Synthesis, Surface Activity, Emulsifiability and Bactericidal Performance of Zwitterionic Tetrameric Surfactants
Abstract
:1. Introduction
2. Results and Discussion
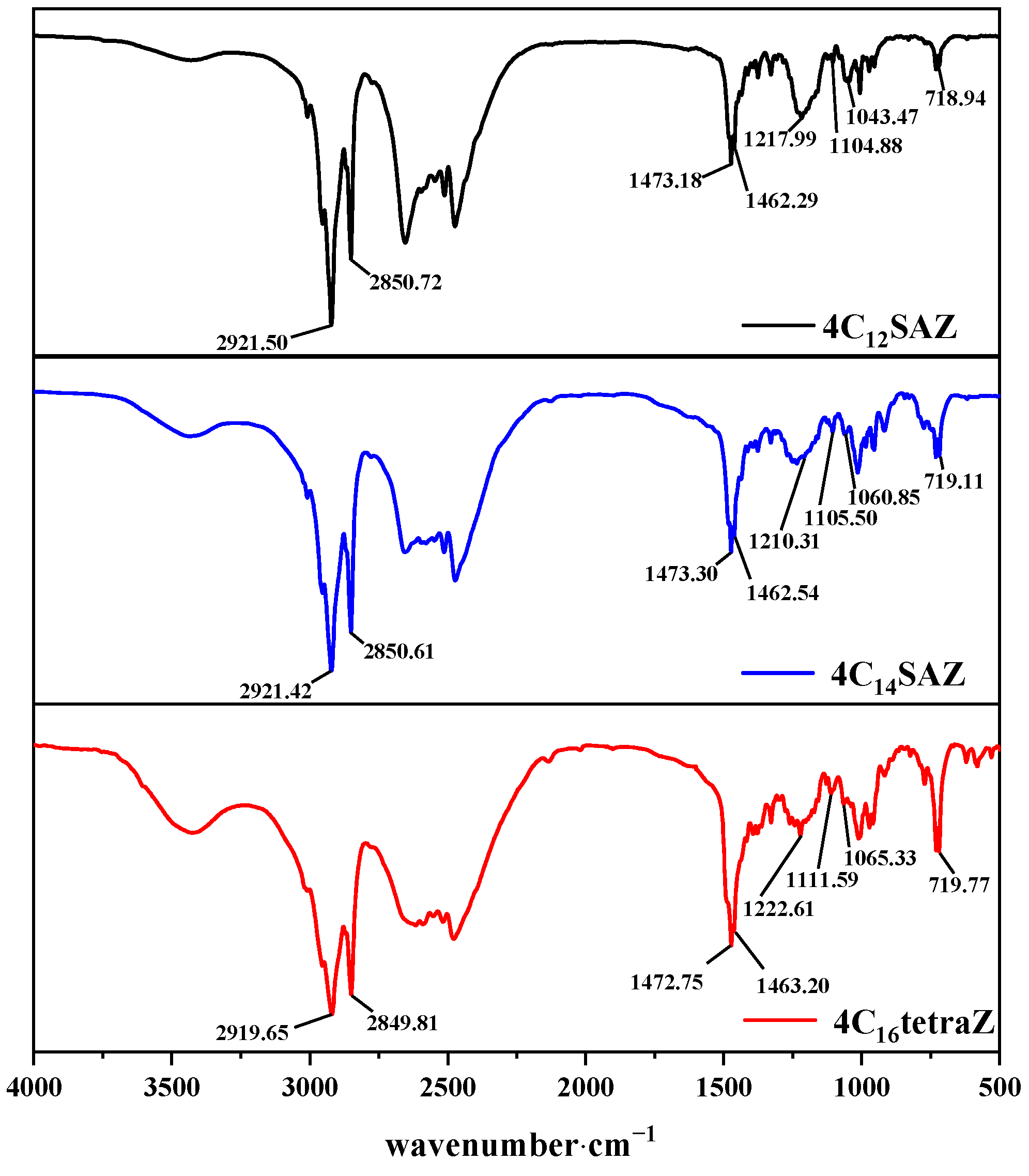
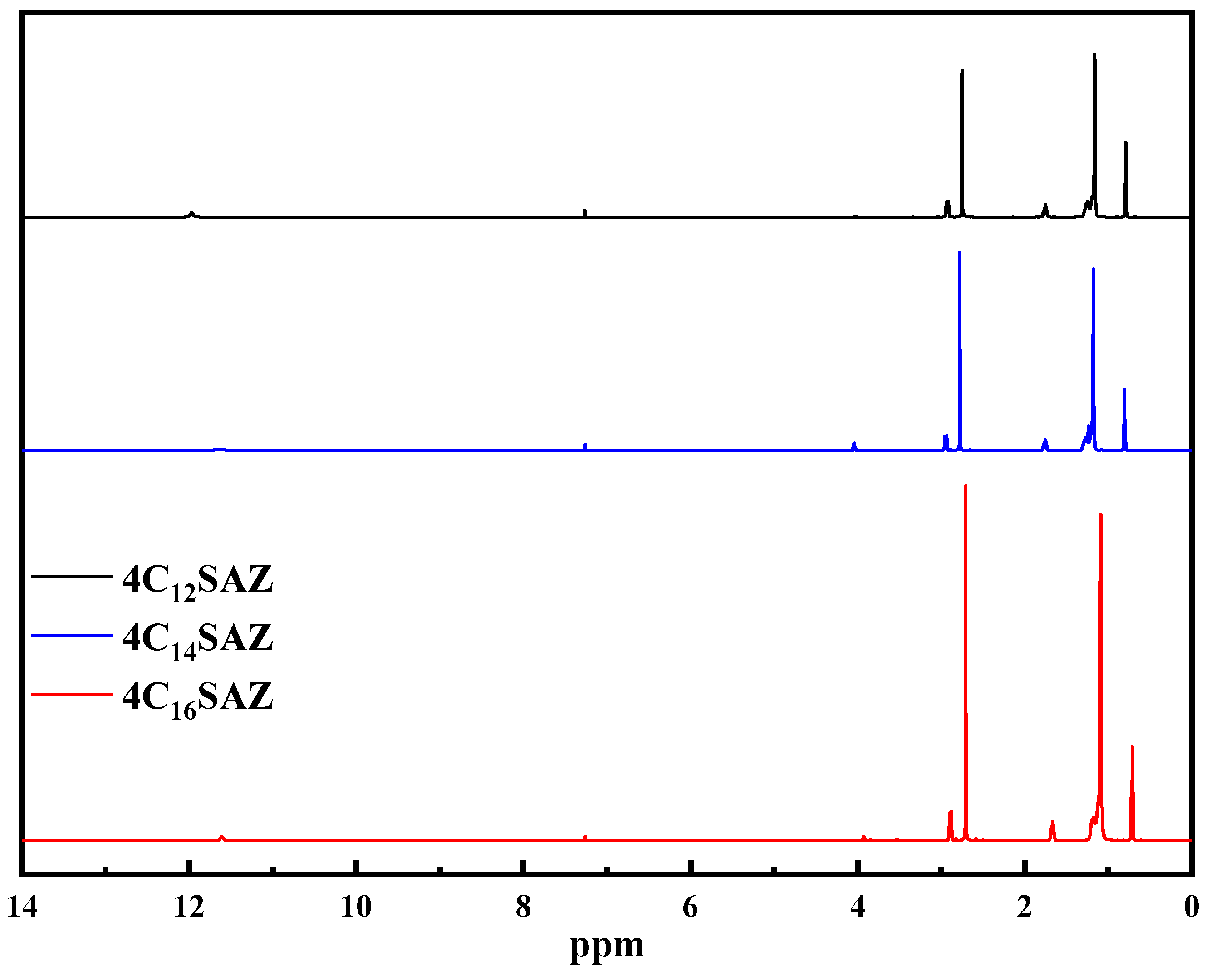
2.1. Structural Characterization
2.2. Surface Activity
2.3. Thermodynamics of Micellization/Thermodynamic Parameters
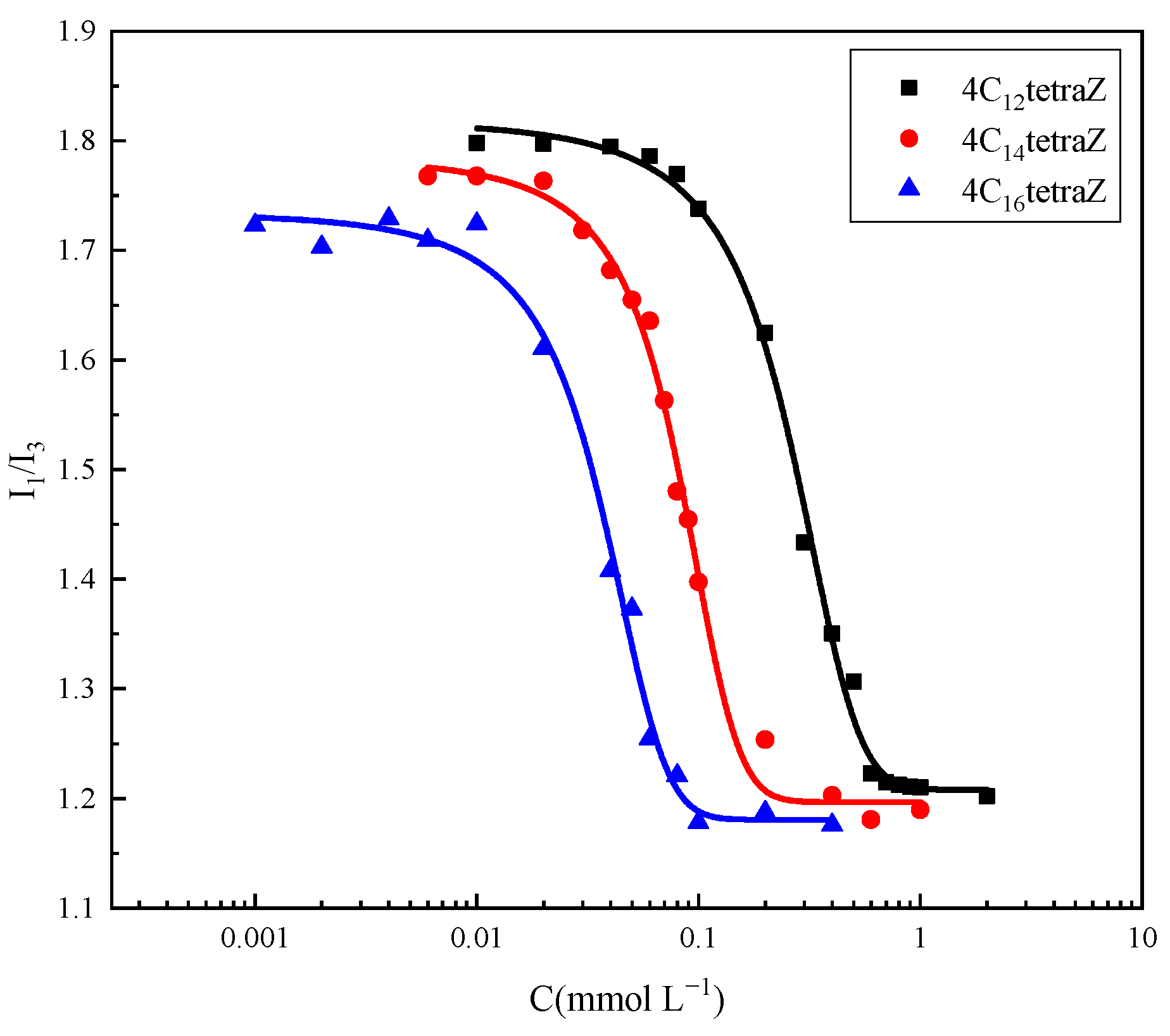
2.4. Micropolarity
2.5. Critical Arrangement Parameters and Aggregate Morphology
2.6. DLS
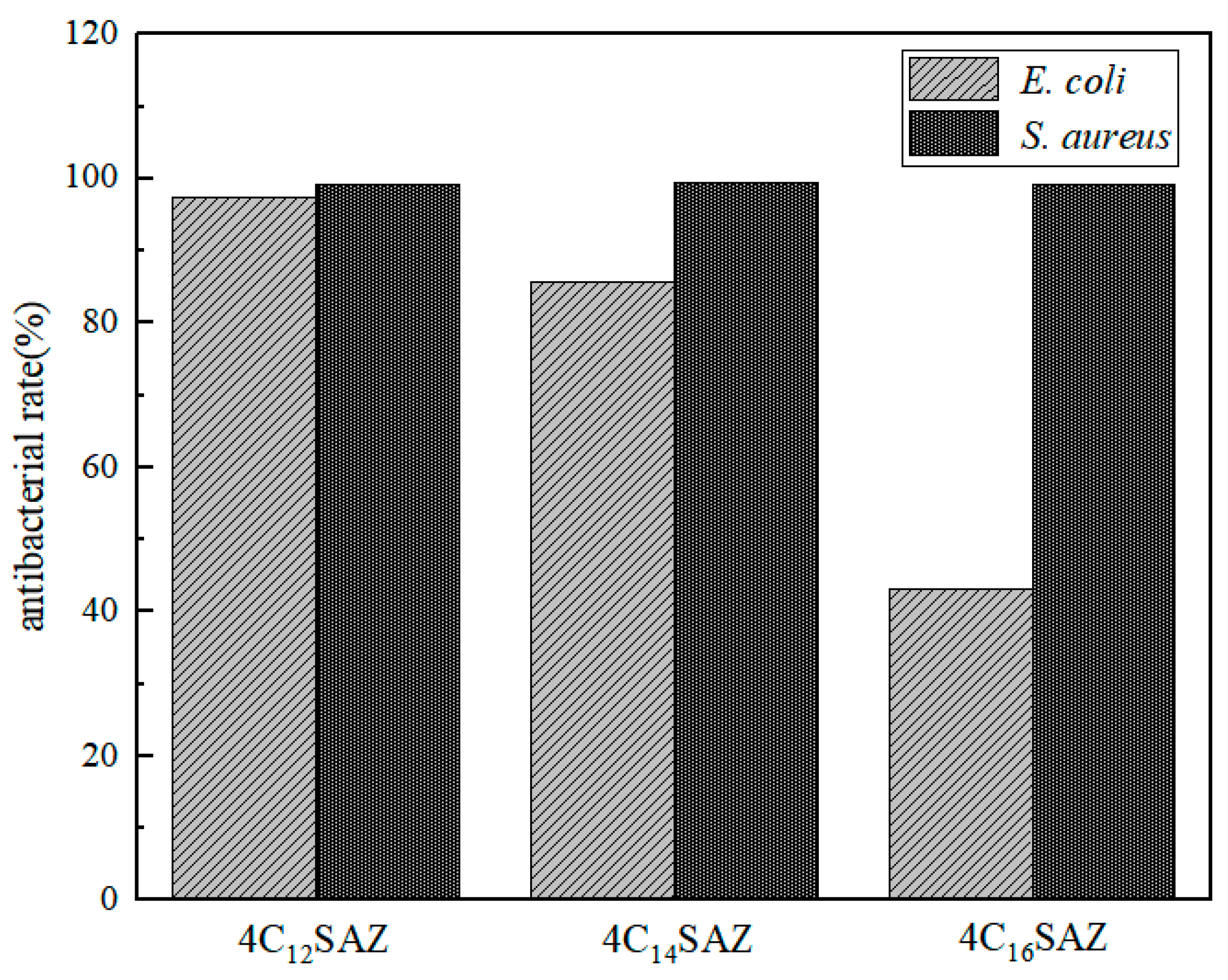
2.7. Antibacterial Performance
2.8. Emulsification Performance
3. Experimental Methods and Materials
3.1. Materials
3.2. Synthesis of Zwitterionic Tetrameric Surfactants
3.2.1. Synthesis of Pentaerythritol Tetraglycidyl Ether (I)
3.2.2. Synthesis of Quaternary Ammonium Tetrameric Surfactant (II)
3.2.3. Synthesis of Zwitterionic Tetrameric Surfactants (4CnSAZs)
3.3. Methods
3.3.1. Surface Tension Measurements
3.3.2. Steady-State Fluorescence Spectrum
3.3.3. Dynamic Light Scattering (DLS)
3.3.4. Antibacterial Activities
3.3.5. Emulsion Stability Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, M.; Huang, Z.; Yu, S.; Yang, Y.; Huang, Y.S.; Qiu, D.; Zhao, J.Z. Synthesis and surface active properties of novel oligomer betaine surfactants. Tenside Surfactants Deterg. 2016, 53, 134–139. [Google Scholar] [CrossRef]
- Liu, J.H.; Liu, Z.; Yuan, T.J.; Wang, C.W.; Gao, R.M.; Hu, G.F.; Xu, J.S.; Zhao, J.S. Synthesis and properties of zwitterionic gemini surfactants for enhancing oil recovery. J. Mol. Liq. 2020, 311, 113179. [Google Scholar] [CrossRef]
- Yoshimura, T.; Nyuta, K.; Esumi, K. Zwitterionic heterogemini surfactants containing ammonium and carboxylate headgroups. 1. Adsorption and micellization. Langmuir 2005, 21, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mandal, A. Synthesis and physiochemical characterization of zwitterionnic surfactant for application in enhanced oil recovery. J. Mol. Liq. 2017, 243, 61–71. [Google Scholar] [CrossRef]
- Yaseen, M.; Wang, Y.; Su, T.J.; Lu, J.R. Surface adsorption of zwitterionic surfactants: N-alkyl phosphocholines characterised by surface tensiometry and neutron reflection. J. Colloid Interface Sci. 2005, 288, 361–370. [Google Scholar] [CrossRef]
- Geng, X.F.; Hu, X.Q.; Jia, X.C.; Luo, L.J. Effects of sodium salicylate on the microstructure of a novel zwitterionic gemini surfactant and its rheological responses. Colloid Polym. Sci. 2014, 292, 915–921. [Google Scholar] [CrossRef]
- Hussain, S.S.; Kamal, M.S.; Fogang, L.T. Synthesis and physicochemical investigation of betaine type polyoxyethylene zwitterionic surfactants containing different ionic headgroups. J. Mol. Struct. 2019, 1178, 83–88. [Google Scholar] [CrossRef]
- Chen, S.Y.; Liu, H.J.; Sun, H.; Yan, X.; Wang, G.H.; Zhou, Y.J.; Zhang, J.N. Synthesis and physiochemical performance evaluation of novel sulphobetaine zwitterionic surfactants from lignin for enhanced oil recovery. J. Mol. Liq. 2018, 249, 73–82. [Google Scholar] [CrossRef]
- Domínguez, R.; Rodríguez, A.; Maestre, A.; Robina, I.; Moyá, M.L. Synthesis and physicochemical characterization of alkanedyil-α-ω-bis (dimethyl dodecylammo-nium) bromide, 12-s-12, 2Br−, surfactants with s= 7, 9, 11 in aqueous medium. J. Colloid Interface Sci. 2012, 386, 228–239. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhang, Y.; Ding, L.M.; Liu, J.; Zhao, B.; Deng, Q.G.; Yan, T. Synthesis and physiochemical properties of novel gemini surfactants with phenyl-1, 4-bis (carbamoylmethyl) spacer. RSC Adv. 2015, 5, 74764–74773. [Google Scholar] [CrossRef]
- Seredyuk, V.; Alami, E.; Nydén, M.; Holmberg, K.; Peresypkin, A.V.; Menger, F.M. Micellization and adsorption properties of novel zwitterionic surfactants. Langmuir 2001, 17, 5160–5165. [Google Scholar] [CrossRef]
- Morita, T.; Yada, S.; Yoshimura, T. Effect of Spacer Structures on the Interfacial Adsorption and Micelle Properties of Quaternary Ammonium Salt-Based Gemini Surfactants. Langmuir 2021, 38, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Singer, O.M.; Campbell, J.W.; Hoare, J.G.; Masuda, J.D.; Marangoni, G.; Singer, R.D. Improved Green Synthesis and Crystal Structures of Symmetrical Cationic Gemini Surfactants. ACS Omega 2022, 7, 35326–35330. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.P.; Yang, W.G.; Jiang, Y.J.; Wang, Y.K.; Ju, H.B.; Geng, T. Studies on physicochemical properties of three Gemini surfactants with different spacer groups. J. Mol. Liq. 2021, 325, 115039. [Google Scholar] [CrossRef]
- Yoshimura, T.; Ohno, A.; Esumi, K. Equilibrium and dynamic surface tension properties of partially fluorinated quaternary ammonium salt gemini surfactants. Langmuir 2006, 22, 4643–4648. [Google Scholar] [CrossRef]
- Xing, F.L.; Gao, Y.Y.; Xu, Q.; Li, X.; Wang, L.Y.; Meng, J.; Wang, P.L. The effects of the organic groups attached at the silicone atoms of the organosilane-based Gemini nonionic surfactants on their surface activities. J. Surfactants Deterg. 2014, 17, 739–745. [Google Scholar] [CrossRef]
- Zana, R. Dimeric (gemini) surfactants: Effect of the spacer group on the association behavior in aqueous solution. J. Colloid Interface Sci. 2002, 248, 203–220. [Google Scholar] [CrossRef]
- Knaebel, A.; Oda, R.; Mendesm, E.; Candau, S.J. Lamellar structures in aqueous solutions of a dimeric surfactant. Langmuir 2000, 16, 2489–2494. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, L.; Xu, Z.C.; Liu, D.D.; Song, X.W.; Cao, X.L.; Zhang, L.; Zhao, S. Effect of zwitterionic surfactants on wetting of quartz surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2013, 430, 110–116. [Google Scholar] [CrossRef]
- Mafi, A.; Hu, D.; Chou, K.C. Interactions of sulfobetaine zwitterionic surfactants with water on water surface. Langmuir 2016, 32, 10905–10911. [Google Scholar] [CrossRef]
- Zhou, M.; Li, S.S.; Zhang, Z.; Wang, C.W.; Luo, G.; Zhao, J. Z Progress in the synthesis of zwitterionic gemini surfactants. J. Surfactants Deterg. 2017, 20, 1243–1254. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Yang, Y.; Niu, C.R.; Feng, Z.; Zhao, W.H.; Lu, S. Progress in synthesis and application of zwitterionic Gemini surfactants. Front. Mater. Sci. 2019, 13, 242–257. [Google Scholar] [CrossRef]
- Xu, W.T.; Han, X.Y.; Fang, B.; He, J.L.; Wu, H.N.; Hui, X. Rheology on novel viscoelastic trimeric octadecyl zwitterionic surfactant micelle solutions. J. Surfactants Deterg. 2023, 26, 667–681. [Google Scholar] [CrossRef]
- Park, K.H.; Lim, J.C. Synthesis of phospholipid based zwitterionic surfactant from coconut oil source and characterization of their interfacial, antiseptic and antiviral propertie. J. Ind. Eng. Chem. 2022, 115, 241–250. [Google Scholar] [CrossRef]
- Yoshimura, T.; Esumi, K. Physicochemical properties of ring-type trimeric surfactants from cyanuric chloride. Langmuir 2003, 19, 3535–3538. [Google Scholar] [CrossRef]
- Murguía, M.C.; Cabrera, M.I.; Guastavino, J.F.; Grau, R.J. New oligomeric surfactants with multiple-ring spacers: Synthesis and tensioactive properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 262, 1–7. [Google Scholar] [CrossRef]
- In, M.; Bec, V.; Aguerre-Chariol, O.; Zana, R. Quaternary ammonium bromide surfactant oligomers in aqueous solution: Self-association and microstructure. Langmuir 2000, 16, 141–148. [Google Scholar] [CrossRef]
- Zana, R.; Levy, H.; Papoutsi, D.; Beinert, G. Micellization of two triquaternary ammonium surfactants in aqueous solution. Langmuir 1995, 11, 3694–3698. [Google Scholar] [CrossRef]
- In, M.; Warr, G.G.; Zana, R. Dynamics of branched threadlike micelles. Phys. Rev. Lett. 1999, 83, 2278–2281. [Google Scholar] [CrossRef]
- Shi, X.D.; Zeng, M.D.; Xu, X.X.; Liu, Y.X.; Kou, J.J.; Bian, Q.; Song, H.; Zhang, J.J.; Wang, Q.M. Promotion of droplet deposition, diffusion-wetting and retention on hydrophobic surfaces by nonionic star-shaped oligomeric surfactants. J. Mol. Liq. 2023, 386, 122521. [Google Scholar] [CrossRef]
- Menger, F.M.; Migulin, V.A. Synthesis and properties of multiarmed geminis. J. Org. Chem. 1999, 64, 8916–8921. [Google Scholar] [CrossRef]
- Karpichev, Y.; Jahan, N.; Paul, N.; Petropolis, C.P.; Mercer, T.; Grindley, T.B.; Marangoni, D.G. The micellar and surface properties of a unique type of two-headed surfactant–Pentaerythritol based di-cationic surfactants. J. Colloid Interface Sci. 2014, 423, 94–100. [Google Scholar] [CrossRef]
- Jahan, N.; Paul, N.; Petropolis, C.J.; Marangoni, D.G.; Grindley, T.B. Synthesis of surfactants based on pentaerythritol. I. Cationic and zwitterionic gemini surfactants. J. Org. Chem. 2009, 74, 7762–7773. [Google Scholar] [CrossRef]
- Tran, T.; Jahan, N.; Marangoni, D.G.; Grindley, T.B. Synthesis of surfactants based on pentaerythritol. II. Anionic gemini surfactants. Can. J. Chem. 2013, 91, 1085–1092. [Google Scholar] [CrossRef]
- Asadov, Z.G.; Ragimov, R.A.; Akhmedova, G.A. Synthesis, physicochemical characteristics and properties of oligomeric surfactants based on pentaerythritol and propylene oxide. Russ. J. Appl. Chem. 2011, 84, 1188–1194. [Google Scholar] [CrossRef]
- Abdel-Raouf, M.E. Biodegradable polyoxyethylenated pentaerythritol Quaternary esters as oil spill dispersants. Tenside Surfactants Deterg. 2012, 49, 114–123. [Google Scholar] [CrossRef]
- Xie, Y.C.; Li, J.; Li, Z.F.; Sun, T.; Wang, Y.P.; Qu, G.M. The adsorption and aggregation properties of dendritic cationic tetrameric surfactants. RSC Adv. 2018, 8, 36015–36024. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.J.; Yu, Y.M.; Jiang, L.D. Studies on micellar behavior of anionic and surface-active monomers with acrylamide type in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2007, 293, 210–216. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Lu, X.B.; Lai, L.; Yu, L.W.; Zheng, H.; Dai, C.Y. The micelle thermodynamics and mixed properties of sulfobetaine-type zwitterionic Gemini surfactant with nonionic and anionic surfactants. J. Mol. Liq. 2020, 299, 112108. [Google Scholar] [CrossRef]
- Guo, J.W.; Zhong, X.; Zhu, H.; Feng, L.J.; Cui, Y.D. Synthesis of novel quaternary ammonium surfactants containing adamantane. Chin. Chem. Lett. 2012, 23, 653–656. [Google Scholar] [CrossRef]
- Xie, Y.C.; Li, J.; Sun, T.; Han, Y.; Qu, G.M.; Niu, R.X. Synthesis, surface activity, and corrosion inhibition of dentritic quaternary ammonium salt-type tetrameric surfactants. J. Dispers. Sci. Technol. 2018, 39, 1153–1159. [Google Scholar] [CrossRef]
- Geng, X.F.; Hu, X.Q.; Xia, J.J.; Jia, X.C. Synthesis and surface activities of a novel di-hydroxyl-sulfate-betaine-type zwitterionic gemini surfactants. Appl. Surf. Sci. 2013, 271, 284–290. [Google Scholar] [CrossRef]
- Elged, A.H.; Shaban, S.M.; Eluskkary, M.M.; Aiad, I.; Soliman, E.A.; Elsharif, A.M.; Kim, D.H. Impact of hydrophobic tails of new phospho-zwitterionic surfactants on the structure, catalytic, and biological activities of AgNPs. J. Ind. Eng. Chem. 2021, 94, 435–447. [Google Scholar] [CrossRef]
- Sayed, G.H.; Ghuiba, F.M.; Abdou, M.I.; Badr, E.A.A.; Tawfik, S.M.; Negm, N.A.M. Synthesis, surface, thermodynamic properties of some biodegradable vanillin-modified polyoxyethylene surfactants. J. Surfact. Deterg. 2012, 15, 735–743. [Google Scholar] [CrossRef]
- Shaban, S.M.; Aiad, I.; Ismail, A.R. Surface parameters and biological activity of N-(3-(dimethyl benzyl ammonio) propyl) alkanamide chloride cationic surfactants. J. Surfact. Deterg. 2016, 19, 501–510. [Google Scholar] [CrossRef]
- Zhou, L.M.; Jiang, X.H.; Li, Y.T.; Chen, Z.; Hu, X.Q. Synthesis and properties of a novel class of gemini pyridinium surfactants. Langmuir 2007, 23, 11404–11408. [Google Scholar] [CrossRef]
- Zhang, S.S.; Ding, S.P.; Yu, J.; Chen, X.R.; Lei, Q.F.; Fang, W.J. Antibacterial activity, in vitro cytotoxicity, and cell cycle arrest of gemini quaternary ammonium surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef]
- Fu, S.Q.; Guo, J.W.; Zhong, X.; Yang, Z.; Lai, X.F. Synthesis, physiochemical property and antibacterial activity of gemini quaternary ammonium salts with a rigid spacer. RSC Adv. 2016, 6, 16507–16515. [Google Scholar] [CrossRef]
- Zhi, L.F.; Shi, X.F.; Zhang, E.Z.; Pan, Y.L.; Li, X.M.; Wang, H.; Liu, W. Synthesis and properties of stellate lactosamide quaternary ammonium surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127317. [Google Scholar] [CrossRef]
- Sun, Y.; Han, F.; Zhou, Y.W.; Xu, B.C. Synthesis and properties of quaternary ammonium gemini surfactants with hydroxyethyl at head groups. J. Surfactants Deterg. 2019, 22, 675–681. [Google Scholar] [CrossRef]
- Chauhan, V.; Kumar, M.; Soni, I.; Shandilya, P.; Singh, S. Synthesis, physical properties and cytotoxic assessment of ester-terminated gemini imidazolium surfactants. J. Mol. Liq. 2023, 387, 122645. [Google Scholar] [CrossRef]
- Patial, P.; Shaheen, A.; Ahmad, I. Gemini pyridinium surfactants: Synthesis and their surface active properties. J. Surfactants Deterg. 2014, 17, 929–935. [Google Scholar] [CrossRef]
- Ray, G.B.; Chakraborty, I.; Ghosh, S.; Moulik, S.P.; Palepu, R. Self-aggregation of alkyltrimethylammonium bromides (C10-, C12-, C14-, and C16TAB) and their binary mixtures in aqueous medium: A critical and comprehensive assessment of interfacial behavior and bulk properties with reference to two types of micelle formation. Langmuir 2005, 21, 10958–10967. [Google Scholar] [CrossRef]
- Wang, G.Y.; Li, P.; Du, Z.P.; Wang, W.X.; Li, G.J. Surface activity and aggregation behavior of siloxane-based ionic liquids in aqueous solution. Langmuir 2015, 31, 8235–8242. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, R.; Singh, S.; Chauhan, V. Synthesis, characterization and surface properties of N-(2-hydroxyalkyl)-N′-(2-hydroxyethyl) imidazolium surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 233–241. [Google Scholar] [CrossRef]
- Negm, N.A. Solubilization, surface active and thermodynamic parameters of Gemini amphiphiles bearing nonionic hydrophilic spacers. J. Surfactants Deterg. 2007, 10, 71–80. [Google Scholar] [CrossRef]
- Ren, C.C.; Wang, F.; Zhang, Z.Q.; Nie, H.H.; Li, N.; Cui, M. Synthesis, surface activity and aggregation behavior of Gemini imidazolium surfactants 1, 3-bis (3-alkylimidazolium-1-yl) propane bromide. Colloids Surf. A Physicochem. Eng. Aspects 2015, 467, 1–8. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Q.; Xia, Y.; Gao, Z.N. Surface properties and aggregation behavior of cationic gemini surfactants with dipropylammonium head-groups. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 211–217. [Google Scholar] [CrossRef]
- Man, Z.Q.; Wu, W.X. Study on the synthesis, surface activity, and self-assembly behavior of anionic non-ionic gemini surfactants. Molecules 2024, 29, 1725. [Google Scholar] [CrossRef]
- Siddiqui, U.S.; Ghosh, G.; Kabir-ud-Din. Dynamic light scattering studies of additive effects on the microstructure of aqueous gemini micelles. Langmuir 2006, 22, 9874–9878. [Google Scholar] [CrossRef]
- Figueira-Gonzalez, M.; Francisco, V.; Garcia-Rio, L.; Marques, E.F.; Parajo, M.; Rodriguez-Dafonte, P. Self-aggregation properties of ionic liquid 1, 3-didecyl-2-methylimidazolium chloride in aqueous solution: From spheres to cylinders to bilayers. J. Phys. Chem. B 2013, 117, 2926–2937. [Google Scholar] [CrossRef]
- Lu, T.; Han, F.; Mao, G.R.; Lin, G.F.; Huang, J.B.; Huang, X.; Wang, Y.L.; Fu, H.L. Effect of hydrocarbon parts of the polar headgroup on surfactant aggregates in gemini and bola surfactant solutions. Langmuir 2007, 23, 2932–2936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, Z.N.; Xu, F.; Tai, S.X.; Liu, X.G.; Mo, S.B.; Niu, F. Surface tension and aggregation properties of novel cationic gemini surfactants with diethylammonium headgroups and a diamido spacer. Langmuir 2012, 28, 11979–11987. [Google Scholar] [CrossRef] [PubMed]
- Taleb, K.; Mohamed-Benkada, M.; Benhamed, N.; Saidi-Besbes, S.; Grohens, Y.; Derdour, A. Benzene ring containing cationic gemini surfactants: Synthesis, surface properties and antibacterial activity. J. Mol. Liq. 2017, 241, 81–90. [Google Scholar] [CrossRef]
- Birnie, C.R.; Malamud, D.; Schnaare, R.L. Antimicrobial evaluation of N-alkyl betaines and N-alkyl-N, N-dimethylamine oxides with variations in chain length. Antimicrob. Agents Chemother. 2000, 44, 2514–2517. [Google Scholar] [CrossRef]
- Wieczorek, D.; Gwiazdowska, D.; Staszak, K.; Chen, Y.L.; Shen, T.L. Surface and antimicrobial activity of sulfobetaines. J. Surfactants Deterg. 2016, 19, 813–822. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Yang, X.Q.; Sun, G.J.; Cheng, Y.M. Study on the synthesis and surface activities of disodium of mono-alkyl polyoxyethylene ether citrate. Tenside Surfactants Deterg. 2007, 44, 287–290. [Google Scholar] [CrossRef]
- Xu, D.Q.; Qi, B.H.; Fang, D.; Zhang, X.M.; Zhang, T. Preparation, characterization and properties of novel cationic gemini surfactants with rigid amido spacer groups. J. Surfactants Deterg. 2016, 19, 91–99. [Google Scholar] [CrossRef]
- Wu, H.R.; Tan, R.; Hong, S.P.; Zhou, Q.; Liu, B.Y.; Chang, J.W.; Luan, T.F.; Kang, N.; Hou, J.R. Synergistic anionic/zwitterionic mixed surfactant system with high emulsification efficiency for enhanced oil recovery in low permeability reservoirs. Petrol Sci. 2024, 21, 936–950. [Google Scholar] [CrossRef]
- Pei, L.J.; Cai, Z.S.; Shang, S.B.; Song, Z.Q. Synthesis and properties of a cationic gemini surfactant with the hydrophenanthrene structure. J. Surfactants Deterg. 2014, 17, 433–439. [Google Scholar] [CrossRef]
- Chen, S.Y.; Li, X.L.; Lei, Q.; Han, Y.H.; Zhou, X.P.; Zhang, J.N. Synthesis, characterization and performance of lignin carboxyl betaine zwitterionic surfactants for application in enhanced oil recovery. RSC Adv. 2023, 13, 16352–16362. [Google Scholar] [CrossRef] [PubMed]
- Dahal, H.; Roy, S.; Dey, J.; Bose Dasgupta, S. Impact of the Hydrocarbon Chain Length of Biodegradable Ester-Bonded Cationic Gemini Surfactants on Self-Assembly, In Vitro Gene Transfection, Cytotoxicity, and Antimicrobial Activity. Langmuir 2024, 40, 2242–2253. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.E.; Xue, Z.; Huang, Y.; Seo, Y.; Lapitsky, Y. Physicochemical and antibacterial properties of surfactant mixtures with quaternized chitosan microgels. Carb. Pol. 2013, 93, 709–717. [Google Scholar] [CrossRef]
- Sadeghi, M.B.; Ramazani SA, A.; Taghikhani, V.; Ghotbi, C. Experimental investigation of rheological and morphological properties of water in crude oil emulsions stabilized by a lipophilic surfactant. J. Dispers. Sci. Technol. 2013, 34, 356–368. [Google Scholar] [CrossRef]
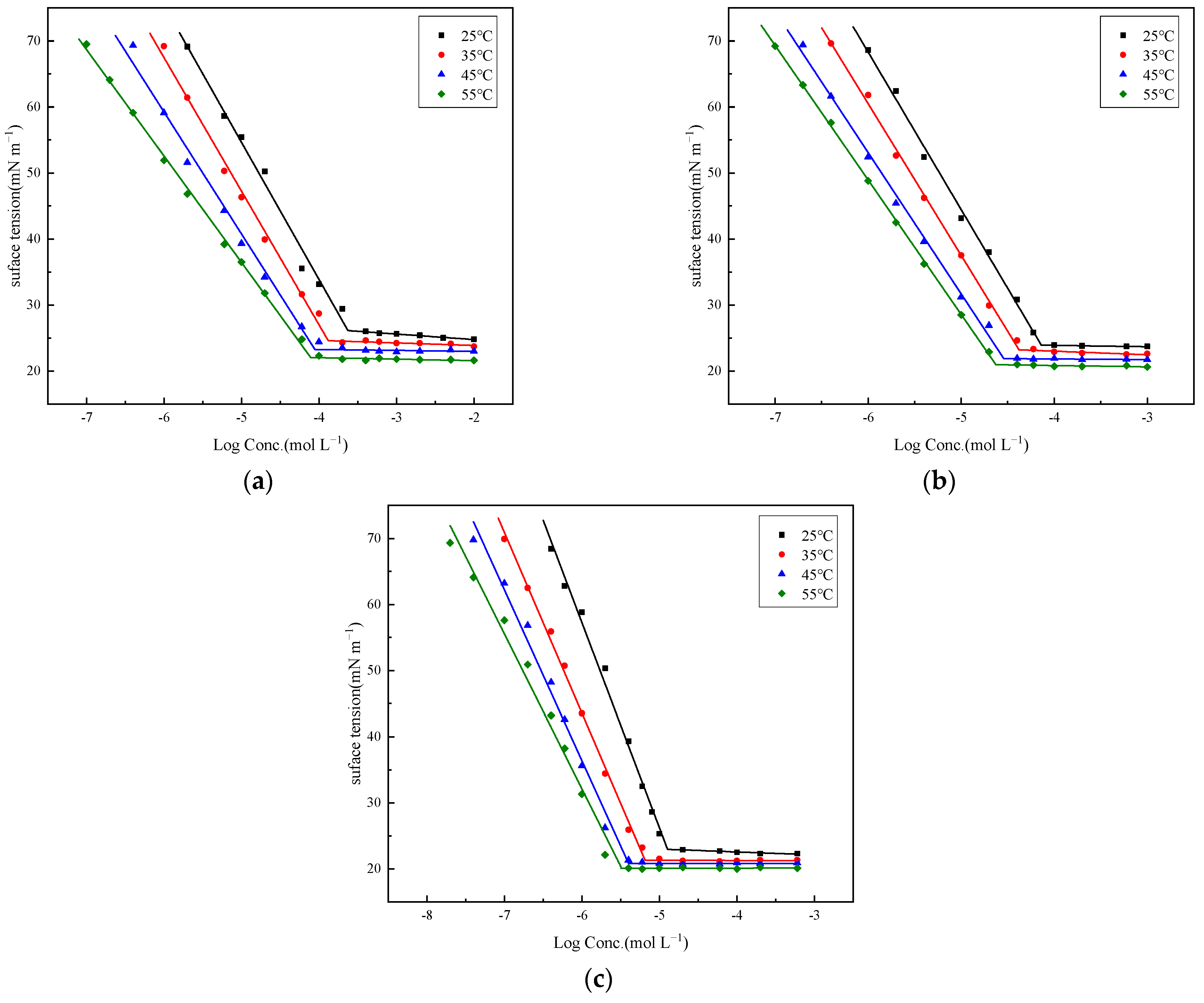




| Surfactants | Temp. (°C) | CAC (mmol L−1) | γCAC (mN m−1) | Γmax (1010 mol cm−2) | Amin (nm2) | πCAC (mN m−1) | pC20 |
|---|---|---|---|---|---|---|---|
| 4C12SAZ | 25 | 0.236 | 26.12 | 3.64 | 0.46 | 45.84 | 4.87 |
| 35 | 0.131 | 24.60 | 3.43 | 0.48 | 45.79 | 5.16 | |
| 45 | 0.088 | 23.26 | 3.03 | 0.55 | 45.47 | 5.43 | |
| 55 | 0.078 | 22.04 | 2.57 | 0.65 | 45.03 | 5.66 | |
| 4C14SAZ | 25 | 0.073 | 23.91 | 4.18 | 0.40 | 48.06 | 5.32 |
| 35 | 0.042 | 23.21 | 3.90 | 0.43 | 47.17 | 5.56 | |
| 45 | 0.029 | 21.89 | 3.51 | 0.47 | 46.85 | 5.80 | |
| 55 | 0.024 | 20.95 | 3.24 | 0.51 | 46.11 | 5.91 | |
| 4C16SAZ | 25 | 0.013 | 22.95 | 5.44 | 0.31 | 49.02 | 5.83 |
| 35 | 0.007 | 21.32 | 4.63 | 0.36 | 49.06 | 6.25 | |
| 45 | 0.004 | 20.83 | 4.24 | 0.39 | 47.91 | 6.48 | |
| 55 | 0.003 | 20.08 | 3.73 | 0.45 | 46.99 | 6.64 | |
| SDS | 25 | 7.25 a | 34.80 a | 3.60 a | 0.48 b | 37.17 a | 2.50 a |
| DTAB | 25 | 15.7 b | 38.90 b | 3.17 b | 0.46 a | 33.20 b | 2.18 b |
| Surfactants | Temp. (K) | ΔGomic (KJ mol−1) | ΔHomic (KJ mol−1) | ΔSomic (KJ mol−1 K−1) | −TΔSomic (KJ mol−1) | ΔGoads (KJ mol−1) |
|---|---|---|---|---|---|---|
| 4C12SAZ | 298.15 | −30.66 | 27.42 | 0.19 | −58.08 | −43.26 |
| 308.15 | −33.18 | 29.29 | 0.20 | −62.47 | −46.53 | |
| 318.15 | −35.31 | 31.22 | 0.21 | −66.53 | −50.30 | |
| 328.15 | −36.75 | 33.21 | 0.21 | −69.97 | −54.30 | |
| 4C14SAZ | 298.15 | −33.57 | 27.71 | 0.21 | −61.29 | −45.08 |
| 308.15 | −36.11 | 29.61 | 0.21 | −65.71 | −48.22 | |
| 318.15 | −38.28 | 31.56 | 0.22 | −69.84 | −51.62 | |
| 328.15 | −40.02 | 33.57 | 0.22 | −73.59 | −54.23 | |
| 4C16SAZ | 298.15 | −37.89 | 33.85 | 0.24 | −71.74 | −46.91 |
| 308.15 | −40.88 | 36.16 | 0.25 | −77.04 | −51.47 | |
| 318.15 | −43.50 | 38.54 | 0.26 | −82.04 | −54.79 | |
| 328.15 | −45.42 | 41.00 | 0.26 | −86.43 | −58.02 |
| Surfactants | a0 (nm2) | l0 (nm) | Vhydrophobic (nm3) | p | R |
|---|---|---|---|---|---|
| 4C12SAZ | 0.46 | 1.67 | 0.35 | 0.46 | 3.05 |
| 4C14SAZ | 0.40 | 1.93 | 0.40 | 0.53 | 4.04 |
| 4C16SAZ | 0.31 | 2.18 | 0.46 | 0.68 | 5.91 |
| Control | 4C12SAZ | 4C14SAZ | 4C16SAZ | |
|---|---|---|---|---|
| E. coli |  | 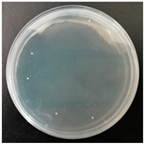 |  | 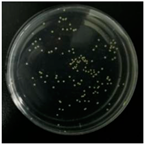 |
| S. aureus |  | 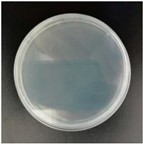 | 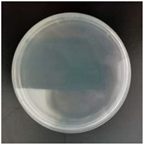 |  |
| Surfactants | Liquid Paraffin (s) | N-Hexane (s) | Kerosene (s) |
|---|---|---|---|
| 4C12SAZ | 683 | 934 | 1012 |
| 4C14SAZ | 741 | 1303 | 2198 |
| 4C16SAZ | 794 | 3247 | 4404 |
| CTAB | 461 | 626 | 768 |
| SDS | 347 | 540 | 712 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Li, J.; Geng, X.; Niu, D.; Wei, Z.; Wang, C.; Sun, Z.; Xie, Y. Synthesis, Surface Activity, Emulsifiability and Bactericidal Performance of Zwitterionic Tetrameric Surfactants. Molecules 2024, 29, 4286. https://doi.org/10.3390/molecules29184286
Wei X, Li J, Geng X, Niu D, Wei Z, Wang C, Sun Z, Xie Y. Synthesis, Surface Activity, Emulsifiability and Bactericidal Performance of Zwitterionic Tetrameric Surfactants. Molecules. 2024; 29(18):4286. https://doi.org/10.3390/molecules29184286
Chicago/Turabian StyleWei, Xin, Jie Li, Xiangfei Geng, Di Niu, Zhenjie Wei, Chenxu Wang, Ziqi Sun, and Yangchun Xie. 2024. "Synthesis, Surface Activity, Emulsifiability and Bactericidal Performance of Zwitterionic Tetrameric Surfactants" Molecules 29, no. 18: 4286. https://doi.org/10.3390/molecules29184286






