein-A-tag-mediated conjugation | Golden hamsters | Stimulated strong innate and adaptive immune responses, inducing cross-neutralizing antibodies and protecting golden hamsters from multiple SARS-CoV-2 variants | [45] |
| Conserved stem domain of hemagglutinin, ectodomain of matrix protein 2 | Influenza Virus | N-terminus | E. coli | Mice | Induces cross-reactive neutralizing antibodies, cellular immunity, and long-lasting memory B cell responses, offering protection against H3N2 and partial protection against H1N1. | [79] |
| Three copies of extracellular domain of the transmembrane protein M2 of H1N1 | Influenza Virus | N-terminus | E. coli | Mice | Significantly activated lung CD11b dendritic cells, increased effector memory T cells and tissue-resident memory T cells in the lungs, and boosted mucosal IgG and IgA antibody production. | [57] |
| Dominant B and T cell epitopes of the highly immunogenic ASFV antigen (p72, CD2v, pB602L and p30) | African swine fever virus (ASFV) | N-terminus | E. coli | Mice | The nanoparticle vaccines can induce a more robust T cell response, and the high-level antibody response against ASFV can last for more than 231 days. | [75] |
| E protein domains I and II (EDI–II) of DTMUV (EDI–II-RFNp) | Duck Tembusu virus (DTMUV) | N-terminus | E. coli | Ducks | The self-assembled ferritin nanoparticles effectively protect ducks against the DTMUV challenge. | [80] |
| Hemagglutinin protein | Peste des Petits Ruminants (PPR) | N-terminus | Silkworm baculovirus | Mice | The immunogenicity and protective immune response of H-Fe nanoparticle antigens expressed by silkworms were improved compared with the H antigen alone. | [81] |
| Ectodomain of BPIV3 hemagglutinin-neuraminidase (HN) | Bovine parainfluenza virus type 3(BPIV3) | N-terminus | Baculovirus | Mice | The nanoparticles induced dendritic cell maturation, upregulated surface molecules, increased inflammatory cytokine secretion, and enhanced T cell activation. In mice, it induced higher titers of specific antibodies and provided better protection against BPIV3. | [82] |
| Hemagglutinin | Canine distemper virus (CDV) | C-terminus | E. coli | Mice | All proteins self-assembled into nanoparticles. Vaccination induced strong, long-lasting antibody responses and anti-CDV neutralizing activity, particularly in YaH4F and YaHF groups, which also enhanced ADCC effects and induced Th1 and Th2 responses. | [83] |
| E2 protein | Hepatitis C virus (HCV) | N-terminus | Drosophila Schneider 2 cell | Mice | The sE2-ferritin nanoparticle not only had nearly natural conformation but also had better affinities than the unfused sE2 to neutralizing antibodies, receptor, and patient serum. Mouse immunization studies showed that sE2-ferritin was more potent than sE2 in inducing anti-HCV broadly neutralizing antibodies. | [84] |
| Inner capsid protein VP6 | Rotavirus | N-terminus | E. coli | Mice | Oral administration in mice induced strong humoral and mucosal immunity. Transgenic expression in mouse milk also induced strong immunogenicity in pups, reducing diarrhea symptoms and growth impacts. | [85] |
| The trimeric N. meningitidis antigen, NadA.Two fragments of NadA(f NadA5 and NadA3) | Neisseria meningitidis | N-terminus | E. coli | Mice | In mice, the two nanoparticles elicited comparable NadA antibody levels that were 10- to 100-fold higher than those elicited by the corresponding NadA trimer subunits. Further, the NadA ferritin nanoparticles potently induced complement-mediated serum bactericidal activity. | [86] |
| Type A flagellin | Pseudomonas aeruginosa (PA) | N-terminus | E. coli | Mice | A-type flagellin- ferritin nanoparticles induced a strong Th1 immune response and enhancing protection against PA strains with A-type and B-type flagellins. | [45] |
| PcrV and OprI of PA | Pseudomonas aeruginosa (PA) | N-terminus | E. coli | Mice | Intramuscular immunization with nanoparticles induced quick and efficient immunity and conferred protection against PA pneumonia in mice. In addition, intranasal immunization with nanoparticles enhanced protective mucosal immunity. | [87] |
| VP1 | Foot-and-mouth disease (FMD) | N-terminus | E. coli | Mice, pig | The results from guinea pigs immunized with Hpf-T34E showed that the immune efficacy was largely consistent with the immunogenicity of the FMD inactivated vaccine (IV) and could confer partial protection against FMDV challenge in guinea pigs. | [42] |
| GP5 Protein | Porcine reproductive and respiratory syndrome virus (PRRSV) | N-terminus | E. coli | Mice | Ferritin (Ft) nanovaccines targeting the major glycoprotein (GP5GP5m-Ft t) exhibited the highest ELISA antibody levels, neutralizing antibody titers, the lymphocyte proliferation index, and IFN-γ levels. Furthermore, vaccination with the GP5m-Ft nanoparticle effectively protected piglets against a highly pathogenic PRRSV challenge. | [88] |
| PEDV HR protein | Porcine epidemic diarrhea virus (PEDV) | N-terminus | E. coli | Mice | HR-Ferritin nanoparticles stimulated the maturation DCs and elevated the secretion of pro-inflammatory cytokines, while enhancing the uptake of antigens by DCs. | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.; Ma, D.; Ji, S.; Zhou, M.; Zhu, S. Self-Assembled Ferritin Nanoparticles for Delivery of Antigens and Development of Vaccines: From Structure and Property to Applications. Molecules 2024, 29, 4221. https://doi.org/10.3390/molecules29174221
Cao S, Ma D, Ji S, Zhou M, Zhu S. Self-Assembled Ferritin Nanoparticles for Delivery of Antigens and Development of Vaccines: From Structure and Property to Applications. Molecules. 2024; 29(17):4221. https://doi.org/10.3390/molecules29174221
Chicago/Turabian StyleCao, Shinuo, Dongxue Ma, Shengwei Ji, Mo Zhou, and Shanyuan Zhu. 2024. "Self-Assembled Ferritin Nanoparticles for Delivery of Antigens and Development of Vaccines: From Structure and Property to Applications" Molecules 29, no. 17: 4221. https://doi.org/10.3390/molecules29174221
APA StyleCao, S., Ma, D., Ji, S., Zhou, M., & Zhu, S. (2024). Self-Assembled Ferritin Nanoparticles for Delivery of Antigens and Development of Vaccines: From Structure and Property to Applications. Molecules, 29(17), 4221. https://doi.org/10.3390/molecules29174221







