Antibiotics Amoxicillin, Ampicillin and Their Mixture—Impact on Bacteria, Fungi, Ostracods and Plants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bacteria and Fungi
2.2. Ostracodtoxkit
2.3. Phytotoxicity
3. Materials and Methods
3.1. Chemical Reagents
3.2. Determination of Total Bacterial and Fungal Counts
- Tryptic Soya Agar to determine total bacterial abundance (TSBA);
- Dichloran Rose Bengal Chloramphenicol (DRBC) to determine total mold and yeast abundance;
- Mannitol Salt Agar (MSA) to determine the abundance of Staphylococcus bacteria;
- Pseudomonas CN Agar to determine the abundance of bacteria of the genus Pseudomonas (PFA);
- Actinomycete Isolation Agar (AIA) to determine the abundance of Actinomycetes;
- Violet Red Bile with Glucose Agar (VRBG) to determine the abundance of bacteria of the genus Enterobacteriaceae.
3.3. Ostracodtoxkit Test
3.4. Phytotoxicity Tests
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Kumar, M.; Singh, D.K. Insight into the amoxicillin resistance, ecotoxicity, and remediation strategies. J. Water Process Eng. 2021, 39, 101858. [Google Scholar] [CrossRef]
- Magdaleno, A.; Saenz, M.E.; Juárez, A.B.; Moretton, J. Effects of six antibiotics and their binary mixtures on growth of Pseudokirchneriella subcapitata. Ecotoxicol. Environ. Saf. 2015, 113, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A.; Tuqan, A.; Assi, H.A. Antibiotic removal from water: Elimination of amoxicillin and ampicillin by microscale and nanoscale iron particles. Environ. Pollut. 2009, 157, 1626–1635. [Google Scholar] [CrossRef]
- Li, L.; Li, T.; Liu, Y.; Li, L.; Huang, X.; Xie, J. Effects of antibiotics stress on root development, seedling growth, antioxidant status and abscisic acid level in wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2023, 252, 114621. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganés, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernández-Pinãs, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef]
- Singh, V.; Pandey, B.; Suthar, S. Phytotoxicity of amoxicillin to the duckweed Spirodela polyrhiza: Growth, oxidative stress, biochemical traits and antibiotic degradation. Chemosphere 2018, 201, 492–502. [Google Scholar] [CrossRef]
- Alexy, R.; Kümpel, T.; Kümmerer, K. Assessment of degradation of 18 antibiotics in the closed bottle test. Chemosphere 2004, 57, 505–512. [Google Scholar] [CrossRef]
- Rosen, W.G. Effects of streptomycin on certain green plants. Ohio J. Sci. 1954, 54, 73–78. [Google Scholar]
- Xie, X.; Zhou, Q.; Lin, D.; Guo, J.; Bao, Y. Toxic effect of tetracycline exposure on growth, antioxidative and genetic indices of wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2011, 18, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Cozzolino, S.; Fiori, M. Phytotoxicity to and uptake of enrofloxacin in crop plants. Chemosphere 2003, 52, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Dąbek-Szreniawska, M.; Hajnos, M.; Stotzky, G.; Collins, Y.; Malicki, J. Numbers of culturable bacteria in soil under mineral or organic cultivation: Comparison of Hattori’s ‘FOR’ and standard dilution plate methods. Int. Agrophys. 2006, 20, 277–288. [Google Scholar]
- Götz, F.; Bannerman, T.; Schleifer, K.L. The genera Staphylococcus and Macrococcus. Prokaryotes 2006, 4, 5–75. [Google Scholar]
- Adamek, E.; Baran, W.; Adamczyk, R. Aerobowa degradacja ampicyliny w glebie. Proc. ECOpole 2020, 14, 59–67. (In Polish) [Google Scholar]
- Akhavan, B.J.; Khanna, N.R.; Vijhani, P. Amoxicillin. In StatPearls; StarPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482250/ (accessed on 15 February 2024).
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Foti, C.; Giuffrè, O. Interaction of Ampicillin and Amoxicillin with Mn2+: A Speciation Study in Aqueous Solution. Molecules 2020, 25, 3110. [Google Scholar] [CrossRef]
- Nolan, K.A.; Ruiz, V.; Burdowski, A.J.; Casares, K.; Brown, O. A simple experiment that reveals overgrowth of fungi as a “side-effect” of antibiotic use. Test. Stud. Lab. Teach. Proc. Assoc. Biol. Lab. Educ. 2019, 40, 69. [Google Scholar]
- Voychuk, S.I.; Gromozova, E.N.; Sadovskiy, M.G. 2010 The model of fungal population dynamics affected by nystatin. Int. J. Quantum Chem. 2010, 110, 242–251. [Google Scholar] [CrossRef]
- Yu-Kyong, S.; Ki-Young, K. Ampicillin activates Mpk1 phosphorylation in Saccharomyces cerevisiae and ERK1/2 phosphorylation in HepG2 cells. Turk. J. Biol. 2017, 41, 600–607. [Google Scholar]
- Spatz, M.; Da Costa, G.; Ventin-Holmberg, R.; Planchais, J.; Michaudel, C.; Wang, Y.; Danne, C.; Lapiere, A.; Michel, M.L.; Kolho, K.L.; et al. Antibiotic treatment using amoxicillin-clavulanic acid impairs gut mycobiota development through modification of the bacterial ecosystem. Microbiome 2023, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Viçosa, G.N.; Botelho, C.V.; Botta, C.; Bertolino, M.; de Carvalho, A.F.; Nero, L.A.; Cocolin, L. Impact of co-cultivation with Enterococcus faecalis over growth, enterotoxin production and gene expression of Staphylococcus aureus in broth and fresh cheeses. Int. J. Food Microbiol. 2019, 308, 108291. [Google Scholar]
- Prithiviraj, B.; Bais, H.P.; Jha, A.K.; Vivanco, J.M. Staphylococcus aureus pathogenicity on Arabidopsis thaliana is mediated either by a direct effect of salicylic acid on the pathogen or by SA-dependent, NPR1-independent host responses. Plant J. 2005, 42, 417–432. [Google Scholar] [CrossRef]
- Byappanahalli, M.N.; Nevers, M.B.; Korajkic, A.; Staley, Z.R.; Harwood, V.J. Enterococci in the environment. Microbiol. Mol. Biol. Rev. 2012, 76, 685–706. [Google Scholar] [CrossRef]
- Preston, G.M. Plant perceptions of plant growth-promoting Pseudomonas. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 907–918. [Google Scholar] [CrossRef]
- Mehmood, N.; Saeed, M.; Zafarullah, S.; Hyder, S.; Rizvi, Z.F.; Gondal, A.S. Multifaceted Impacts of Plant-Beneficial Pseudomonas spp. in Managing Various Plant Diseases and Crop Yield Improvement. ACS Omega 2023, 8, 22296–22315. [Google Scholar] [CrossRef]
- Malik, A.; Aleem, A. 2011 Incidence of metal and antibiotic resistance in Pseudomonas spp. from the river water, agricultural soil irrigated with wastewater and groundwater. Environ. Monit. Assess. 2011, 178, 293–308. [Google Scholar] [CrossRef]
- Bellin, N.; Spezzano, R.; Rossi, V. Assessing the Extinction Risk of Heterocypris incongruens (Crustacea: Ostracoda) in Climate Change with Sensitivity and Uncertainty Analysis. Water 2021, 13, 1828. [Google Scholar] [CrossRef]
- Wieczerzak, M.; Namieśnik, J.; Kudłak, B. Bioassays as one of the Green Chemistry tools for assessing environmental quality: A review. Environ. Int. 2016, 94, 341–361. [Google Scholar] [CrossRef]
- Perrodin, Y.; Bazin, C.; Bony, S.; Devaux, A.; Bertrand-Krajewski, J.-L.; Cren-Olivé, C.; Roch, A.; Brelot, E. A priori assessment of ecotoxicological risks linked to building a hospital. Chemosphere 2013, 90, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Kudłak, B.; Wieczerzak, M.; Namiesnik, J. Determination of toxicological parameters of selected bioactive organic chemicals using the Ostracodtoxkit FTM. Chem. Didact. Ecol. Metrol. 2018, 23, 113–126. [Google Scholar] [CrossRef]
- Mukhtar, A.; Manzoor, M.; Gul, I.; Zafar, R.; Jamil, H.I.; Niazi, A.K.; Ali, M.A.; Park, T.J.; Arshad, M. Phytotoxicity of different antibiotics to rice and stress alleviation upon application of organic amendments. Chemosphere 2020, 258, 127353. [Google Scholar]
- Bellino, A.; Lofrano, G.; Carotenuto, M.; Libralato, G.; Baldantoni, D. Antibiotic effects on seed germination and root development of tomato (Solanum lycopersicum L.). Ecotoxicol. Environ. Saf. 2018, 148, 135–141. [Google Scholar] [CrossRef]
- Riaz, L.; Mahmood, T.; Coyne, M.S.; Khalid, A.; Rashid, A.; Hayat, M.T.; Gulzar, A.; Amjad, M. Physiological and antioxidant response of wheat (Triticum aestivum) seedlings to fluoroquinolone antibiotics. Chemosphere 2017, 177, 250–257. [Google Scholar] [CrossRef]
- Pierattini, E.C.; Francini, A.; Raffaelli, A.; Sebastiani, L. Morpho-physiological response of Populus alba to erythromycin: A timeline of the health status of the plant. Sci. Total Environ. 2016, 569–570, 540–547. [Google Scholar] [CrossRef]
- Fu, L.; Huang, T.; Wang, S.; Wang, X.; Su, L.; Li, C.; Zhao, Y. Toxicity of 13 different antibiotics towards freshwater green algae Pseudokirchneriella subcapitata and their modes of action. Chemosphere 2017, 168, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Ilesanmi, O.I.; Adekunle, E.A.; Omolaiye, J.A.; Olorode, E.M.; Ogunkanmi, L.A. Isolation, optimization and molecular characterization of lipase producing bacteria from contaminated soil. Sci. Afr. 2020, 8, e00279. [Google Scholar] [CrossRef]
- Cycoń, M.; Borymski, S.; Żołnierczyk, B.; Piotrowska-Seget, Z. Variable Effects of Non-steroidal Anti-inflammatory Drugs (NSAIDs) on Selected Biochemical Processes Mediated by Soil Microorganisms. Front. Microbiol. 2016, 7, 1969. [Google Scholar] [CrossRef]
- ISO 14371:2012; Water Quality—Determination of Fresh Water Sediment Toxicity to Heterocypris incongruens (Crustacea, Ostracoda). ISO: Geneva, Switzerland, 2012.
- OECD/OCDE 208, 2006; Guidelines for the Testing of Chemical. Terrestrial Plant: Seedling Test: Seedlings Emergence and Seedling Growth Test. OECD: Paris, France, 2006. Available online: https://www.oecd-ilibrary.org/environment/test-no-208-terrestrial-plant-test-seedling-emergence-and-seedling-growth-test_9789264070066-en (accessed on 8 January 2024).
- PN-EN ISO 11269:2; Determination of the Effects of Pollutants on Soil Flora—Part 2: Effects of Contaminated Soil on the Emergence and Early Growth of Higher Plants. ISO: Geneva, Switzerland, 2012.
- Liu, T.; Zhu, L.; Xie, H.; Wang, J.; Wang, J.; Sun, F.; Wang, F. Effects of the ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate on the growth of wheat seedlings. Environ. Sci. Pollut. Res. 2014, 21, 3936–3945. [Google Scholar] [CrossRef]
- Wang, L.-S.; Wang, L.; Wang, L.; Wang, G.; Li, Z.-H.; Wang, J.-J. Effect of 1-butyl- 3-methylimidazolium tetrafluoroborate on the wheat (Triticum aestivum L.) seedlings. Environ. Toxicol. 2009, 24, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, I. Content of Selected Components of Spinach (Spinacia oleraceae L.) Grown with Different Calcium Content. Rocz. AR W Pozn. 2004, CCCLX, 105–110. (In Polish) [Google Scholar]

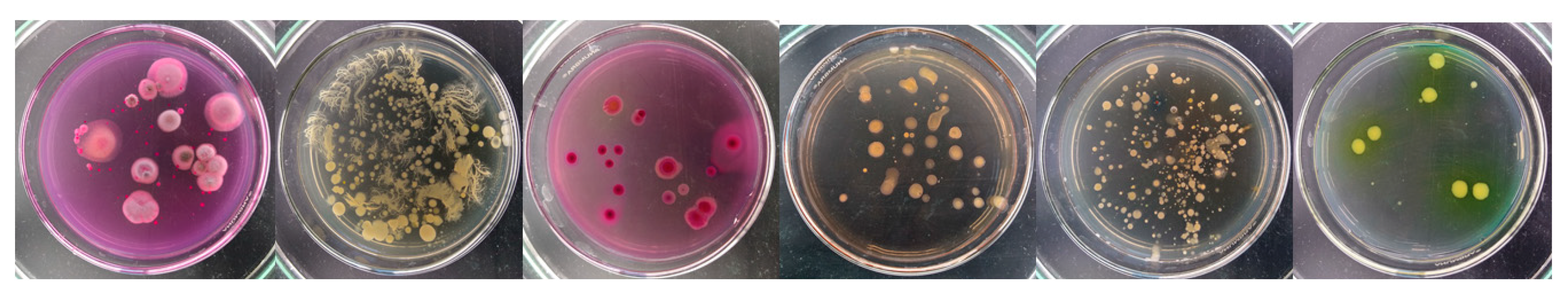
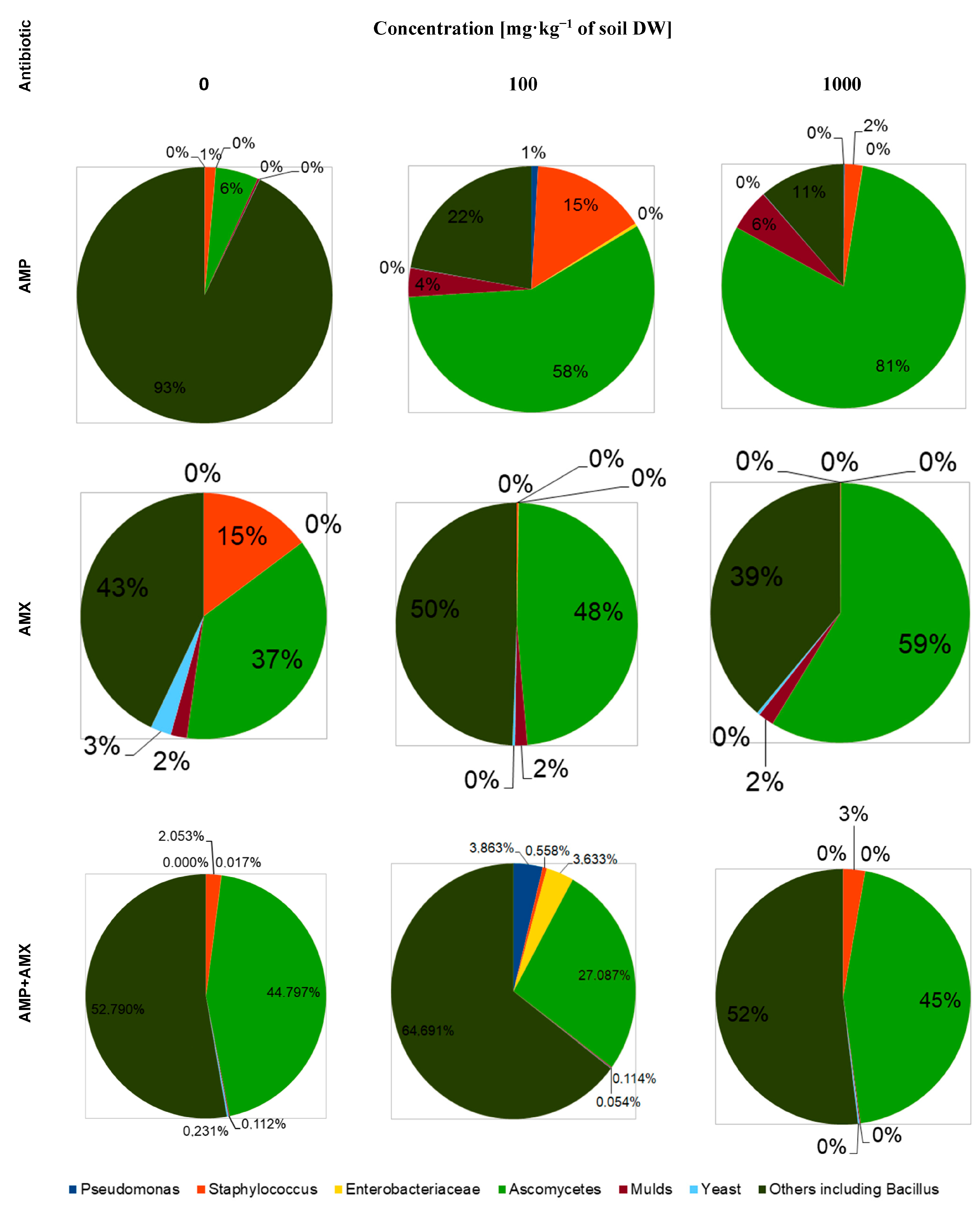
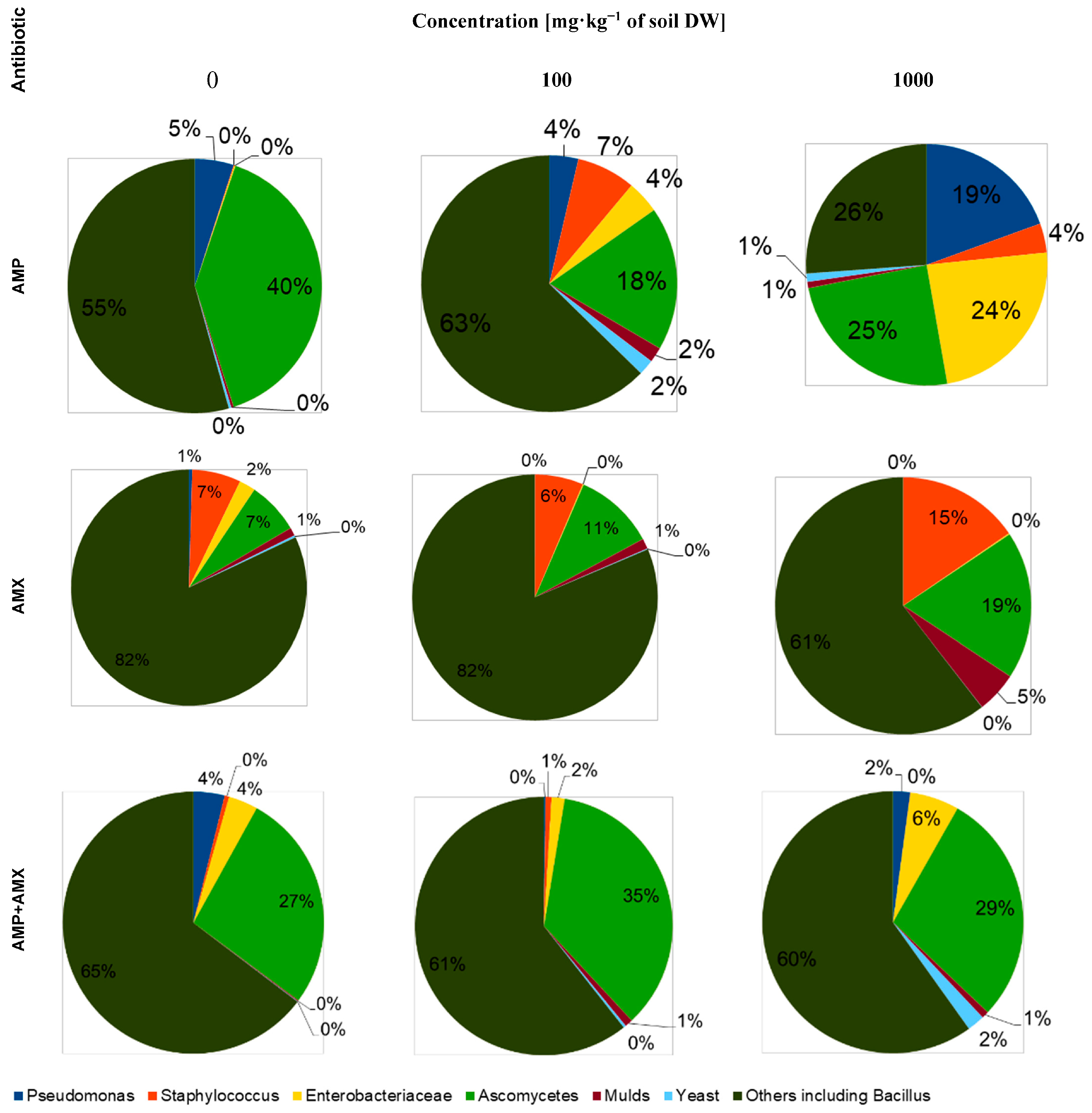
| Concentration of Antibiotics [mg·kg−1 of Soil DW] | AMP | AMX | AMP + AMX | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Medium Length [µm] | Growth Inhibition [%] | Mortality [%] | Medium Length [µm] | Growth Inhibition [%] | Mortality [%] | Medium Length [µm] | Growth Inhibition [%] | Mortality [%] | |
| Reference soil | 980.56 ab | - | 0 | 980.56 ab | - | 0 | 980.56 ab | - | 0 |
| 0.1 | 984.88 ab | 5.03 | 0 | 982.19 ab | 5.40 | 0 | 973.69 a | 6.58 | 0 |
| 1 | 983.88 ab | 5.17 | 0 | 978.84 ab | 5.87 | 0 | 972.37 a | 6.77 | 0 |
| 10 | 985.70 ab | 4.91 | 0 | 987.40 ab | 4.68 | 0 | 970.55 a | 7.02 | 0 |
| 50 | 980.85 ab | 5.59 | 0 | 1025.95 a | −0.68 | 0 | 986.53 a | 4.80 | 0 |
| 100 | 947.60 ab | 10.20 | 0 | 984.89 ab | 5.03 | 0 | 1000.94 a | 2.80 | 0 |
| 1000 | 917.13 b | 14.44 | 0 | 924.34 b | 13.44 | 0 | 933.51 a | 12.16 | 0 |
| Concentration of Antibiotics (mg‧kg−1 of Soil DW) | AMP | AMX | AMP + AMX | |||
|---|---|---|---|---|---|---|
| GP (%) | GR (%) | GP (%) | GR (%) | GP (%) | GR (%) | |
| 0 | 88.3 ± 2.9 b | 95.0 ± 0.0 ab | 95.0 ± 8.7 a | 95.0 ± 8.7 a | 96.7 ± 5.8 a | 98.3 ± 2.9 a |
| 0.1 | 95.0 ± 5.0 ab | 96.7 ± 5.8 ab | 95.0 ± 5.0 a | 95.0 ± 5.0 a | 93.3 ± 5.8 a | 93.3 ± 7.6 a |
| 1 | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 98.3 ± 2.9 a | 98.3 ± 2.9 a | 93.3 ± 5.8 a | 93.3 ± 5.8 a |
| 10 | 95.0 ± 0.0 ab | 96.7 ± 2.9 ab | 95.0 ± 5.0 a | 96.7 ± 5.8 a | 91.7 ± 2.9 a | 93.3 ± 2.9 a |
| 50 | 90.0 ± 5.0 ab | 90.0 ± 5.00 b | 98.3 ± 2.9 a | 98.3 ± 2.9 a | 91.7 ± 2.9 a | 95.0 ± 0.0 a |
| 100 | 95.0 ± 5.0 ab | 96.7 ± 2.9 ab | 96.7 ± 2.9 a | 98.3 ± 2.9 a | 96.7 ± 2.9 a | 98.3 ± 2.9 a |
| 1000 | 98.3 ± 2.9 ab | 98.3 ± 2.9 ab | 98.3 ± 2.9 a | 100.0 ± 0.0 a | 90.0 ± 5.0 a | 96.7 ± 5.8 a |
| Concentration of Antibiotics (mg‧kg−1 of soil DW) | AMP | AMX | AMP + AMX |
|---|---|---|---|
| Yield [g pot−1] | |||
| 0 | 2.507 ± 0.104 c | 2.951 ± 0.594 a | 2.492 ± 0.146 a |
| 0.1 | 2.543 ± 0.084 c | 3.125 ± 0.132 a | 2.440 ± 0.258 a |
| 1 | 2.924 ± 0.157 ab | 3.207 ± 0.228 a | 2.495 ± 0.237 a |
| 10 | 2.729 ± 0.012 bc | 3.197 ± 0.121 a | 2.654 ± 0.084 a |
| 50 | 2.736 ± 0.072 bc | 3.142 ± 0.231 a | 2.599 ± 0.196 a |
| 100 | 2.943 ± 0.144 ab | 3.080 ± 0.123 a | 2.554 ± 0.007 a |
| 1000 | 3.192 ± 0.233 a | 3.086 ± 0.126 a | 2.520 ± 0.117 a |
| Dry weight [g g−1 FW] | |||
| 0 | 0.0936 ± 0.0007 a | 0.0956 ± 0.0006 b | 0.0943 ± 0.0010 a |
| 0.1 | 0.0941 ± 0.0031 a | 0.0925 ± 0.0006 d | 0.0931 ± 0.0028 a |
| 1 | 0.0926 ± 0.0011 ab | 0.0929 ± 0.0005 cd | 0.0945 ± 0.0005 a |
| 10 | 0.0940 ± 0.0025 a | 0.0930 ± 0.0006 cd | 0.0925 ± 0.0009 a |
| 50 | 0.0902 ± 0.0014 ab | 0.0948 ± 0.0005 bc | 0.0942 ± 0.0004 a |
| 100 | 0.0914 ± 0.0019 ab | 0.0944 ± 0.0010 bcd | 0.0959 ± 0.0003 a |
| 1000 | 0.0882 ± 0.0011 b | 0.0989 ± 0.0011 a | 0.0947 ± 0.0010 a |
| Shoot length [cm] | |||
| 0 | 14.3 ± 1.1 d | 18.6 ± 1.0 a | 15.5 ± 1.0 b |
| 0.1 | 15.0 ± 0.6 cd | 17.7 ± 0.4 a | 15.7 ± 0.7 ab |
| 1 | 15.9 ± 0.8 ab | 18.0 ± 0.7 a | 15.9 ± 0.9 ab |
| 10 | 15.7 ± 0.9 bc | 17.4 ± 3.2 a | 16.4 ± 1.0 a |
| 50 | 16.1 ± 0.9 ab | 17.7 ± 2.6 a | 16.2 ± 0.6 ab |
| 100 | 15.8 ± 0.8 abc | 18.0 ± 0.8 a | 15.7 ± 0.7 ab |
| 1000 | 16.7± 0.8 a | 18.4 ± 0.6 a | 15.9 ± 0.6 ab |
| Root length [cm] | |||
| 0 | 13.1 ± 1.8 ab | 12.9 ± 1.2 a | 12.6 ± 1.6 ab |
| 0.1 | 13.0 ± 1.2 ab | 14.0 ± 1.1 a | 12.2 ± 0.8 b |
| 1 | 13.9 ± 1.1 a | 13.7 ± 1.2 a | 13.7 ± 1.0 a |
| 10 | 13.6 ± 1.1 ab | 14.1 ± 1.1 a | 13.8 ± 1.2 a |
| 50 | 14.0 ± 1.2 bc | 13.0 ± 1.0 a | 13.1 ± 1.0 ab |
| 100 | 12.3 ± 1.2 bc | 14.2 ± 1.3 a | 13.3 ± 1.2 ab |
| 1000 | 11.4 ± 0.9 c | 11.1 ± 1.3 b | 12.4 ± 0.9 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowska, B.; Sysa, M.; Godela, A.; Biczak, R. Antibiotics Amoxicillin, Ampicillin and Their Mixture—Impact on Bacteria, Fungi, Ostracods and Plants. Molecules 2024, 29, 4301. https://doi.org/10.3390/molecules29184301
Pawłowska B, Sysa M, Godela A, Biczak R. Antibiotics Amoxicillin, Ampicillin and Their Mixture—Impact on Bacteria, Fungi, Ostracods and Plants. Molecules. 2024; 29(18):4301. https://doi.org/10.3390/molecules29184301
Chicago/Turabian StylePawłowska, Barbara, Marcin Sysa, Agnieszka Godela, and Robert Biczak. 2024. "Antibiotics Amoxicillin, Ampicillin and Their Mixture—Impact on Bacteria, Fungi, Ostracods and Plants" Molecules 29, no. 18: 4301. https://doi.org/10.3390/molecules29184301
APA StylePawłowska, B., Sysa, M., Godela, A., & Biczak, R. (2024). Antibiotics Amoxicillin, Ampicillin and Their Mixture—Impact on Bacteria, Fungi, Ostracods and Plants. Molecules, 29(18), 4301. https://doi.org/10.3390/molecules29184301









