Characterization of Camphene- and Fenchol-Based Hydrophobic Eutectic Solvents and Their Application in Aldehyde Extraction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Eutectic Solvents and Appearance after 48 h
2.2. SLE and Eutectic Compositions
2.3. Hydrogen Bond Determinations by Nuclear Magnetic Resonance Spectroscopy (NMR)
2.4. Physicochemical Properties: Density and Viscosity
2.5. Extraction Efficiency for Aromatic Aldehydes
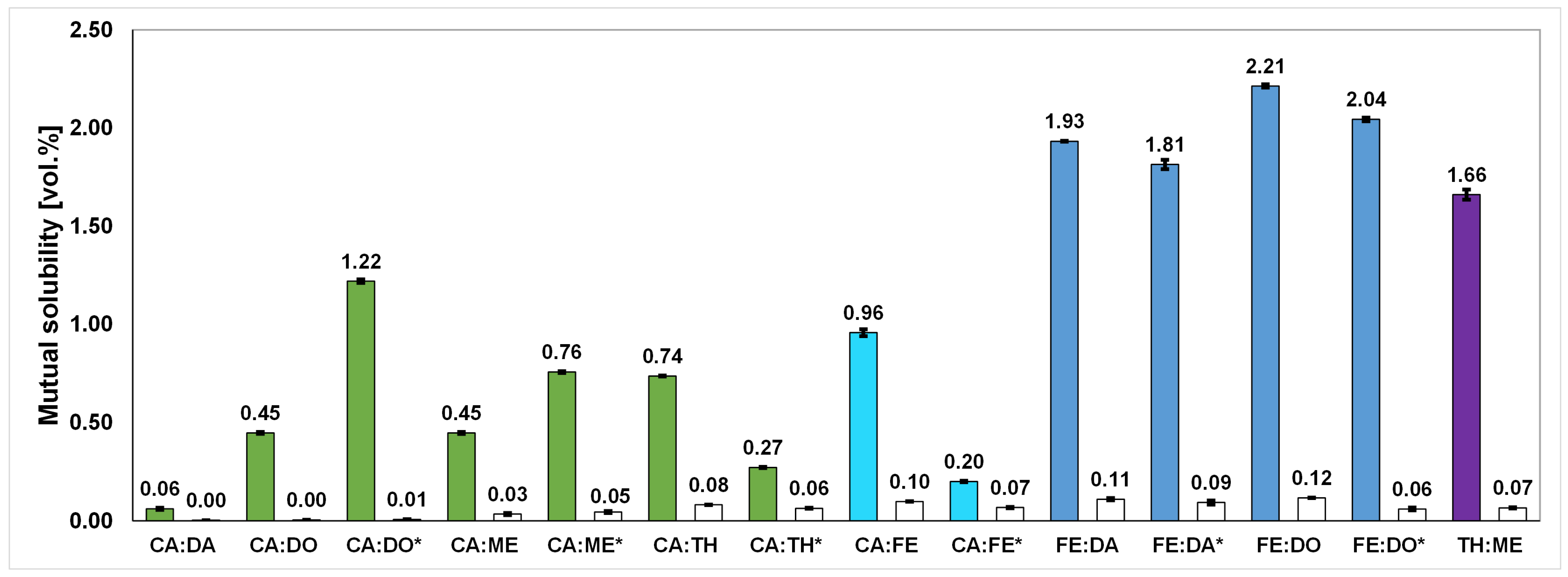
2.6. Mutual Solubility
2.7. Deviation in Solvent Composition (Stability) during Solvent Extraction
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Eutectic Mixtures
3.3. Determination of SLE
3.4. Nuclear Magnetic Resonance Spectroscopy (NMR)
3.5. Physicochemical Properties: Density and Viscosity
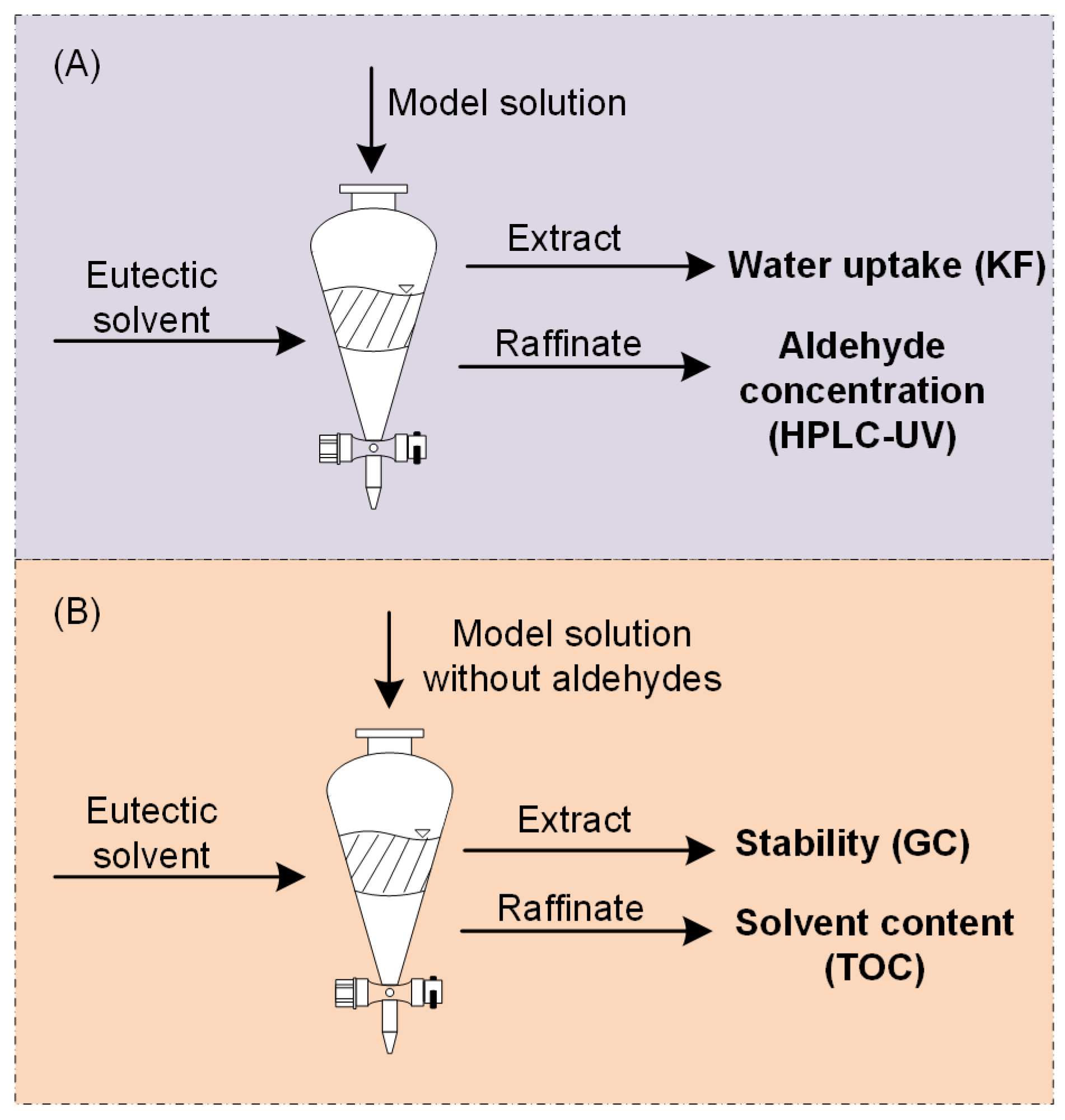
3.6. Solvent Extraction and Mutual Solubility
3.7. Determination of Extraction Efficiency with HPLC-UV
3.8. Stability Analysis by GC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cañadas, R.; González-Miquel, M.; González, E.J.; Núñez de Prado, A.; Díaz, I.; Rodríguez, M. Sustainable Recovery of High Added-Value Vanilla Compounds from Wastewater Using Green Solvents. ACS Sustain. Chem. Eng. 2021, 9, 4850–4862. [Google Scholar] [CrossRef]
- Rodríguez-Llorente, D.; Cañada-Barcala, A.; Muñoz, C.; Pascual-Muñoz, G.; Navarro, P.; Santiago, R.; Águeda, V.I.; Álvarez-Torrellas, S.; García, J.; Larriba, M. Separation of phenols from aqueous streams using terpenoids and hydrophobic eutectic solvents. Sep. Purif. Technol. 2020, 251, 117379. [Google Scholar] [CrossRef]
- Hibbert, H.; Tomlinson, J.G.H. Manufacture of Vanillin from Waste Sulphite Pulp Liquor. U.S. Patent US2069185A, 27 December 1934. [Google Scholar]
- Gao, P.; Li, C.; Wang, H.; Wang, X.; Wang, A. Perovskite hollow nanospheres for the catalytic wet air oxidation of lignin. Chin. J. Catal. 2013, 34, 1811–1815. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Statista. Global Carbon Project; Annual Carbon Dioxide (CO2) Emissions Worldwide from 1940 to 2023 (in Billion Metric Tons). 2023. Available online: https://www.statista.com/statistics/276629/global-co2-emissions/ (accessed on 13 May 2024).
- Ivanković, A. Review of 12 Principles of Green Chemistry in Practice. IJSGE 2017, 6, 39–48. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goals (SDGs). Available online: https://sdgs.un.org/goals (accessed on 10 July 2024).
- Cañadas, R.; González-Miquel, M.; González, E.J.; Díaz, I.; Rodríguez, M. Overview of neoteric solvents as extractants in food industry: A focus on phenolic compounds separation from liquid streams. Food Res. Int. 2020, 136, 109558. [Google Scholar] [CrossRef]
- Sequeira, R.A.; Bhatt, J.; Prasad, K. Recent Trends in Processing of Proteins and DNA in Alternative Solvents: A Sustainable Approach. Sustain. Chem. 2020, 1, 116–137. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; De la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Abranches, D.O.; Martins, M.A.R.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A.P. Phenolic hydrogen bond donors in the formation of non-ionic deep eutectic solvents: The quest for type V DES. Chem. Commun. 2019, 55, 10253–10256. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; AlNashef, I.M.; Mirghani, M.E.S. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Abranches, D.O.; Coutinho, J.A. Type V deep eutectic solvents: Design and applications. Curr. Opin. Green Sustain. Chem. 2022, 35, 100612. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I.M. Menthol-based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- van Osch, D.J.; Zubeir, L.F.; van den Bruinhorst, A.; Rocha, M.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef]
- Kozhevnikova, A.V.; Zinov’eva, I.V.; Zakhodyaeva, Y.A.; Baranovskaya, V.B.; Voshkin, A.A. Application of Hydrophobic Deep Eutectic Solvents in Extraction of Metals from Real Solutions Obtained by Leaching Cathodes from End-of-Life Li-Ion Batteries. Processes 2022, 10, 2671. [Google Scholar] [CrossRef]
- MK, S.B.; Katchala, N.; Natarajan, T.S.; Suresh, S.; Kancharla, S. Nd(III) and Dy(III) extraction from discarded NdFeB magnets using TOPO-based hydrophobic eutectic solvents. J. Mol. Liq. 2024, 402, 124697. [Google Scholar] [CrossRef]
- Tang, N.; Liu, L.; Yin, C.; Zhu, G.; Huang, Q.; Dong, J.; Yang, X.; Wang, S. Environmentally benign hydrophobic deep eutectic solvents for palladium(II) extraction from hydrochloric acid solution. J. Taiwan Inst. Chem. Eng. 2021, 121, 92–100. [Google Scholar] [CrossRef]
- Lalikoglu, M. Intensification of formic acid from dilute aqueous solutions using menthol based hydrophobic deep eutectic solvents. J. Indian Chem. Soc. 2022, 99, 100303. [Google Scholar] [CrossRef]
- Demmelmayer, P.; Steiner, L.; Weber, H.; Kienberger, M. Thymol-menthol-based deep eutectic solvent as a modifier in reactive liquid–liquid extraction of carboxylic acids from pretreated sweet sorghum silage press juice. Sep. Purif. Technol. 2023, 310, 123060. [Google Scholar] [CrossRef]
- Naik, P.K.; Kundu, D.; Bairagya, P.; Banerjee, T. Phase behavior of water-menthol based deep eutectic solvent-dodecane system. Chem. Thermodyn. Therm. Anal. 2021, 3–4, 100011. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Development of hydrophobic deep eutectic solvents for extraction of pesticides from aqueous environments. Fluid Phase Equilibria 2017, 448, 135–142. [Google Scholar] [CrossRef]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Tailor-made hydrophobic deep eutectic solvents for cleaner extraction of polyprenyl acetates from Ginkgo biloba leaves. J. Clean. Prod. 2017, 152, 399–405. [Google Scholar] [CrossRef]
- Zubeir, L.F.; Van Osch, D.J.; Rocha, M.A.; Banat, F.; Kroon, M.C. Carbon Dioxide Solubilities in Decanoic Acid-Based Hydrophobic Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 913–919. [Google Scholar] [CrossRef]
- Patra, A.; Khokhar, V.; Pandey, S. Decanoic Acid Based Deep Eutectic Solvents: Constituent-Controlled Physicochemical Properties and Solvatochromic Probe Behavior. Ind. Eng. Chem. Res. 2022, 61, 14702–14710. [Google Scholar] [CrossRef]
- Fan, C.; Liu, Y.; Sebbah, T.; Cao, X. A Theoretical Study on Terpene-Based Natural Deep Eutectic Solvent: Relationship between Viscosity and Hydrogen-Bonding Interactions. Glob. Chall. 2021, 5, 2000103. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Monteiro, N.V.; Ribeiro, B.D.; Branco, L.C.; Marrucho, I.M. Hydrophobic deep eutectic solvents for purification of water contaminated with Bisphenol-A. J. Mol. Liq. 2020, 297, 111841. [Google Scholar] [CrossRef]
- Ortega-Zamora, C.; Jiménez-Skrzypek, G.; González-Sálamo, J.; Mazzapioda, L.; Navarra, M.A.; Gentili, A.; Hernández-Borges, J. Extraction of Emerging Contaminants from Environmental Waters and Urine by Dispersive Liquid-Liquid Microextraction with Solidification of the Floating Organic Droplet Using Fenchol: Acetic Acid Deep Eutectic Mixtures. ACS Sustain. Chem. Eng. 2022, 10, 15714–15725. [Google Scholar] [CrossRef]
- Rodríguez-Llorente, D.; Navarro, P.; Santiago, R.; Águeda, V.I.; Álvarez-Torrellas, S.; García, J.; Larriba, M. Extractive removal and recovery of bisphenol A from aqueous solutions using terpenoids and hydrophobic eutectic solvents. J. Environ. Chem. Eng. 2021, 9, 106128. [Google Scholar] [CrossRef]
- Cañadas, R.; González-Miquel, M.; González, E.J.; Díaz, I.; Rodríguez, M. Hydrophobic eutectic solvents for extraction of natural phenolic antioxidants from winery wastewater. Sep. Purif. Technol. 2021, 254, 117590. [Google Scholar] [CrossRef]
- Schaeffer, N.; Abranches, D.O.; Silva, L.P.; Martins, M.A.; Carvalho, P.J.; Russina, O.; Triolo, A.; Paccou, L.; Guinet, Y.; Hedoux, A.; et al. Non-Ideality in Thymol + Menthol Type V Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2021, 9, 2203–2211. [Google Scholar] [CrossRef]
- Brouwer, T.; Dielis, B.C.; Bock, J.M.; Schuur, B. Hydrophobic Deep Eutectic Solvents for the Recovery of Bio-Based Chemicals: Solid–Liquid Equilibria and Liquid–Liquid Extraction. Processes 2021, 9, 796. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Silva, L.P.; Schaeffer, N.; Abranches, D.O.; Maximo, G.J.; Pinho, S.P.; Coutinho, J.A.P. Greener Terpene–Terpene Eutectic Mixtures as Hydrophobic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 17414–17423. [Google Scholar] [CrossRef]
- Fan, C.; Liu, Y.; Shan, Y.; Cao, X. A priori design of new natural deep eutectic solvent for lutein recovery from microalgae. Food Chem. 2021, 376, 131930. [Google Scholar] [CrossRef]
- Fan, Y.; Luo, H.; Zhu, C.; Li, W.; Wu, D.; Wu, H. Hydrophobic natural alcohols based deep eutectic solvents: Effective solvents for the extraction of quinine. Sep. Purif. Technol. 2021, 275, 119112. [Google Scholar] [CrossRef]
- Fan, C.; Shan, Y.; Wen, L.; Cao, X. Extraction of artemisinin using natural deep eutectic solvent selected by COSMO-RS. Sustain. Chem. Pharm. 2023, 33, 101096. [Google Scholar] [CrossRef]
- Zheng, X.; Ma, W.; Wang, Q.; Xu, Y.; Yang, Y.; Qin, S.; Jing, X. Development of self-dispersion ferrofluid-based dispersive liquid–liquid microextraction for determining chiral fungicide hexaconazole in water, tea, and juice using high-performance liquid chromatography. Microchem. J. 2024, 196, 109593. [Google Scholar] [CrossRef]
- Audeh, D.J.S.A.; Carniel, A.; Borges, C.P.; Coelho, M.A.Z.; Buarque, F.S.; Ribeiro, B.D. Hydrophobic Deep Eutectic Solvents for Ethanol, Propan-1-ol, and Propan-2-ol Recovery from Aqueous Solutions. Processes 2024, 12, 1255. [Google Scholar] [CrossRef]
- Okuniewski, M.; Paduszyński, K.; Domańska, U. Thermodynamic Study of Molecular Interactions in Eutectic Mixtures Containing Camphene. J. Phys. Chem. B 2016, 120, 12928–12936. [Google Scholar] [CrossRef]
- Wei, D.; Wang, L.; Zhang, C. Solid-Liquid Equilibria in Binary Mixtures of 1,8-Cineole with p -Cymene, β-Pinene, and Camphene. J. Chem. Eng. Data 2010, 55, 1456–1458. [Google Scholar] [CrossRef]
- Satterwhite, D.M.; Wheeler, C.J.; Croteau, R. Biosynthesis of monoterpenes. Enantioselectivity in the enzymatic cyclization of linalyl pyrophosphate to (−)-endo-fenchol. J. Biol. Chem. 1985, 260, 13901–13908. [Google Scholar] [CrossRef] [PubMed]
- Volzone, C.; Masini, O.; Comelli, N.A.; Grzona, L.M.; Ponzi, E.N.; Ponzi, M.I. Production of camphene and limonene from pinene over acid di-and trioctahedral smectite clays. Appl. Catal. A Gen. 2001, 214, 213–218. [Google Scholar] [CrossRef]
- Sánchez-Velandia, J.E.; Pájaro, E.; Villa, A.L.; Martínez-O, F. Selective synthesis of camphene from isomerization of α- and β-pinene over heterogeneous catalysts. Microporous Mesoporous Mater. 2021, 324, 111273. [Google Scholar] [CrossRef]
- Adhar, M.; HadjKacem, B.; Périno-Issartier, S.; Ben Amor, I.; Feki, A.; Gargouri, J.; Gargouri, A.; Tounsi, S.; Chemat, F.; Allouche, N. Thymol-enriched extract from Thymus vulgaris L leaves: Green extraction processes and antiaggregant effects on human platelets. Bioorg. Chem. 2022, 125, 105858. [Google Scholar] [CrossRef] [PubMed]
- Villanueva Bermejo, D.; Angelov, I.; Vicente, G.; Stateva, R.P.; Rodriguez García-Risco, M.; Reglero, G.; Ibañez, E.; Fornari, T. Extraction of thymol from different varieties of thyme plants using green solvents. J. Sci. Food Agric. 2015, 95, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Dylong, D.; Hausoul, P.J.C.; Palkovits, R.; Eisenacher, M. Synthesis of (−)-menthol: Industrial synthesis routes and recent development. Flavour Fragr. J. 2022, 37, 195–209. [Google Scholar] [CrossRef]
- Mesbah, M.; Soltanali, S.; Bahranifard, Z.; Hosseinzadeh, A.; Karami, H. Production of thymol from alkylation of m-cresol with isopropanol over ZSM-5 catalysts: Artificial Neural Network (ANN) modelling. J. Indian Chem. Soc. 2023, 100, 100882. [Google Scholar] [CrossRef]
- Noweck, K.; Grafahrend, W. Fatty Alcohols. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Tambun, R.; Ferani, D.G.; Afrina, A.; Tambun, J.A.A.; Tarigan, I.A.A. Fatty Acid Direct Production from Palm Kernel Oil. IOP Conf. Ser. Mater. Sci. Eng. 2019, 505, 12115. [Google Scholar] [CrossRef]
- Hsieh, S.-C.; Wang, J.-H.; Lai, Y.-C.; Su, C.-Y.; Lee, K.-T. Production of 1-Dodecanol, 1-Tetradecanol, and 1,12-Dodecanediol through Whole-Cell Biotransformation in Escherichia coli. Appl. Environ. Microbiol. 2018, 84, e01806-17. [Google Scholar] [CrossRef]
- Funk, I.; Rimmel, N.; Schorsch, C.; Sieber, V.; Schmid, J. Production of dodecanedioic acid via biotransformation of low cost plant-oil derivatives using Candida tropicalis. J. Ind. Microbiol. Biotechnol. 2017, 44, 1491–1502. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498754286. [Google Scholar]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- Bergua, F.; Castro, M.; Lafuente, C.; Artal, M. Thymol+l-menthol eutectic mixtures: Thermophysical properties and possible applications as decontaminants. J. Mol. Liq. 2022, 368, 120789. [Google Scholar] [CrossRef]
- Soltani, S.; Sereshti, H.; Nouri, N. Deep eutectic solvent-based clean-up/vortex-assisted emulsification liquid-liquid microextraction: Application for multi-residue analysis of 16 pesticides in olive oils. Talanta 2021, 225, 121983. [Google Scholar] [CrossRef]
- Altshuller, A.P.; Everson, H.E. The Solubility of Ethyl Acetate in Water. J. Am. Chem. Soc. 1953, 75, 1727. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Gmehling, J.; Kleiber, M.; Kolbe, B.; Rarey, J. Chemical Thermodynamics for Process Simulation: Second, Completely Revised and Enlarged Edition, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; ISBN 978-3-527-34325-6. [Google Scholar]
- Joback, K.G.; Reid, R.C. Estimation of pure-component properties from group-contributions. Chem. Eng. Commun. 1987, 57, 233–243. [Google Scholar] [CrossRef]
- Kaufmann, A.; Maier, L.; Kienberger, M. Solvent screening for the extraction of aromatic aldehydes. Sep. Purif. Technol. 2024, 340, 126780. [Google Scholar] [CrossRef]
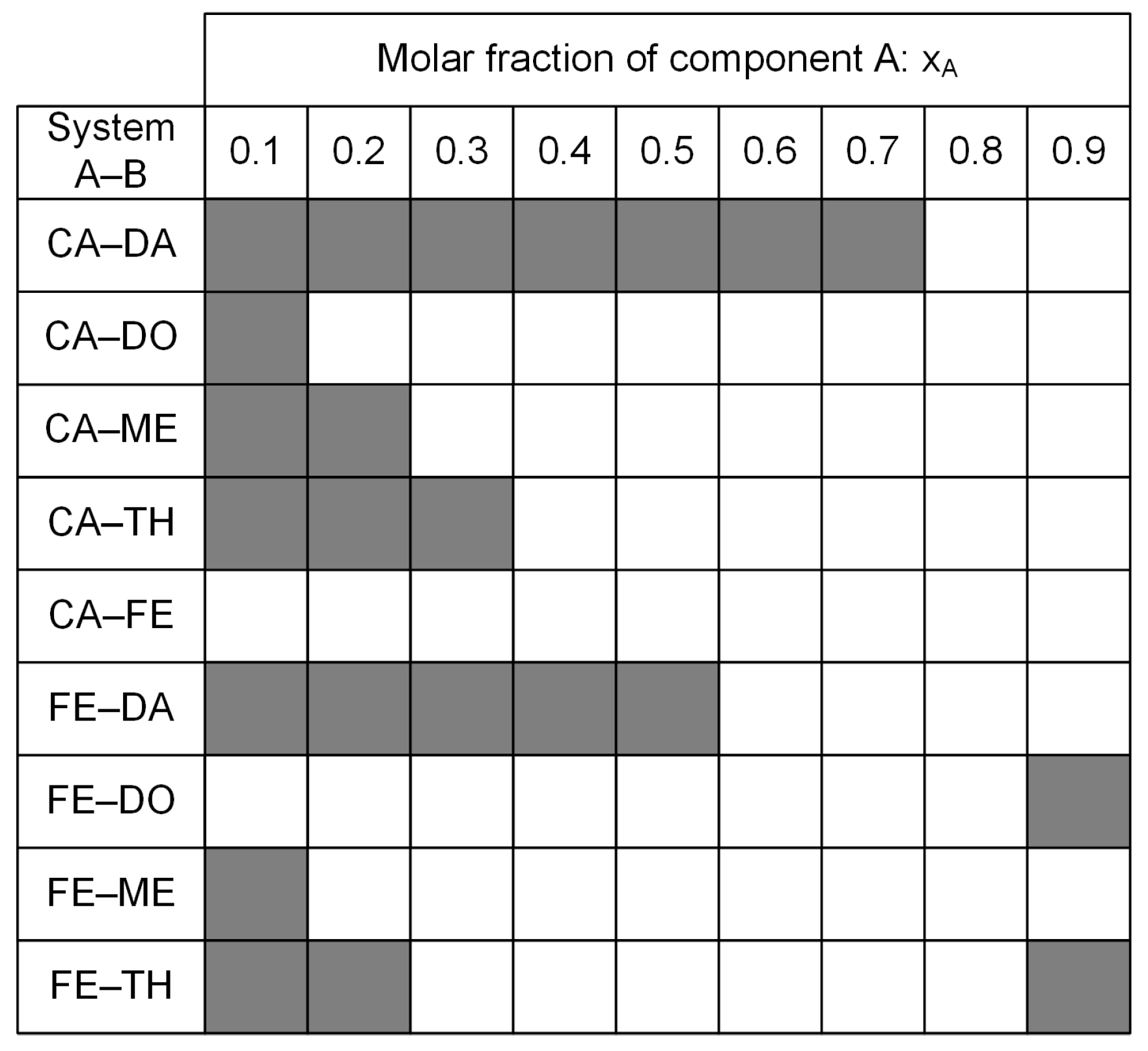
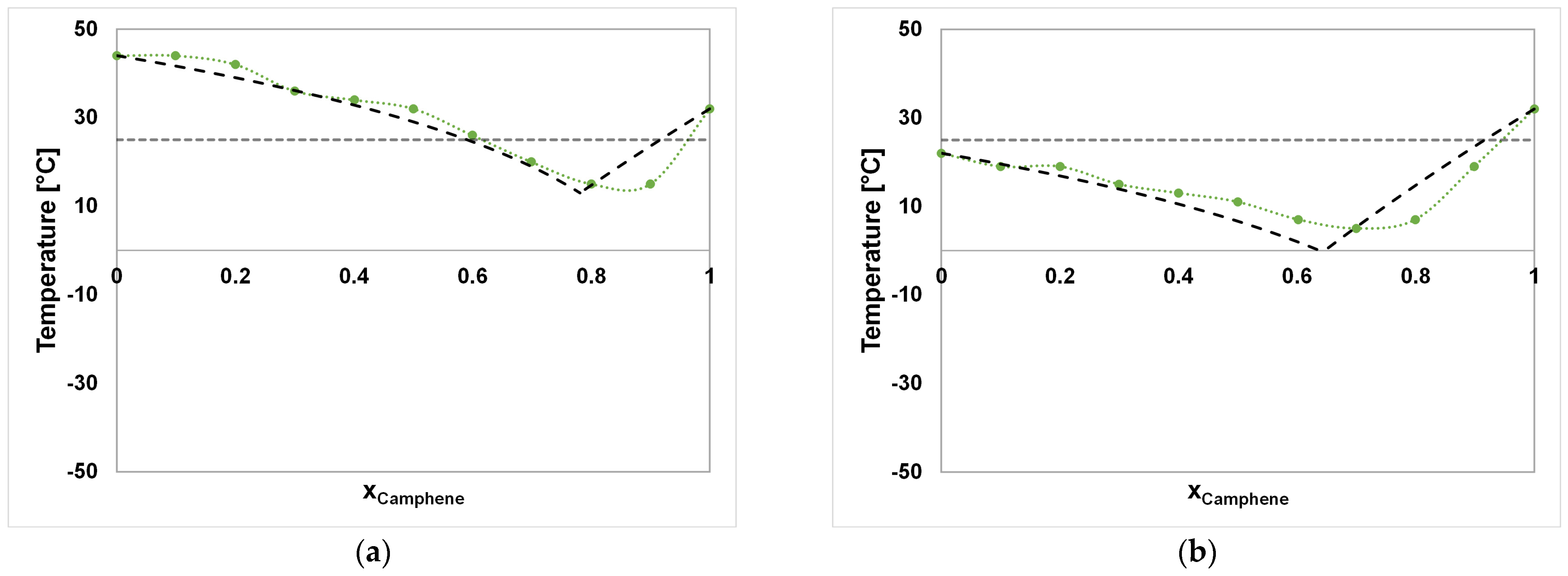
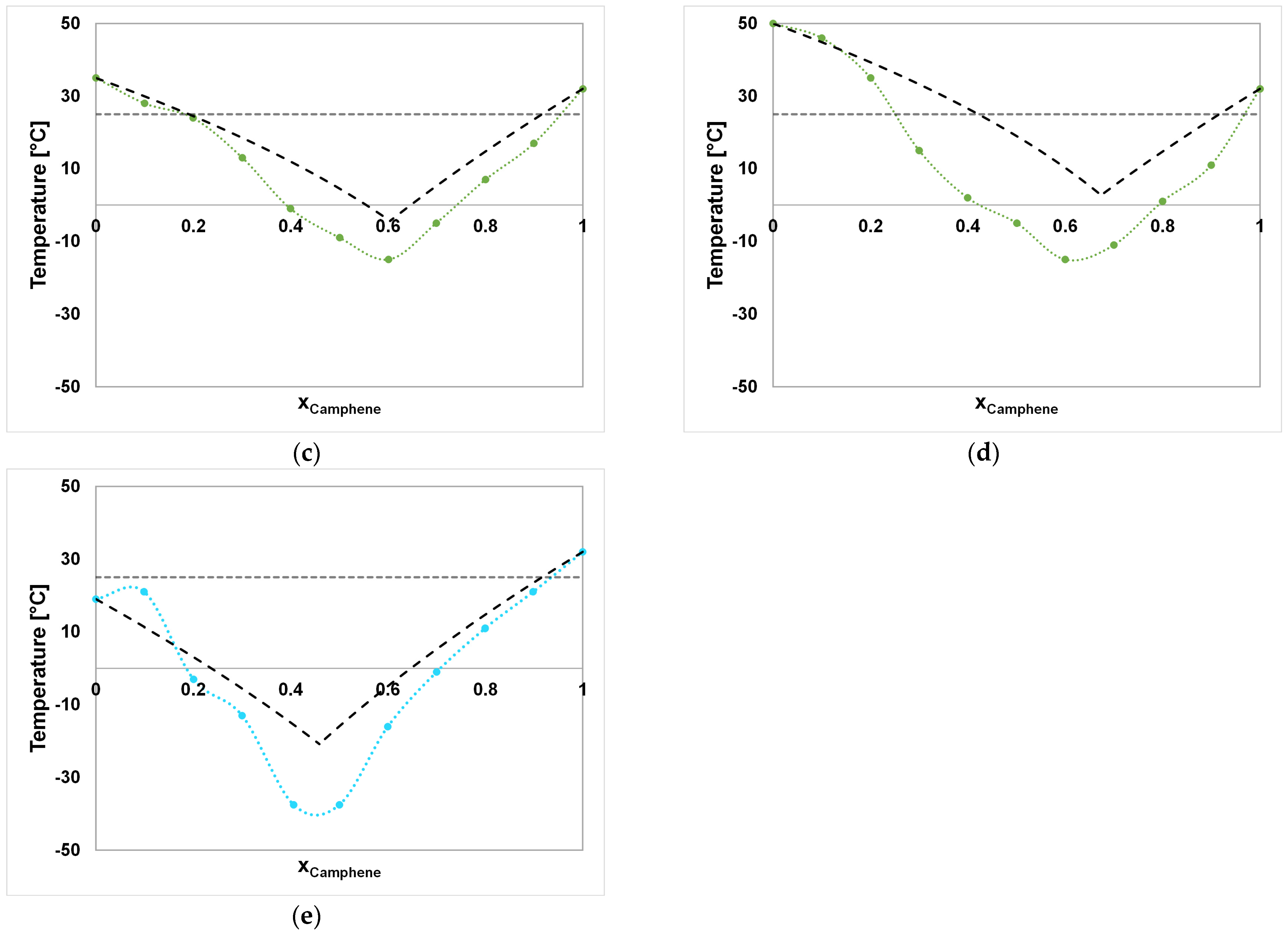
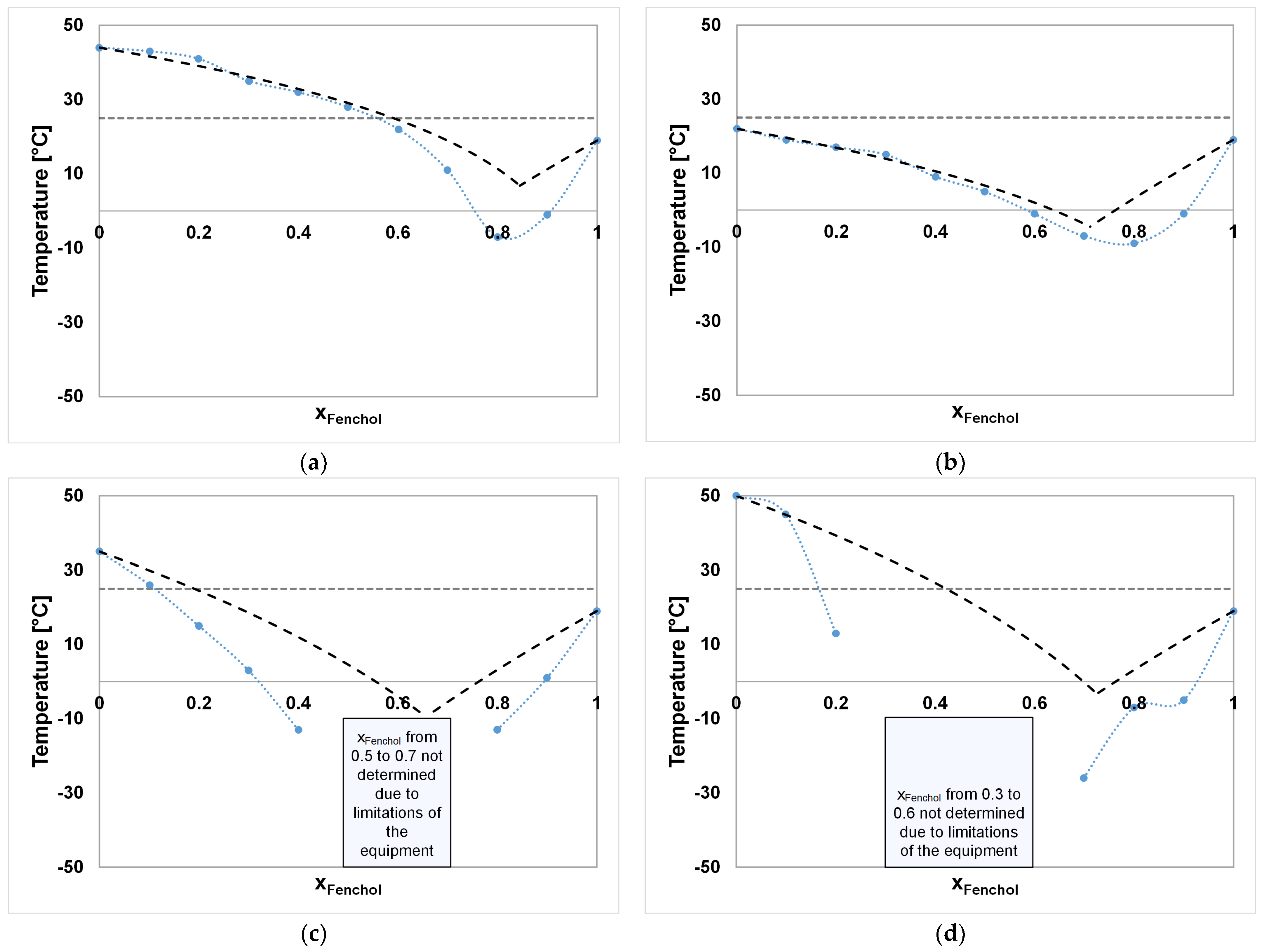


| System A–B | Component A | Component B |
|---|---|---|
| CA–DA | Camphene (CA) | Dodecanoic Acid (DA) |
| CA–DO | Camphene (CA) | 1-Dodecanol (DO) |
| CA–ME | Camphene (CA) | L-Menthol (ME) |
| CA–TH | Camphene (CA) | Thymol (TH) |
| CA–FE | Camphene (CA) | Fenchol (FE) |
| FE–DA | Fenchol (FE) | Dodecanoic Acid (DA) |
| FE–DO | Fenchol (FE) | 1-Dodecanol (DO) |
| FE–ME | Fenchol (FE) | L-Menthol (ME) |
| FE–TH | Fenchol (FE) | Thymol (TH) |
| System A–B | Ideal SLE xA [ ] | Ideal SLE Tm [°C] | Experimental SLE xA [ ] | Experimental SLE Tm [°C] |
|---|---|---|---|---|
| CA–DA | 0.78 | 13.00 | 0.85 | ≤15 |
| CA–DO | 0.64 | −0.33 | 0.70 | ≤7 |
| CA–ME | 0.60 | −4.40 | 0.60 | −15 |
| CA–TH | 0.67 | 2.73 | 0.60 | −15 |
| CA–FE | 0.46 | −20.81 | 0.45 | ≤−37.5 |
| FE–DA | 0.84 | 6.85 | 0.80 | −7 |
| FE–DO | 0.71 | −4.49 | 0.80 | −9 |
| FE–ME | 0.66 | −9.52 | n.a. | n.a. |
| FE–TH | 0.73 | −3.28 | n.a. | n.a. |
| Solvent A:B | Composition xA:xB | Solvent * | Composition xA:xB |
|---|---|---|---|
| CA:DA | 0.85:0.15 | n.a. | n.a. |
| CA:DO | 0.70:0.30 | CA:DO * | 0.40:0.60 |
| CA:ME | 0.60:0.40 | CA:ME * | 0.42:0.58 |
| CA:TH | 0.60:0.40 | CA:TH * | 0.80:0.20 |
| CA:FE | 0.44:0.56 | CA:FE * | 0.80:0.20 |
| FE:DA | 0.80:0.20 | FE:DA * | 0.70:0.30 |
| FE:DO | 0.80:0.20 | FE:DO * | 0.40:0.60 |
| TH:ME | 0.50:0.50 [36] | n.a. | n.a. |
| Solvent | xA:xB | ρ [g cm−3] | η [mPas] | ν [mm2 s−1] |
|---|---|---|---|---|
| CA:DA | 0.85:0.15 | 0.868 ± 0.000 | 3.136 ± 0.006 | 3.612 ± 0.006 |
| CA:DO | 0.70:0.30 | 0.850 ± 0.001 | 4.454 ± 0.023 | 5.238 ± 0.025 |
| CA:DO * | 0.40:0.60 | 0.840 ± 0.001 | 8.476 ± 0.035 | 10.085 ± 0.039 |
| CA:ME | 0.60:0.40 | 0.876 ± 0.000 | 4.894 ± 0.011 | 5.585 ± 0.011 |
| CA:ME * | 0.42:0.58 | 0.881 ± 0.000 | 8.944 ± 0.074 | 10.147 ± 0.083 |
| CA:TH | 0.60:0.40 | 0.905 ± 0.000 | 3.436 ± 0.002 | 3.796 ± 0.002 |
| CA:TH * | 0.80:0.20 | 0.884 ± 0.001 | 2.526 ± 0.011 | 2.867 ± 0.020 |
| CA:FE | 0.44:0.56 | 0.913 ± 0.000 | 8.465 ± 0.068 | 9.269 ± 0.074 |
| CA:FE * | 0.80:0.20 | 0.882 ± 0.000 | 3.067 ± 0.019 | 3.479 ± 0.022 |
| FE:DA | 0.80:0.20 | 0.937 ± 0.001 | 34.617 ± 0.813 | 36.944 ± 0.846 |
| FE:DA * | 0.70:0.30 | 0.929 ± 0.000 | 29.514 ± 0.396 | 31.765 ± 0.428 |
| FE:DO | 0.80:0.20 | 0.923 ± 0.001 | 31.801 ± 0.532 | 34.466 ± 0.551 |
| FE:DO * | 0.40:0.60 | 0.871 ± 0.000 | 21.131 ± 0.124 | 24.257 ± 0.135 |
| TH:ME | 0.50:0.50 | 0.933 ± 0.000 | 34.741 ± 0.32 | 37.252 ± 0.343 |
| Solvent | xA:xB | EE_HYD [%] | EE_VAN [%] | EE_SYR [%] |
|---|---|---|---|---|
| CA:DA | 0.85:0.15 | 8.43 ± 0.13 | 44.59 ± 0.36 | 26.50 ± 0.21 |
| CA:DO | 0.70:0.30 | 81.48 ± 0.32 | 79.51 ± 0.35 | 66.90 ± 0.64 |
| CA:DO * | 0.40:0.60 | 89.80 ± 0.29 | 87.81 ± 0.63 | 80.03 ± 1.44 |
| CA:ME | 0.60:0.40 | 84.15 ± 0.50 | 81.26 ± 0.67 | 68.83 ± 1.23 |
| CA:ME * | 0.42:0.58 | 90.07 ± 0.19 | 87.14 ± 0.32 | 77.75 ± 0.73 |
| CA:TH | 0.60:0.40 | 71.92 ± 0.26 | >95.00 | >93.00 |
| CA:TH * | 0.80:0.20 | 42.69 ± 0.49 | 90.50 ± 0.11 | >93.00 |
| CA:FE | 0.44:0.56 | 85.85 ± 0.40 | 89.09 ± 0.31 | 84.60 ± 0.21 |
| CA:FE * | 0.80:0.20 | 55.55 ± 1.12 | 71.77 ± 0.85 | 59.73 ± 0.00 |
| FE:DA | 0.80:0.20 | 88.42 ± 0.12 | 90.71 ± 0.01 | 87.25 ± 0.41 |
| FE:DA * | 0.70:0.30 | 84.86 ± 0.19 | 88.31 ± 0.38 | 85.03 ± 0.79 |
| FE:DO | 0.80:0.20 | 93.08 ± 0.05 | 93.37 ± 0.10 | 90.75 ± 0.32 |
| FE:DO * | 0.40:0.60 | 92.49 ± 0.14 | 91.35 ± 0.15 | 86.92 ± 0.07 |
| TH:ME | 0.50:0.50 | 92.61 ± 0.16 | >95.00 | >93.00 |
| Solvent A:B | xA:xB | AA:AB before Extraction | AA:AB after Extraction | Absolute Deviation [%] | Preferably Dissolved Component: |
|---|---|---|---|---|---|
| CA:DA | 0.85:0.15 | 14.66 | 14.30 | 2.47 | CA |
| CA:DO | 0.70:0.30 | 2.75 | 2.78 | <1 | DO |
| CA:DO * | 0.40:0.60 | 0.79 | 0.80 | 1.67 | DO |
| CA:ME | 0.60:0.40 | 1.79 | 1.89 | 5.55 | ME |
| CA:ME * | 0.42:0.58 | 0.76 | 0.80 | 5.49 | ME |
| CA:TH | 0.60:0.40 | 1.80 | 1.80 | <0.5 | - |
| CA:TH * | 0.80:0.20 | 5.27 | 5.16 | 2.07 | CA |
| CA:FE | 0.44:0.56 | 0.99 | 1.01 | 2.19 | FE |
| CA:FE * | 0.80:0.20 | 5.19 | 5.22 | <1 | FE |
| FE:DA | 0.80:0.20 | 6.91 | 6.57 | 4.91 | FE |
| FE:DA * | 0.70:0.30 | 3.83 | 3.50 | 8.63 | FE |
| FE:DO | 0.80:0.20 | 4.28 | 4.28 | <0.5 | - |
| FE:DO * | 0.40:0.60 | 0.70 | 0.70 | <0.5 | - |
| TH:ME | 0.50:0.50 | 0.99 | 0.99 | <0.5 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaufmann, A.; Häcker, L.; Mayer, J.M.; Weber, H.; Kienberger, M. Characterization of Camphene- and Fenchol-Based Hydrophobic Eutectic Solvents and Their Application in Aldehyde Extraction. Molecules 2024, 29, 4232. https://doi.org/10.3390/molecules29174232
Kaufmann A, Häcker L, Mayer JM, Weber H, Kienberger M. Characterization of Camphene- and Fenchol-Based Hydrophobic Eutectic Solvents and Their Application in Aldehyde Extraction. Molecules. 2024; 29(17):4232. https://doi.org/10.3390/molecules29174232
Chicago/Turabian StyleKaufmann, Alexander, Lars Häcker, Jacob Michael Mayer, Hansjörg Weber, and Marlene Kienberger. 2024. "Characterization of Camphene- and Fenchol-Based Hydrophobic Eutectic Solvents and Their Application in Aldehyde Extraction" Molecules 29, no. 17: 4232. https://doi.org/10.3390/molecules29174232
APA StyleKaufmann, A., Häcker, L., Mayer, J. M., Weber, H., & Kienberger, M. (2024). Characterization of Camphene- and Fenchol-Based Hydrophobic Eutectic Solvents and Their Application in Aldehyde Extraction. Molecules, 29(17), 4232. https://doi.org/10.3390/molecules29174232







