Advancements in Inorganic Membrane Filtration Coupled with Advanced Oxidation Processes for Wastewater Treatment
Abstract
:1. Introduction
2. Inorganic Membrane Filtration
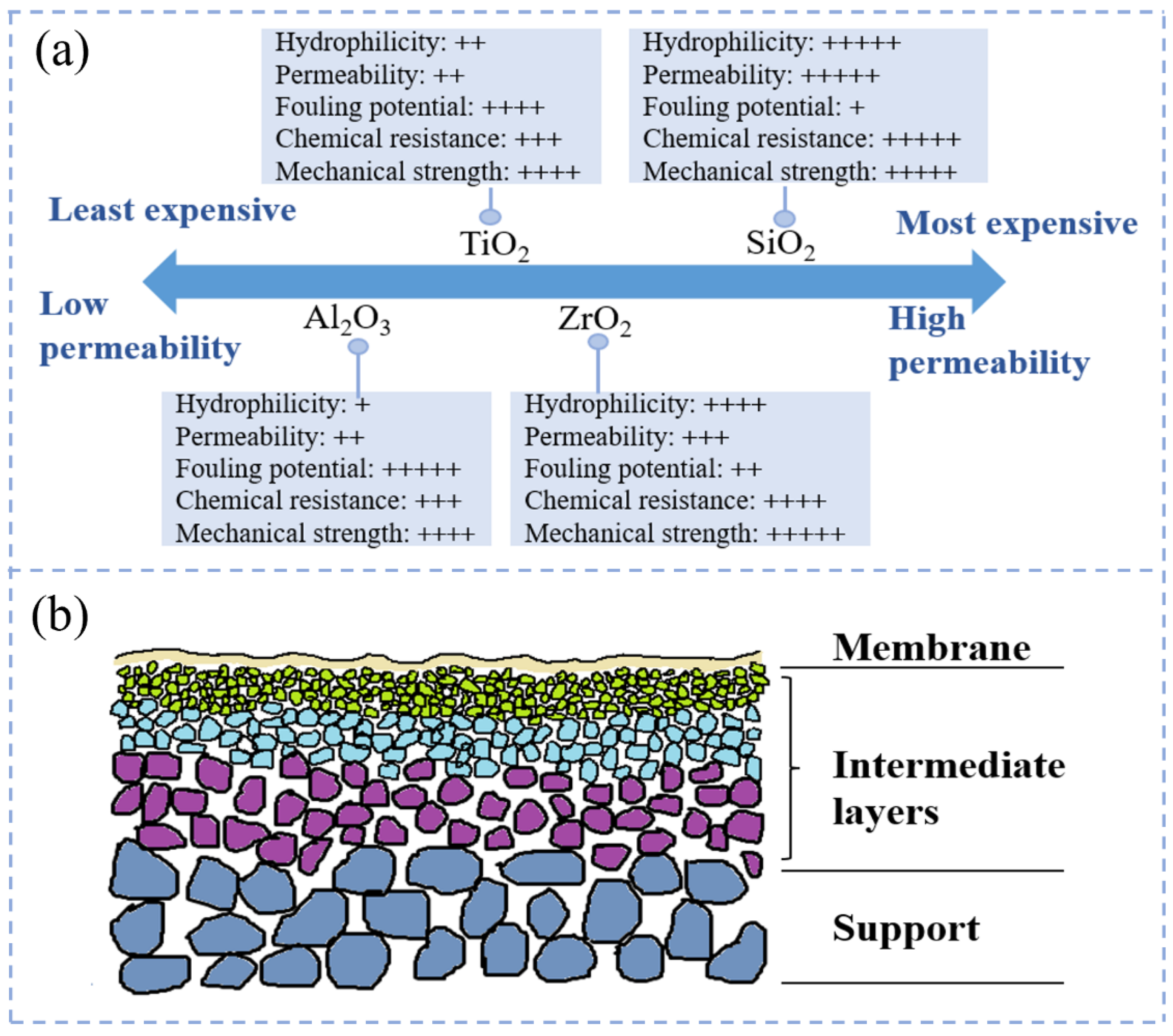
2.1. Inorganic Membrane Types
2.1.1. Ceramic Membranes
2.1.2. Carbon-Based Membranes
- (1)
- Carbon nanotube membranes
- (2)
- Graphene membranes
2.2. Influences
2.2.1. Coexisting Substances in Water
2.2.2. Inorganic Membrane Properties
3. Inorganic Membrane Filtration Coupled with AOPs
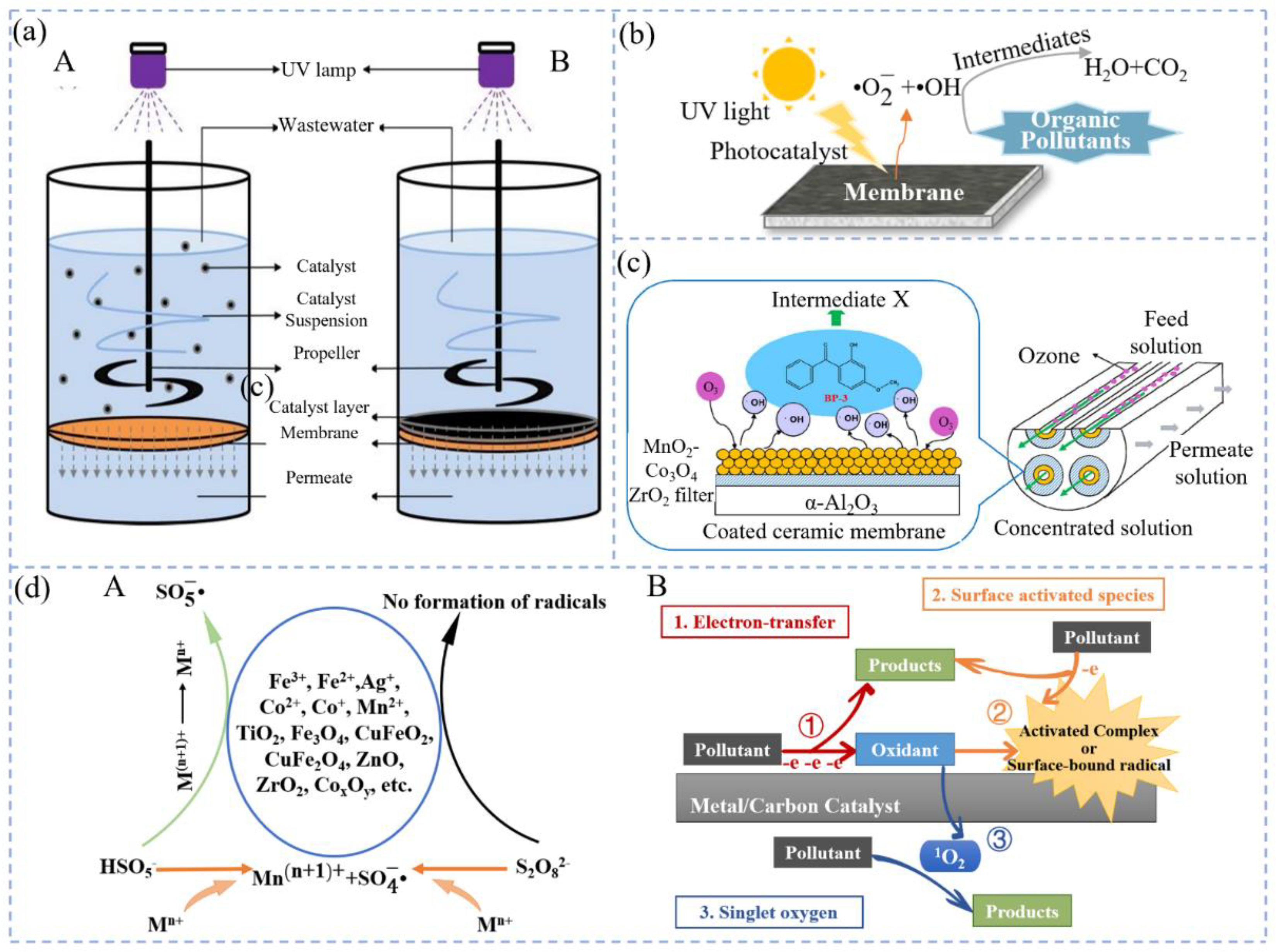
3.1. IMF Coupled with Photocatalytic Oxidation System
3.2. IMF Coupled with Ozonation System
3.3. IMF Coupled with Persulfate Oxidation System
3.4. IMF Coupled with Fenton and Composite Fenton Oxidation Systems
3.4.1. IMF Coupled with Fenton Oxidation Systems
3.4.2. IMF Coupled with Composite Fenton Oxidation Systems
3.5. Comparison of Different IMF Coupled with AOPs
4. Challenges and Prospects
- (1)
- Higher cost of inorganic membranes. Despite the longer service life of inorganic membranes, both ceramic and carbon material membranes have high manufacturing costs, which limits the practical application of inorganic membranes. For CM, cost-effective natural materials such as kaolin, pyroxene, and dolomite can be used as raw materials [230,231]. Carbon nanomaterials can be used in combination with other materials to reduce the proportion of carbon materials while ensuring membrane performance [14,232]. All of these can be used as a means to reduce costs. The use of natural materials will inevitably bring other components to inorganic membranes, leading to membrane defects [14]. In the future, it will be necessary to optimize the preparation method of inorganic membranes and adjust the material ratios to create more cost-effective membrane materials.
- (2)
- Membrane fouling affects the service life of the membrane. Some IMFs are prone to membrane fouling. Reversible membrane fouling could be removed by cleaning the membrane, but excessive cleaning times will inevitably reduce the service life of the membrane. Irreversible membrane fouling cannot be eliminated [115] and will inevitably affect the permeation flux of the membrane. The membrane can be modified by loading other materials to alter its surface properties [64,233], such as roughness, hydrophilicity, stability, membrane surface charge, etc. Currently, many materials have been applied to the preparation of catalytic membranes. Materials that can provide high permeability and selectivity as well as low energy consumption, will be one of the most effective materials for doping membranes.
- (3)
- Low catalytic membrane recovery. The use of O3 or the use of tiny electric field cleaning can effectively recover membranes during wastewater treatment [120,133,234]. But research is only at the laboratory stage and practical water treatment applications are not always feasible. The toxic waste generated after cleaning requires specialized treatment and disposal. Therefore, new environmentally friendly cleaning agents should be prepared to achieve sustainable long-term membrane operation.
- (4)
- The application examples of IMF-AOPs are scarce [31]. In the literature on using membrane filtration coupling with the AOP process to remove pollutants, most of them are laboratory-simulated water samples instead of real wastewater samples. Real wastewater environments are very complex. In order to better apply catalytic membranes to long-term real-world processes, the stability and effectiveness of these catalytic processes need to be explored using real water quality.
- (5)
- In the future, it is necessary to further improve the performance of the IMF coupled with -AOPs system through optimal design. The utilization rate of oxidizer and the removal efficiency of pollutant can be improved in the coupling system while maintaining low energy consumption. And it is best to minimize the membrane pollution and improve the reuse performance of the membrane [197]. CBMs have high electrical conductivity [64,235], and catalytic CMs with electrical conductivity can reduce internal resistance due to their large thickness. The application of inorganic membranes to electrocatalysis is a future direction [220,236]. On the other hand, multifunctional catalytic membranes can be developed in the future, considering the trade-off between processing efficiency and energy consumption. The developed catalytic membranes can be applied to the removal of different pollutants under one or even multiple AOP systems.
- (6)
- The mechanism of interaction between membranes and contaminants in the IMF coupled with AOPs process has not been clearly described so far. The current mechanism of the membrane filtration and AOP coupling process is based on the removal of pollutants by AOPs. The mechanisms of synergism and interaction between contaminant degradation intermediates and membrane skeleton surfaces remain unclear. Elucidating the dynamic application of inorganic membranes in contaminant degradation can provide a clearer understanding of the membrane fouling mechanism, thus effectively preventing membrane contamination and improving membrane lifetime.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, M.; Xu, Z.; Hou, D.; Gao, B.; Cao, X.; Ok, Y.S.; Rinklebe, J.; Bolan, N.S.; Tsang, D.C.W. Waste-derived biochar for water pollution control and sustainable development. Nat. Rev. Earth Environ. 2022, 3, 444–460. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Al-Khateeb, L.A.; Almotiry, S.; Salam, M.A. Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem. Eng. J. 2014, 248, 191–199. [Google Scholar] [CrossRef]
- Bukusoglu, E.; Koku, H.; Çulfaz-Emecen, P.Z. Addressing challenges in the ultrafiltration of biomolecules from complex aqueous environments. Curr. Opin. Colloid Interface Sci. 2020, 46, 52–64. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Hu, X.; Hua, T. Electrochemical advanced oxidation processes coupled with membrane filtration for degrading antibiotic residues: A review on its potential applications, advances, and challenges. Sci. Total Environ. 2021, 784, 146912. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. Review: Is interplay between nanomaterial and membrane technology the way forward for desalination? J. Chem. Technol. Biotechnol. 2014, 90, 971–980. [Google Scholar] [CrossRef]
- Zhu, R.; Diaz, A.J.; Shen, Y.; Qi, F.; Chang, X.; Durkin, D.P.; Sun, Y.; Solares, S.D.; Shuai, D. Mechanism of humic acid fouling in a photocatalytic membrane system. J. Membr. Sci. 2018, 563, 531–540. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.; Wang, Y.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A review on oil/water emulsion separation membrane material. J. Environ. Chem. Eng. 2022, 10, 107257. [Google Scholar] [CrossRef]
- Alduraiei, F.; Kumar, S.; Liu, J.; Nunes, S.P.; Szekely, G. Rapid fabrication of fluorinated covalent organic polymer membranes for organic solvent nanofiltration. J. Membr. Sci. 2022, 648, 120345. [Google Scholar] [CrossRef]
- Lau, W.J.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Chen, J.P.; Ismail, A.F. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Pan, Y.; Di, H.; Zeng, G.; Zhang, L.; Zhang, C. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Membr. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Geise, G.M.; Lee, H.-S.; Miller, D.J.; Freeman, B.D.; McGrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1685–1718. [Google Scholar] [CrossRef]
- Ghandashtani, M.B.; Zokaee Ashtiani, F.; Karimi, M.; Fouladitajar, A. A novel approach to fabricate high performance nano-SiO2 embedded PES membranes for microfiltration of oil-in-water emulsion. Appl. Surf. Sci. 2015, 349, 393–402. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Tan, Y.H.; Abdullah, E.C.; Rahman, M.E.; Karri, R.R. A comprehensive review on micropollutants removal using carbon nanotubes-based adsorbents and membranes. J. Environ. Chem. Eng. 2021, 9, 106647. [Google Scholar] [CrossRef]
- Ghernaout, D. Advanced Oxidation Processes for Wastewater Treatment: Facts and Future Trends. OALib 2020, 07, 1–15. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. Int. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- He, Z.; Ong, J.H.; Bao, Y.; Hu, X. Chemocatalytic ceramic membranes for removing organic pollutants in wastewater: A review. J. Environ. Chem. Eng. 2023, 11, 109548. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Liu, T.; Aniagor, C.O.; Ejimofor, M.I.; Menkiti, M.C.; Tang, K.H.D.; Chin, B.L.F.; Chan, Y.H.; Yiin, C.L.; Cheah, K.W.; Ho Chai, Y.; et al. Technologies for removing pharmaceuticals and personal care products (PPCPs) from aqueous solutions: Recent advances, performances, challenges and recommendations for improvements. J. Mol. Liq. 2023, 374, 121144. [Google Scholar] [CrossRef]
- Li, N.; Lu, X.; He, M.; Duan, X.; Yan, B.; Chen, G.; Wang, S. Catalytic membrane-based oxidation-filtration systems for organic wastewater purification: A review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef]
- Bao, Y.; Lee, W.J.; Wang, P.; Xing, J.; Liang, Y.N.; Lim, T.-T.; Hu, X. A novel molybdenum-based nanocrystal decorated ceramic membrane for organics degradation via catalytic wet air oxidation (CWAO) at ambient conditions. Catal. Today 2021, 364, 276–284. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Dumée, L.F. Photo-catalytic membrane reactors for the remediation of persistent organic pollutants—A review. Sep. Purif. Technol. 2020, 230, 115878. [Google Scholar] [CrossRef]
- Ly, Q.V.; Cui, L.; Asif, M.B.; Khan, W.; Nghiem, L.D.; Hwang, Y.; Zhang, Z. Membrane-based nanoconfined heterogeneous catalysis for water purification: A critical review. Water Res. 2023, 230, 119577. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, N.; Han, Y.; Wang, X.; Liu, S.; Zhang, L.; Sun, Y.; Jiang, B. Development of polyacrylonitrile/perovskite catalytic membrane with abundant channel-assisted reaction sites for organic pollutant removal. Chem. Eng. J. 2022, 437, 135163. [Google Scholar] [CrossRef]
- Zuo, K.; Wang, K.; DuChanois, R.M.; Fang, Q.; Deemer, E.M.; Huang, X.; Xin, R.; Said, I.A.; He, Z.; Feng, Y.; et al. Selective membranes in water and wastewater treatment: Role of advanced materials. Mater. Today 2021, 50, 516–532. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Chadha, U.; Selvaraj, S.K.; Vishak Thanu, S.; Cholapadath, V.; Abraham, A.M.; Zaiyan, M.; Manikandan, M.; Paramasivam, V. A review of the function of using carbon nanomaterials in membrane filtration for contaminant removal from wastewater. Mater. Res. Express 2022, 9, 012003. [Google Scholar] [CrossRef]
- Akash, F.A.; Shovon, S.M.; Rahman, W.; Rahman, M.A.; Chakraborty, P.; Prasetya, T.A.E.; Monir, M.U. Advancements in ceramic membrane technology for water and wastewater treatment: A comprehensive exploration of current utilizations and prospective horizons. Desalination Water Treat. 2024, 319, 100569. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Da, X.; Chen, X.; Sun, B.; Wen, J.; Qiu, M.; Fan, Y. Preparation of zirconia nanofiltration membranes through an aqueous sol–gel process modified by glycerol for the treatment of wastewater with high salinity. J. Membr. Sci. 2016, 504, 29–39. [Google Scholar] [CrossRef]
- DeFriend, K.A.; Wiesner, M.R.; Barron, A.R. Alumina and aluminate ultrafiltration membranes derived from alumina nanoparticles. J. Membr. Sci. 2003, 224, 11–28. [Google Scholar] [CrossRef]
- Ewis, D.; Ismail, N.A.; Hafiz, M.; Benamor, A.; Hawari, A.H. Nanoparticles functionalized ceramic membranes: Fabrication, surface modification, and performance. Environ. Sci. Pollut. Res. 2021, 28, 12256–12281. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Z.; Sheng, M.; Qiao, Z.; Wang, J. Scaling up of defect-free flat membrane with ultra-high gas permeance used for intermediate layer of multi-layer composite membrane and oxygen enrichment. Sep. Purif. Technol. 2020, 239, 116580. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef]
- Issaoui, M.; Limousy, L. Low-cost ceramic membranes: Synthesis, classifications, and applications. Comptes Rendus Chim. 2019, 22, 175–187. [Google Scholar] [CrossRef]
- Das, N.; Maiti, H.S. Ceramic membrane by tape casting and sol–gel coating for microfiltration and ultrafiltration application. J. Phys. Chem. Solids 2009, 70, 1395–1400. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Rahman, M.A.; Jaafar, J.; Ismail, A.F.; Amin, S.Z.M. Green silica-based ceramic hollow fiber membrane for seawater desalination via direct contact membrane distillation. Sep. Purif. Technol. 2018, 205, 22–31. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Guan, K.; Peng, C.; Wu, J. Freeze-casting of alumina ultra-filtration membranes with good performance for anionic dye separation. Ceram. Int. 2018, 44, 11901–11904. [Google Scholar] [CrossRef]
- Du, Z.; Yao, D.; Xia, Y.; Zuo, K.; Yin, J.; Liang, H.; Zeng, Y.-P. Highly porous silica foams prepared via direct foaming with mixed surfactants and their sound absorption characteristics. Ceram. Int. 2020, 46, 12942–12947. [Google Scholar] [CrossRef]
- Oun, A.; Tahri, N.; Mahouche-Chergui, S.; Carbonnier, B.; Majumdar, S.; Sarkar, S.; Sahoo, G.C.; Ben Amar, R. Tubular ultrafiltration ceramic membrane based on titania nanoparticles immobilized on macroporous clay-alumina support: Elaboration, characterization and application to dye removal. Sep. Purif. Technol. 2017, 188, 126–133. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Gopal, S.; Nambikkattu, J.; Rambabu, K.; Aboulella, A.M.; Ranil Wickramasinghe, S.; Banat, F. Recent developments in porous ceramic membranes for wastewater treatment and desalination: A review. J. Environ. Manag. 2021, 293, 112925. [Google Scholar] [CrossRef]
- Li, Y.; Richardson, J.B.; Mark Bricka, R.; Niu, X.; Yang, H.; Li, L.; Jimenez, A. Leaching of heavy metals from E-waste in simulated landfill columns. Waste Manag. 2009, 29, 2147–2150. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, S.; Wang, Z.; Yu, S. Composite NF membranes with anti-bacterial activity prepared by electrostatic self-assembly for dye recycle. J. Taiwan Inst. Chem. Eng. 2020, 106, 34–50. [Google Scholar] [CrossRef]
- Fujiwara, M.; Imura, T. Photo Induced Membrane Separation for Water Purification and Desalination Using Azobenzene Modified Anodized Alumina Membranes. ACS Nano 2015, 9, 5705–5712. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, A.; Gad, A. Chemical and microstructural analyses for heavy metals removal from water media by ceramic membrane filtration. Water Sci. Technol. 2017, 75, 439–450. [Google Scholar] [CrossRef]
- Asif, M.B.; Ren, B.; Li, C.; Maqbool, T.; Zhang, X.; Zhang, Z. Powdered activated carbon—Membrane bioreactor (PAC-MBR): Impacts of high PAC concentration on micropollutant removal and microbial communities. Sci. Total Environ. 2020, 745, 141090. [Google Scholar] [CrossRef]
- Ağtaş, M.; Dilaver, M.; Koyuncu, İ. Ceramic membrane overview and applications in textile industry: A review. Water Sci. Technol. 2021, 84, 1059–1078. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, X.; Fan, Y.; Xing, W. 1.11 Ceramic Membranes. In Comprehensive Membrane Science and Engineering, 2nd ed.; Drioli, E., Giorno, L., Fontananova, E., Eds.; Elsevier: Oxford, UK, 2017; pp. 270–297. [Google Scholar]
- Zhong, J.; Sun, X.; Wang, C. Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration. Sep. Purif. Technol. 2003, 32, 93–98. [Google Scholar] [CrossRef]
- Cui, Z.; Xing, W.; Fan, Y.; Xu, N. Pilot study on the ceramic membrane pre-treatment for seawater desalination with reverse osmosis in Tianjin Bohai Bay. Desalination 2011, 279, 190–194. [Google Scholar] [CrossRef]
- Kim, S.; Park, C. Potential of ceramic ultrafiltration membranes for the treatment of anionic surfactants in laundry wastewater for greywater reuse. J. Water Process Eng. 2021, 44, 102373. [Google Scholar] [CrossRef]
- Ji, Y.; Qian, W.; Yu, Y.; An, Q.; Liu, L.; Zhou, Y.; Gao, C. Recent developments in nanofiltration membranes based on nanomaterials. Chin. J. Chem. Eng. 2017, 25, 1639–1652. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Yuan, S.; Zhou, J.; Wang, B. Challenges and recent advances in MOF-polymer composite membranes for gas separation. Inorg. Chem. Front. 2016, 3, 896–909. [Google Scholar] [CrossRef]
- Yuan, J.; Hung, W.-S.; Zhu, H.; Guan, K.; Ji, Y.; Mao, Y.; Liu, G.; Lee, K.-R.; Jin, W. Fabrication of ZIF-300 membrane and its application for efficient removal of heavy metal ions from wastewater. J. Membr. Sci. 2019, 572, 20–27. [Google Scholar] [CrossRef]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Al-anzi, B.S.; Siang, O.C. Recent developments of carbon based nanomaterials and membranes for oily wastewater treatment. RSC Adv. 2017, 7, 20981–20994. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed]
- Thines, R.K.; Mubarak, N.M.; Nizamuddin, S.; Sahu, J.N.; Abdullah, E.C.; Ganesan, P. Application potential of carbon nanomaterials in water and wastewater treatment: A review. J. Taiwan Inst. Chem. Eng. 2017, 72, 116–133. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Zhang, L.; Li, S.; Yuan, Y.; Xiao, X.; Fan, X.; Song, C. Carbon-based membrane materials and applications in water and wastewater treatment: A review. Environ. Chem. Lett. 2020, 19, 1457–1475. [Google Scholar] [CrossRef]
- Corry, B. Designing carbon nanotube membranes for efficient water desalination. J. Phys. Chem. B 2008, 112, 1427–1434. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, C.; Seo, D.K.; Kim, T.; Lee, J.S.; Kim, Y.H.; Ahn, K.H.; Bae, S.S.; Lee, S.C.; Lim, J.; et al. High performance and antifouling vertically aligned carbon nanotube membrane for water purification. J. Membr. Sci. 2014, 460, 171–177. [Google Scholar] [CrossRef]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef]
- Jame, S.A.; Zhou, Z. Electrochemical carbon nanotube filters for water and wastewater treatment. Nanotechnol. Rev. 2016, 5, 41–50. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Kumar, P.; Kim, K.H.; Ok, Y.S. Designer carbon nanotubes for contaminant removal in water and wastewater: A critical review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, J.; Zhu, C.; Zeng, X.C.; Francisco, J.S. Water desalination through rim functionalized carbon nanotubes. J. Mater. Chem. A 2019, 7, 3583–3591. [Google Scholar] [CrossRef]
- Chen, Q.; Meng, L.; Li, Q.; Wang, D.; Guo, W.; Shuai, Z.; Jiang, L. Water transport and purification in nanochannels controlled by asymmetric wettability. Small 2011, 7, 2225–2231. [Google Scholar] [CrossRef]
- Corry, B. Water and ion transport through functionalised carbon nanotubes: Implications for desalination technology. Energy Environ. Sci. 2011, 4, 751–759. [Google Scholar] [CrossRef]
- Kim, E.S.; Hwang, G.; Gamal El-Din, M.; Liu, Y. Development of nanosilver and multi-walled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J. Membr. Sci. 2012, 394, 37–48. [Google Scholar] [CrossRef]
- Singh, R.; Samuel, M.S.; Ravikumar, M.; Ethiraj, S.; Kirankumar, V.S.; Kumar, M.; Arulvel, R.; Suresh, S. Processing of Carbon-Based Nanomaterials for the Removal of Pollutants from Water/Wastewater Application. Water 2023, 15, 3003. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoon, H.W.; Yoon, S.M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective gas transport through few-layered graphene and graphene oxide membranes. Science 2013, 342, 91–95. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Grossman, J.C. Nanoporous graphene as a reverse osmosis membrane: Recent insights from theory and simulation. Desalination 2015, 366, 59–70. [Google Scholar] [CrossRef]
- Kabiri, S.; Tran, D.N.H.; Cole, M.A.; Losic, D. Functionalized three-dimensional (3D) graphene composite for high efficiency removal of mercury. Environ. Sci. Water Res. Technol. 2016, 2, 390–402. [Google Scholar] [CrossRef]
- O’Hern, S.C.; Boutilier, M.S.H.; Idrobo, J.-C.; Song, Y.; Kong, J.; Laoui, T.; Atieh, M.; Karnik, R. Selective Ionic Transport through Tunable Subnanometer Pores in Single-Layer Graphene Membranes. Nano Lett. 2014, 14, 1234–1241. [Google Scholar] [CrossRef]
- An, Y.-C.; Gao, X.-X.; Jiang, W.-L.; Han, J.-L.; Ye, Y.; Chen, T.-M.; Ren, R.-Y.; Zhang, J.-H.; Liang, B.; Li, Z.-L.; et al. A critical review on graphene oxide membrane for industrial wastewater treatment. Environ. Res. 2023, 223, 115409. [Google Scholar] [CrossRef]
- Makhija, G.; Sharma, V.; Singh, S.; Sharma, N.; Vyas, R.; Sachdev, K. Investigation on the suitability of water/polyethylene glycol solutions for GO layer deposition in GO/Ag/GO films for transparent conducting electrode. Appl. Nanosci. 2019, 9, 1671–1683. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, F.; Zhang, P.; An, Z.; Zhao, Y.; Chen, L. The photodegradation of methylene blue in water with PVDF/GO/ZnO composite membrane. Mater. Sci. Eng. C 2019, 96, 684–692. [Google Scholar] [CrossRef]
- Li, G.; Shi, L.; Zeng, G.; Zhang, Y.; Sun, Y. Efficient dehydration of the organic solvents through graphene oxide (GO)/ceramic composite membranes. RSC Adv. 2014, 4, 52012–52015. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, H.; Wang, H.; Rassu, P.; Wang, Z.; Song, P.; Rao, D. Free-standing graphene oxide membrane with tunable channels for efficient water pollution control. J. Hazard. Mater. 2019, 366, 659–668. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, X.; Wei, Y.; Wang, X.; Wang, J.; Zhang, Y.; Gao, C. Enhanced desalination performance of carboxyl functionalized graphene oxide nanofiltration membranes. Desalination 2017, 405, 29–39. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Z.; Sun, D.D.; Ng, W.J. The efficient separation of surfactant-stabilized oil–water emulsions with a flexible and superhydrophilic graphene–TiO2composite membrane. J. Mater. Chem. A 2014, 2, 14082–14088. [Google Scholar] [CrossRef]
- Akhondi, E.; Wu, B.; Sun, S.; Marxer, B.; Lim, W.; Gu, J.; Liu, L.; Burkhardt, M.; McDougald, D.; Pronk, W.; et al. Gravity-driven membrane filtration as pretreatment for seawater reverse osmosis: Linking biofouling layer morphology with flux stabilization. Water Res. 2015, 70, 158–173. [Google Scholar] [CrossRef]
- Wang, W.; Li, R.; Bu, F.; Gao, Y.; Gao, B.; Yue, Q.; Yang, M.; Li, Y. Coagulation and membrane fouling mechanism of Al species in removing humic acid: Effect of pH and a dynamics process analysis. Sep. Purif. Technol. 2023, 309, 123130. [Google Scholar] [CrossRef]
- Kramer, F.C.; Shang, R.; Rietveld, L.C.; Heijman, S.J.G. Influence of pH, multivalent counter ions, and membrane fouling on phosphate retention during ceramic nanofiltration. Sep. Purif. Technol. 2019, 227, 115675. [Google Scholar] [CrossRef]
- Luo, J.; Wan, Y. Effects of pH and salt on nanofiltration—A critical review. J. Membr. Sci. 2013, 438, 18–28. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Susanto, H.; Nasseri, S.; Ulbricht, M. Influences of solution chemistry and polymeric natural organic matter on the removal of aquatic pharmaceutical residuals by nanofiltration. Water Res. 2009, 43, 3270–3280. [Google Scholar] [CrossRef]
- Wang, L.-F.; He, D.-Q.; Chen, W.; Yu, H.-Q. Probing the roles of Ca2+ and Mg2+ in humic acids-induced ultrafiltration membrane fouling using an integrated approach. Water Res. 2015, 81, 325–332. [Google Scholar] [CrossRef]
- Verliefde, A.R.D.; Cornelissen, E.R.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Amy, G.L.; Van der Bruggen, B.; van Dijk, J.C. The role of electrostatic interactions on the rejection of organic solutes in aqueous solutions with nanofiltration. J. Membr. Sci. 2008, 322, 52–66. [Google Scholar] [CrossRef]
- Al-Amoudi, A.S. Factors affecting natural organic matter (NOM) and scaling fouling in NF membranes: A review. Desalination 2010, 259, 1–10. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, W. Efficient faulty variable selection and parsimonious reconstruction modelling for fault isolation. J. Process Control 2016, 38, 31–41. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, Y.; Xu, X.; Peng, X.; Niu, Y.; Xu, P.; Li, T. Research on the factors influencing nanofiltration membrane fouling and the prediction of membrane fouling. J. Water Process Eng. 2024, 59, 104876. [Google Scholar] [CrossRef]
- Su, Z.; Liu, T.; Li, X.; Graham, N.J.D.; Yu, W. Tracking metal ion-induced organic membrane fouling in nanofiltration by adopting spectroscopic methods: Observations and predictions. Sci. Total Environ. 2020, 708, 135051. [Google Scholar] [CrossRef]
- Laitinen, N.; Michaud, D.; Piquet, C.; Teilleria, N.; Luonsi, A.; Levänen, E.; Nyström, M. Effect of filtration conditions and backflushing on ceramic membrane ultrafiltration of board industry wastewaters. Sep. Purif. Technol. 2001, 24, 319–328. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.; Huang, S.; Mai, S.; Yang, C.; Wang, X.; Zhou, Z. Effects of various factors on critical flux in submerged membrane bioreactors for municipal wastewater treatment. Sep. Purif. Technol. 2008, 62, 56–63. [Google Scholar] [CrossRef]
- Ji, X.; Huang, J.; Teng, L.; Li, S.; Li, X.; Cai, W.; Chen, Z.; Lai, Y. Advances in particulate matter filtration: Materials, performance, and application. Green Energy Environ. 2023, 8, 673–697. [Google Scholar] [CrossRef]
- Wu, B.; Hochstrasser, F.; Akhondi, E.; Ambauen, N.; Tschirren, L.; Burkhardt, M.; Fane, A.G.; Pronk, W. Optimization of gravity-driven membrane (GDM) filtration process for seawater pretreatment. Water Res. 2016, 93, 133–140. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, Q.; Meng, B.; Zhou, K.; Liu, G.; Gugliuzza, A.; Drioli, E.; Jin, W. Roughness-enhanced hydrophobic graphene oxide membrane for water desalination via membrane distillation. J. Membr. Sci. 2020, 611, 118364. [Google Scholar] [CrossRef]
- Sun, C.; Lin, B.; Zheng, X.; Dong, Y.; Zhao, M.; Tang, C.Y. Robust ceramic-based graphene membrane for challenging water treatment with enhanced fouling and scaling resistance. Water Res. 2023, 243, 120348. [Google Scholar] [CrossRef]
- Baig, U.; Faizan, M.; Sajid, M. Multifunctional membranes with super-wetting characteristics for oil-water separation and removal of hazardous environmental pollutants from water: A review. Adv. Colloid Interface Sci. 2020, 285, 102276. [Google Scholar] [CrossRef]
- Wae AbdulKadir, W.A.F.; Ahmad, A.L.; Seng, O.B.; Che Lah, N.F. Biomimetic hydrophobic membrane: A review of anti-wetting properties as a potential factor in membrane development for membrane distillation (MD). J. Ind. Eng. Chem. 2020, 91, 15–36. [Google Scholar] [CrossRef]
- Mallya, D.S.; Yang, G.; Lei, W.; Muthukumaran, S.; Baskaran, K. Tuning nanofiltration membrane performance: OH–MoS2 nanosheet engineering and divalent cation influence on fouling and organic removal. Discov. Nano 2023, 18, 131. [Google Scholar] [CrossRef]
- Bortot Coelho, F.E.; Kaiser, N.N.; Magnacca, G.; Candelario, V.M. Corrosion resistant ZrO2/SiC ultrafiltration membranes for wastewater treatment and operation in harsh environments. J. Eur. Ceram. Soc. 2021, 41, 7792–7806. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse osmosis pretreatment technologies and future trends: A comprehensive review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef]
- Duan, J.; Wilson, F.; Graham, N.; Tay, J.H. Adsorption of humic acid by powdered activated carbon in saline water conditions. Desalination 2003, 151, 53–66. [Google Scholar] [CrossRef]
- Huang, H.; Schwab, K.; Jacangelo, J.G. Pretreatment for low pressure membranes in water treatment: A review. Environ. Sci. Technol. 2009, 43, 3011–3019. [Google Scholar] [CrossRef]
- Huang, H.; Young, T.A.; Jacangelo, J.G. Chlorine-induced permeability recovery for low-pressure membrane filtration of natural waters. J. Membr. Sci. 2008, 325, 50–57. [Google Scholar] [CrossRef]
- Sakamoto, H.; Hafuka, A.; Tsuchiya, T.; Kimura, K. Intensive routine cleaning for mitigation of fouling in flat-sheet ceramic membranes used for drinking water production: Unique characteristics of resulting foulants. Sep. Purif. Technol. 2022, 301, 121950. [Google Scholar] [CrossRef]
- Hube, S.; Hauser, F.; Burkhardt, M.; Brynjólfsson, S.; Wu, B. Ultrasonication-assisted fouling control during ceramic membrane filtration of primary wastewater under gravity-driven and constant flux conditions. Sep. Purif. Technol. 2023, 310, 123083. [Google Scholar] [CrossRef]
- Ghadimkhani, A.; Zhang, W.; Marhaba, T. Ceramic membrane defouling (cleaning) by air Nano Bubbles. Chemosphere 2016, 146, 379–384. [Google Scholar] [CrossRef]
- Omar, N.M.A.; Othman, M.H.D.; Tai, Z.S.; Milad, M.; Sokri, M.N.M.; Puteh, M.H. Recent progress and technical improvement strategies for mitigating ceramic membrane bottlenecks in water purification processes: A review. Int. J. Appl. Ceram. Technol. 2023, 20, 3327–3356. [Google Scholar] [CrossRef]
- Wu, H.; Sun, C.; Huang, Y.; Zheng, X.; Zhao, M.; Gray, S.; Dong, Y. Treatment of oily wastewaters by highly porous whisker-constructed ceramic membranes: Separation performance and fouling models. Water Res. 2022, 211, 118042. [Google Scholar] [CrossRef]
- Sun, H.; Liu, H.; Han, J.; Zhang, X.; Cheng, F.; Liu, Y. Chemical cleaning-associated generation of dissolved organic matter and halogenated byproducts in ceramic MBR: Ozone versus hypochlorite. Water Res. 2018, 140, 243–250. [Google Scholar] [CrossRef]
- Shang, R.; Verliefde, A.R.; Hu, J.; Zeng, Z.; Lu, J.; Kemperman, A.J.; Deng, H.; Nijmeijer, K.; Heijman, S.G.; Rietveld, L.C. Tight ceramic UF membrane as RO pre-treatment: The role of electrostatic interactions on phosphate rejection. Water Res. 2014, 48, 498–507. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Liu, Z.; Zhang, X. Integration of ferrate (VI) pretreatment and ceramic membrane reactor for membrane fouling mitigation in reclaimed water treatment. J. Membr. Sci. 2018, 552, 315–325. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, H.; Ding, A.; Tang, X.; Liu, B.; Zhu, X.; Gan, Z.; Wu, D.; Li, G. Ferrous iron/peroxymonosulfate oxidation as a pretreatment for ceramic ultrafiltration membrane: Control of natural organic matter fouling and degradation of atrazine. Water Res. 2017, 113, 32–41. [Google Scholar] [CrossRef]
- Mao, H.; Qiu, M.; Chen, X.; Verweij, H.; Fan, Y. Fabrication and in-situ fouling mitigation of a supported carbon nanotube/γ-alumina ultrafiltration membrane. J. Membr. Sci. 2018, 550, 26–35. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, T.; Chang, Q.; Ma, C.; Yang, Y.; Wang, S.; Pan, Z.; Sun, Y.; Ding, G. Performance Stability and Regeneration Property of Catalytic Membranes Coupled with Advanced Oxidation Process: A Comprehensive Review. Sustainability 2023, 15, 7556. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Ait Akbour, R.; Nidheesh, P.V.; Hamdani, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process. Process Intensif. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Chang, Q.; Zhou, J.-E.; Wang, Y.; Liang, J.; Zhang, X.; Cerneaux, S.; Wang, X.; Zhu, Z.; Dong, Y. Application of ceramic microfiltration membrane modified by nano-TiO2 coating in separation of a stable oil-in-water emulsion. J. Membr. Sci. 2014, 456, 128–133. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Ghalamchi, L.; Vatanpour, V.; Khataee, A. Photocatalytic-membrane technology: A critical review for membrane fouling mitigation. J. Ind. Eng. Chem. 2021, 93, 101–116. [Google Scholar] [CrossRef]
- Vatanpour, V.; Karami, A.; Sheydaei, M. Central composite design optimization of Rhodamine B degradation using TiO 2 nanoparticles/UV/PVDF process in continuous submerged membrane photoreactor. Chem. Eng. Process. Process Intensif. 2017, 116, 68–75. [Google Scholar] [CrossRef]
- Khataee, A.; Arefi-Oskoui, S.; Abdollahi, B.; Hanifehpour, Y.; Joo, S.W. Synthesis and characterization of Pr x Zn1−x Se nanoparticles for photocatalysis of four textile dyes with different molecular structures. Res. Chem. Intermed. 2015, 41, 8425–8439. [Google Scholar] [CrossRef]
- Adeleke, J.T.; Theivasanthi, T.; Thiruppathi, M.; Swaminathan, M.; Akomolafe, T.; Alabi, A.B. Photocatalytic degradation of methylene blue by ZnO/NiFe2O4 nanoparticles. Appl. Surf. Sci. 2018, 455, 195–200. [Google Scholar] [CrossRef]
- Ghalamchi, L.; Aber, S.; Vatanpour, V.; Kian, M. Development of an antibacterial and visible photocatalytic nanocomposite microfiltration membrane incorporated by Ag3PO4/CuZnAl NLDH. Sep. Purif. Technol. 2019, 226, 218–231. [Google Scholar] [CrossRef]
- Fan, H.; Li, G.; Yang, F.; Yang, L.; Zhang, S. Photodegradation of cellulose under UV light catalysed by TiO2. J. Chem. Technol. Biotechnol. 2011, 86, 1107–1112. [Google Scholar] [CrossRef]
- Huang, T.; Huang, W.; Zhou, C.; Situ, Y.; Huang, H. Superhydrophilicity of TiO2/SiO2 thin films: Synergistic effect of SiO2 and phase-separation-induced porous structure. Surf. Coat. Technol. 2012, 213, 126–132. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Chen, M.; Heijman, S.G.J.; Rietveld, L.C. State-of-the-Art Ceramic Membranes for Oily Wastewater Treatment: Modification and Application. Membranes 2021, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Lai, C.; Wang, D.; Liu, S.; Li, X.; Zhou, X.; Yi, H.; Li, B.; Zhang, M.; Li, L.; et al. In situ chemical oxidation: Peroxide or persulfate coupled with membrane technology for wastewater treatment. J. Mater. Chem. A 2021, 9, 11944–11960. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, B.; Qi, F. A novel ceramic membrane coated with MnO2–Co3O4 nanoparticles catalytic ozonation for benzophenone-3 degradation in aqueous solution: Fabrication, characterization and performance. Chem. Eng. J. 2016, 287, 381–389. [Google Scholar] [CrossRef]
- Szymański, K.; Morawski, A.W.; Mozia, S. Humic acids removal in a photocatalytic membrane reactor with a ceramic UF membrane. Chem. Eng. J. 2016, 305, 19–27. [Google Scholar] [CrossRef]
- Liu, X.; Cao, L.; Sun, W.; Zhou, Z.; Yang, J. A P/N type compounded Cu2O/TiO2 photo-catalytic membrane for organic pollutant degradation. Res. Chem. Intermed. 2016, 42, 6289–6300. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, X.; Chen, S.; Zhao, H.; Zhao, Y. The removal of sodium dodecylbenzene sulfonate surfactant from water using silica/titania nanorods/nanotubes composite membrane with photocatalytic capability. Appl. Surf. Sci. 2006, 252, 8598–8604. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Wang, Z.-b.; Guan, Y.-j.; Chen, B.; Bai, S.-l. Retention and separation of 4BS dye from wastewater by the N-TiO2 ceramic membrane. Desalination Water Treat. 2015, 57, 16963–16969. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef]
- Kandy, M.M.; Gaikar, V.G. Photocatalytic reduction of CO2 using CdS nanorods on porous anodic alumina support. Mater. Res. Bull. 2018, 102, 440–449. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, J.; Kang, P.; Tang, Q.; Sun, Q.; Ma, M. Ag nanocrystals decorated g-C3N4/Nafion hybrid membranes: One-step synthesis and photocatalytic performance. Mater. Lett. 2018, 213, 218–221. [Google Scholar] [CrossRef]
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic degradation of rhodamine B and real textile wastewater using Fe-doped TiO2 anchored on reduced graphene oxide (Fe-TiO2/rGO): Characterization and feasibility, mechanism and pathway studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Kwon, B.G. Characterization of the hydroperoxyl/superoxide anion radical (HO2/O2−) formed from the photolysis of immobilized TiO2 in a continuous flow. J. Photochem. Photobiol. A Chem. 2008, 199, 112–118. [Google Scholar] [CrossRef]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 2007, 202, 199–206. [Google Scholar] [CrossRef]
- Kumakiri, I.; Diplas, S.; Simon, C.; Nowak, P. Photocatalytic Membrane Contactors for Water Treatment. Ind. Eng. Chem. Res. 2011, 50, 6000–6008. [Google Scholar] [CrossRef]
- Kirk, C.H.; Wang, P.; Chong, C.Y.D.; Zhao, Q.; Sun, J.; Wang, J. TiO2 photocatalytic ceramic membranes for water and wastewater treatment: Technical readiness and pathway ahead. J. Mater. Sci. Technol. 2024, 183, 152–164. [Google Scholar] [CrossRef]
- Babu, S.G.; Karthik, P.; John, M.C.; Lakhera, S.K.; Ashokkumar, M.; Khim, J.; Neppolian, B. Synergistic effect of sono-photocatalytic process for the degradation of organic pollutants using CuO-TiO2/rGO. Ultrason. Sonochemistry 2019, 50, 218–223. [Google Scholar] [CrossRef]
- Khader, E.H.; Mohammed, T.J.; Albayati, T.M.; Harharah, H.N.; Amari, A.; Saady, N.M.C.; Zendehboudi, S. Current trends for wastewater treatment technologies with typical configurations of photocatalytic membrane reactor hybrid systems: A review. Chem. Eng. Process. Process Intensif. 2023, 192, 109503. [Google Scholar] [CrossRef]
- Jagadevan, S.; Graham, N.J.; Thompson, I.P. Treatment of waste metalworking fluid by a hybrid ozone-biological process. J Hazard. Mater. 2013, 244, 394–402. [Google Scholar] [CrossRef]
- Kim, J.; Davies, S.H.R.; Baumann, M.J.; Tarabara, V.V.; Masten, S.J. Effect of ozone dosage and hydrodynamic conditions on the permeate flux in a hybrid ozonation–ceramic ultrafiltration system treating natural waters. J. Membr. Sci. 2008, 311, 165–172. [Google Scholar] [CrossRef]
- Chiang, Y.P.; Liang, Y.Y.; Chang, C.N.; Chao, A.C. Differentiating ozone direct and indirect reactions on decomposition of humic substances. Chemosphere 2006, 65, 2395–2400. [Google Scholar] [CrossRef]
- Ghuge, S.P.; Saroha, A.K. Catalytic ozonation for the treatment of synthetic and industrial effluents—Application of mesoporous materials: A review. J Environ. Manage. 2018, 211, 83–102. [Google Scholar] [CrossRef]
- Byun, S.; Davies, S.H.; Alpatova, A.L.; Corneal, L.M.; Baumann, M.J.; Tarabara, V.V.; Masten, S.J. Mn oxide coated catalytic membranes for a hybrid ozonation–membrane filtration: Comparison of Ti, Fe and Mn oxide coated membranes for water quality. Water Res. 2011, 45, 163–170. [Google Scholar] [CrossRef]
- Park, H.; Kim, Y.; An, B.; Choi, H. Characterization of natural organic matter treated by iron oxide nanoparticle incorporated ceramic membrane-ozonation process. Water Res. 2012, 46, 5861–5870. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y. Ceramic membrane separation coupled with catalytic ozonation for tertiary treatment of dyestuff wastewater in a pilot-scale study. Chem. Eng. J. 2016, 301, 19–26. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, H.; Qu, F.; Ding, A.; Chang, H.; Liu, B.; Tang, X.; Wu, D.; Li, G. Fabrication of Mn oxide incorporated ceramic membranes for membrane fouling control and enhanced catalytic ozonation of p -chloronitrobenzene. Chem. Eng. J. 2017, 308, 1010–1020. [Google Scholar] [CrossRef]
- Chen, S.; Yu, J.; Wang, H.; Yu, H.; Quan, X. A pilot-scale coupling catalytic ozonation–membrane filtration system for recirculating aquaculture wastewater treatment. Desalination 2015, 363, 37–43. [Google Scholar] [CrossRef]
- Guo, Y.; Song, Z.; Xu, B.; Li, Y.; Qi, F.; Croue, J.P.; Yuan, D. A novel catalytic ceramic membrane fabricated with CuMn(2)O(4) particles for emerging UV absorbers degradation from aqueous and membrane fouling elimination. J Hazard. Mater. 2018, 344, 1229–1239. [Google Scholar] [CrossRef]
- Lee, W.J.; Bao, Y.; Hu, X.; Lim, T.-T. Hybrid catalytic ozonation-membrane filtration process with CeOx and MnOx impregnated catalytic ceramic membranes for micropollutants degradation. Chem. Eng. J. 2019, 378, 121670. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, K.; Wang, H.; Wang, X.-m.; Zhang, X.-h.; Huang, X. Incorporating catalytic ceramic membrane into the integrated process of in situ ozonation, membrane filtration and biological degradation: Enhanced performance and underlying mechanisms. J. Membr. Sci. 2022, 652, 120509. [Google Scholar] [CrossRef]
- Li, P.; Miao, R.; Wang, P.; Sun, F.; Li, X.-y. Bi-metal oxide-modified flat-sheet ceramic membranes for catalytic ozonation of organic pollutants in wastewater treatment. Chem. Eng. J. 2021, 426, 131263. [Google Scholar] [CrossRef]
- Szymański, K.; Mozia, S.; Ayral, A.; Brosillon, S.; Mendret, J. Hybrid system coupling ozonation and nanofiltration with functionalized catalytic ceramic membrane for ibuprofen removal. Environ. Sci. Pollut. Res. 2023, 30, 69042–69053. [Google Scholar] [CrossRef]
- Sui, M.; Sheng, L.; Lu, K.; Tian, F. FeOOH catalytic ozonation of oxalic acid and the effect of phosphate binding on its catalytic activity. Appl. Catal. B Environ. 2010, 96, 94–100. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, H.; Peng, P.; Bo, H. Degradation and transformation of atrazine under catalyzed ozonation process with TiO2 as catalyst. J Hazard. Mater. 2014, 279, 444–451. [Google Scholar] [CrossRef]
- Liu, J.; He, K.; Zhang, J.; Li, C.; Zhang, Z. Coupling ferrate pretreatment and in-situ ozonation/ceramic membrane filtration for wastewater reclamation: Water quality and membrane fouling. J. Membr. Sci. 2019, 590, 117310. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Jiang, P.; Wang, G.; Zhang, J.; Zhang, C. ZnAl2O4 as a novel high-surface-area ozonation catalyst: One-step green synthesis, catalytic performance and mechanism. Chem. Eng. J. 2015, 260, 623–630. [Google Scholar] [CrossRef]
- Masten, S.J.; Tarabara, V.V.; Corneal, L.M.; Byun, S.; Baumann, M.J.; Davies, S.H. Fabrication of catalytic ceramic membranes for water filtration. Water Supply 2010, 10, 81–86. [Google Scholar] [CrossRef]
- Chen, X.; Ma, J.; Chen, J.; Wang, Z. Ceramic membrane filtration coupled with ozonation for water purification: Principles, applications and perspectives. J. Water Process Eng. 2023, 55, 104127. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M.; Gohari, F. Degradation of 2,4,6-trichlorophenol in aqueous solutions using peroxymonosulfate/activated carbon/UV process via sulfate and hydroxyl radicals. J. Water Process Eng. 2016, 9, 22–28. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Xiao, H.; Hong, C.; Zhu, F.; Yao, Y.; Xue, Z. Accelerated TiO2 photocatalytic degradation of Acid Orange 7 under visible light mediated by peroxymonosulfate. Chem. Eng. J. 2012, 193, 290–295. [Google Scholar] [CrossRef]
- Furman, O.S.; Teel, A.L.; Ahmad, M.; Merker, M.C.; Watts, R.J. Effect of Basicity on Persulfate Reactivity. J. Environ. Eng. 2011, 137, 241–247. [Google Scholar] [CrossRef]
- Zou, J.; Ma, J.; Chen, L.; Li, X.; Guan, Y.; Xie, P.; Pan, C. Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine. Environ. Sci. Technol. 2013, 47, 11685–11691. [Google Scholar] [CrossRef]
- Luo, X.; Liang, H.; Qu, F.; Ding, A.; Cheng, X.; Tang, C.Y.; Li, G. Free-standing hierarchical alpha-MnO(2)@CuO membrane for catalytic filtration degradation of organic pollutants. Chemosphere 2018, 200, 237–247. [Google Scholar] [CrossRef]
- Bao, Y.; Oh, W.-D.; Lim, T.-T.; Wang, R.; Webster, R.D.; Hu, X. Surface-nucleated heterogeneous growth of zeolitic imidazolate framework—A unique precursor towards catalytic ceramic membranes: Synthesis, characterization and organics degradation. Chem. Eng. J. 2018, 353, 69–79. [Google Scholar] [CrossRef]
- Bao, Y.; Lim, T.-T.; Wang, R.; Webster, R.D.; Hu, X. Urea-assisted one-step synthesis of cobalt ferrite impregnated ceramic membrane for sulfamethoxazole degradation via peroxymonosulfate activation. Chem. Eng. J. 2018, 343, 737–747. [Google Scholar] [CrossRef]
- Wang, S.; Tian, J.; Wang, Q.; Xiao, F.; Gao, S.; Shi, W.; Cui, F. Development of CuO coated ceramic hollow fiber membrane for peroxymonosulfate activation: A highly efficient singlet oxygen-dominated oxidation process for bisphenol a degradation. Appl. Catal. B Environ. 2019, 256, 117783. [Google Scholar] [CrossRef]
- Chen, L.; Maqbool, T.; Nazir, G.; Hou, C.; Yang, Y.; Guo, J.; Zhang, X. Developing the large-area manganese-based catalytic ceramic membrane for peroxymonosulfate activation: Applications in degradation of endocrine disrupting compounds in drinking water. J. Membr. Sci. 2022, 655, 120602. [Google Scholar] [CrossRef]
- Hirani, R.A.K.; Wu, H.; Asif, A.H.; Rafique, N.; Shi, L.; Zhang, S.; Wu, Z.; Zhang, L.-C.; Wang, S.; Yin, Y.; et al. Cobalt oxide functionalized ceramic membrane for 4-hydroxybenzoic acid degradation via peroxymonosulfate activation. J. Hazard. Mater. 2023, 448, 130874. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Y.; Guan, Z.; Li, Q.; Xia, D. Development of CuCo2O4 integrated ceramic membrane for peroxymonosulfate activation: An efficient oxidation process for bisphenol A degradation. J. Environ. Chem. Eng. 2023, 11, 109500. [Google Scholar] [CrossRef]
- Wang, Z.; Nengzi, L.-c.; Zhang, X.; Zhao, Z.; Cheng, X. Novel NiCo2S4/CS membranes as efficient catalysts for activating persulfate and its high activity for degradation of nimesulide. Chem. Eng. J. 2020, 381, 122571. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, H.; Duan, X.; Sun, H.; Tan, X.; Liu, S.; Wang, S. Magnetic Ni-Co alloy encapsulated N-doped carbon nanotubes for catalytic membrane degradation of emerging contaminants. Chem. Eng. J. 2019, 362, 251–261. [Google Scholar] [CrossRef]
- Sheng, J.; Yin, H.; Qian, F.; Huang, H.; Gao, S.; Wang, J. Reduced graphene oxide-based composite membranes for in-situ catalytic oxidation of sulfamethoxazole operated in membrane filtration. Sep. Purif. Technol. 2020, 236, 116275. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Yu, S.; Chen, B.; Chen, C.; Shen, L.; Li, B.; Lin, H. The coupling of persulfate activation and membrane separation for the effective pollutant degradation and membrane fouling alleviation. Chem. Eng. J. 2023, 451, 139009. [Google Scholar] [CrossRef]
- Liangdy, A.; Lee, W.J.; Bao, Y.; Oh, W.-D.; Lim, T.-T. Hybrid process of persulfate-based advanced oxidation with MeOx-functionalized catalytic ceramic membrane for synergistic removal of micropollutants: Recent developments, new insights, and prospects. Chem. Eng. J. 2023, 466, 143280. [Google Scholar] [CrossRef]
- Shan, H.; Dong, X.; Cheng, X.; Si, Y.; Yu, J.; Ding, B. Highly flexible, mesoporous structured, and metallic Cu-doped C/SiO2 nanofibrous membranes for efficient catalytic oxidative elimination of antibiotic pollutants. Nanoscale 2019, 11, 14844–14856. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, D.; Xu, C.; Zhong, J.; Chen, M.; Xu, S.; Cao, Y.; Zhao, Q.; Yang, M.; Ma, J. Synergistic oxidation—Filtration process analysis of catalytic CuFe2O4—Tailored ceramic membrane filtration via peroxymonosulfate activation for humic acid treatment. Water Res. 2020, 171, 115387. [Google Scholar] [CrossRef]
- Wu, H.; Xu, X.; Shi, L.; Yin, Y.; Zhang, L.C.; Wu, Z.; Duan, X.; Wang, S.; Sun, H. Manganese oxide integrated catalytic ceramic membrane for degradation of organic pollutants using sulfate radicals. Water Res. 2019, 167, 115110. [Google Scholar] [CrossRef]
- Fan, X.; Li, S.; Sun, M.; Song, C.; Xiao, J.; Du, J.; Tao, P.; Sun, T.; Shao, M.; Wang, T. Degradation of phenol by coal-based carbon membrane integrating sulfate radicals-based advanced oxidation processes. Ecotoxicol. Environ. Saf. 2019, 185, 109662. [Google Scholar] [CrossRef]
- Wang, Y.; Ao, Z.; Sun, H.; Duan, X.; Wang, S. Activation of peroxymonosulfate by carbonaceous oxygen groups: Experimental and density functional theory calculations. Appl. Catal. B Environ. 2016, 198, 295–302. [Google Scholar] [CrossRef]
- Yu, C.; Xiong, Z.; Zhou, H.; Zhou, P.; Zhang, H.; Huang, R.; Yao, G.; Lai, B. Marriage of membrane filtration and sulfate radical-advanced oxidation processes (SR-AOPs) for water purification: Current developments, challenges and prospects. Chem. Eng. J. 2022, 433, 133802. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, K.; Zhou, Y.; Yu, W.; Tao, S.; Le, C.; Lu, D.; Yu, Z.; Liang, S.; Hu, J.; et al. Profiling of amino acids and their interactions with proteinaceous compounds for sewage sludge dewatering by Fenton oxidation treatment. Water Res. 2020, 175, 115645. [Google Scholar] [CrossRef]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Effect of matrix components on UV/H2O2 and UV/S2O82− advanced oxidation processes for trace organic degradation in reverse osmosis brines from municipal wastewater reuse facilities. Water Res. 2016, 89, 192–200. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. An overview of the application of Fenton oxidation to industrial wastewaters treatment. J. Chem. Technol. Biotechnol. 2008, 83, 1323–1338. [Google Scholar] [CrossRef]
- Yang, T.; Yu, D.; Wang, D.; Yang, T.; Li, Z.; Wu, M.; Petru, M.; Crittenden, J. Accelerating Fe(III)/Fe(II) cycle via Fe(II) substitution for enhancing Fenton-like performance of Fe-MOFs. Appl. Catal. B Environ. 2021, 286, 119859. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Sun, J.; Wang, Y.; Zhao, X. Electrospun flexible self-standing Cu–Al2O3 fibrous membranes as Fenton catalysts for bisphenol A degradation. J. Mater. Chem. A 2017, 5, 19151–19158. [Google Scholar] [CrossRef]
- Wang, X.; Dou, L.; Yang, L.; Yu, J.; Ding, B. Hierarchical structured MnO2@SiO2 nanofibrous membranes with superb flexibility and enhanced catalytic performance. J. Hazard. Mater. 2017, 324, 203–212. [Google Scholar] [CrossRef]
- Shi, F.; Shan, H.; Li, D.; Yin, X.; Yu, J.; Ding, B. A general strategy to fabricate soft magnetic CuFe(2)O(4)@SiO(2) nanofibrous membranes as efficient and recyclable Fenton-like catalysts. J. Colloid Interface Sci. 2019, 538, 620–629. [Google Scholar] [CrossRef]
- Plakas, K.V.; Mantza, A.; Sklari, S.D.; Zaspalis, V.T.; Karabelas, A.J. Heterogeneous Fenton-like oxidation of pharmaceutical diclofenac by a catalytic iron-oxide ceramic microfiltration membrane. Chem. Eng. J. 2019, 373, 700–708. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, Z.; Cui, K.; Tang, Y.; Du, X.; He, B.; Li, M.; Feng, J.; Yu, B.; Xiong, W. Catalytic wet peroxide oxidation of phenolic wastewater on novel Cu/Mn-UiO-66@Al2O3 ceramic tube membrane catalysts. Chem. Eng. J. 2022, 430, 132787. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Graphene oxide/Fe(III)-based metal-organic framework membrane for enhanced water purification based on synergistic separation and photo-Fenton processes. Appl. Catal. B Environ. 2020, 264, 118548. [Google Scholar] [CrossRef]
- Li, Q.; Kong, H.; Li, P.; Shao, J.; He, Y. Photo-Fenton degradation of amoxicillin via magnetic TiO2-graphene oxide-Fe3O4 composite with a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). J. Hazard. Mater. 2019, 373, 437–446. [Google Scholar] [CrossRef]
- Jiang, W.-L.; Xia, X.; Han, J.-L.; Ding, Y.-C.; Haider, M.R.; Wang, A.-J. Graphene Modified Electro-Fenton Catalytic Membrane for in Situ Degradation of Antibiotic Florfenicol. Environ. Sci. Technol. 2018, 52, 9972–9982. [Google Scholar] [CrossRef]
- Huong Le, T.X.; Dumée, L.F.; Lacour, S.; Rivallin, M.; Yi, Z.; Kong, L.; Bechelany, M.; Cretin, M. Hybrid graphene-decorated metal hollow fibre membrane reactors for efficient electro-Fenton— Filtration co-processes. J. Membr. Sci. 2019, 587, 117182. [Google Scholar] [CrossRef]
- Li, Z.; Shen, C.; Liu, Y.; Ma, C.; Li, F.; Yang, B.; Huang, M.; Wang, Z.; Dong, L.; Wolfgang, S. Carbon nanotube filter functionalized with iron oxychloride for flow-through electro-Fenton. Appl. Catal. B Environ. 2020, 260, 118204. [Google Scholar] [CrossRef]
- Sharma, V.K.; Feng, M. Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater. 2019, 372, 3–16. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, S.; Xiong, W.; Liu, D.; Li, M.; He, B.; Fan, X.; Luo, D. Supported CuO catalysts on metal-organic framework (Cu-UiO-66) for efficient catalytic wet peroxide oxidation of 4-chlorophenol in wastewater. Microporous Mesoporous Mater. 2020, 291, 109703. [Google Scholar] [CrossRef]
- Ahile, U.J.; Wuana, R.A.; Itodo, A.U.; Sha’Ato, R.; Dantas, R.F. A review on the use of chelating agents as an alternative to promote photo-Fenton at neutral pH: Current trends, knowledge gap and future studies. Sci. Total Environ. 2020, 710, 134872. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Li, Y.; Chen, X.; Yao, Y.; Zhang, P.; Ren, S.; Li, M.; Luo, Y.; Chen, N.; et al. Heterogeneous electro-Fenton catalytic FeOCl@NCNT/ceramic membrane filtration for in-situ membrane fouling control: Performance and mechanism. Sep. Purif. Technol. 2023, 316, 123845. [Google Scholar] [CrossRef]
- Jiang, W.-L.; Ding, Y.-C.; Haider, M.R.; Han, J.-L.; Liang, B.; Xia, X.; Yang, L.-M.; Wang, H.-c.; Peng, Y.-Z.; Wang, A.-J. A novel TiO2/graphite felt photoanode assisted electro-Fenton catalytic membrane process for sequential degradation of antibiotic florfenicol and elimination of its antibacterial activity. Chem. Eng. J. 2020, 391, 123503. [Google Scholar] [CrossRef]
- Inchaurrondo, N.; Font, J.; Ramos, C.P.; Haure, P. Natural diatomites: Efficient green catalyst for Fenton-like oxidation of Orange II. Appl. Catal. B Environ. 2016, 181, 481–494. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, S.; Meng, X.; Yu, S. Research progress of MOF-based membrane reactor coupled with AOP technology for organic wastewater treatment. Environ. Sci. Pollut. Res. 2023, 30, 104958–104975. [Google Scholar] [CrossRef]
- Ye, Z.; Oriol, R.; Yang, C.; Sirés, I.; Li, X.-Y. A novel NH2-MIL-88B(Fe)-modified ceramic membrane for the integration of electro-Fenton and filtration processes: A case study on naproxen degradation. Chem. Eng. J. 2022, 433, 133547. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Wang, H.; Wang, Z.; Zeng, G.; Xu, P.; Huang, D.; Chen, M.; Song, B.; Qin, H.; et al. Metal-organic framework-derived nanomaterials in environment related fields: Fundamentals, properties and applications. Coord. Chem. Rev. 2021, 429, 213618. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, S.; Zhou, J.; Quan, X. A novel porous-carbon-based hollow fiber membrane with electrochemical reduction mediated by in-situ hydroxyl radical generation for fouling control and water treatment. Appl. Catal. B Environ. 2019, 255, 117772. [Google Scholar] [CrossRef]
- Zhu, X.; Jassby, D. Electroactive Membranes for Water Treatment: Enhanced Treatment Functionalities, Energy Considerations, and Future Challenges. Acc. Chem. Res. 2019, 52, 1177–1186. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Li, C.; Guo, X.; Wang, X.; Fan, S.; Zhou, Q.; Shao, H.; Hu, W.; Li, C.; Tong, L.; Kumar, R.R.; et al. Membrane fouling mitigation by coupling applied electric field in membrane system: Configuration, mechanism and performance. Electrochim. Acta 2018, 287, 124–134. [Google Scholar] [CrossRef]
- Tao, P.; Xu, Y.; Song, C.; Yin, Y.; Yang, Z.; Wen, S.; Wang, S.; Liu, H.; Li, S.; Li, C.; et al. A novel strategy for the removal of rhodamine B (RhB) dye from wastewater by coal-based carbon membranes coupled with the electric field. Sep. Purif. Technol. 2017, 179, 175–183. [Google Scholar] [CrossRef]
- Sun, M.; Feng, G.; Zhang, M.; Song, C.; Tao, P.; Wang, T.; Shao, M. Enhanced removal ability of phenol from aqueous solution using coal-based carbon membrane coupled with electrochemical oxidation process. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 186–193. [Google Scholar] [CrossRef]
- Li, C.; Feng, G.; Pan, Z.; Song, C.; Fan, X.; Tao, P.; Wang, T.; Shao, M.; Zhao, S. High-performance electrocatalytic microfiltration CuO/Carbon membrane by facile dynamic electrodeposition for small-sized organic pollutants removal. J. Membr. Sci. 2020, 601, 117913. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Mansas, C.; Mendret, J.; Brosillon, S.; Ayral, A. Coupling catalytic ozonation and membrane separation: A review. Sep. Purif. Technol. 2020, 236, 116221. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Bao, C.; Xu, X.; Li, D.; Chen, J.; Hong, M.; Peng, B.; Zhang, Q. Self-cleaning catalytic membrane for water treatment via an integration of Heterogeneous Fenton and membrane process. J. Membr. Sci. 2021, 624, 119121. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.F.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar] [CrossRef]
- Jeong, Y.; Cho, K.; Kwon, E.E.; Tsang, Y.F.; Rinklebe, J.; Park, C. Evaluating the feasibility of pyrophyllite-based ceramic membranes for treating domestic wastewater in anaerobic ceramic membrane bioreactors. Chem. Eng. J. 2017, 328, 567–573. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Kwiecińska-Mydlak, A. New generation of semipermeable membranes with carbon nanotubes for water and wastewater treatment: Critical review. Arch. Environ. Prot. 2021, 47, 3–27. [Google Scholar] [CrossRef]
- Yi, Q.; Li, Z.; Li, J.; Zhou, J.; Li, X.; Dai, R.; Wang, X. Enhancing oxidants activation by transition metal-modified catalytic membranes for wastewater treatment. Res. Chem. Intermed. 2023, 49, 655–678. [Google Scholar] [CrossRef]
- Dong, Z.; Shang, W.; Dong, W.; Zhao, L.; Li, M.; Wang, R.; Sun, F. Suppression of membrane fouling in the ceramic membrane bioreactor (CMBR) by minute electric field. Bioresour. Technol. 2018, 270, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiao, S.; Jin, R.; Zhou, J.; Quan, X. Novel Anaerobic Electrochemical Membrane Bioreactor with a CNTs Hollow Fiber Membrane Cathode to Mitigate Membrane Fouling and Enhance Energy Recovery. Environ. Sci. Technol. 2019, 53, 1014–1021. [Google Scholar] [CrossRef]
- Duan, W.; Ronen, A.; de Leon, J.V.; Dudchenko, A.; Yao, S.; Corbala-Delgado, J.; Yan, A.; Matsumoto, M.; Jassby, D. Treating anaerobic sequencing batch reactor effluent with electrically conducting ultrafiltration and nanofiltration membranes for fouling control. J. Membr. Sci. 2016, 504, 104–112. [Google Scholar] [CrossRef]


| Membrane Properties | Operating Conditions | Strategy | Fouling Mitigation Effect | Comments | Ref. |
|---|---|---|---|---|---|
| Flat ceramic membrane (200 nm) | HRT: 0.5 h, Flux: 30 LMH, MLSS: 1 g/L, O3 and NaClO: 0~5 mg/L | Chemical cleaning | O3 effectively degraded larger biopolymers to low molecular weight substances. | O3 cleaning produced less TOCl, which was more environmental. | [117] |
| Flat ceramic membrane | HA: 50 ± 1 g/L, Air flow rate: 5.83 × 10−6~7.50 × 10−6 m3/s, TMP: 413.7 kPa | Air NBs cleaning | The permeation flux of CM basically recovered to 99%. | NBs successfully unclogged the pores of the membrane. | [114] |
| ZrO2/SiC UF membrane | Cross flow: 1527 L/h, HRT: 80 min, TMP: 1 bar | Dip coating a SiC support with a ZrO2 slurry | The test of olive oil/water emulsion removed 99.91% of oil without fouling. | Long-term corrosion tests did not cause change in morphology. | [107] |
| TiO2 ceramic membrane | HRT: 3 h, pH: 6~9, Temp.: 20 ± 1 °C, Flux: 62 LMH | SWRO pretreatment | SWRO pretreatment limited biofouling in RO by inducing phosphate limitation. | Phosphate removal was up to 87%. | [118] |
| Al2O3 UF membrane | Flux: 90 LMH, Temp.: 22~25 °C, Ferrate: 0.15 mM | Integration of ferrate (VI) pretreatment | Cake layer scaling decreased significantly, TMP decreased by 81.8%. | Cake layer became more porous with increase in ferrate. | [119] |
| Ceramic UF membrane | pH: 7.0 ± 0.1, TMP: 50 kPa, Fe(II): 15 or 50 μΜ, PMS: 15 or 50 μΜ, Temp.: 25 ± 1 °C | Fe(II)/PMS pretreatment | The rate of reversible and irreversible fouling was reduced by 83.5% and 96.5%. | Low dose of Fe(II)/PMS aggravated membrane fouling caused by BSA. | [120] |
| CNTs-Al2O3 composite membrane | Electric field: >3 × 103 kV/m, Flux: 75 LMH, Temp.: 25 ± 2 °C, TMP: 1.5 bar | Coupled with ultrasound | The recovered water permeance of the membrane which coupled with ultrasound. | Microcurrents or vibrations resulted in reduced concentration polarization. | [121] |
| Catalyst | Support Material/Membrane | Exp. Conditions | Efficiency | Comments | Ref. |
|---|---|---|---|---|---|
| TiO2 | Tubular Al2O3 UF membrane | UV intensity: 1.54 W/m2, TOC: 5 ± 0.5 mg/dm3, CFV: 3~6 m/s, TMP: 0.1 MPa, HA: 5 ± 0.5 mg TOC/dm3 | Removal rate: >95%, Mineralization rate: 70%. | Pure water flux: 367 dm3/(m2·h). Stable operation for 400 h. | [136] |
| Cu2O/TiO2 | FTO conducting glass | Mercury lamp: 125 W, Light intensity: 1.8 mW/cm2, MB: 10 ppm, pH: 4 or 8 | MB: 80%. | Cu2O/TiO2 film could utilize visible light. | [137] |
| SiO2/TiO2 nanorods/nanotubes | Alumina template membrane (200 nm) | Flow velocity: 12.7 L/h, Temp: 298 K, UV: 400 μW/cm2, pH: 3.5 | SDBS: 89%. | The composite membrane had better removal. | [138] |
| rGO/TiO2 and N-TiO2-10 | Tubular γ-Al2O3 UF membrane | UV intensity: 2.1, 7.2 mW/cm2, Flow rate: 1.5 mL/min, MO: 6.4 mg/L, MB: 2 mg/L | MB: 63%. | MB removal was superior to MO. | [139] |
| N-TiO2 | Ceramic UF membrane | Xenon lamp intensity: 300 W, Water flow rate: 4 L/min, TMP: 0.4 MPa, pH: 7, Dye: 10 mg/L | The Retention rate: 99%. Water flux: 20 LMH. | The visible light effect was poor. Could be reused 7 times. | [140] |
| N-TiO2 | Commercial α-Al2O3 UF membrane | UV intensity: 712.3 W/m2, CBZ: 4.24 × 10−3 mM, pH: 7 | CBZ: 90% | N-doped composite films utilized sunlight more efficiently. | [141] |
| CdS | PAA | Visible light intensity: 2.53 mW/cm2, CO2 flow velocity: 3 cm3/min | 45.4% | - | [142] |
| AgNCs | g-C3N4/NF hybrid membrane | Xenon lamp intensity: 420 W, AgNCs: 4~5 nm, RhB: 10 mg/L | RhB: 86%. | Maintained good stability after 5 cycles. | [143] |
| Fe-doped TiO2 | rGO | pH: 6, RhB: 20 mg/L, TOC: 930 mg/L, COD: 1550 mg/L | The removal of TOC: 66.7%. The removal of COD: 59.1%. | The removal rate decreased slightly (about 12%) after 5 cycles. | [144] |
| Catalyst(s) | Membrane | Exp. Conditions | Efficiency | Comments | Ref. | ||
|---|---|---|---|---|---|---|---|
| Membrane | O3 | Coupling | |||||
| – | Tubular Al2O3 UF membrane (pore size: 0.5 μm) | O3: 9.5 g/m3, O3 flow rate: 0.2 L/min, Temp: 22.5 °C, TOC: 11.8 mg/L | - | - | 15% reduction in flux. | Osmotic flux increased with increasing O3 concentration. | [152] |
| Fe2O3 or TiO2 or MnO2 | CM (MWCO: 5 kDa) | O3 flow rate: 10 mL/min, O3: 10 g/m3, TMP: 1.9~2.2 bar, TOC: 10.4 mg/L | - | - | THM: 39%, HAA: 55%. | The fouling behavior: Fe2O3 > TiO2 > MnO2 | [155] |
| Fe2O3 | γ-Al2O3 UF membrane | O3: 10 mg/L, pCBA: 3.4 mg/L, pH: 7, TMP: 80 kPa Water flux: 0.02 L/min | 8% | 28% | 46% | Effective in controlling NOM pollution. | [156] |
| TiMn2O3 | CM | O3: 2.5 mg/L, CODCr: 100 ± 20 mg/L, SS: 20 ± 5 mg/L, E. coli: >2.4 × 106 MPN/L | 65% | 60% | Reduced membrane flux: 53%. | Chroma, SS, and E. coli were entirely removed. | [157] |
| Three types of MnO2 | TiO2 membrane | O3: 0.5 mg/L, Water flux: 1.0 mL/min, TMP: 100 kPa, SA: 1.0 g/L, p-CNB: 100 μg/L | 15% | 41.4% | (a) 51.7%, (b) 61.5%, (c) 68%. | S-MnO2 has the best ozonation effect. | [158] |
| Ti–Mn/TiO2 | Tubular γ-Al2O3 membrane | O3 flow rate: 52 mg/min, TMP: 2.22~2.30 bar, Water flux: 1.04 ± 0.04 m/s | 55%. | - | SS removal rate: 100%. | Short start-up times and stable running conditions. | [159] |
| CuMn2O4 | Tubular CM | O3: 1.0 mg/L, BP-3: 2 mg/L, pH: 7.2 | 51.6% | 47.4% | 71.2% | Decreased the UV254 and DOC of effluent. | [160] |
| CeOx/MnOx | Tubular α-Al2O3 membrane | O3: 500 mL/min, Feed flux: 20 mL/min, HRT: 13.7 S, BPA/BTZ: 3 mg/L, | BPA: 55% | - | BPA: 84%, BTZ: 57% | Higher ozone utilization of Ce-CCM. | [161] |
| MnOx | CM | Ozone: 5 mg/L, Water flux: 80 LMH, HRT: 4 h | 31.2% | - | 39.5% | - | [162] |
| MgO, CeOx, and MnO2 | Flat-sheet CM | Water flux: 62.5 LMH, 4BS: 12 mg/L, HRT: 30 s, O3 flow rate: 3.3 g/h | - | 38% | Mg-Ce: 85%, Mg-Mn: 88%. | Effective reduction of membrane fouling. | [163] |
| Fe2O3 | Tubular CM | Flow rate: 69 L/h, O3 flow rate: 20 L/h, pH: 8.5, IBU:10 mg/L | 76% | - | 99% | The ozone-NF system reduced the toxicity of pollutants. | [164] |
| Catalyst(s) | Membrane | Exp. Conditions | Efficiency | Comments | Ref. |
|---|---|---|---|---|---|
| CuO | α-MnO2 nanowire membrane | Flow rate: 20 mL/min, pH: 7.4, PMS: 1.0 mM, MB: 0.1 mM | MB: >99%. | It also had a high degradation effect on other kinds of dyes. | [176] |
| Co3O4 | Al2O3 CM | PMS: 0.1 g/L, TMP: 0.07 bar, SMX: 10 mg/L, pH: 5 | SMX: 90%. | Maintained 95% of initial flow rate after 3 cycles. | [177] |
| CoFe2O4 | Porous Al2O3-based filter substrate | PMS: 0.1 g/L, SMX: 10 mg/L, Temp: indoor temperature, P: 0.09 bar, pH: 5 | SMX: 98%. | It was well tolerated in a wide pH range (3–11) and different anions. | [178] |
| CuO | CHFMs | PMS: 0.5 mM, BPA: 10 mg/L, T: 25 °C, pH: 7 | BPA: >98.5%. | 1O2 dominated non-radical pathway. | [179] |
| - | Mn2O3-Al2O3 membrane | EDCs: 0.1 mg/L, PMS: 0.3 mM, Flux: 60 LMH | Trace EDCs: >95%. | Reduced manganese ion leaching. | [180] |
| Co3O4 | Al2O3 membrane | PMS: 4 mM, TMP: 2 bar, CFV: 5 mL/s, HBA: 20 ppm, pH: 7 | HBA: >95%. | Good water flux even at HA concentration of 200 ppm. | [181] |
| CuCo2O4 | α-Al2O3 membrane | BPA: 30 mg/L, pH: 7.0, PMS: 2 mM, Flux: 650 LMH | BPA: >92.1%. | Ion leaching of Co and Cu within a safe range. | [182] |
| NiCo2S4 | CS | Nim: 5 mg/L, PDS: 0.4 g/L, Temp: 25 °C, V: 50 mL | Nim: >94%. | The degradation rate was 81% when repeated 6 times. | [183] |
| Ni-Co | NCNTs | PMS: 0.65 mM, IBP: 20 mg/L, Temp: 25 °C, Catalyst: 0.05 g/L | IBP: 98%. | Metal leaching was extremely low. | [184] |
| rGO | CNTs | PS: 5 mM, Flow rate: 1.0 mL/min, SMX: 500 μg/L, Temp.: 25 ± 2 °C | SMX: 98%. | The optimal C/O ratio was estimated at 2.9. | [185] |
| AOPs | Catalyst(s) | Membrane | Exp. Conditions | Efficiency | Comments | Ref. |
|---|---|---|---|---|---|---|
| Fenton | – | Cu-Al2O3 fibrous membrane | BPA: 20 mg/L, pH: 7 H2O2: 12 mM, Water flux: 0.3 mL/min, | BPA: >87% | High Fenton catalytic activity at neutral pH. | [200] |
| MnO2 | SNM | MB: 10 mg/L, Temp: 23 ± 3 °C, P: 5 kPa, pH: 6 | Fenton: 6%, SiO2/Fenton: 9%, Mn-NFM/Fenton: 90%. | High degradation properties after five cycles. | [201] | |
| CuFe2O4 | SNM | MB: 10 mg/L, pH: 6 | MB: 96%. | The degradation degree was 85.7%. | [202] | |
| Fe3O4 | Tubular α-Al2O3 membrane | H2O2: 8.7 mg/L, pH: 3.0 Water flux: 0.5 L/min, DCF: 282.4 μg/L, | Removal rate: 65.1% Mineralization rate: 47.9%. | Wide pH range, low ferrous ion leaching. | [203] | |
| Cu-UiO-66 or Mn-UiO-66 | Tubular Al2O3 membrane | Phenol: 100 mg/L, H2O2: 510 mg/L, V: 200 mL, T: 60 °C | Phenol: 99%. | Higher catalytic activity of the Cu-UiO-66@CM. | [204] | |
| Photo-Fenton | M88A (Fe) | GO | MB: 10 mg/L, H2O2: 10 mM, Light intensity: 104 mW/cm2 | Dark: 42.1%, PF: 98.8%. | The MB degradation rate was 97.87% after 12 cycles. | [205] |
| TiO2-GO-Fe3O4 | Flat CM | H2O2: 20mM, AMX: 20 mg/L, Flux: 100 mL/min | AMX: 88.5%. | Composites exhibited PF catalytic property. | [206] | |
| Electro-Fenton | GO | PTFE | Florfenicol: 1 mg/L, H2O2: 5 mg/L, Potential: −0.6 V, Na2SO4: 20 mM | Single filtration: 27%, EF: 90%. | The process can be used for advanced water purification. | [207] |
| Graphene | SS membrane | Potential: −0.5 V, PCM: 0.1 mM, I: 170 mA, pH: 3 | Mineralized current efficiency values increased by 165% | Remained stable for 3 cycles. | [208] | |
| FeOCl | CNT | Potential: −0.8 V, TC: 0.04 mM, pH: 6.5, Na2SO4: 10 mM | TC: >95%. | Wider range of pH applications. | [209] |
| Coupling Process | Advantages | Disadvantages | References |
|---|---|---|---|
| IMF coupled with photocatalytic oxidation | Increases the hydrophilicity of the catalytic membrane. Mild reaction conditions. No additional chemical reagents are required. Easy to combine with other AOPs. | Requires illumination. Low efficiency of visible light utilization. Poor treatment of high suspended solids and high-turbidity wastewater. | [19,24,125,136,226] |
| IMF coupled with ozonation | Quickly oxidizes contaminants. Wider pH range. | Requires an ozone generator with high operating costs. Easy to generate DBP in the reaction process. Low ozone utilization rate. | [20,31,227] |
| IMF coupled with persulfate oxidation | Higher oxidation potential for greater oxidizing power. Cheaper persulfate oxidizer. | PS price is high. The remaining needs to be processed later. The mechanism of non-radical reactions is not yet clear. | [135,194,228] |
| IMF coupled with Fenton oxidation | Low cost. Convenient operation. Mild reaction conditions. Widely applicable. | More oxidant consumption and relatively weaker oxidation ability. Generate a large amount of iron sludge. Narrow pH range. | [21,135,229] |
| IMF coupled with composite Fenton oxidation | A wider range of pH applications. The amount of iron mud generated decreases. A decrease in iron loss. Improves the utilization rate of oxidants. | Requires lighting or electricity to increase energy consumption. The practical operation process requires higher requirements. | [209,215] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yuan, R.; Chen, H.; Zhou, B.; Cui, Z.; Zhu, B. Advancements in Inorganic Membrane Filtration Coupled with Advanced Oxidation Processes for Wastewater Treatment. Molecules 2024, 29, 4267. https://doi.org/10.3390/molecules29174267
Zhang C, Yuan R, Chen H, Zhou B, Cui Z, Zhu B. Advancements in Inorganic Membrane Filtration Coupled with Advanced Oxidation Processes for Wastewater Treatment. Molecules. 2024; 29(17):4267. https://doi.org/10.3390/molecules29174267
Chicago/Turabian StyleZhang, Chaoying, Rongfang Yuan, Huilun Chen, Beihai Zhou, Zexin Cui, and Boyun Zhu. 2024. "Advancements in Inorganic Membrane Filtration Coupled with Advanced Oxidation Processes for Wastewater Treatment" Molecules 29, no. 17: 4267. https://doi.org/10.3390/molecules29174267
APA StyleZhang, C., Yuan, R., Chen, H., Zhou, B., Cui, Z., & Zhu, B. (2024). Advancements in Inorganic Membrane Filtration Coupled with Advanced Oxidation Processes for Wastewater Treatment. Molecules, 29(17), 4267. https://doi.org/10.3390/molecules29174267






