Wide pH, Adaptable High Internal Phase Pickering Emulsion Stabilized by a Crude Polysaccharide from Thesium chinense Turcz.
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the TTP Extractive Conditions
2.2. Characterization of TTP
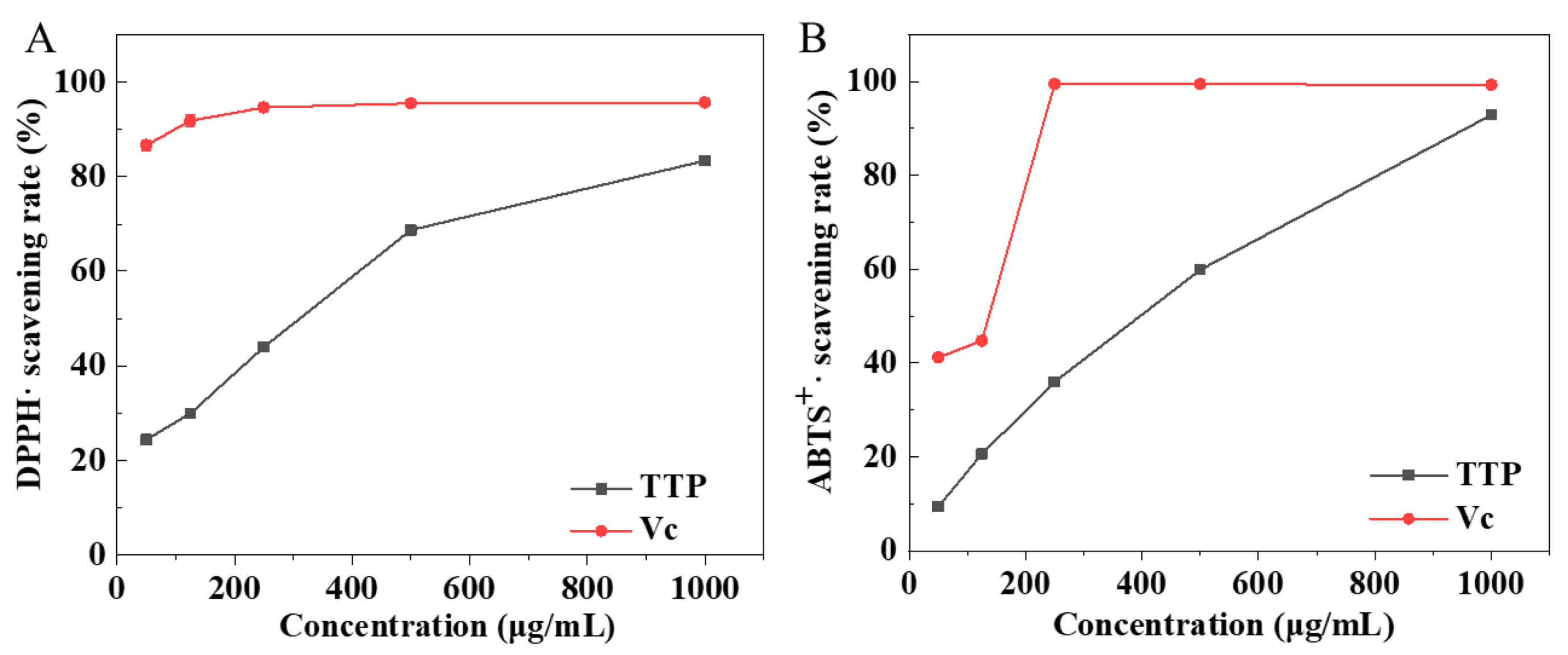
2.3. Antioxidant Activity of TTP
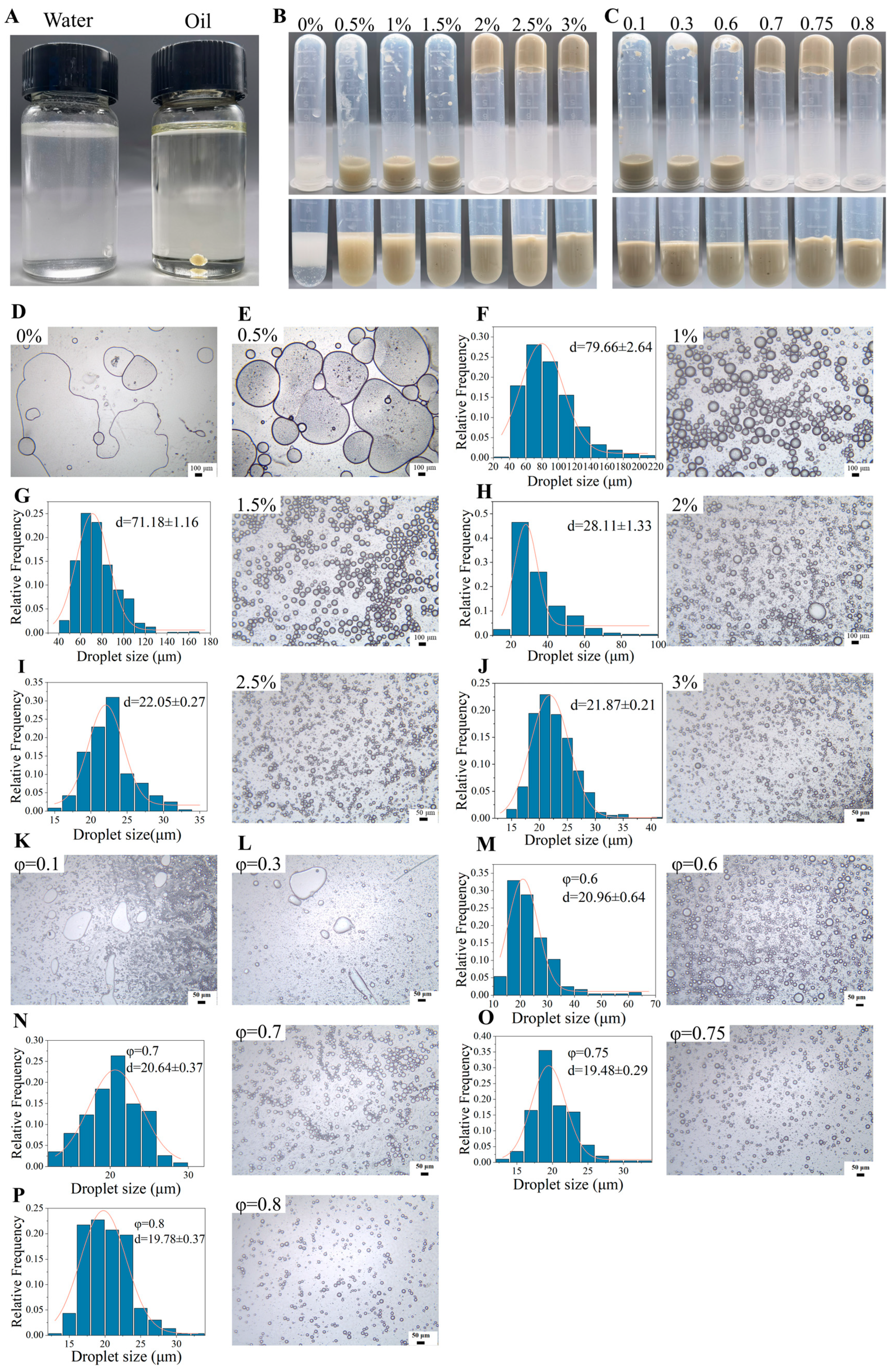
2.4. Preparation and Characteristics of Emulsions Stabilized by TTP
2.5. Stability of HIPEs Stabilized by TTP
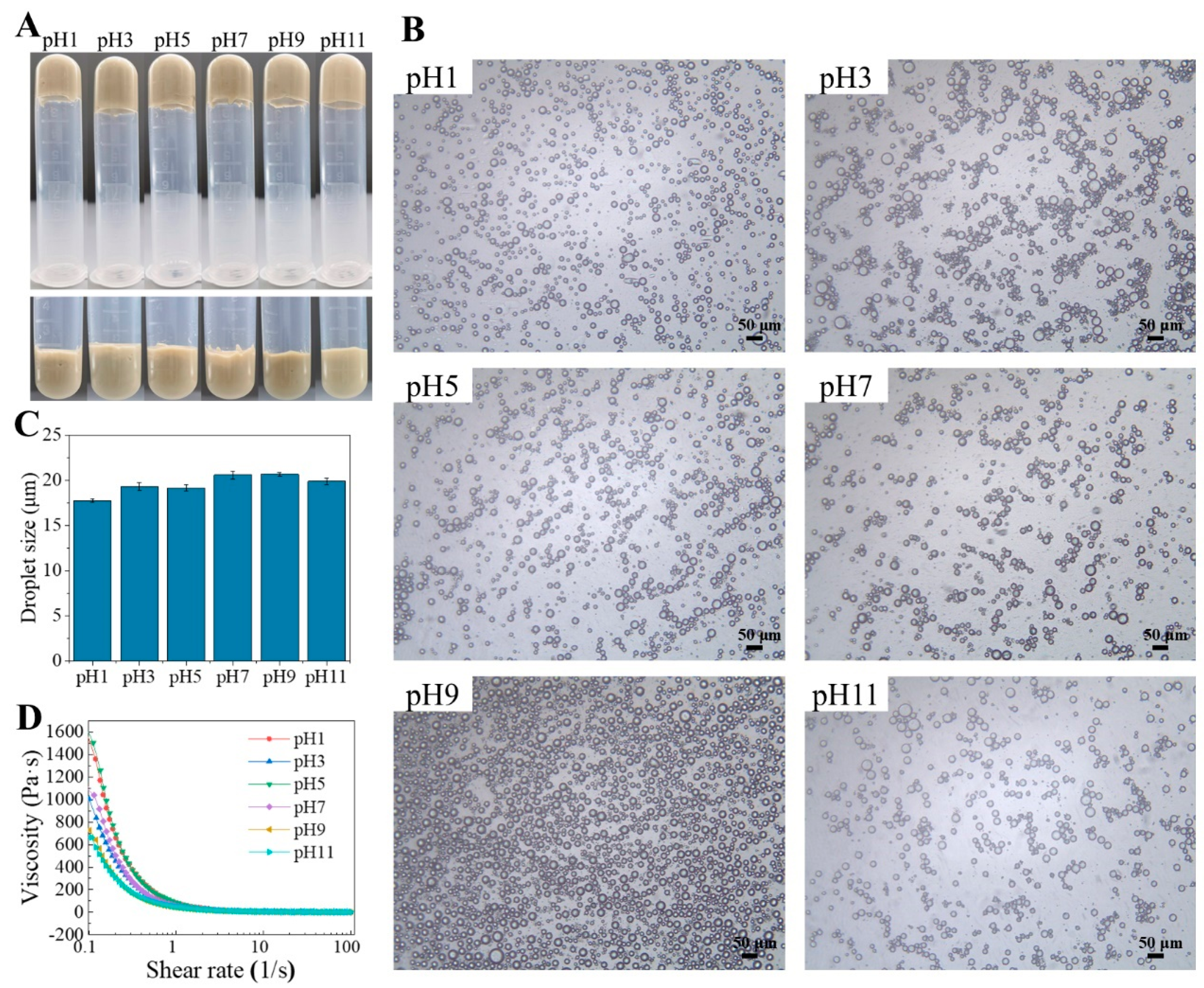
2.5.1. Effects of pH
2.5.2. Effects of Ionic Strengths
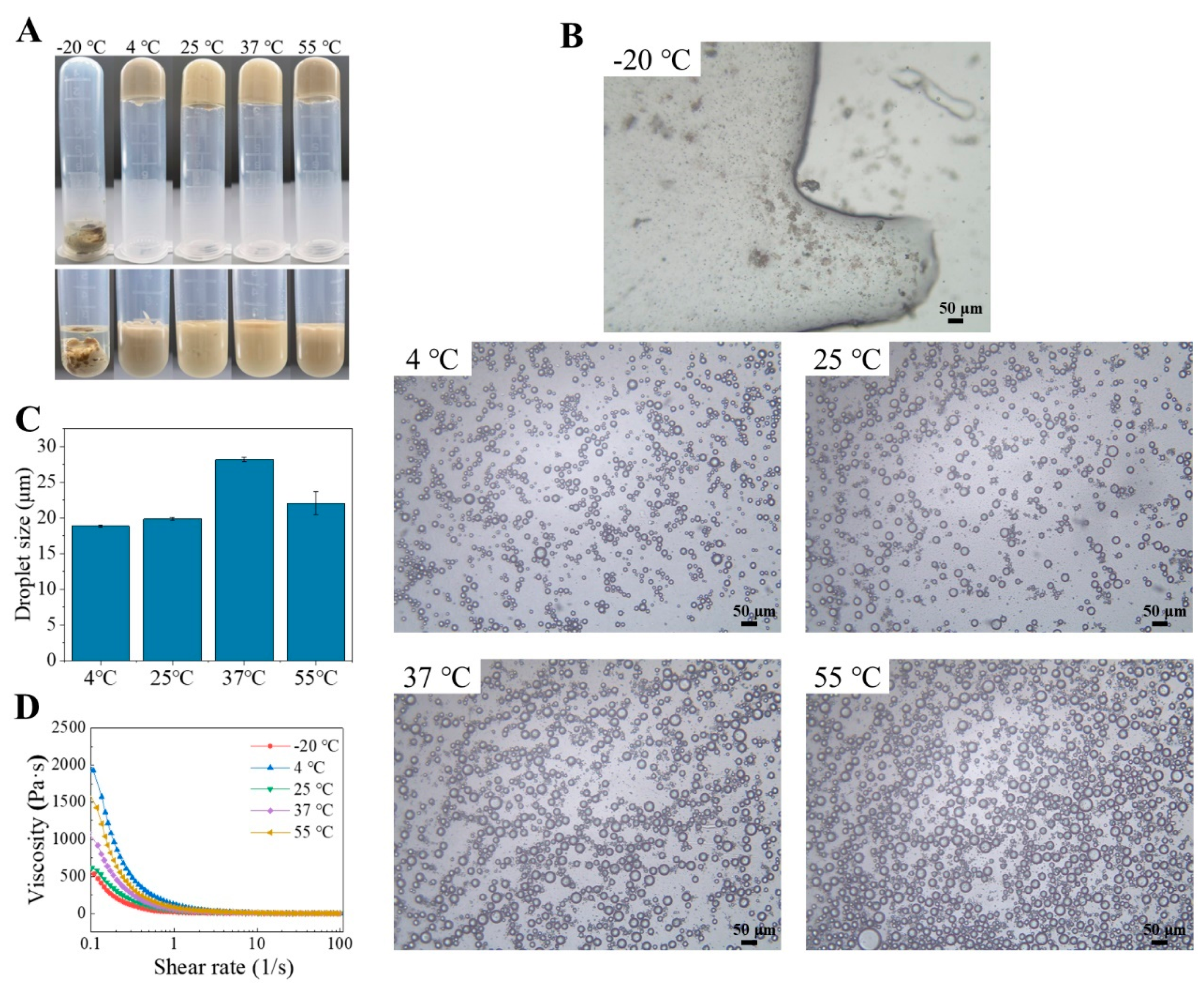
2.5.3. Effects of Temperature
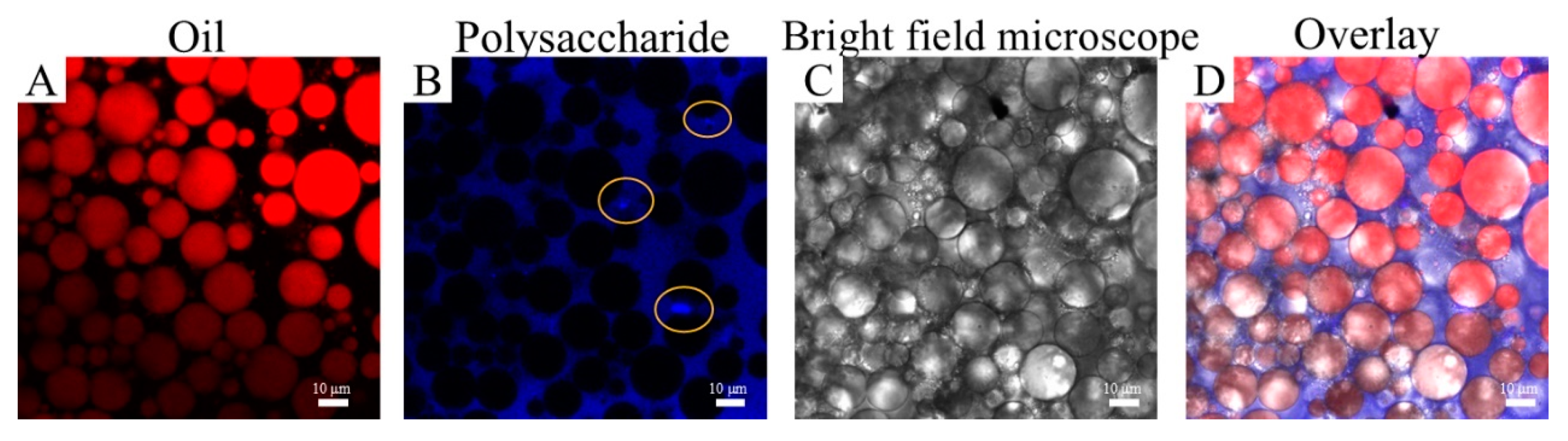
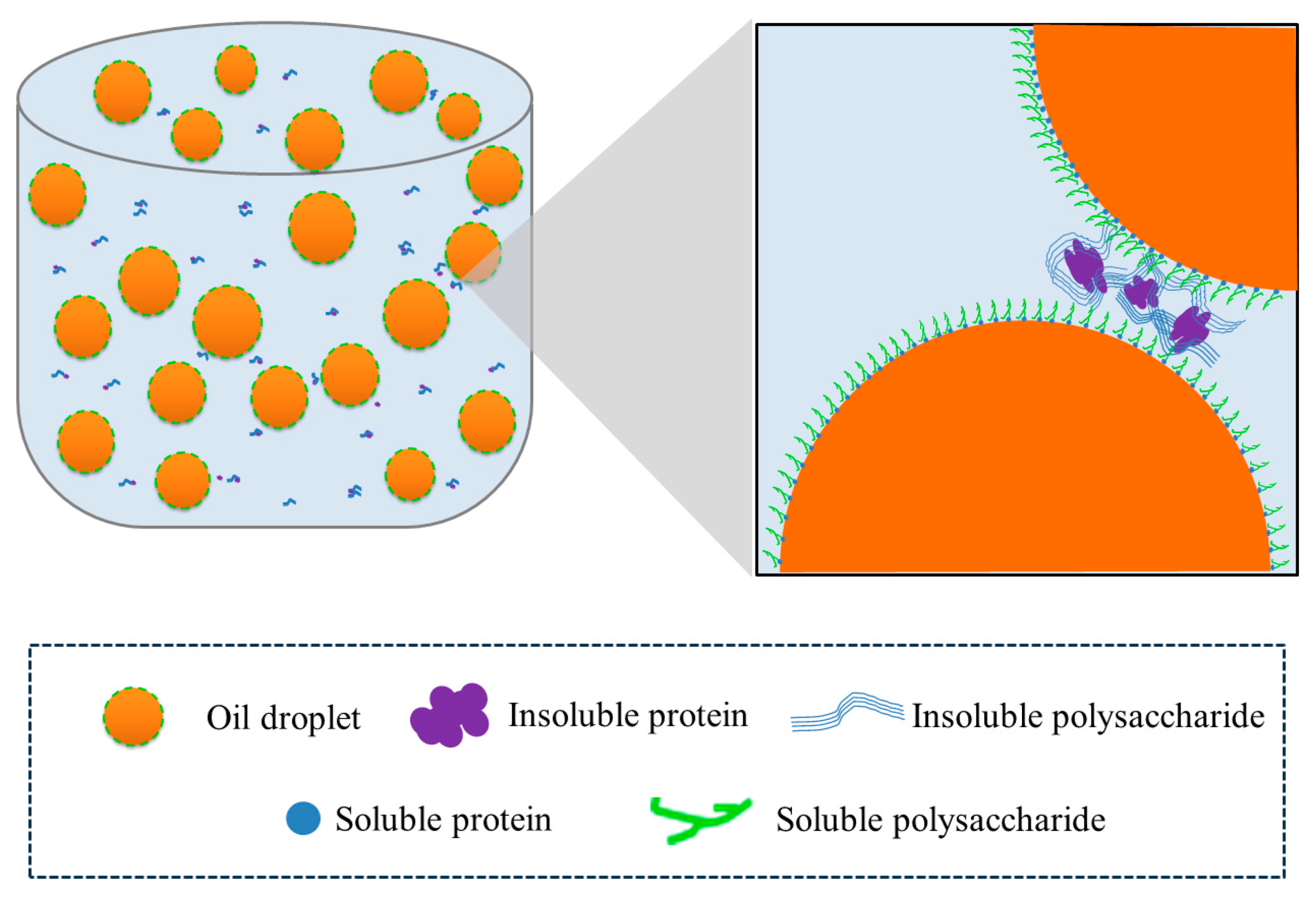
2.6. Microstructure of HIPEs Stabilized by TTP
3. Materials and Methods
3.1. Materials and Reagents
3.2. Pretreatment of Raw Materials
3.3. Ultrasound-Assisted Extraction of TTP
3.4. Chemical Composition and Water Solubility of TTP Analysis
3.5. FTIR Spectra of TTP Analysis
3.6. Antioxidant Activity of TTP Assay
3.7. Preparation of Pickering Emulsions Stabilized by TTP
3.8. Characterization of the Pickering Emulsions Stabilized by TTP
3.9. Stability of Pickering Emulsions Stabilized by TTP
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, F.; Zhao, S.; Guan, X.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Polysaccharide-based Pickering emulsions: Formation, stabilization and applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Harman, C.L.; Patel, M.A.; Guldin, S.; Davies, G.-L. Recent developments in Pickering emulsions for biomedical applications. Curr. Opin. Colloid 2019, 39, 173–189. [Google Scholar] [CrossRef]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-grade Pickering emulsions: Preparation, stabilization and applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Chitosan-based Pickering emulsions and their applications: A review. Carbohydr. Polym. 2020, 250, 116885. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Lissant, K.J. The geometry of high-internal-phase-ratio emulsions. J. Colloid Interface Sci. 1966, 22, 462–468. [Google Scholar] [CrossRef]
- Dimitrova, T.D.; Leal-Calderon, F.J.L. Bulk elasticity of concentrated protein-stabilized emulsions. Langmuir 2001, 17, 3235–3244. [Google Scholar] [CrossRef]
- Zhao, Q.; Zaaboul, F.; Liu, Y.; Li, J. Recent advances on protein-based Pickering high internal phase emulsions (Pickering HIPEs): Fabrication, characterization, and applications. Compr. Rev. Food Sci. 2020, 19, 1934–1968. [Google Scholar] [CrossRef]
- Jiang, H.; Hong, L.; Li, Y.; Ngai, T. All-Silica Submicrometer Colloidosomes for Cargo Protection and Tunable Release. Angew. Chem. 2018, 130, 11836–11840. [Google Scholar] [CrossRef]
- Fessi, N.; Nsib, M.F.; Chevalier, Y.; Guillard, C.; Dappozze, F.; Houas, A.; Palmisano, L.; Parrino, F. Photocatalytic degradation enhancement in Pickering emulsions stabilized by solid particles of bare TiO2. Langmuir 2019, 35, 2129–2136. [Google Scholar] [CrossRef]
- Douliez, J.P.; Martin, N.; Beneyton, T.; Eloi, J.C.; Chapel, J.P.; Navailles, L.; Baret, J.C.; Mann, S.; Béven, L. Preparation of Swellable Hydrogel-Containing Colloidosomes from Aqueous Two-Phase Pickering Emulsion Droplets. Angew. Chem. 2018, 130, 7906–7910. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.; Ji, Z.; Zhang, H. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, A.; Qiu, S.; Liu, Y.; Zhu, Q.; Yin, L. Covalent conjugation of bovine serum album and sugar beet pectin through Maillard reaction/laccase catalysis to improve the emulsifying properties. Food Hydrocoll. 2018, 76, 173–183. [Google Scholar] [CrossRef]
- Yu, X.; Han, L.; Liu, J.; Jiang, W.; Pan, J.; Yu, C.; Dong, X. Preparation and 3D printing of high internal-phase Pickering emulsions stabilized by chicken egg white microgel. Food Hydrocoll. 2024, 147, 109393. [Google Scholar] [CrossRef]
- Tang, C.-H. Globular proteins as soft particles for stabilizing emulsions: Concepts and strategies. Food Hydrocoll. 2020, 103, 105664. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Zhao, G.J. Fabricating a Pickering stabilizer from okara dietary fibre particulates by conjugating with soy protein isolate via Maillard reaction. Foods 2020, 9, 143. [Google Scholar] [CrossRef]
- Zhu, F. Starch based Pickering emulsions: Fabrication, properties, and applications. Trends Food Sci. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Yang, H.; Su, Z.; Meng, X.; Zhang, X.; Kennedy, J.F.; Liu, B.J. Fabrication and characterization of Pickering emulsion stabilized by soy protein isolate-chitosan nanoparticles. Carbohydr. Polym. 2020, 247, 116712. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, Q.; Shen, X.J. Quinoa protein/polysaccharide electrostatic complex stabilized vegan high internal phase emulsions for 3D printing: Role of complex state and gelling-type polysaccharides. Food Chem. 2024, 434, 137447. [Google Scholar] [CrossRef]
- Wang, M.; Yin, Z.; Zeng, M.J. Construction of 3D printable Pickering emulsion gels using complexes of fiber polysaccharide-protein extracted from Haematococcus pluvialis residues and gelatin for fat replacer. Food Hydrocoll. 2023, 137, 108350. [Google Scholar] [CrossRef]
- Shao, L.; Sun, Y.; Liang, J.; Li, M.; Li, X. Decolorization affects the structural characteristics and antioxidant activity of polysaccharides from Thesium chinense Turcz: Comparison of activated carbon and hydrogen peroxide decolorization. Int. J. Biol. Macromol. 2020, 155, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Li, C.; Shi, X.; Xiao, H.J. Naturally occurring protein/polysaccharide hybrid nanoparticles for stabilizing oil-in-water Pickering emulsions and the formation mechanism. Food Chem. 2022, 395, 133641. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Li, Q.; Liu, J.; Ye, Z.; Feng, T.; Wang, G.; Wang, W.; Zhang, Y. Ultrasonic–assisted extraction of polysaccharides from Auricularia auricula and effects of its acid hydrolysate on the biological function of Caenorhabditis elegans. Int. J. Biol. Macromol. 2021, 167, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Dan, M.; Li, W.; Zhang, P. Characterizations and immunostimulatory activities of a polysaccharide from Arnebia euchroma (Royle) Johnst. roots. Int. J. Biol. Macromol. 2019, 125, 791–799. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Barros, A.; Barros, M.; Rutledge, D.N.; Delgadillo, I. Multivariate analysis of uronic acid and neutral sugars in whole pectic samples by FT-IR spectroscopy. Carbohydr. Polym. 1998, 37, 241–248. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Jiménez-Escrig, A.; Rupérez, P. Multifunctional antioxidant activity of polysaccharide fractions from the soybean byproduct okara. Carbohydr. Polym. 2010, 82, 245–250. [Google Scholar] [CrossRef]
- Gu, F.-L.; Kim, J.M.; Abbas, S.; Zhang, X.-M.; Xia, S.-Q.; Chen, Z.-X. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein–glucose. Food Chem. 2010, 120, 505–511. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, S.W.; Gong, J.; Guo, Q.; Wang, Q.; Hua, Y. A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll. 2015, 48, 155–164. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Luo, Y. Caseinate-zein-polysaccharide complex nanoparticles as potential oral delivery vehicles for curcumin: Effect of polysaccharide type and chemical cross-linking. Food Hydrocoll. 2017, 72, 254–262. [Google Scholar] [CrossRef]
- Jafari, S.M.; Sedaghat Doost, A.; Nikbakht Nasrabadi, M.; Boostani, S.; Van der Meeren, P. Phytoparticles for the stabilization of Pickering emulsions in the formulation of novel food colloidal dispersions. Trends Food Sci. Technol. 2020, 98, 117–128. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Chen, Z.; Wang, T.; Lu, Z.; Hu, W.; Wang, L.J. Zein/gum Arabic nanoparticle-stabilized Pickering emulsion with thymol as an antibacterial delivery system. Carbohydr. Polym. 2018, 200, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Wu, Z.-L.; Zhu, J.-Y.; Yin, S.-W.; Tang, C.-H.; Wu, L.-Y.; Yang, X.-Q. Development of antioxidant Pickering high internal phase emulsions (HIPEs) stabilized by protein/polysaccharide hybrid particles as potential alternative for PHOs. Food Chem. 2017, 231, 122–130. [Google Scholar] [CrossRef]
- Lv, P.; Wang, D.; Chen, Y.; Zhu, S.; Zhang, J.; Mao, L.; Gao, Y.; Yuan, F. Pickering emulsion gels stabilized by novel complex particles of high-pressure-induced WPI gel and chitosan: Fabrication, characterization and encapsulation. Food Hydrocoll. 2020, 108, 105992. [Google Scholar] [CrossRef]
- Li, M.-F.; He, Z.-Y.; Li, G.-Y.; Zeng, Q.-Z.; Su, D.-X.; Zhang, J.-L.; Wang, Q.; Yuan, Y.; He, S. The formation and characterization of antioxidant pickering emulsions: Effect of the interactions between gliadin and chitosan. Food Hydrocoll. 2019, 90, 482–489. [Google Scholar] [CrossRef]
- Tong, Q.; Yi, Z.; Ran, Y.; Chen, X.; Chen, G.; Li, X.J. Green tea polyphenol-stabilized gel-like high internal phase pickering emulsions. ACS Sustain. Chem. 2021, 9, 4076–4090. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Chen, J.; Li, B.; Li, Y.; Liu, S. Edible foam based on pickering effect of bacterial cellulose nanofibrils and soy protein isolates featuring interfacial network stabilization. Food Hydrocoll. 2020, 100, 105440. [Google Scholar] [CrossRef]
- Lomolino, G.; Vincenzi, S.; Zannoni, S.; Marangon, M.; De Iseppi, A.; Curioni, A. Emulsifying activity of potato proteins in the presence of k-carrageenan at different pH conditions. Food Chem. X 2022, 13, 100232. [Google Scholar] [CrossRef]
- Du Le, H.; Loveday, S.M.; Singh, H.; Sarkar, A. Pickering emulsions stabilised by hydrophobically modified cellulose nanocrystals: Responsiveness to pH and ionic strength. Food Hydrocoll. 2020, 99, 105344. [Google Scholar] [CrossRef]
- Tan, C.; Pajoumshariati, S.; Arshadi, M.; Abbaspourrad, A. A simple route to renewable high internal phase emulsions (HIPEs) strengthened by successive cross-linking and electrostatics of polysaccharides. Chem. Commun. 2019, 55, 1225–1228. [Google Scholar] [CrossRef]
- Huang, M.; Wang, J.; Tan, C. Tunable high internal phase emulsions stabilized by cross-linking/electrostatic deposition of polysaccharides for delivery of hydrophobic bioactives. Food Hydrocoll. 2021, 118, 106742. [Google Scholar] [CrossRef]
- Zhu, Y.; Ren, X.; Bao, Y.; Li, S.; Peng, Z.; Zhang, Y.; Zhou, G. Emulsification of oil-in-water emulsions with eggplant (Solanum melongena L.). J. Colloid Interface Sci. 2020, 563, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Liu, H.; Zhang, K. Recent progress on Pickering emulsions stabilized by polysaccharides-based micro/nanoparticles. Adv. Colloid Interface Sci. 2021, 296, 102522. [Google Scholar] [CrossRef]
- Mao, G.; Zou, Y.; Feng, W.; Wang, W.; Zhao, T.; Ye, C.; Zhu, Y.; Wu, X.; Yang, L.; Wu, X. Extraction, preliminary characterization and antioxidant activity of Se-enriched Maitake polysaccharide. Carbohydr. Polym. 2014, 101, 213–219. [Google Scholar] [CrossRef]
- Lin, S.; Guo, H.; Gong, J.D.B.; Lu, M.; Lu, M.-Y.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D.-T. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cereal Sci. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, G.; Li, J.; Feng, Y.; Zhang, L.; Peng, Y.; Tang, Y.; Wang, L. Eco-friendly Pickering emulsion stabilized by silica nanoparticles dispersed with high-molecular-weight amphiphilic alginate derivatives. ACS Sustain. Chem. 2018, 6, 4105–4114. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, P.; Wang, M.; Wang, F.; Wang, N. Effect of konjac glucomannan with different molecular weights on physicochemical properties of corn starch. Food Hydrocoll. 2019, 96, 663–670. [Google Scholar] [CrossRef]
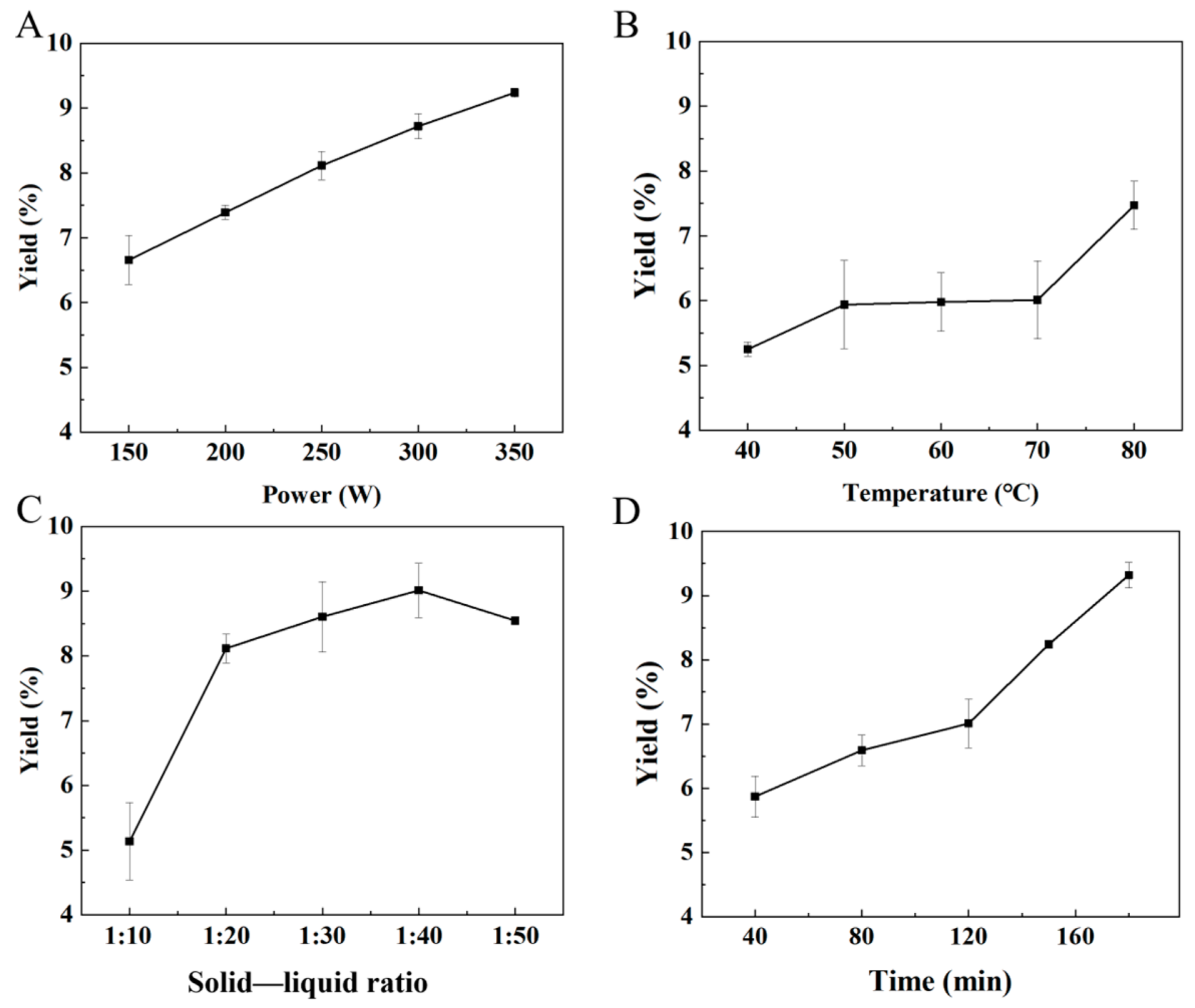


| Levels | Factor | Yield (%) | |||
|---|---|---|---|---|---|
| Solid–Liquid Ratio | Time (min) | Temperature (°C) | Power (W) | ||
| 1 | 20 | 120 | 60 | 250 | 4.74 |
| 2 | 20 | 150 | 70 | 300 | 8.05 |
| 3 | 20 | 180 | 80 | 350 | 9.24 |
| 4 | 30 | 120 | 60 | 250 | 6.35 |
| 5 | 30 | 150 | 70 | 300 | 8.19 |
| 6 | 30 | 180 | 80 | 350 | 7.52 |
| 7 | 40 | 120 | 60 | 250 | 9.77 |
| 8 | 40 | 150 | 70 | 300 | 6.73 |
| 9 | 40 | 180 | 80 | 350 | 8.10 |
| K1 | 7.34 | 6.95 | 6.33 | 7.01 | / |
| K2 | 7.35 | 7.66 | 7.50 | 8.4 | / |
| K3 | 8.20 | 8.29 | 9.07 | 7.44 | / |
| R | 0.86 | 1.33 | 2.73 | 1.43 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, B.; Shao, L.; Jiang, H.; Wu, S. Wide pH, Adaptable High Internal Phase Pickering Emulsion Stabilized by a Crude Polysaccharide from Thesium chinense Turcz. Molecules 2024, 29, 4312. https://doi.org/10.3390/molecules29184312
Ling B, Shao L, Jiang H, Wu S. Wide pH, Adaptable High Internal Phase Pickering Emulsion Stabilized by a Crude Polysaccharide from Thesium chinense Turcz. Molecules. 2024; 29(18):4312. https://doi.org/10.3390/molecules29184312
Chicago/Turabian StyleLing, Borong, Lijun Shao, Huicong Jiang, and Shufang Wu. 2024. "Wide pH, Adaptable High Internal Phase Pickering Emulsion Stabilized by a Crude Polysaccharide from Thesium chinense Turcz." Molecules 29, no. 18: 4312. https://doi.org/10.3390/molecules29184312
APA StyleLing, B., Shao, L., Jiang, H., & Wu, S. (2024). Wide pH, Adaptable High Internal Phase Pickering Emulsion Stabilized by a Crude Polysaccharide from Thesium chinense Turcz. Molecules, 29(18), 4312. https://doi.org/10.3390/molecules29184312






