Experimental Study for the Sorption and Diffusion of Supercritical Carbon Dioxide into Polyetherimide
Abstract
:1. Introduction
2. Results
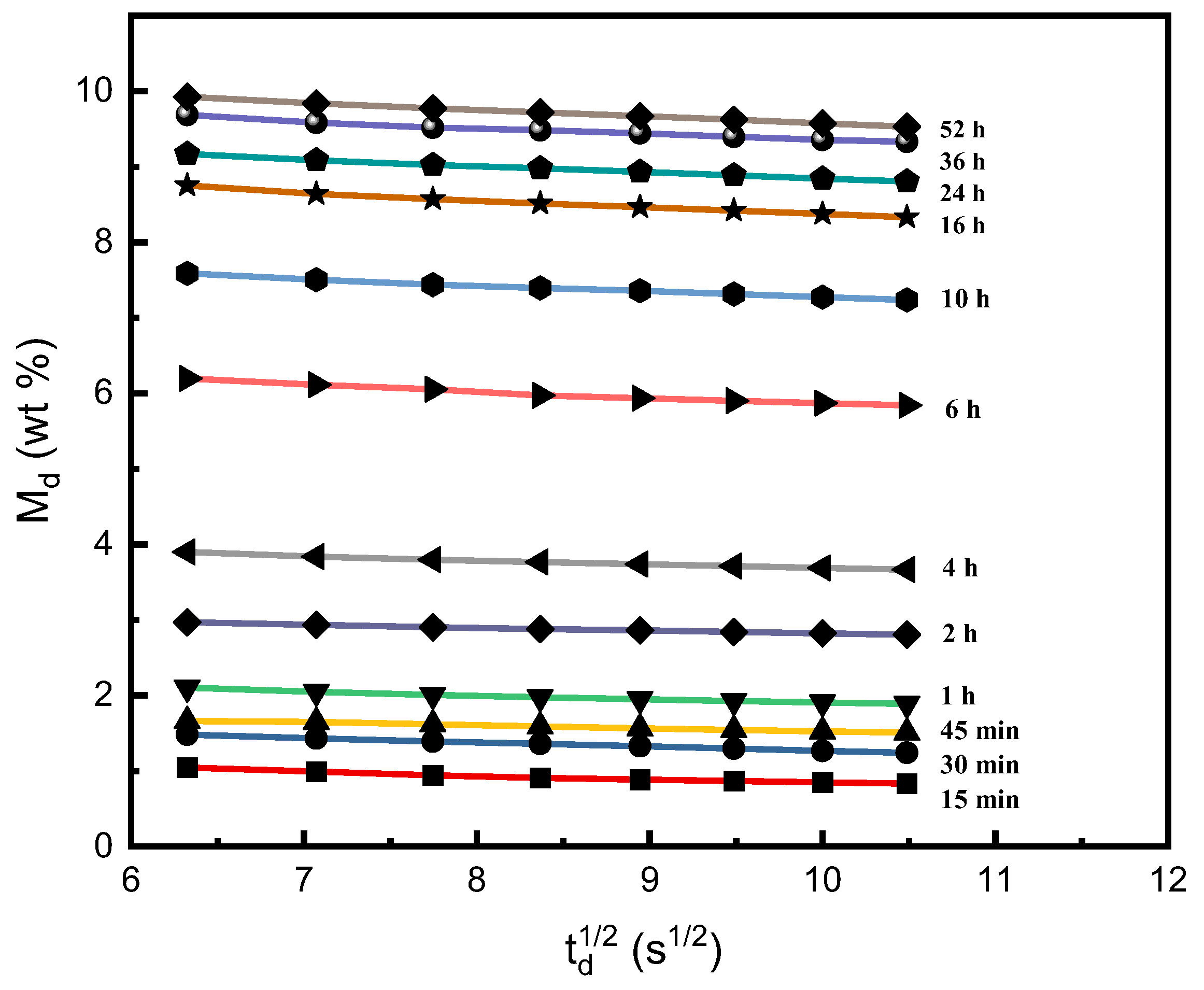
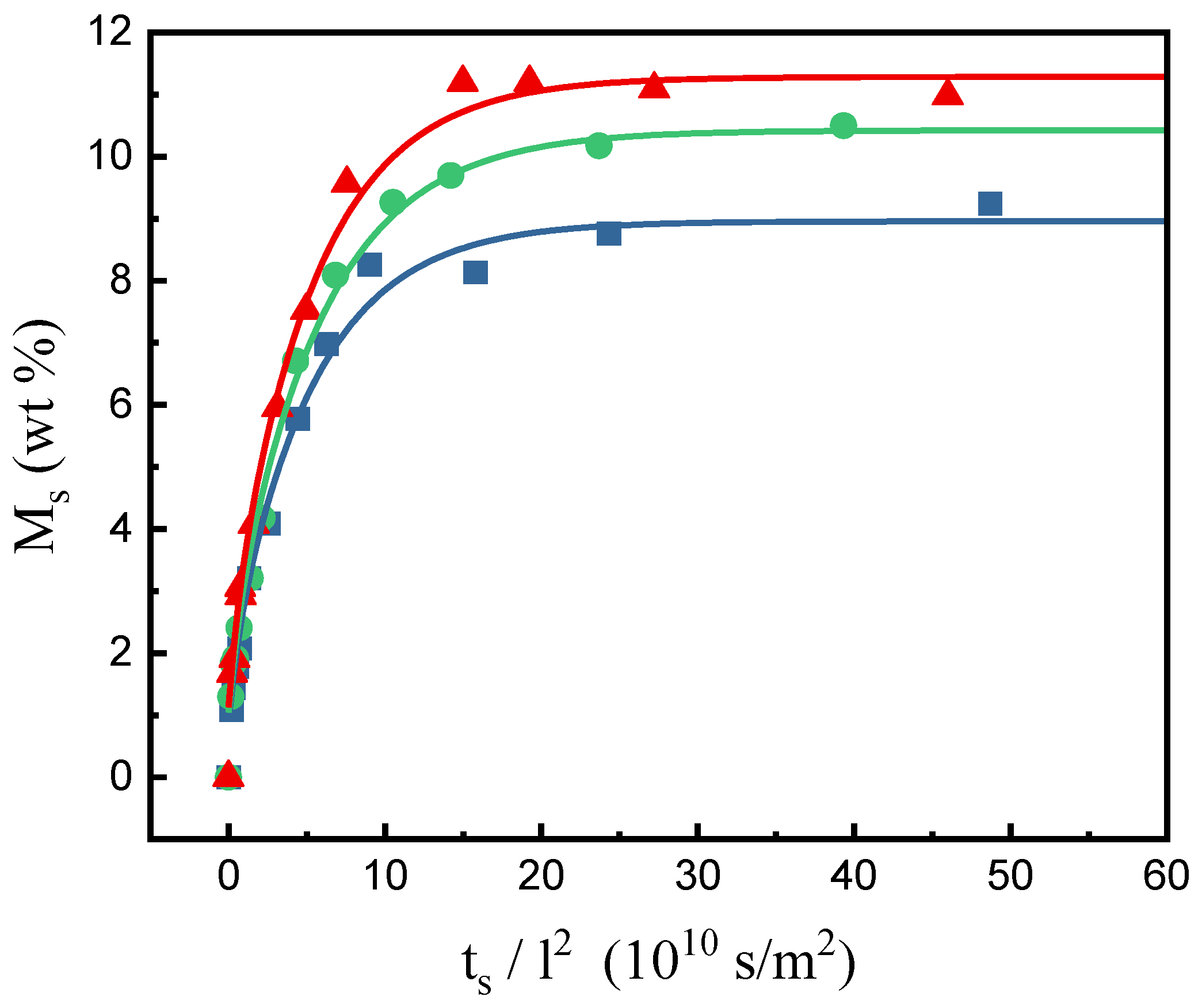
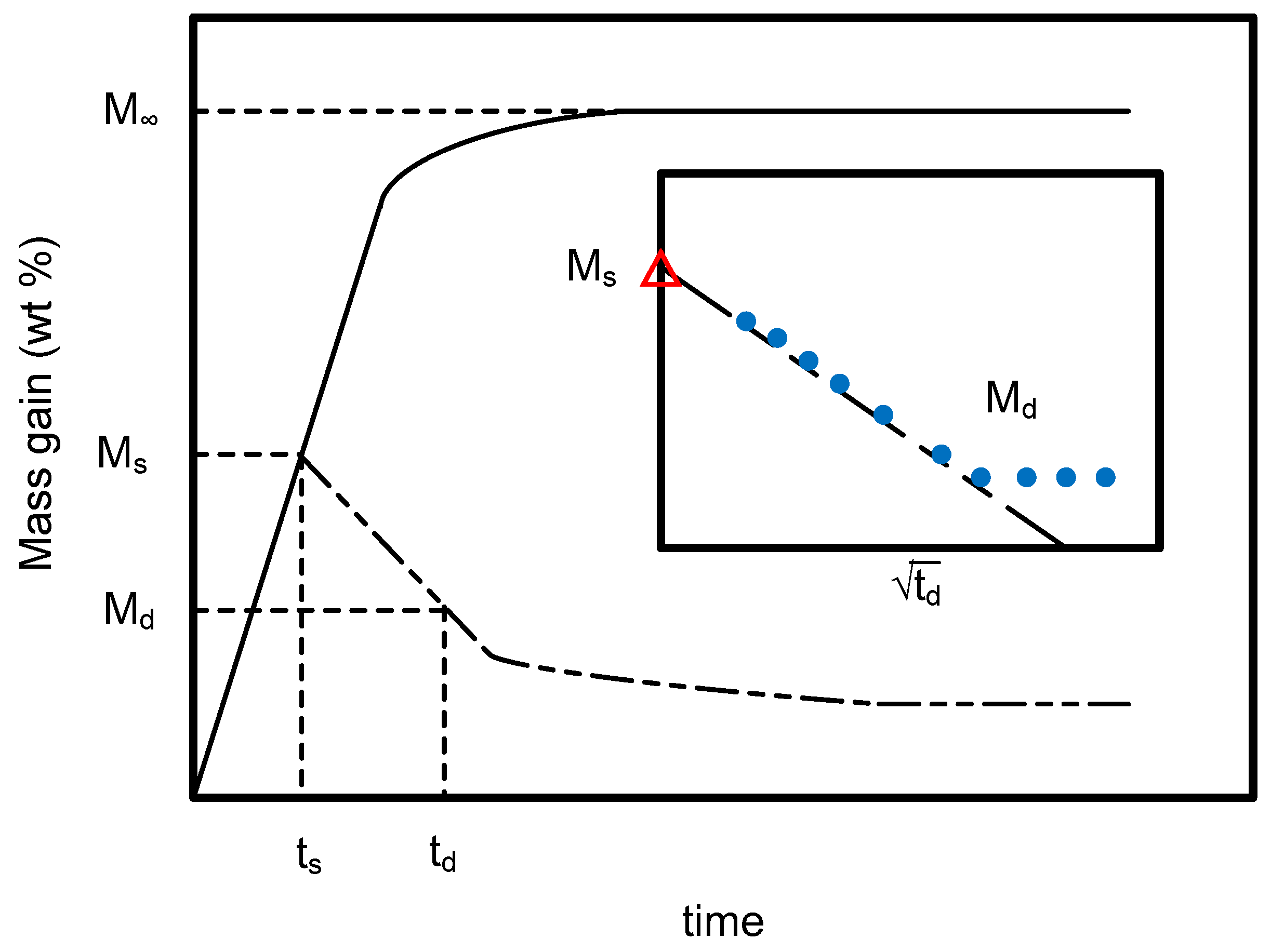
2.1. Determination of the Sorption and Desorption Diffusivities of SCCO2 in PEI
2.2. The Sorption Mechanism of SCCO2 in PEI
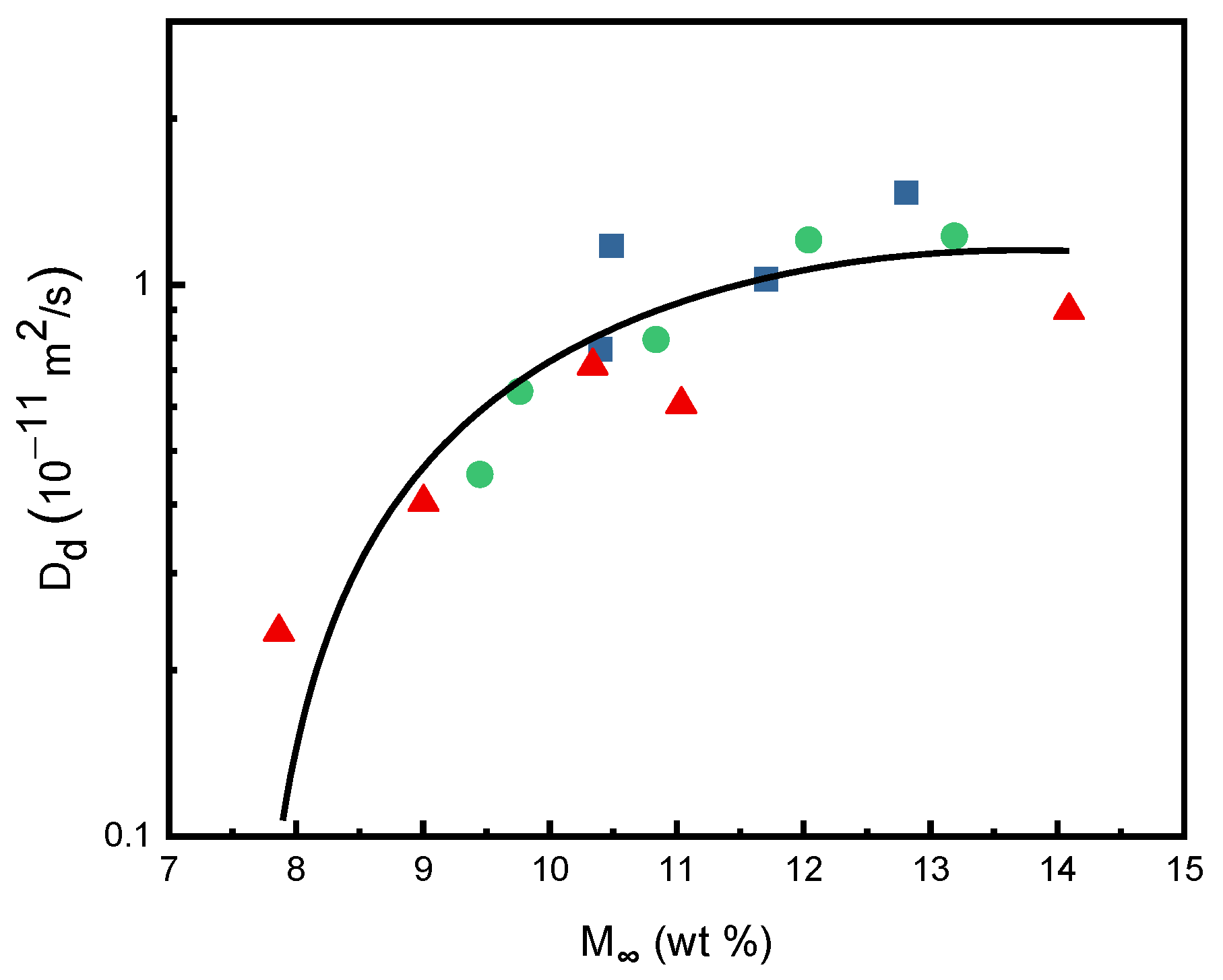
2.3. Comparison of SCCO2 Diffusivities in Various Polymers
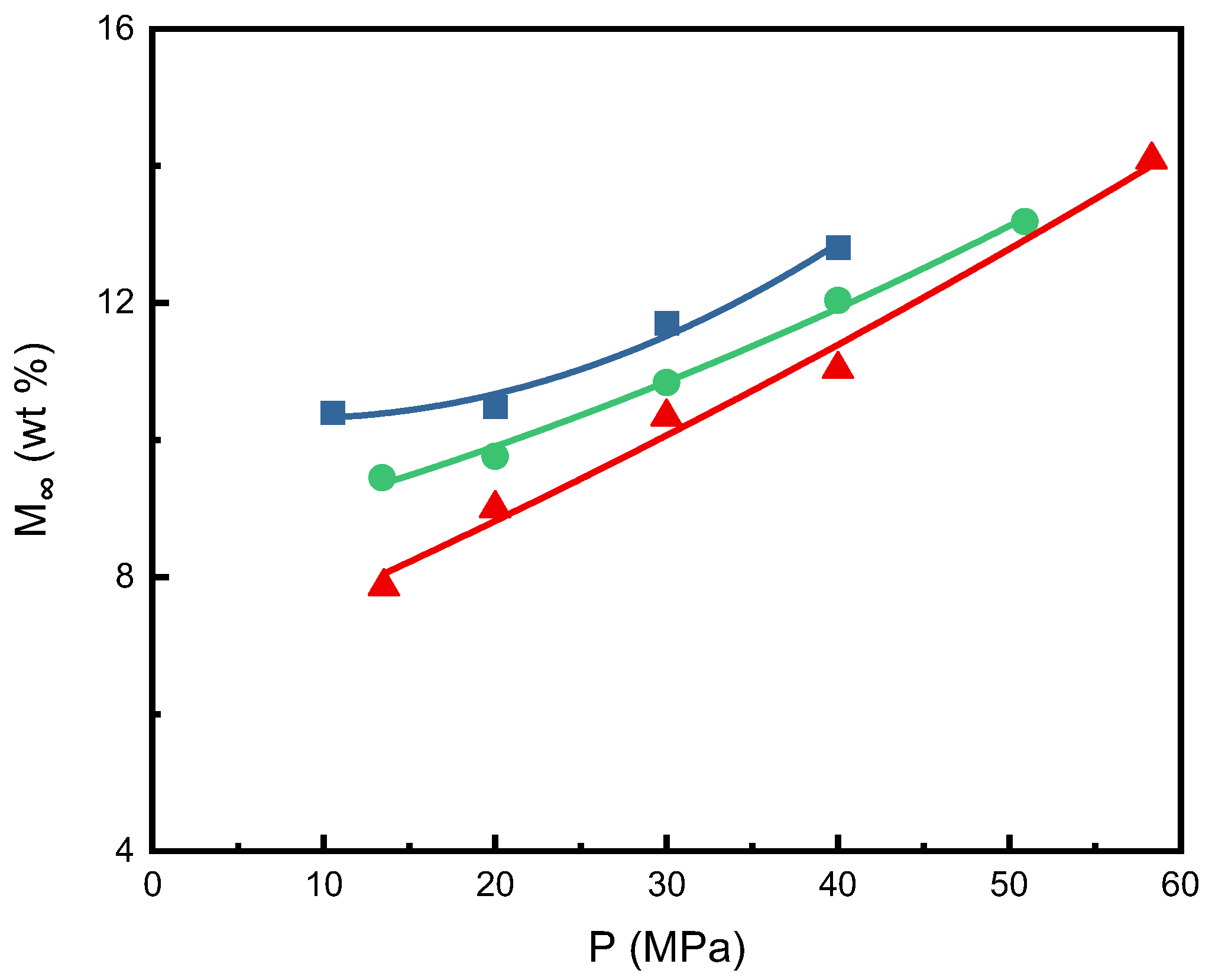
2.4. Plasticization Effect for the Sorption of SCCO2 in PEI
3. Materials and Methods
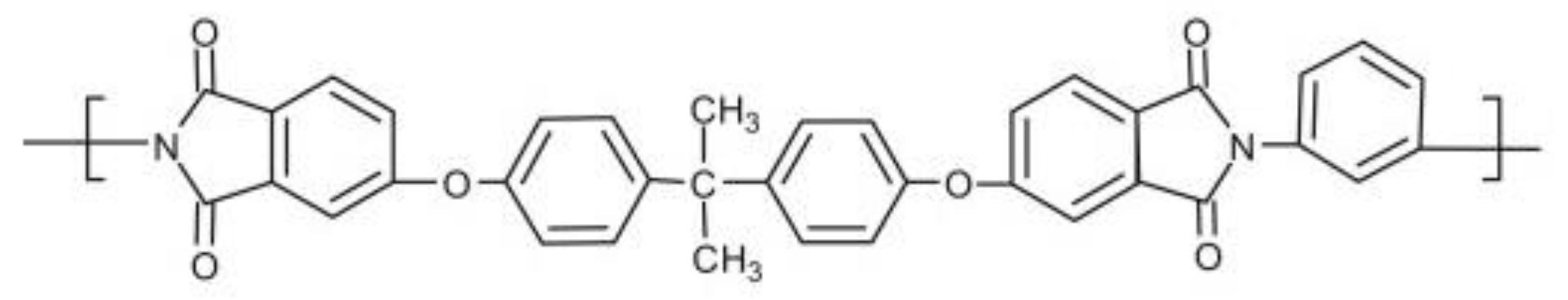
3.1. Material
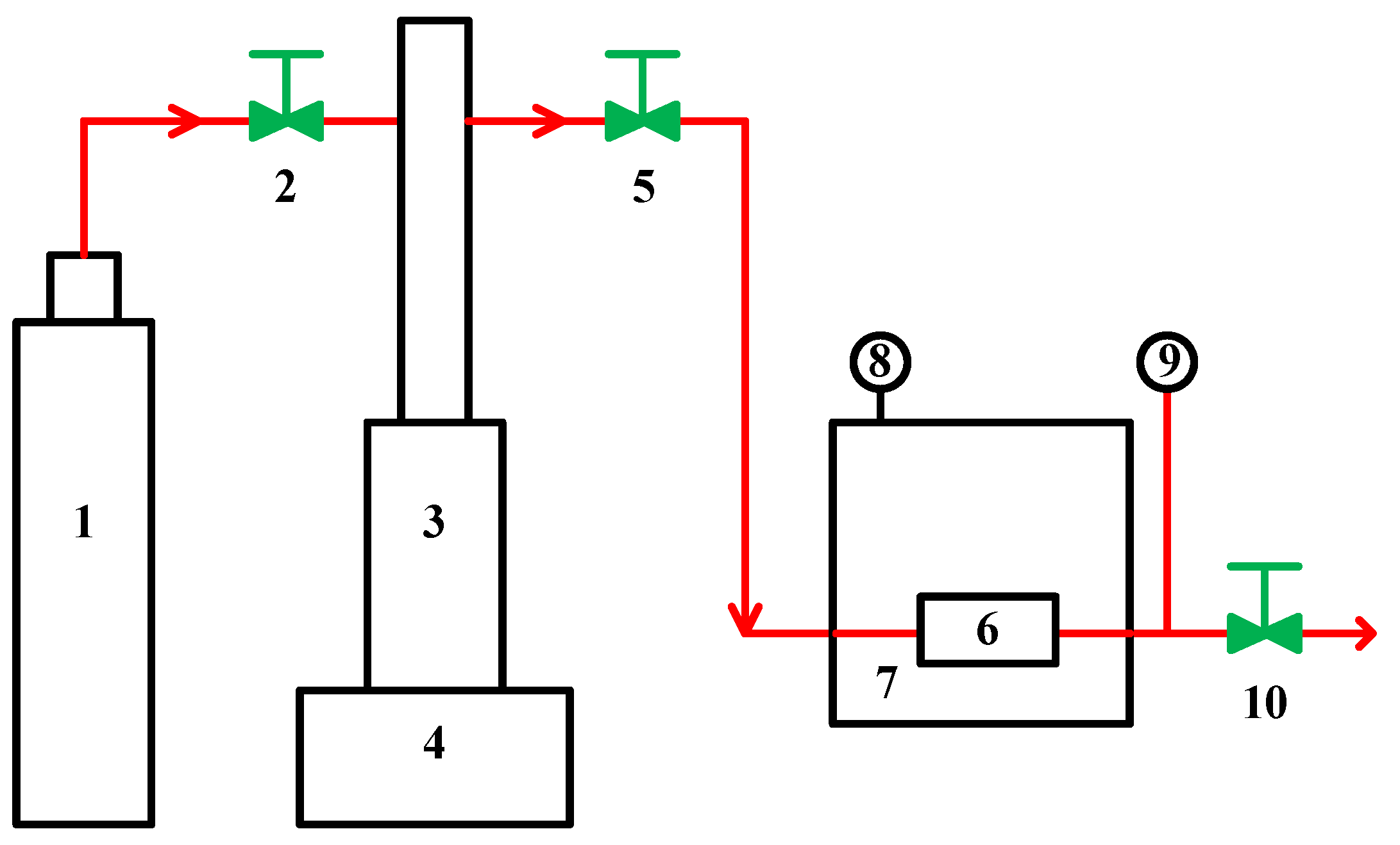
3.2. Apparatus and Procedures
3.3. Data Analysis Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensitieri, G.; Scherillo, G.; La Manna, P.; Musto, P. Sorption thermodynamics of CO2, H2O, and CH3OH in a glassy polyetherimide: A molecular perspective. Membranes 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary perspective: Polymers and mixed matrix membranes for gas and vapor separation: A review and prospective opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Edebali, S. (Ed.) Advanced Sorption Process Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Machado, N.D.; Mosquera, J.E.; Raquel, E.; Martini, R.E.; Goňi, M.L.; Nicol’as, A.; Gaňán, N.A. Supercritical CO2-assisted impregnation/deposition of polymeric materials with pharmaceutical, nutraceutical, and biomedical applications: A review (2015–2021). J. Supercrit. Fluids 2022, 191, 105763. [Google Scholar] [CrossRef]
- Sattari, A.; Ramazani, A.; Aghahosseini, H.; Aroua, M.K. The application of polymer containing materials in CO2 capturing via absorption and adsorption methods. J. CO2 Util. 2021, 48, 101526. [Google Scholar] [CrossRef]
- Ansaloni, L.; Alcock, B.; Peters, T.A. Effects of CO2 on polymeric materials in the CO2 transport chain: A review. Int. J. Greenh. Gas Control. 2020, 94, 102930. [Google Scholar] [CrossRef]
- Kang, X.; Mao, L.; Shi, J.; Liu, Y.; Zha, B.; Xu, J.; Yuzhou Jiang, Y.; Lichtfouse, E.; Jin, H.; Guo, L. Supercritical carbon dioxide systems for sustainable and efcient dissolution of solutes: A review. Environ. Chem. Lett. 2024, 22, 815–839. [Google Scholar] [CrossRef]
- Prasad, S.K.; Sangwai, J.S.; Byun, H.-S. A review of the supercritical CO2 fluid applications for improved oil and gas production and associated carbon storage. J. CO2 Util. 2023, 72, 102479. [Google Scholar] [CrossRef]
- Sarver, J.A.; Kiran, E. Foaming of polymers with carbon dioxide—The year-in-review—2019. J. Supercrit. Fluids 2021, 173, 105166. [Google Scholar] [CrossRef]
- Neděla, O.; Slepčka, P.; Švorčík, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef]
- Khongnakorn, W.; Bootluck, W.; Jutaporn, P. Surface modification of FO membrane by plasma-grafting polymerization to minimize protein fouling. J. Water Process Eng. 2020, 38, 101633. [Google Scholar] [CrossRef]
- Liu, Z.; Song, L.; Dai, X.; Yang, G.; Han, B.; Xu, J. Grafting of methyl methylacrylate onto isotactic polypropylene film using supercritical CO2 as a swelling agent. Polymer 2002, 43, 1183–1188. [Google Scholar] [CrossRef]
- He, T.; He, J.; Wang, Z.; Cui, Z. Modification strategies to improve the membrane hemocompatibility in extracorporeal membrane oxygenator (ECMO). Adv. Compos. Mater. 2021, 4, 847–864. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Iun, C.-P.; Tsai, J.-C.; Tang, M. Grafting of 2-hydroxyethyl methacrylate onto polyacrylonitrile using supercritical carbon dioxide. J. Supercrit. Fluids 2022, 186, 105589. [Google Scholar] [CrossRef]
- Ricci, E.; De Angelis, M.G.; Minelli, M. A comprehensive theoretical framework for the sub and supercritical sorption and transport of CO2 in polymers. Chem. Eng. J. 2022, 435, 135013. [Google Scholar] [CrossRef]
- Wang, D.; Cai, Z.; Huang, X.; Wang, L. Study on the dissolution and diffusion of supercritical carbon dioxide in polystyrene melts based on adsorption and diffusion mechanism. ACS Omega 2021, 6, 1971–1984. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Martini, R.E.; Strumia, M.C. Mass transfer kinetics and diffusion coefficient estimation of bioinsecticide terpene ketones in LDPE films obtained by supercritical CO2-assisted impregnation. J. Appl. Polym. Sci. 2017, 134, 45558. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, G.; Shi, X.; Yang, Q.; Li, J.; Liu, Y.; Fan, X. Microcellular foaming of poly(phenylene sulfide)/poly(ether sulfones) blends using supercritical carbon dioxide. J. Appl. Polym. Sci. 2015, 132, 42634. [Google Scholar] [CrossRef]
- Burges, S.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Lin, S.; Yang, J.; Yan, J.; Zhao, Y.; Yang, B. Sorption and diffusion of supercritical carbon dioxide in a biodegradable polymer. J. Macromol. Sci. Phys. 2010, 49, 286–300. [Google Scholar] [CrossRef]
- Zhao, J.J.; Zhao, Y.P.; Yang, B. Investigation of sorption and diffusion of supercritical carbon dioxide in polycarbonate. J. Appl. Polym. Sci. 2008, 109, 1661–1666. [Google Scholar] [CrossRef]
- Pantoula, M.; Panayitou, C. Sorption and swelling in glassy polymer/carbon dioxide systems: Part I. Sorption. J. Supercrit. Fluids 2006, 37, 254–262. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Martins, C.; Coimbra, P.; Gil, M.H.M.; de Sousa, H.C.; Duarte, C.M.M. Sorption and diffusion of dense carbon dioxide in a biocompatible polymer. J. Supercrit. Fluids 2006, 38, 392–398. [Google Scholar] [CrossRef]
- Tang, M.; Du, T.B.; Chen, Y.P. Sorption and diffusion of supercritical carbon dioxide in polycarbonate. J. Supercrit. Fluids 2004, 28, 207–218. [Google Scholar] [CrossRef]
- Tang, M.; Huang, Y.C.; Chen, Y.P. Sorption and diffusion of supercritical carbon dioxide into polysulfone. J. Appl. Polym. Sci. 2004, 94, 474–482. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Li, H.; Liu, D.; Han, X.; Wingert, M.J.; Lee, L.J.; Koelling, K.W. A review of CO2 applications in the processing of polymers. Ind. Eng. Chem. Res. 2003, 42, 6431–6456. [Google Scholar] [CrossRef]
- Seifert, B.; Mihanetzis, G.; Groth, T.; Albrecht, W.; Richau, K.; Missirlis, Y.; Paul, D.; von Sengbrusch, G. Polyetherimide: A new membrane-forming polymer for biomedical applications. Artif. Organs 2002, 26, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Li, L.; Wang, Q. Fabrication of three-dimensional polyetherimide bead foams via supercritical CO2/ethanol cofoaming technology. RSC Adv. 2019, 9, 4072–4081. [Google Scholar] [CrossRef]
- Aher, B.; Olson, N.M.; Kumar, V. Production of bulk solid-state PEI nanofoams using supercritical CO2. J. Mater. Res. 2013, 28, 2366–2373. [Google Scholar] [CrossRef]
- Zhou, C.; Vaccaro, N.; Sundarram, S.S.; Li, W. Fabrication and characterization of polyetherimide nanofoams using supercritical CO2. J. Cell. Plast. 2012, 48, 239–255. [Google Scholar] [CrossRef]
- Liu, W.; Ma, J.; Wang, D.; Wang, P.; Zhao, J.; Wenzheng Wu, W.; Song, W. Performance modulation and 3D printing parameters optimization of implantable medical tricalcium-silicate/polyetherimide composite. Ceram. Int. 2021, 47, 10679–10687. [Google Scholar] [CrossRef]
- Crank, J. Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK; Elsevier: Amsterdam, The Netherlands, 1975. [Google Scholar]
- NIST Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C124389 (accessed on 20 August 2024).
- Brantley, N.H.; Kazarian, S.G.; Eckert, C.A. In situ FTIR measurement of carbon dioxide sorption into poly(ethylene terephthalate) at elevated pressures. J. Apply. Polym. Sci. 2000, 77, 764–775. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L.; Eckert, C.A. Specific inter-molecular interaction of carbon dioxide with polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Nelson, M.R.; Borkman, R.F. Ab initio calculations on CO2 binding to carbonyl groups. J. Phys. Chem. 1998, 102, 7860–7863. [Google Scholar] [CrossRef]
- Muth, O.; Hirth, T.; Vogel, H. Investigation of sorption and diffusion of supercritical carbon dioxide into poly(vinyl chloride). J. Supercrit. Fluids 2001, 19, 299–306. [Google Scholar] [CrossRef]
- Bos, A.; Punt, I.G.M.; Wessling, M. CO2-induced plasticization phenomena in glassy polymers. J. Membr. Sci. 1999, 155, 67–78. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Su, J.H.; Manivannan, G.; Lee, P.H.C.; Sawan, S.P.; Spall, W.D. Interaction of supercritical carbon dioxide with polymers. II. Amorphous polymers J. Apply. Polym. Sci. 1996, 59, 707–717. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Hao, J.; Fujita, S.-I.; Arai, M.; Wu, Z.; Zhao, F. Theoretical study on interaction between CO2 and carbonyl compounds: Influence of CO2 on infrared spectroscopy and activity of C=O. J. Supercrit. Fluids 2010, 54, 9–15. [Google Scholar] [CrossRef]
- Yuan, Y.; Teja, A.S. Quantification of specific interactions between CO2 and the carbonyl group in polymers via ATR-FTIR measurements. J. Supercrit. Fluids 2011, 56, 208–212. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Ewing, A.V.; Andrey, M.; Chibiryaev, A.M.; Alexander, M.; Agafontsev, A.M.; Konstantin, A.; Dubkov, K.A.; Kazarian, S.G. New insights into the mechanism of interaction between CO2 and polymers from thermodynamic parameters obtained by in situ ATR-FTIR spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 6465–6475. [Google Scholar] [CrossRef]
- von Schnitzler, J.; Eggers, R. Mass transfer in polymers in a supercritical CO2-atmosphere. J. Supercrit. Fluids 1999, 16, 81–92. [Google Scholar] [CrossRef]
- Pierleon, D.; Minelli, M.; Scherillo, G.; Mensitieri, G.; Loianno, W.; Bonavolonta, F.; Doghieri, F. Analysis of a polystyrene—Toluene system through “dynamic” sorption tests: Glass transition and retrograde vitrification. J. Phys. Chem. B 2017, 121, 9969–9981. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.H.; Ryu, Y.; Cha, S.W. Modeling and experiment for the diffusion coefficient of subcritical carbon dioxide in poly(methyl methacrylate) to predict gas sorption and desorption. Polymers 2022, 14, 596. [Google Scholar] [CrossRef]
- Kiran, E.; Sarver, J.A.; Hassler, J.C. Solubility and diffusivity of CO2 and N2 in polymers and polymer swelling, glass transition, melting, and crystallization at high pressure: A critical review and perspectives on experimental methods, data, and modeling. J. Supercrit. Fluids 2022, 185, 105378. [Google Scholar] [CrossRef]
- Minelli, M.; Sarti, G.C. 110th Anniversary: Gas and vapor sorption in glassy polymeric membranes-critical review of different physical and mathematical models. Ind. Eng. Chem. Res. 2020, 59, 341–365. [Google Scholar] [CrossRef]
- Wang, L.; Corriou, J.-P.; Castel, C.; Favre, E. Transport of gases in glassy polymers under transient conditions: Limit-behavior investigations of dual-mode sorption theory. Ind. Eng. Chem. Res. 2013, 52, 1089–1101. [Google Scholar] [CrossRef]
- Silva, E.; Fedel, M.; Deflorian, F.; Cotting, F.; Lins, V. Properties of post-consumer polyethylene terephthalate coating mechanically deposited on mild steels. Coatings 2019, 9, 28. [Google Scholar] [CrossRef]
- Song, P.; Trivedi, A.R.; Siviour, C.R. Mechanical response of four polycarbonates at a wide range of strain rates and temperatures. Polym. Test. 2023, 121, 107986. [Google Scholar] [CrossRef]
- Palczynski, K.; Wilke, A.; Paeschke, M.; Dzubiella, J. Molecular modeling of polycarbonate materials: Glass transition and mechanical properties. Phys. Rev. Mater. 2017, 1, 043804. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Zhang, B.; Zhang, T.; Lu, W.; Chen, Z.; Liu, Z.; Kim, S.H.; Donovan, B.; Warzoha, R.J.; et al. High-temperature polymers with record-high breakdown strength enabled by rationally designed chain-packing behavior in blends. Matter 2021, 4, 2448–2459. [Google Scholar] [CrossRef]
- Murakami, K.; Kudo, H. Gamma-rays irradiation effects on polysulfone at high temperature. Nucl. Instrum. Methods Phys. Res. B 2007, 265, 125–129. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mukhtar, H.B.; Shariff, A.M. Effect of Glass Transition Temperature in Enhanced Polymeric Blend Membranes. Procedia Eng. 2016, 148, 11–17. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Ye, L.; Yan, C. Fracture Behavior of Polyetherimide (PEI) and Interlaminar Fracture of CF/PEI Laminates at Elevated Temperatures. Polym. Compos. 2005, 26, 20–28. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.Q.; Huang, P.; Cao, H.-J.; Zeng, W.; Li, J.; Li, F.; Sun, B.-G.; Shi, H.-Q.; Zhou, Z.-L.; et al. Temperature-dependent mechanical properties of polyetherimide composites reinforced by graphene oxide-coated short carbon fibers. Compos. Struct. 2021, 270, 114075. [Google Scholar] [CrossRef]
- Cao, K.; Ma, X.; Zhang, B.; Wang, Y.; Wang, Y. Tensile behavior of polycarbonate over a wide range of strain rates. Mater. Sci. Eng. A 2010, 527, 4056–4061. [Google Scholar] [CrossRef]
- Chukov, D.; Nematulloev, S.; Zadorozhnyy, M.; Victor Tcherdyntsev, V.; Stepashkin, A.; Zherebtsov, D. Structure, mechanical and thermal properties of polyphenylene sulfide and polysulfone impregnated carbon fiber composites. Polymers 2019, 11, 684. [Google Scholar] [CrossRef]
- Xie, X.-L.; Liu, Q.-X.; Li, R.K.-Y.; Zhou, X.-P.; Qing-Xin Zhang, Q.-X.; Yu, Z.-Z.; Mai, Y.-W. Rheological and mechanical properties of PVC/CaCO3 nanocomposites prepared by in situ polymerization. Polymer 2004, 45, 6665–6673. [Google Scholar] [CrossRef]

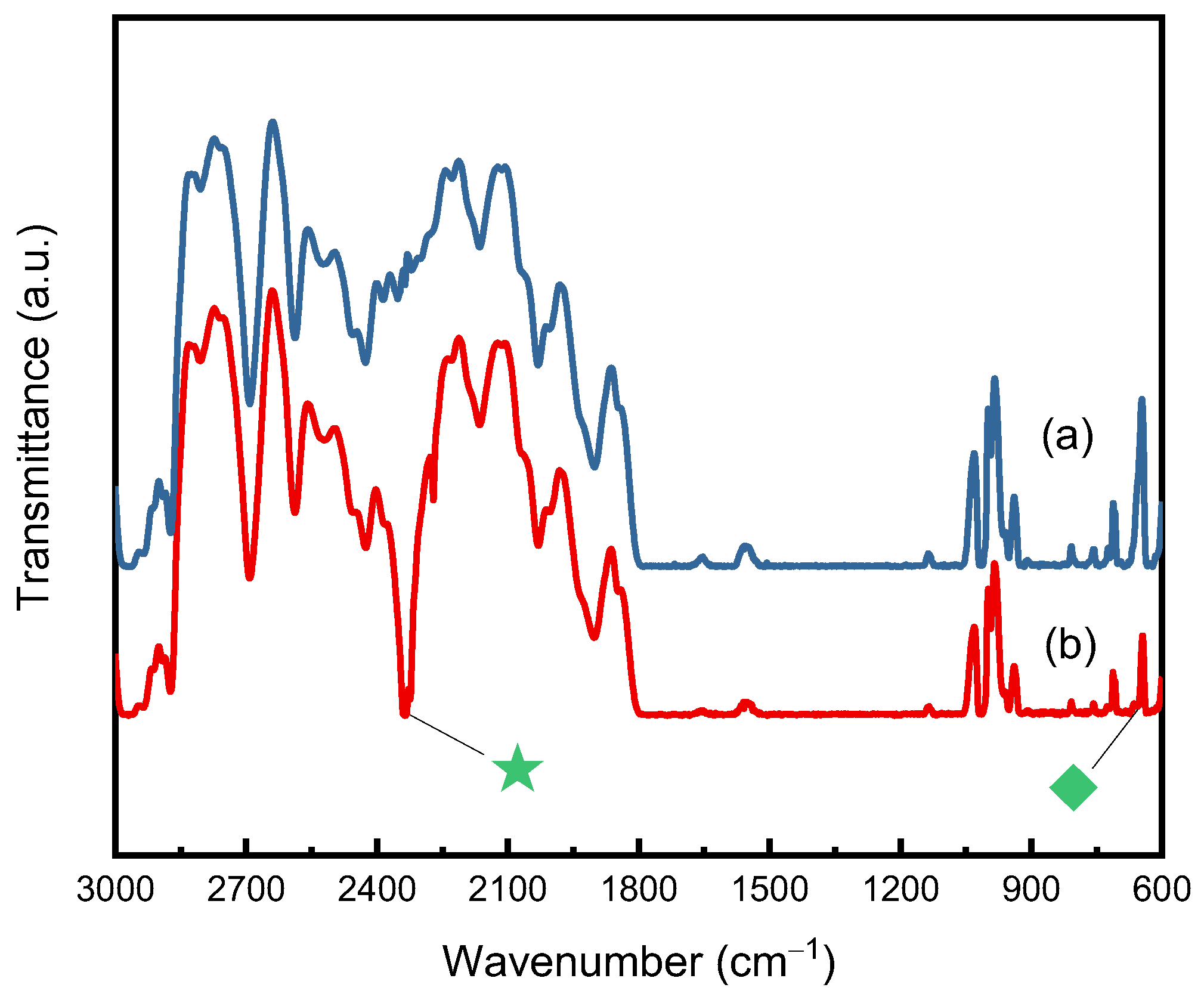
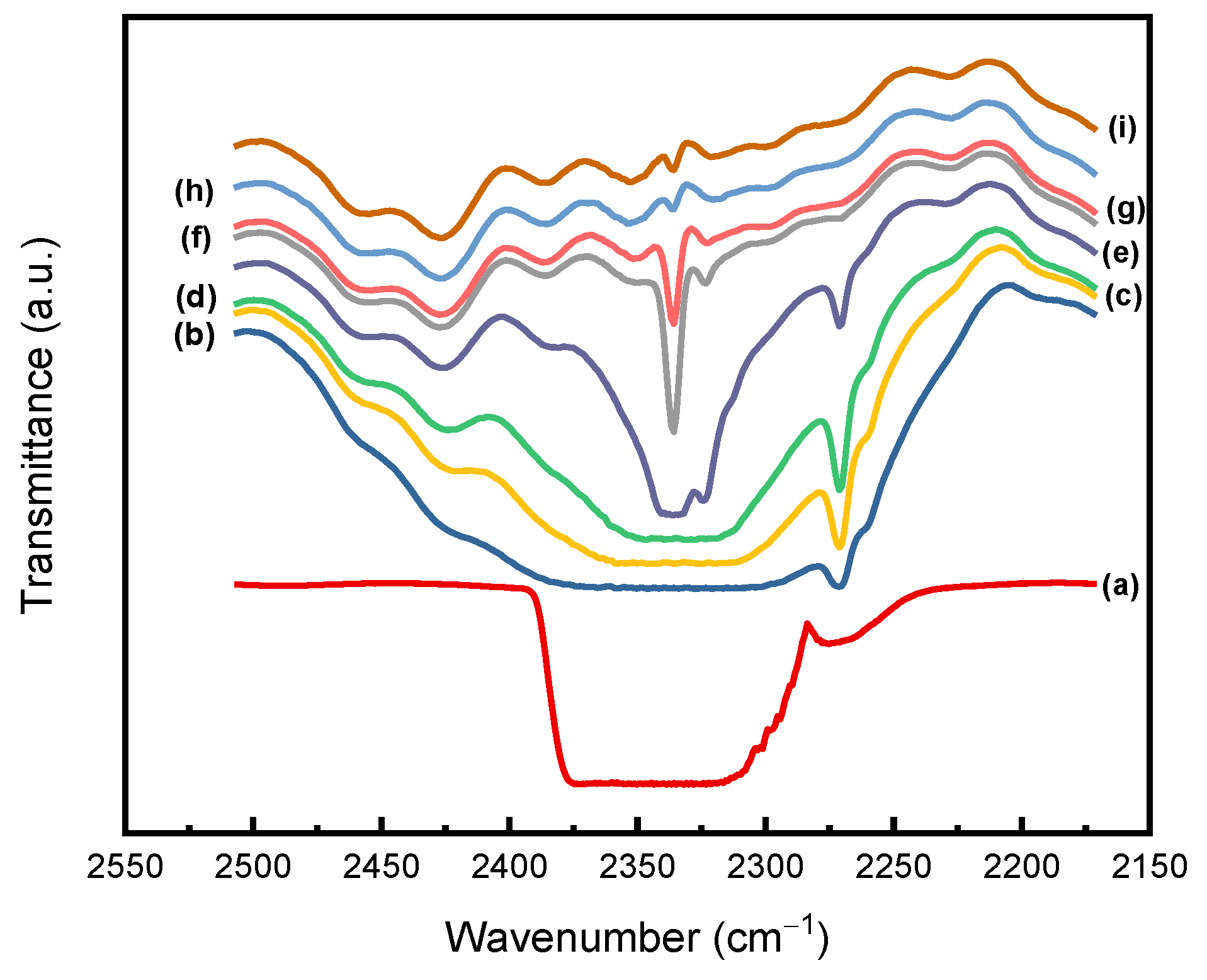

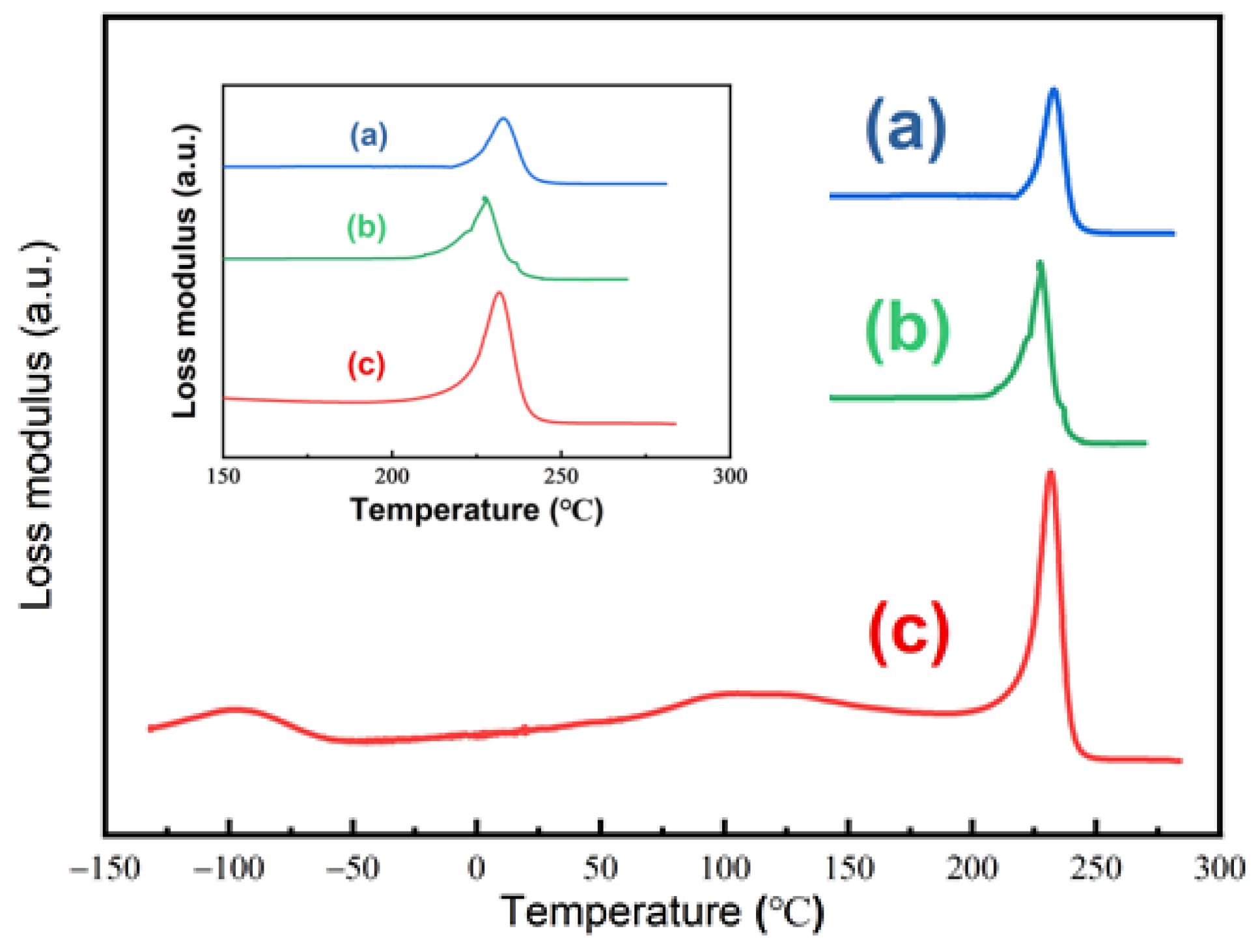
| Pressure (MPa) | Temperature (°C) | SCCO2 Density (kg/m3) | M∞ (wt%) | Dd (10−11m2/s) | Ds (10−11m2/s) |
|---|---|---|---|---|---|
| 20 | 40 | 839.8 | 10.5 | 1.18 | 0.13 |
| 20 | 50 | 784.3 | 9.8 | 0.64 | 0.18 |
| 20 | 60 | 723.7 | 9.0 | 0.40 | 0.30 |
| 30 | 40 | 909.9 | 11.7 | 1.02 | 0.11 |
| 30 | 50 | 870.4 | 10.8 | 0.79 | 0.24 |
| 30 | 60 | 829.7 | 10.3 | 0.71 | 0.22 |
| 40 | 40 | 956.1 | 12.8 | 1.47 | 0.12 |
| 40 | 50 | 923.3 | 12.0 | 1.20 | 0.12 |
| 40 | 60 | 890.1 | 11.0 | 0.61 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-H.; Chen, P.-H.; Chen, C.-W.; Su, C.-S.; Tang, M.; Tsai, J.-C.; Chen, Y.-P.; Lin, F.-H. Experimental Study for the Sorption and Diffusion of Supercritical Carbon Dioxide into Polyetherimide. Molecules 2024, 29, 4233. https://doi.org/10.3390/molecules29174233
Huang W-H, Chen P-H, Chen C-W, Su C-S, Tang M, Tsai J-C, Chen Y-P, Lin F-H. Experimental Study for the Sorption and Diffusion of Supercritical Carbon Dioxide into Polyetherimide. Molecules. 2024; 29(17):4233. https://doi.org/10.3390/molecules29174233
Chicago/Turabian StyleHuang, Wei-Heng, Pei-Hua Chen, Chin-Wen Chen, Chie-Shaan Su, Muoi Tang, Jung-Chin Tsai, Yan-Ping Chen, and Feng-Huei Lin. 2024. "Experimental Study for the Sorption and Diffusion of Supercritical Carbon Dioxide into Polyetherimide" Molecules 29, no. 17: 4233. https://doi.org/10.3390/molecules29174233








