Abstract
Tribolium castaneum is a challenging pest of stored products, causing significant economic losses. The present study explored the efficacy of Coridothymus capitatus essential oil and its primary constituent, carvacrol, as eco-friendly alternatives for managing this pest. To evaluate their insecticidal potential, repellency, fumigant toxicity, and antifeedant properties, progeny inhibition assays were performed. Carvacrol exhibited superior repellency compared to the essential oil, achieving a 92% repellency rate at 2 mg/cm2. Both compounds demonstrated significant fumigant toxicity against T. castaneum, with LC50 values of 168.47 and 106.5 μL/L for the essential oil and carvacrol, respectively, after 24 h. Carvacrol also outperformed the essential oil in antifeedant activity, inducing an 80.7% feeding deterrence at 1.17 mg/g. Moreover, both treatments effectively suppressed the development of the pest’s progeny. These results collectively underscore the potent insecticidal properties of C. capitatus essential oil and carvacrol, particularly carvacrol, as promising candidates for the sustainable management of T. castaneum in stored product protection.
1. Introduction
The red flour beetle, Tribolium castaneum (Herbs), is considered one of the world’s most destructive pests for processed foods and stored products [1]. It has a wide range of target products, including cookies, beans, spices, pasta, cake mixes, dry pet food, dried flowers, chocolate, and almonds [2]. Tribolium castaneum is primarily found in tropical and subtropical regions and causes significant economic losses, posing a challenge to the value chain for grains [2,3]. Both the adults and larvae of this insect feed on broken and finely powdered grains, causing serious damage to stored seeds. These pests have a rapid population growth rate, which could lead to large economic losses if not properly managed [4]. The abdomen glands secrete benzoquinones and emit a large quantity of exuviae throughout their metamorphosis, resulting in unpleasant odors and colors in the products. As a result, it is classified as a pest that must be constantly monitored and managed [2,3].
The management of this pest in stored products has traditionally relied on chemical insecticides, particularly organophosphates, phosphine, and pyrethroids [5]. However, the excessive reliance on these synthetic compounds has resulted in a range of negative consequences. Environmental pollution, escalating treatment costs, and the development of pest resistance have become significant [6]. Moreover, the insect’s tolerance to multiple pesticides, including malathion and phosphine, has further complicated control efforts [7]. To overcome resistance, higher pesticide doses and more frequent applications are often employed, exacerbating environmental contamination, increasing economic burdens, and posing heightened risks to human health [8].
Thus, it is crucial to develop novel insecticides with reduced environmental and human health impacts [9,10]. Plant-derived compounds, characterized by their diverse biological activities, emerge as promising alternatives to synthetic insecticides [11,12]. A shift to plant-based products and their bioactive ingredients is consistent with sustainable development objectives. These botanical insecticides offer the advantages of being environmentally friendly, biodegradable, and are often effective against pest insects [13].
Coridothymus capitatus, also known by several synonyms such as Thymus capitatus and Thymbra capitata, is a plant native to Mediterranean Europe and Turkey. Commonly referred to as conehead thyme, Persian-hyssop, and Spanish oregano, it is widely recognized for its essential oils (EOs), which have proven effectiveness in pest control. The essential oils extracted from these plants are known for their potent antimicrobial, insecticidal, and acaricidal properties, making them valuable in natural pest management strategies [14]. The Thymus genus, to which C. capitatus belongs, is renowned for its diverse biological properties attributed to compounds like carvacrol, thymol, p-cymene, and γ-terpinene, known for their potent antibacterial activity [15]. Several studies have demonstrated the repellent and insecticidal effects of the members of the genus Thymus species against the red flour beetle, Tribolium castaneum. However, a comprehensive comparison of their toxicity, repellency, and fumigant properties remains scarce. Therefore, the main objective of the present study was to assess the insecticidal efficacy of C. capitatus extract and its major component, carvacrol, against T. castaneum.
2. Results
2.1. GC-MS of Coridiothymus capitatus Extract
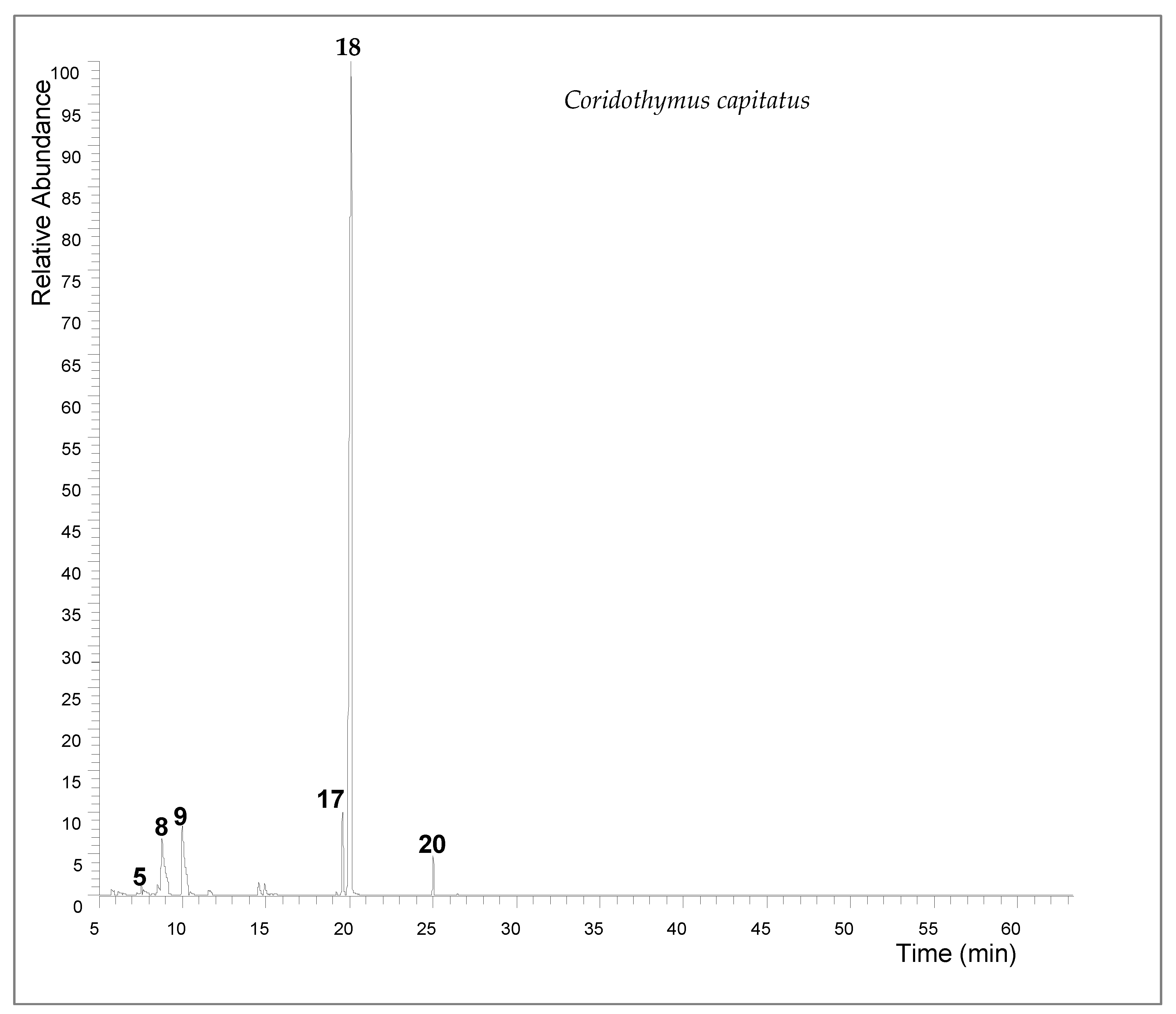
The extraction yield of Coridothymus capitatus essential oil was 2.7% (v/w). Gas chromatographic analysis revealed a complex composition, with carvacrol being the predominant constituent, making up 71.14% of the oil. Other significant components included γ-terpinene (8.3%), p-cymene (7.69%), and thymol (5.02%) (Table 1 and Figure 1).

Table 1.
Chemical composition of the Coridothymus capitatus essential oil.
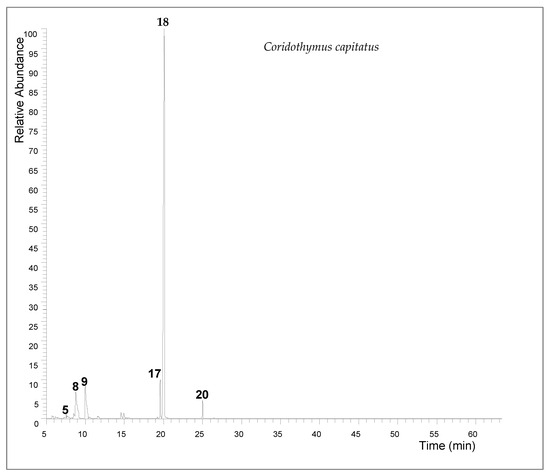
Figure 1.
The total ion chromatogram (TIC) of Coridothymus capitatus essential oil (5 myrcene; 8 p-cymene; 9 γ-terpinene; 17 thymol; 18 carvacrol; 20 β-caryophyllene).
2.2. Repellency Assay
The repellency against T. castaneum was found to be concentration-dependent for both C. capitatus essential oil and carvacrol. Carvacrol exhibited significantly greater repellency than C. capitatus extract (p < 0.001, F = 51.66), while both essential oils showed significantly higher repellency compared to the control (p < 0.001, F = 50.38). At the highest concentration (2 mg/cm2), DEET, carvacrol, and C. capitatus essential oil achieved repellency percentages of 100%, 92%, and 72%, respectively, after three hours. The concentration required to repel 50% of insects (RC50) was 0.42 mg/cm2 for carvacrol and 1.05 mg/cm2 for C. capitatus extract (Table 2).

Table 2.
Repellent effect of C. capitatus essential oil and carvacrol against adults of T. castaneum adults (n = 5).
2.3. Fumigant Toxicity Assay
Both C. capitatus essential oil (F = 312.62, p < 0.001) and carvacrol (F = 165.01, p < 0.001) exhibited highly significant fumigant toxicity against T. castaneum adults compared to untreated controls and the chlorpyrifos treated group. Carvacrol demonstrated significantly greater fumigant efficacy than C. capitatus essential oil. The LC50 values for C. capitatus essential oil and carvacrol were 168.5 mg/L and 106.5 mg/L, respectively (Table 3).

Table 3.
Fumigant toxicity effect of Coridothymus capitatus essential oil and carvacrol against T. castaneum adults (n = 5).
2.4. Effects of Coridothymus capitatus and Carvacrol on F1 Progeny Production of T. castaneum (30–45 Days)
Both C. capitatus essential oil and carvacrol showed potent insecticidal activity against adult Tribolium castaneum, with 24 h LC50 values of 0.53 mg/g for both compounds. The LC90 value was also 0.53 mg/g for C. capitatus essential oil, while for carvacrol, it was slightly higher at 1.17 mg/g (Table 4). Both essential oils significantly inhibited progeny production compared to control treatments (p < 0.001, F = 24,373.3 for C. capitatus and F = 41,339.3 for carvacrol). The complete absence of F1 adults at the LC90 level indicated the sterilizing effects of both compounds. Even at the LC50, C. capitatus and carvacrol demonstrated strong inhibitory effects on progeny production, reducing it by 96% and 98%, respectively (Table 5).

Table 4.
LC50 and LC90 of the Coridothymus capitatus and carvacrol against Tribolium castaneum in wheat grains after 24 h.

Table 5.
The inhibition rates of progeny by the effect of C. capitatus and carvacrol against T. castaneum adults (n = 5).
2.5. Antifeedant Effects (Grains Weight Loss)
Both essential oils exhibited a highly significant antifeedant effect (p < 0.001; F = 514.7) compared to the untreated or chlorpyrifos-treated control insects. When dried wheat grains were treated with LC90 concentrations of C. capitatus and carvacrol, the weight loss decreased from 29.02% to 10.4% and 5.6%, respectively (Table 6). Carvacrol demonstrated a greater feeding deterrent index (FDI%) than C. capitatus, with an FDI% of 80.70% (Table 6).

Table 6.
Antifeedant activity and feeding deterrent index of C. capitatus and carvacrol at LD90 against adult T. castaneum adults (n = 5).
2.6. Residual Toxicity of Carvacrol on Wheat Grains against Adult T. castaneum
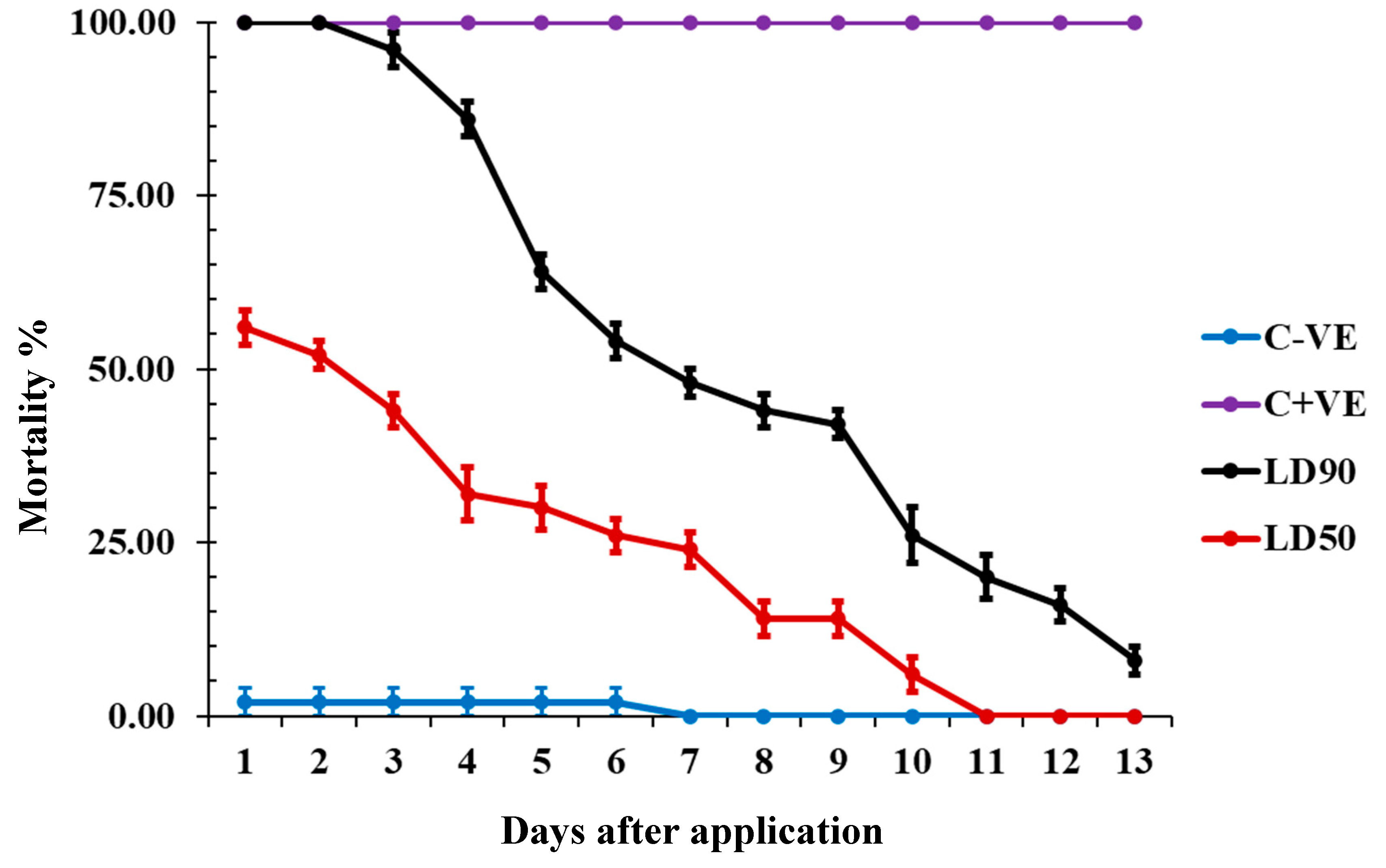
The LD90 and LD50 of carvacrol had distinct effects on insect mortality over time. The LD90 caused a significant increase in total mortality for the first two days, followed by a sharp reduction in mortality, eventually reaching only 10% after 12 days. In contrast, the LD50 showed a decrease in mortality beginning on the fourth day, with a complete loss of toxicity by the twelfth day after application. This indicates that while higher doses of carvacrol maintain strong initial efficacy, the toxic effects diminish over time, particularly at lower doses. (Figure 2).
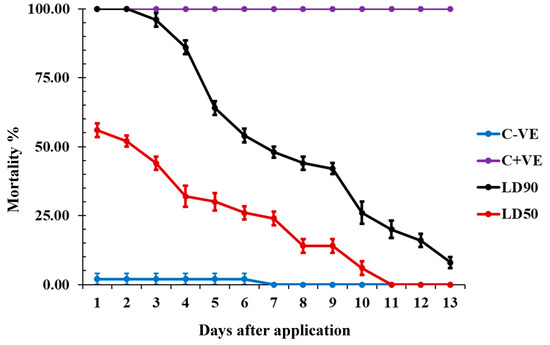
Figure 2.
The mortality % recorded from residual toxicity effect of carvacrol against T. castaneum adults, where (C − ve) refers to control untreated insects and (C + ve) is control treated with chlorpyrifos.
3. Discussion
The fundamental requirements of any grain storage method or structure are to secure the grains from insects and rodents and to prevent spoilage caused by the activities of micro-organisms [16]. Chemical control methods, such as those employing pyrethroids and organophosphates, offer rapid, cost-effective, and efficient management of stored product pests. However, they also present significant drawbacks, including environmental damage, product contamination, human and animal health risks, pesticide residues, and the development of pest resistance [17]. Consequently, the pursuit of alternative, non-toxic grain protectants has become a paramount objective. This research focused on assessing the insecticidal potential of C. capitatus and carvacrol as promising candidates for the control of the red flour beetle, T. castaneum, a notorious pest of stored grains.
The present data reveled that C. capitatus exhibited pronounced fumigant toxicity, repellent, and antifeedant properties against the red flour beetle, T. castaneum. Furthermore, a significant reduction in the beetle’s progeny (F1 generation) was observed, indicating a pronounced impact on reproductive capacity. Previous studies have established the fumigant toxicity of Lamiaceae-derived essential oils against stored product beetles [18]. The present study represents the first investigation into the fumigant toxicity of C. capitatus against T. castaneum. The current GC-MC analysis revealed the present of several components of C. capitatus, e.g., carvacrol, thymol, p-cymene, linalool, and caryophyllene, which were previously documented to exhibit contact toxicity [18]. For instance, p-cymene has demonstrated contact toxicity against Sitophilus oryzae with an LC50 of 0.8 mg/cm2 [19]. Similarly, thymol has been reported to exhibit contact toxicity against Sitophilus oryzae and Rhyzopertha dominica with LC50 values of 8.8 and 24.07 μg/cm2, respectively [20]. Linalool has demonstrated potent contact toxicity against four key stored product pests: Sitophilus oryzae, Sitophilus zeamais, Lasioderma serricorne, and Tribolium castaneum. The reported LC50 values for these pests are 66.74 μg/cm2, 2.45 μL/cm2, 27.41 μg/cm2, and 45.96 μg/cm2, respectively [21,22,23]. These findings underscore the contact toxicity of these bioactive compounds and, consequently, the broader insecticidal properties of C. capitatus extract against a range of stored product pests.
In the present study, carvacrol exhibited pronounced fumigant, repellent, and antifeedant effects against T. castaneum, significantly suppressing adult populations and progeny production, even at low concentrations. Similarly, carvacrol showed significant toxicity against Sitophilus oryzae and T. castaneum with LC50 values of 17.15 and 21.16 μg/cm2, respectively [20]. Remarkably, carvacrol revealed significant toxicity against Lasioderma serricorne and Rhyzopertha dominica with very low LC50 values of 0.012 and 0.019 mg/cm2, respectively [24].
The present study demonstrated that both EOs exhibited significant repellent effects against adult T. castaneum. While the repellent properties of C. capitatus EO have not been previously reported, other Thymus species have shown repellent activity against stored product insects. For instance, Thymus vulgaris has been shown to strongly repel Plodia interpunctella and Sitophilus zeamais [25,26,27]. Additionally, Thymus quinquecostatus effectively repelled T. castaneum, Lasioderma serricorne, and Liposcelis bostrychophila [28], and Thymus persicus EO strongly repelled T. castaneum and Callosobruchus maculatus [29]. Furthermore, carvacrol, a major component of both EOs, has been shown to repel Rhyzopertha dominica and Lasioderma serricorne with high repellency rates after three hours of exposure at 12.5 μg/cm2 [30].
Significantly, both essential oils revealed antifeedant effect against T. castaneum. This pronounced response is likely attributed to the high concentration of carvacrol within the oils. Previous research has established carvacrol’s potent antifeedant properties against a broad spectrum of insect pests, including stored product coleopterans. For instance, a 30% feeding deterrence against Rhyzopertha dominica was observed at a maximal concentration of 1 mg/g [20]. Moreover, carvacrol exhibited 50% feeding repellency against Aedes aegypti mosquitoes at a dosage of 22.51 μg/cm2 [31].
The current study represents the first evaluation of the progeny inhibition effects of both essential oils (EOs) against T. castaneum. A significant reduction in T. castaneum progeny was observed following exposure to both EOs. Previous research by [32] demonstrated a similar effect of carvacrol on S. zeamais progeny, with decreasing progeny production at higher concentrations. The noticeable reduction in offspring is likely attributed to disruptions in adult mating and communication, leading to decreased oviposition and potential mortality during the egg and larval stages [33].
Overall, the essential oil (EO) from C. capitatus is highly insecticidal in its various modes of action, which are dependent on its chemical composition. This is notably due to the presence of carvacrol, which has insects’ toxic properties [34]. The current results showed that C. capitatus’s toxicity can be explained by a very high concentration of carvacrol, reaching 71%. There are two suggested main mechanisms for the observed insecticidal properties of carvacrol. Firstly, at the central nervous system level, octopaminergic and gamma-aminobutyric acid (GABA) receptors are considered as the main targets for the neurotoxic effects of carvacrol. As a result, this interaction induces significant alterations in the neuromuscular transmission in insects [35]. Secondly, carvacrol inhibits acetylcholinesterase activity [36]. In addition, it has been found out that carvacrol exhibits a significant inhibitory effect on insects’ detoxification system by specifically targeting cytochrome P450s [37]. Importantly, thymol, as one of seven monoterpenes investigated, has been proven to be the most effective inhibitor of food intake within the EO composition of C. capitatus [38]. The neurotoxic potential of EOs of Thymus capitatus is not limited to its insecticidal activity, but includes other symptoms such as hyperactivity, convulsions, and paralysis. The aforementioned symptoms can result in death because the essential oil affects indigenous physiological mechanisms in the insect [34].
Thyme essential oils offer numerous advantages as insecticides, positioning them as a viable alternative to synthetic pesticides as they are eco-friendly. Thyme essential oils have a low environmental persistence due to their volatility, reducing the likelihood of harmful residues [39]. Furthermore, essential oils of thyme demonstrate enhanced targeted efficacy, as they encompass insecticidal properties that selectively target specific pests, while leaving beneficial organisms unaffected, thus contributing to the preservation of the ecological equilibrium. Also, EOs tend to be less harmful to mammals, making them a safer option for environments where humans and pets are present [40]. In addition, thyme essential oils are officially approved by agencies such as the EPA and FDA as safe for inclusion in food products, which further increases their attractiveness as a pest control solution [41]. On the other hand, there are some disadvantages of thyme oil, e.g., their effectiveness, which can vary widely based on several factors, including the specific type of oil, its concentration, and the environmental conditions. This variability can make them less trustworthy than conventional insecticides [42]. Moreover, exposing essential oil of thyme to the various environmental conditions may encounter different stability issues. This instability can lead to a decrease in their efficacy over time, making them less reliable compared to synthetic insecticides [43].
4. Materials and Methods
4.1. Chemicals
Acetone (99.9%) and chlorpyrifos (25%) were purchased (KZ Chemical Industries, Giza, Egypt), while both N, N-diethyl-m-toluamide (DEET, 97%), and purified oil of carvacrol (98%) were purchased form Sigma Aldrich in Cairo, Egypt.
4.2. Insect Culture
This colony of Tribolium castaneum was maintained in a dark incubator at a relative humidity of 70–80% and a temperature of 28 °C for five years, without exposure to any insecticide. The insects were reared on a mixture of active yeast and wheat flour (10:1, w/w), with the moisture level of the wheat flour being 12–13% according to [44]. One-week-old, unsexed adult insects were used for the following bioassays.
4.3. Coridothymus capitatus Essential Oil Extraction
Coridothymus capitatus was collected from the vicinity of Institute of Agriculture nearby Izmir and identified by Dr. Aşkın Akpulat, a plant taxonomist at Cumhuriyet University, Sivas, Turkey. The leaves were separated from the stems and blossoms, dried in the shade for two weeks, and then crushed to pass through a 2 mm mesh. A total of 100 g of the dried, ground leaves were placed in a Clevenger-type distillation apparatus with 2 L of double-distilled water and hydrodistilled for 3 h [45]. The extracted oil was dried over anhydrous sodium sulfate, filtered, and stored at 4 °C until use.
4.4. GC-MS Analysis of the Essential Oil
The essential oil (EO) was analyzed using a Trace Ultra gas chromatograph (GC) coupled with a DSQ II mass spectrometer (MS; Thermo Scientific, Waltham, MA, USA). The compounds were isolated on a Thermo-5MS (30 m × 0.25 mm ΙD, film thickness 0.25 μm) capillary column (Thermo Scientific) with a temperature program ranging from 60 to 250 °C at a rate of 3 °C/min and a helium flow rate of 1 mL/min. The injector and MS transfer line temperatures were set at 220 and 250 °C, respectively. Samples were prepared by diluting 1 mg of EO in 1 mL of acetone, and 1 µL of the diluted sample was injected manually in splitless mode. The MS operated in EI mode at 70 eV, with an ion source temperature of 230 °C, and mass spectra were acquired in scan mode for the mass range 40–400. The identification of the compounds was achieved by the comparison of their relative retention indexes and mass spectra with corresponding data reported in the literature and instrument’s databases (Adams Book 07, Nist 98, Xcalibur). A series of n-alkanes (C8–C24) was used to determine the relative retention index (RRI). Relative percentages of the compounds were obtained electronically from area percentage data.
4.5. Repellency Assay
This assay was conducted in Petri dishes based on the choice behavior of the insects. Glass Petri dishes (9 cm in diameter) were used, with Whatman filter papers (9 cm in diameter) divided into two halves. A pipette was used to evenly distribute 0.5 mL of each dilution onto one half of the filter paper at concentrations of 0.125, 0.25, 0.5, 1, and 2 mg/cm2, while the other half of the filter paper was treated with acetone only. The solvent was allowed to evaporate for 10 min. C. capitatus extract and carvacrol were prepared in an acetone solvent at different dilution rates to produce the required solutions. The positive control treatment was prepared in the same manner using DEET (2 mg/cm2) [24] on one-half of the filter paper and acetone on the other. After the solvent had evaporated, the Petri dishes were covered and 10 adult T. castaneum were placed within them, between the two filter paper halves. The bioassay was conducted at room temperature (26 ± 2 °C) and in daylight (10 am) with five replicates of each dilution [46]. After 3 h, the total numbers of T. castaneum on the treated (Nt) and untreated halves (Nc) were counted, recorded, and the repellency percentages (PR) were calculated using the following equation [47]: PR = [(Nc − Nt)/(Nc + Nt)] × 100.
4.6. Fumigant Toxicity Assay
A 2 cm diameter Filter sheets was placed to cover the inner surfaces of a 20 mL glass vials. With a micropipette, 5 μL/L of the tested concentrations was applied to each piece of paper as follows: 6.25, 12.5, 25, 50, and 100 μL/L of C. capitatus extract and carvacrol. Five replications were used for every concentration. A positive control using chlorpyrifos (5 μL/L) and an acetone-treated piece of paper was utilized as a negative control. Following the placement of each vial with its 10 insects, the tops were tightly secured. The vials were maintained under controlled conditions at 30 ± 1 °C and 60–65% relative humidity for 24 h [48]. Insect mortality was determined, and insects were considered dead if there was no movement of legs or antennae after 15 s of stimulation with an entomological pin. The mortality rate was determined using [49] the following correction:
where (Mt) is mortality on treatment and (Mc) is mortality on control.
4.7. Effects of C. capitatus and Carvacrol on F1 Progeny Production of T. castaneum (30–45 Days)
To assess the impact of C. capitatus and carvacrol on the progeny production of Tribolium castaneum, a modified method of [50] was followed. Wheat grains were treated with Coridothymus capitatus and carvacrol at LC50 and LC90 concentrations and dissolved in acetone. A control group was treated with acetone alone. Ten adult beetles were introduced into plastic cups containing 10 g of treated wheat. The beetles were allowed to oviposit for seven days. After removing the adults, the cups were incubated until the F1 generation emerged (days 30–45). The number of adult F1 beetles was counted in each cup. The inhibition rate of progeny was calculated using the formula provided by [51]:
where Cn and Tn refers to number of Fl progeny on control and treated cups, respectively.
4.8. Antifeedant Effects (Grains Weight Loss) of C. capitatus and Carvacrol
Dried wheat grains weighing 10 g were divided into five replicates and placed in plastic cups. Each cup was then treated with an LC90 concentration of C. capitatus extract and carvacrol, with acetone used as a control. The cups were covered with muslin cloth and incubated under controlled conditions for 30 days. The percentage weight loss of the grains was calculated using the following formula: Weight loss% = (W1 − W2)/W1 × 100. This formula compares the grain’s initial weight before the experiment (W1) to its weight after 30 days of the experiment (W2). The antifeeding effect of the essential oils was assessed by measuring the weight loss in the grains and calculating the feeding deterrent index (FDI%). The FDI% was calculated using the following equation: FDI% = (C − T)/C × 100, where C = weight loss in the control untreated grains and T = weight loss in the treated grains [51].
4.9. Residual Toxicity of Carvacrol on Wheat Grains against Adult T. castaneum
The long-term pesticide residual impact of the investigated essential oils was evaluated using a modified method based on [52]. Thirty grams of wheat grains were mixed with carvacrol at the LC50 and LC90 levels in a glass jar measuring 25 cm in diameter and 20 cm in height. To ensure even oil distribution throughout the grains, the mixture was thoroughly shaken and then allowed to sit for an hour to let the solvent evaporate. A total of 10 pairs of adult T. castaneum from the stock culture were introduced to 10 g of the treated grains, which had been transferred to clean plastic cups. A parallel control treatment using untreated grains was also conducted. Three replicates of the experiment were performed [52]. Ten adult insects were released every twenty-four hours until minimal mortality of less than 10% was observed [53].
4.10. Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics version 26. One-way ANOVA followed by Tukey’s post hoc test was employed to identify significant differences among treatments at a significance level of p < 0.05. Probit analysis, as described by [54], was used to calculate lethal concentrations (LC50 and LC90) and their respective 95% confidence intervals. Data are presented as mean ± standard error.
5. Conclusions
The present observation confirmed that both C. capitatus essential oil and carvacrol exhibited substantial insecticidal efficacy against Tribolium castaneum, demonstrating superior performance in repellency, feeding deterrence, progeny inhibition, and mortality rates exceeding 70%. These findings underscore the potential of these natural compounds as a promising, ecofriendly strategy for managing stored product insect pests. The appropriateness of their safety profile and approval by the European Food Safety Authority (EFSA) for use in animal feed applications further boosts them in terms of sustainable grain protection.
Author Contributions
Conceptualization: A.-A.S.A.-B. and S.M.A.; data curation: S.A.-Q. and H.E.-F.; formal analysis: A.M.E.-M., A.S. and D.D.; funding acquisition: A.-A.S.A.-B. and S.A.-Q.; investigation: H.M.E., F.T. and D.D.; methodology: H.M.E., F.T. and A.S.; supervision: H.E.-F. and A.-A.S.A.-B.; validation: S.A.-Q. and F.T.; visualization: A.M.E.-M.; roles/writing—original draft: H.M.E. and S.A.-Q.; writing—review and editing: A.-A.S.A.-B. and S.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Researcher-Supporting Project (RSP-2024/3), King Saud University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data to this work are available in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bingham, A.C.; Subramanyam, B.; Mahroof, R.; Alavi, S. Development and validation of a model for predicting survival of young larvae of Tribolium castaneum exposed to elevated temperatures during heat treatment of grain-processing facilities. J. Stored Prod. Res. 2017, 72, 143–152. [Google Scholar] [CrossRef]
- Hu, J.; Wang, W.; Dai, J.; Zhu, L. Chemical composition and biological activity against Tribolium castaneum (Coleoptera: Tenebrionidae) of Artemisia brachyloba essential oil. Ind. Crops Prod. 2019, 128, 29–37. [Google Scholar] [CrossRef]
- Abou-Taleb, H.K.; Mohamed, M.I.; Shawir, M.S.; Abdelgaleil, S.A. Insecticidal properties of essential oils against Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase and adenosine triphosphatases. Natural Product. Res. 2015, 30, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; El-Aziz, S.E.A.; Omar, E.; Fahim, S.F. Olfaction cues of food odours influencing behavior response of the red flour beetle, Tribolium castanium. Bull. NRC 2014, 39, 1–11. [Google Scholar]
- Awan, D.A.; Saleem, M.A.; Nadeem, M.S.; Shakoori, A.R. Toxicological and biochemical studies on spinosad and synergism with piperonyl butoxide in susceptible and resistant strains of Tribolium castaneum. Pak. J. Zool. 2012, 44, 649–662. [Google Scholar]
- Paranagama, P.; Abeysekera, K.; Abeywickrama, K.; Nugaliyadde, L. Fungicidal and anti-aflatoxigenic effects of the essential oil of Cymbopogon citratus (DC.) Stapf. (lemongrass) against Aspergillus flavus Link. isolated from stored rice. Lett. Appl. Microbiol. 2003, 37, 86–90. [Google Scholar] [CrossRef]
- Talukder, F. Pesticide Resistance in Stored-Product Insects and Alternative Biorational Management: A Brief review. J. Agric. Mar. Sci. 2009, 14, 9. [Google Scholar] [CrossRef]
- Feroz, A. Efficacy and cytotoxic potential of deltamethrin, essential oils of Cymbopogon citratus and Cinnamonum camphora and their synergistic combinations against stored product pest, Trogoderma granarium (Everts). J. Stored Prod. Res. 2020, 87, 101614. [Google Scholar] [CrossRef]
- Boukouvala, M.C.; Kavallieratos, N.G.; Athanassiou, C.G.; Losic, D.; Hadjiarapoglou, L.P.; Elemes, Y. Laboratory evaluation of five novel pyrrole derivatives as grain protectants against Tribolium confusum and Ephestia kuehniella larvae. J. Pest. Sci. 2016, 90, 569–585. [Google Scholar] [CrossRef]
- Boukouvala, M.; Kavallieratos, N.; Athanassiou, C.; Hadjiarapoglou, L. Biological activity of two new pyrrole derivatives against stored-product species: Influence of temperature and relative humidity. Bull. Entomol. Res. 2016, 106, 446–456. [Google Scholar] [CrossRef]
- Boyer, S.; Zhang, H.; Lempérière, G. A review of control methods and resistance mechanisms in stored-product insects. Bull. Entomol. Res. 2011, 102, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: A systematic review. Parasitol. Res. 2015, 114, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Pirintsos, S.A.; Bariotakis, M.; Kampa, M.; Sourvinos, G.; Lionis, C.; Castanas, E. The Therapeutic Potential of the Essential Oil of Thymbra capitata (L.) Cav., Origanum dictamnus L. and Salvia fruticosa Mill. And a Case of Plant-Based Pharmaceutical Development. Front. Pharmacol. 2020, 11, 522213. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef]
- Mobolade, A.J.; Bunindro, N.; Sahoo, D.; Rajashekar, Y. Traditional methods of food grains preservation and storage in Nigeria and India. Ann. Agric. Sci. 2019, 64, 196–205. [Google Scholar] [CrossRef]
- Römbke, J.; Moltmann, J.F. Applied Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Shaaya, E.; Kostyukovsky, M.; Demchenko, N. Alternative Fumigants for the Control of Stored-Product Insects; CABI: Wallingford, UK, 2003; pp. 556–560. [Google Scholar] [CrossRef]
- Saad, M.M.G.; Abou-Taleb, H.K.; Abdelgaleil, S.A.M. Insecticidal activities of monoterpenes and phenylpropenes against Sitophilus oryzae and their inhibitory effects on acetylcholinesterase and adenosine triphosphatases. Appl. Entomol. Zool. 2018, 53, 173–181. [Google Scholar] [CrossRef]
- Kanda, D.; Kaur, S.; Koul, O. A comparative study of monoterpenoids and phenylpropanoids from essential oils against stored grain insects: Acute toxins or feeding deterrents. J. Pest. Sci. 2016, 90, 531–545. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Badawy, M.E.I.; El-Arami, S.A.A. Fumigant and Contact Toxicities of Monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their Inhibitory Effects on Acetylcholinesterase Activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef]
- Yildirim, E.; Emsen, B.; Kordali, S. Insecticidal Effects of Monoterpenes on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). DOAJ Dir. Open Access J. 2013, 86, 198–204. [Google Scholar] [CrossRef]
- Cao, J.; Guo, S.; Wang, Y.; Pang, X.; Geng, Z.; Du, S. Toxicity and repellency of essential oil from Evodia lenticellata Huang fruits and its major monoterpenes against three stored-product insects. Ecotoxicol. Environ. Saf. 2018, 160, 342–348. [Google Scholar] [CrossRef]
- Ramadan, G.R.; Abdelgaleil, S.A.; Shawir, M.S.; El-Bakary, A.S.; Zhu, K.Y.; Phillips, T.W. Terpenoids, DEET and short chain fatty acids as toxicants and repellents for Rhyzopertha dominica (coleoptera: Bostrichidae) and Lasioderma serricorne (Coleoptera: Ptinidae). J. Stored Prod. Res. 2020, 87, 101610. [Google Scholar] [CrossRef]
- Karahroodi, Z.R.; Moharramipour, S.; Rahbarpour, A. Investigated repellency effect of some essential oils of 17 native medicinal plants on adults Plodia interpunctella. Am.-Eurasian J. Sustain. Agric. 2009, 3, 181–184. [Google Scholar]
- Yang, N.; Li, A.; Wan, F.; Liu, W.; Johnson, D. Effects of plant essential oils on immature and adult sweetpotato whitefly, Bemisia tabaci biotype B. Crop Prot. 2010, 29, 1200–1207. [Google Scholar] [CrossRef]
- Barros, F.A.; Radünz, M.; Scariot, M.A.; Camargo, T.M.; Nunes, C.F.; De Souza, R.R.; Gilson, I.K.; Hackbart, H.C.; Radünz, L.L.; Oliveira, J.V.; et al. Efficacy of encapsulated and non-encapsulated thyme essential oil (Thymus vulgaris L.) in the control of Sitophilus zeamais and its effects on the quality of corn grains throughout storage. Crop Prot. 2022, 153, 105885. [Google Scholar] [CrossRef]
- Bounoua-Fraoucene, S.; Kellouche, A.; Debras, J. Toxicity of Four Essential Oils Against Two Insect Pests of Stored Grains, Rhyzopertha dominica (Coleoptera: Bostrychidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Afr. Entomol. 2019, 27, 344. [Google Scholar] [CrossRef]
- Moharramipour, S.; Taghizadeh, A.; Meshkatalsadat, M.H.; Fathipour, Y.; Talebi, A.A. Repellent Activity and Persistence of Essential Oil Extracted from Prangos acaulis to Three Stored-Product Beetles. Am. -Eurasian J. Sustain. Agric. 2009, 3, 202–204. [Google Scholar]
- Saad, M.M.G.; El-Deeb, D.A.; Abdelgaleil, S.A.M. Insecticidal potential and repellent and biochemical effects of phenylpropenes and monoterpenes on the red flour beetle, Tribolium castaneum Herbst. Environ. Sci. Pollut. Res. Int. 2019, 26, 6801–6810. [Google Scholar] [CrossRef]
- Huang, T.; Tien, N.; Luo, Y. An in vitro bioassay for the quantitative evaluation of mosquito repellents against Stegomyia aegypti (=Aedes aegypti) mosquitoes using a novel cocktail meal. Med. Vet. Entomol. 2015, 29, 238–244. [Google Scholar] [CrossRef]
- Chaubey, M.K.; Kumar, N. Role of carvacrol and menthone in maize weevil Sitophilus zeamais (Coleoptera: Curculionidae) management. Eur. J. Biol. Res. 2023, 13, 181–190. [Google Scholar]
- Chaubey, M.K. Insecticidal property of terpenes against maize weevil, sitophilus zeamais (motschulsky). J. Biopestic. 2022, 15, 91–102. [Google Scholar] [CrossRef]
- Bachrouch, O.; Nefzi, H.; Belloumi, S.; Horchani-Naifer, K.; Eljazi, J.S.; Hamdi, S.H.; Msaada, K.; Labidi, J.; Abderrabba, M.; Jemaa, J.M.B. Insecticidal effects of two Tunisian diatomaceous earth loaded with Thymus capitatus (L.) Hoffmans and Links as an ecofriendly approach for stored coleopteran pest control. Int. J. Environ. Health Res. 2022, 33, 398–412. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Bartolucci, F.; Canale, A.; Maggi, F. Origanum syriacum subsp. syriacum: From an ingredient of Lebanese ‘manoushe’ to a source of effective and eco-friendly botanical insecticides. Ind. Crops Prod. 2019, 134, 26–32. [Google Scholar] [CrossRef]
- López, V.; Pavela, R.; Gómez-Rincón, C.; Les, F.; Bartolucci, F.; Galiffa, V.; Petrelli, R.; Cappellacci, L.; Maggi, F.; Canale, A.; et al. Efficacy of Origanum syriacum Essential Oil against the Mosquito Vector Culex quinquefasciatus and the Gastrointestinal Parasite Anisakis simplex, with Insights on Acetylcholinesterase Inhibition. Molecules 2019, 24, 2563. [Google Scholar] [CrossRef]
- Gaire, S.; Zheng, W.; Scharf, M.E.; Gondhalekar, A.D. Plant essential oil constituents enhance deltamethrin toxicity in a resistant population of bed bugs (Cimex lectularius L.) by inhibiting cytochrome P450 enzymes. Pestic. Biochem. Physiol. 2021, 175, 104829. [Google Scholar] [CrossRef]
- Pavela, R. Insecticidal and repellent activity of selected essential oils against of the pollen beetle, Meligethes aeneus (Fabricius) adults. Ind. Crops Prod. 2011, 34, 888–892. [Google Scholar] [CrossRef]
- Moutassem, D.; Boubellouta, T.; Bellik, Y.; Rouis, Z.; Kucher, D.E.; Utkina, A.O.; Kucher, O.D.; Mironova, O.A.; Kavhiza, N.J.; Rebouh, N.Y. Insecticidal activity of Thymus pallescens de Noë and Cymbogon citratus essential oils against Sitophilus zeamais and Tribolium castaneum. Sci. Rep. 2024, 14, 13951. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huo, X.; Zhou, X.; Zhao, D.; He, W.; Liu, S.; Liu, H.; Feng, T.; Wang, C. Acaricidal Activity and Synergistic Effect of Thyme Oil Constituents against Carmine Spider Mite (Tetranychus Cinnabarinus (Boisduval)). Molecules 2017, 22, 1873. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, J.; Jevremović, S.; Kostić, I.; Kostić, M.; Vuleta, A.; Jovanović, S.M.; Jovanović, D.Š. Toxic, Oviposition Deterrent and Oxidative Stress Effects of Thymus vulgaris Essential Oil against Acanthoscelides obtectus. Insects 2020, 11, 563. [Google Scholar] [CrossRef]
- Isman, M.B. Bioinsecticides based on plant essential oils: A short overview. Zeitschrift Für Naturforschung C 2020, 75, 179–182. [Google Scholar] [CrossRef]
- Gupta, P.; Preet, S.; Ananya, N.; Singh, N. Preparation of Thymus vulgaris (L.) essential oil nanoemulsion and its chitosan encapsulation for controlling mosquito vectors. Sci. Rep. 2022, 12, 4335. [Google Scholar] [CrossRef] [PubMed]
- Aboelhadid, S.M.; Youssef, I.M.I. Control of red flour beetle (Tribolium castaneum) in feeds and commercial poultry diets via using a blend of clove and lemongrass extracts. Environ. Sci. Pollut. Res. Int. 2021, 28, 30111–30120. [Google Scholar] [CrossRef] [PubMed]
- Sokmen, A.; Abdel-Baki, A.S.; Al-Malki, E.S.; Al-Quraishy, S.; Abdel-Haleem, H.M. Constituents of essential oil of Origanum minutiflorum and its in vitro antioxidant, scolicidal and anticancer activities. J. King Saud. Univ.-Sci. 2020, 32, 2377–2382. [Google Scholar] [CrossRef]
- Obeng-Ofori, D.; Reichmuth, C.H.; Bekele, A.J.; Hassanali, A. Toxicity and protectant potential of camphor, a major component of essential oil of Ocimum kilimandscharicum, against four stored product beetles. Int. J. Pest. Manag. 1998, 44, 203–209. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E.E. Repellent activity of essential oils from seven aromatic plants grown in Colombia against Sitophilus zeamais Motschulsky (Coleoptera). J. Stored Prod. Res. 2009, 45, 212–214. [Google Scholar] [CrossRef]
- Negahban, M.; Moharramipour, S.; Sefidkon, F. Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored-product insects. J. Stored Prod. Res. 2007, 43, 123–128. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Islam, M.S.; Talukder, F.A. Toxic and residual effects of Azadirachta indica, Tagetes erecta and Cynodon dactylon seed extracts and leaf powders towards Tribolium castaneum. J. Plant Dis. Prot. 2005, 112, 594–601. [Google Scholar] [CrossRef]
- Shukla, R.; Singh, P.; Prakash, B.; Kumar, A.; Mishra, P.K.; Dubey, N.K. Efficacy of essential oils of Lippia alba (Mill.) N.E. Brown and Callistemon lanceolatus (Sm.) Sweet and their major constituents on mortality, oviposition and feeding behaviour of pulse beetle, Callosobruchus chinensis L. J. Sci. Food Agric. J. Sci. Food Agric. 2011, 91, 2277–2283. [Google Scholar] [CrossRef]
- Elbrense, H.; Elmasry, A.M.A.; Seleiman, M.F.; Al-Harbi, M.S.; El-Raheem, A.M.A. Can Symbiotic Bacteria (Xenorhabdus and Photorhabdus) Be More Efficient than Their Entomopathogenic Nematodes against Pieris rapae and Pentodon algerinus Larvae? Biology 2021, 10, 999. [Google Scholar] [CrossRef]
- Reddy, D.S.; Srivastava, C.; Paul, B. Residual toxicity of impregnated insecticides on gunnybags against red flour beetle (Tribolium castaneum). Indian J. Agric. Sci. 2005, 75, 532–534. [Google Scholar]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971; 333p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).