Enzyme Inhibitors as Multifaceted Tools in Medicine and Agriculture
Abstract
1. Introduction
2. Protease Inhibitors in Plant Research
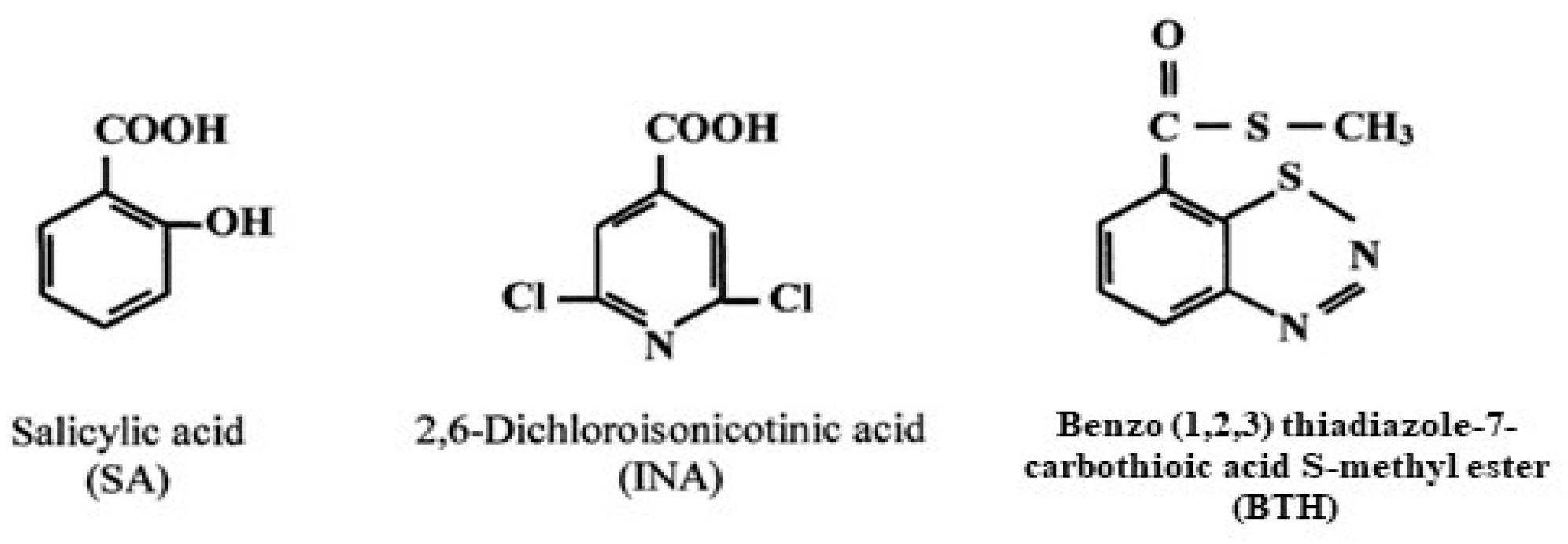
2.1. Plant Defenses
2.2. Definition and Classification of Protease Inhibitors
2.3. Plant Protease Inhibitors
2.4. Examples of Applications of Plant Protease Inhibitors
3. Alpha-Glucosidase Inhibitors from Plants as Potential Candidates for the Treatment of Type 2 Diabetes
3.1. Diabetes Mellitus Type 2: Causes and Symptoms
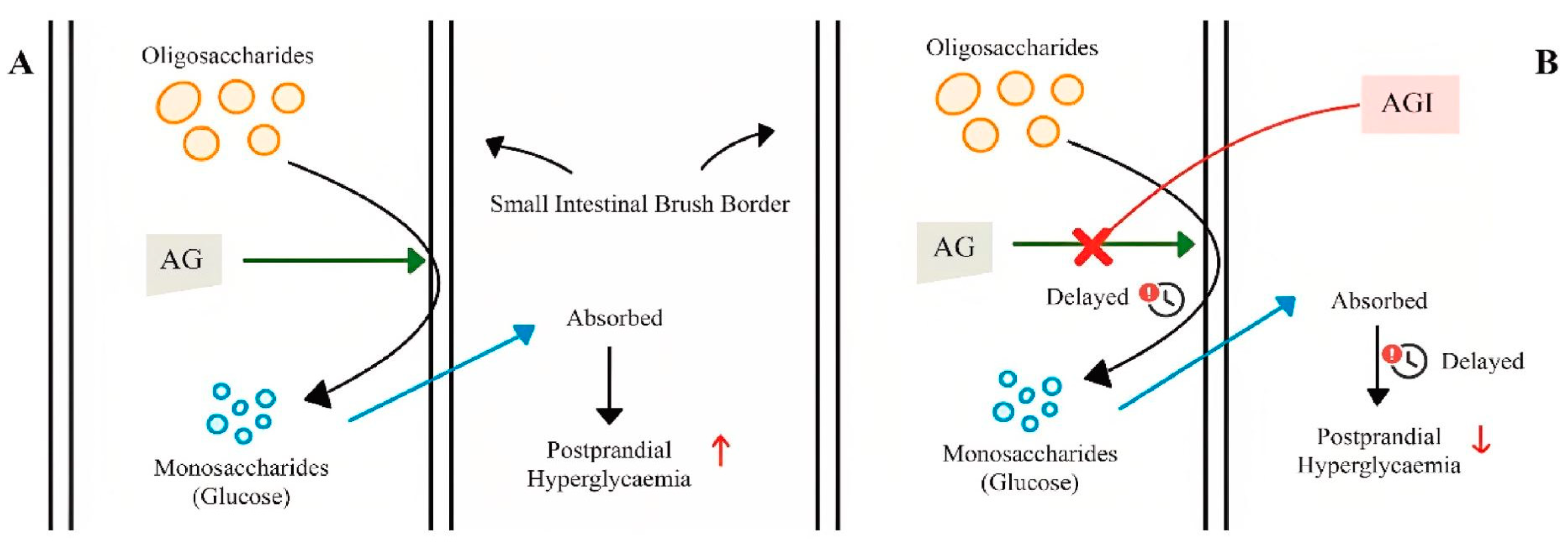
3.2. Alpha-Glucosidase: Structures and Biological Roles
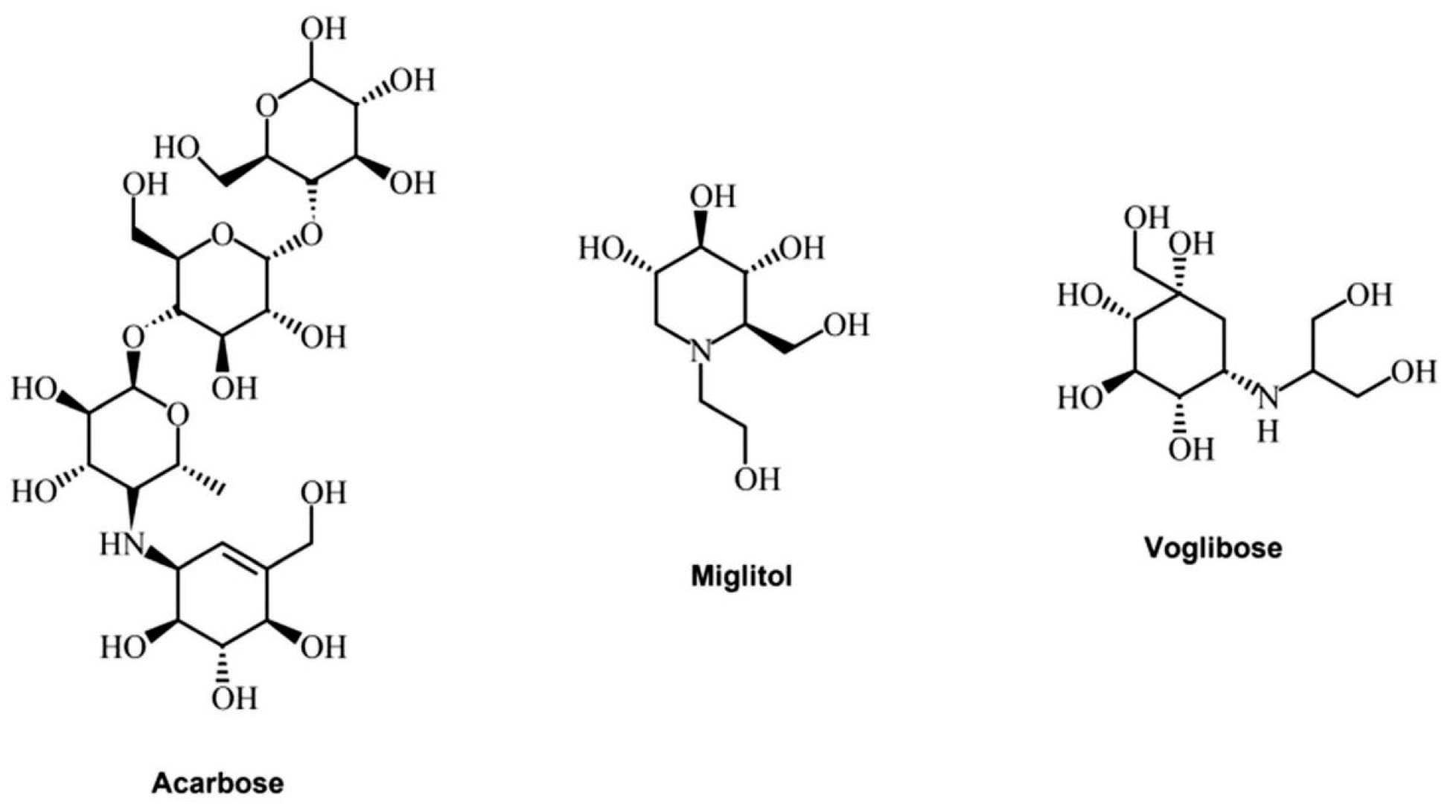
3.3. Alpha-Glucosidase Extracted from Plants as Inhibitors
4. Carbonic Anhydrase Inhibitors as Candidates for the Treatment of Glaucoma and Obesity
4.1. Mechanisms of Action of Carbonic Anhydrases
4.2. Carbonic Anhydrase: Their Isoforms and Inhibitors
4.3. Inhibitors of Carbonic Anhydrases in the Treatment of Glaucoma
4.4. Inhibitors of Carbonic Anhydrases in the Treatment of Obesity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGI | (alpha-glucosidase) |
| API | (aspartic protease inhibitors) |
| BABA | (beta amminobutyric acid) |
| BTH | (benzothiazole) |
| CA | (carbonic anhydrase) |
| GH 31 | (glycoside hydrolase domains) |
| HR | (hypersensitive reaction) |
| INA | (2,6 dichlorosomicotomic acid) |
| IOP | (intraocular pressure) |
| PI | (protease inhibitor) |
| SAR | (systemic acquired resistance) |
| SGLT-2 | (sodium–glucose 6-transporter) |
| T2DM | (type 2 diabetes mellitus) |
References
- Robinson, P.K. Enzymes: Principles and Biotechnological Applications. Essays Biochem. 2015, 59, 1. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Tipton, K.F. Assessment of Enzyme Inhibition: A Review with Examples from the Development of Monoamine Oxidase and Cholinesterase Inhibitory Drugs. Molecules 2017, 22, 1192. [Google Scholar] [CrossRef]
- Patadiya, N.; Panchal, N.; Vaghela, V. A Review on Enzyme Inhibitors. Int. Res. J. Pharm 2021, 12, 60–66. [Google Scholar] [CrossRef]
- Faridoon; Ng, R.; Zhang, G.; Li, J.J. An Update on the Discovery and Development of Reversible Covalent Inhibitors. Med. Chem. Res. 2023, 32, 1039–1062. [Google Scholar] [CrossRef] [PubMed]
- Kiely-Collins, H.; Winter, G.E.; Bernardes, G.J. The Role of Reversible and Irreversible Covalent Chemistry in Targeted Protein Degradation. Cell Chem. Biol. 2021, 28, 952–968. [Google Scholar] [CrossRef] [PubMed]
- Gohara, D.W.; Di Cera, E. Allostery in Trypsin-like Proteases Suggests New Therapeutic Strategies. Trends Biotechnol. 2011, 29, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Rodrigues, J.V.; Tonddast-Navaei, S.; Shakhnovich, E.; Skolnick, J. Rational Design of Novel Allosteric Dihydrofolate Reductase Inhibitors Showing Antibacterial Effects on Drug-Resistant Escherichia Coli Escape Variants. ACS Chem. Biol. 2017, 12, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.L.V.; Silva, M.C.; Sallai, R.C.; Brito, M.V.; Sampaio, M.U. A Novel Subclassification for Kunitz Proteinase Inhibitors from Leguminous Seeds. Biochimie 2010, 92, 1667–1673. [Google Scholar] [CrossRef]
- Bonturi, C.R.; Silva Teixeira, A.B.; Rocha, V.M.; Valente, P.F.; Oliveira, J.R.; Filho, C.M.B.; Fátima Correia Batista, I.; Oliva, M.L.V. Plant Kunitz Inhibitors and Their Interaction with Proteases: Current and Potential Pharmacological Targets. Int. J. Mol. Sci. 2022, 23, 4742. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Considerations When Using Alpha-Glucosidase Inhibitors in the Treatment of Type 2 Diabetes. Expert Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef]
- Bischoff, H. The Mechanism of Alpha-Glucosidase Inhibition in the Management of Diabetes. Clin. Investig. Med. Med. Clin. Et Exp. 1995, 18, 303–311. [Google Scholar]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P.A. Indian Herbs and Herbal Drugs Used for the Treatment of Diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Krungkrai, J.; Scozzafava, A.; Reungprapavut, S.; Krungkrai, S.R.; Rattanajak, R.; Kamchonwongpaisan, S.; Supuran, C.T. Carbonic Anhydrase Inhibitors. Inhibition of Plasmodium Falciparum Carbonic Anhydrase with Aromatic Sulfonamides: Towards Antimalarials with a Novel Mechanism of Action? Bioorg. Med. Chem. 2005, 13, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Minakuchi, T.; Morimoto, K.; Sano, S.; Onishi, S.; Takeuchi, H.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Carbonic Anhydrase Inhibitors: DNA Cloning and Inhibition Studies of the α-Carbonic Anhydrase from Helicobacter Pylori, a New Target for Developing Sulfonamide and Sulfamate Gastric Drugs. J. Med. Chem. 2006, 49, 2117–2126. [Google Scholar] [CrossRef]
- Klengel, T.; Liang, W.-J.; Chaloupka, J.; Ruoff, C.; Schröppel, K.; Naglik, J.R.; Eckert, S.E.; Mogensen, E.G.; Haynes, K.; Tuite, M.F. Fungal Adenylyl Cyclase Integrates CO2 Sensing with cAMP Signaling and Virulence. Curr. Biol. 2005, 15, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-K.; Alkon, D.L. Impairment of Hippocampal CA1 Heterosynaptic Transformation and Spatial Memory by β-Amyloid25–35. J. Neurophysiol. 2002, 87, 2441–2449. [Google Scholar] [CrossRef]
- Conrath, U. Systemic Acquired Resistance. Plant Signal. Behav. 2006, 1, 179–184. [Google Scholar] [CrossRef]
- Kessmann, H.; Staub, T.; Hofmann, C.; Maetzke, T.; Herzog, J.; Ward, E.; Uknes, S.; Ryals, J. Induction of Systemic Acquired Disease Resistance in Plants by Chemicals. Annu. Rev. Phytopathol. 1994, 32, 439–459. [Google Scholar] [CrossRef]
- Lyon, G.; Reglinski, T.; Newton, A. Novel Disease Control Compounds: The Potential to ‘immunize’ plants against Infection. Plant Pathol. 1995, 44, 407. [Google Scholar] [CrossRef]
- Schneider, M.; Schweizer, P.; Meuwly, P.; Métraux, J. Systemic Acquired Resistance in Plants. Int. Rev. Cytol. 1996, 168, 303–340. [Google Scholar]
- Benhamou, N.; Picard, K. La Résistance Induite: Une Nouvelle Stratégie de Défense Des Plantes Contre Les Agents Pathogènes. Phytoprotection 1999, 80, 137–168. [Google Scholar] [CrossRef]
- Cohen, Y. The BABA Story of Induced Resistance. Phytoparasitica 2001, 29, 375–378. [Google Scholar] [CrossRef]
- Kuć, J. Translocated Signals for Plant Immunization a. Ann. N. Y. Acad. Sci. 1987, 494, 221–223. [Google Scholar] [CrossRef]
- Hilder, V.A.; Gatehouse, A.M.; Sheerman, S.E.; Barker, R.F.; Boulter, D. A Novel Mechanism of Insect Resistance Engineered into Tobacco. Nature 1987, 330, 160–163. [Google Scholar] [CrossRef]
- Koiwa, H.; Shade, R.E.; Zhu-Salzman, K.; Subramanian, L.; Murdock, L.L.; Nielsen, S.S.; Bressan, R.A.; Hasegawa, P.M. Phage Display Selection Can Differentiate Insecticidal Activity of Soybean Cystatins. Plant J. 1998, 14, 371–379. [Google Scholar] [CrossRef]
- Schuler, T.H.; Poppy, G.M.; Kerry, B.R.; Denholm, I. Insect-Resistant Transgenic Plants. Trends Biotechnol. 1998, 16, 168–175. [Google Scholar] [CrossRef]
- Mosolov, V.; Grigor’eva, L.; Valueva, T. Involvement of Proteolytic Enzymes and Their Inhibitors in Plant Protection. Appl. Biochem. Microbiol. 2001, 37, 115–123. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Koiwa, H.; Salzman, R.; Shade, R.; Ahn, J. Cowpea Bruchid Callosobruchus Maculatus Uses a Three-component Strategy to Overcome a Plant Defensive Cysteine Protease Inhibitor. Insect Mol. Biol. 2003, 12, 135–145. [Google Scholar] [CrossRef]
- Lingaraju, M.; Gowda, L.R. A Kunitz Trypsin Inhibitor of Entada Scandens Seeds: Another Member with Single Disulfide Bridge. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2008, 1784, 850–855. [Google Scholar] [CrossRef]
- Srikanth, S.; Chen, Z. Plant Protease Inhibitors in Therapeutics-Focus on Cancer Therapy. Front. Pharmacol. 2016, 7, 202270. [Google Scholar] [CrossRef]
- Leipoldt, F.; Santos-Aberturas, J.; Stegmann, D.P.; Wolf, F.; Kulik, A.; Lacret, R.; Popadić, D.; Keinhörster, D.; Kirchner, N.; Bekiesch, P. Warhead Biosynthesis and the Origin of Structural Diversity in Hydroxamate Metalloproteinase Inhibitors. Nat. Commun. 2017, 8, 1965. [Google Scholar] [CrossRef] [PubMed]
- Osmani, Z.; Sabet, M.S.; Nakahara, K.S. Aspartic Protease Inhibitor Enhances Resistance to Potato Virus Y and A in Transgenic Potato Plants. BMC Plant Biol. 2022, 22, 241. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.; Goldman, R.C.; Abad-Zapatero, C. The Secreted Proteinases from Candida: Challenges for Structure-Aided Drug Design. In Proteases of Infectious Agents; Elsevier: Amsterdam, The Netherlands, 1999; pp. 117–138. [Google Scholar]
- Cater, S.A.; Lees, W.E.; Hill, J.; Brzin, J.; Kay, J.; Phylip, L.H. Aspartic Proteinase Inhibitors from Tomato and Potato Are More Potent against Yeast Proteinase A than Cathepsin D. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 2002, 1596, 76–82. [Google Scholar] [CrossRef]
- Rosu, A.; Eremia, M.-C.; Spiridon, M.; Guidea, S.; Lupescu, I.; Jurcoane, S. In Search of Plant Sources for Serine Protease Inhibitors: I. Detection of Serine Protease Inhibitors in Callus Cultures Induced from Somatic Explants of Flax (Linum usitatissimum L.). Biotechnol. Lett 2010, 15, 5669–5674. [Google Scholar]
- Ryan, C.A. Protease Inhibitors in Plants: Genes for Improving Defenses against Insects and Pathogens. Annu. Rev. Phytopathol. 1990, 28, 425–449. [Google Scholar] [CrossRef]
- Lawrence, P.K.; Koundal, K.R. Plant Protease Inhibitors in Control of Phytophagous Insects. Electron. J. Biotechnol. 2002, 5, 5–6. [Google Scholar] [CrossRef]
- Clemente, M.; Corigliano, M.G.; Pariani, S.A.; Sánchez-López, E.F.; Sander, V.A.; Ramos-Duarte, V.A. Plant Serine Protease Inhibitors: Biotechnology Application in Agriculture and Molecular Farming. Int. J. Mol. Sci. 2019, 20, 1345. [Google Scholar] [CrossRef]
- Haq, S.K.; Atif, S.M.; Khan, R.H. Protein Proteinase Inhibitor Genes in Combat against Insects, Pests, and Pathogens: Natural and Engineered Phytoprotection. Arch. Biochem. Biophys. 2004, 431, 145–159. [Google Scholar] [CrossRef]
- Schaller, A. A Cut above the Rest: The Regulatory Function of Plant Proteases. Planta 2004, 220, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Valueva, T.; Mosolov, V. Role of Inhibitors of Proteolytic Enzymes in Plant Defense against Phytopathogenic Microorganisms. Biochemistry 2004, 69, 1305–1309. [Google Scholar] [CrossRef]
- van der Hoorn, R.A.; Jones, J.D. The Plant Proteolytic Machinery and Its Role in Defence. Curr. Opin. Plant Biol. 2004, 7, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Mosolov, V.; Valueva, T. Proteinase Inhibitors and Their Function in Plants: A Review. Appl. Biochem. Microbiol. 2005, 41, 227–246. [Google Scholar] [CrossRef]
- Salas, C.E.; Gomes, M.T.; Hernandez, M.; Lopes, M.T. Plant Cysteine Proteinases: Evaluation of the Pharmacological Activity. Phytochemistry 2008, 69, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Cambra, I.; Martinez, M.; Pozancos, C.; González-Melendi, P.; Grbic, V.; Castañera, P.; Ortego, F.; Diaz, I. Gene Pyramiding of Peptidase Inhibitors Enhances Plant Resistance to the Spider Mite Tetranychus urticae. PLoS ONE 2012, 7, e43011. [Google Scholar] [CrossRef] [PubMed]
- Hörger, A.C.; Van der Hoorn, R.A. The Structural Basis of Specific Protease–Inhibitor Interactions at the Plant–Pathogen Interface. Curr. Opin. Struct. Biol. 2013, 23, 842–850. [Google Scholar] [CrossRef]
- Laskowski Jr, M.; Kato, I. Protein Inhibitors of Proteinases. Annu. Rev. Biochem. 1980, 49, 593–626. [Google Scholar] [CrossRef]
- Birk, Y. Plant Protease Inhibitors: Significance in Nutrition, Plant Protection, Cancer Prevention and Genetic Engineering; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003; ISBN 3-540-00118-2. [Google Scholar]
- Rustgi, S.; Boex-Fontvieille, E.; Reinbothe, C.; von Wettstein, D.; Reinbothe, S. The Complex World of Plant Protease Inhibitors: Insights into a Kunitz-Type Cysteine Protease Inhibitor of Arabidopsis Thaliana. Commun. Integr. Biol. 2018, 11, e1368599. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Tolle, D.P.; Barrett, A.J. Evolutionary Families of Peptidase Inhibitors. Biochem. J. 2004, 378, 705–716. [Google Scholar] [CrossRef]
- Rawlings, N.D. Peptidase Inhibitors in the MEROPS Database. Biochimie 2010, 92, 1463–1483. [Google Scholar] [CrossRef]
- Gitlin-Domagalska, A.; Maciejewska, A.; Dębowski, D. Bowman-Birk Inhibitors: Insights into Family of Multifunctional Proteins and Peptides with Potential Therapeutical Applications. Pharmaceuticals 2020, 13, 421. [Google Scholar] [CrossRef]
- Geisslitz, S.; Weegels, P.; Shewry, P.; Zevallos, V.; Masci, S.; Sorrells, M.; Gregorini, A.; Colomba, M.; Jonkers, D.; Huang, X. Wheat Amylase/Trypsin Inhibitors (ATIs): Occurrence, Function and Health Aspects. Eur. J. Nutr. 2022, 61, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Belenghi, B.; Delledonne, M.; Menachem, E.; Levine, A. The Involvement of Cysteine Proteases and Protease Inhibitor Genes in the Regulation of Programmed Cell Death in Plants. Plant Cell 1999, 11, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Manara, A.; Fasani, E.; Molesini, B.; DalCorso, G.; Pennisi, F.; Pandolfini, T.; Furini, A. The Tomato Metallocarboxypeptidase Inhibitor I, Which Interacts with a Heavy Metal-Associated Isoprenylated Protein, Is Implicated in Plant Response to Cadmium. Molecules 2020, 25, 700. [Google Scholar] [CrossRef]
- De Leo, F.; Bonadé-Bottino, M.; Ceci, L.R.; Gallerani, R.; Jouanin, L. Effects of a Mustard Trypsin Inhibitor Expressed in Different Plants on Three Lepidopteran Pests. Insect Biochem. Mol. Biol. 2001, 31, 593–602. [Google Scholar] [CrossRef]
- Turra, D.; Lorito, M. Potato Type I and II Proteinase Inhibitors: Modulating Plant Physiology and Host Resistance. Curr. Protein Pept. Sci. 2011, 12, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.S.; Mazarei, M.; Jurat-Fuentes, J.L.; Hewezi, T.; Millwood, R.J.; Stewart, C.N., Jr. Overexpression of Soybean Trypsin Inhibitor Genes Decreases Defoliation by Corn Earworm (Helicoverpa zea) in Soybean (Glycine max) and Arabidopsis thaliana. Front. Plant Sci. 2023, 14, 1129454. [Google Scholar] [CrossRef] [PubMed]
- Chiche, L.; Heitz, A.; Gelly, J.-C.; Gracy, J.; Chau, P.T.; Ha, P.T.; Hernandez, J.-F.; Le-Nguyen, D. Squash Inhibitors: From Structural Motifs to Macrocyclic Knottins. Curr. Protein Pept. Sci. 2004, 5, 341–349. [Google Scholar] [CrossRef]
- Michaud, D.; Cantin, L.; Vrain, T.C. Carboxy-Terminal Truncation of Oryzacysatin II by Oryzacytatin-Insensitive Insect Digestive Proteinases. Arch. Biochem. Biophys. 1995, 322, 469–474. [Google Scholar] [CrossRef]
- Orr, G.L.; Strickland, J.A.; Walsh, T.A. Inhibition of Diabrotica Larval Growth by a Multicystatin from Potato Tubers. J. Insect Physiol. 1994, 40, 893–900. [Google Scholar] [CrossRef]
- Zhao, Y.; Botella, M.A.; Subramanian, L.; Niu, X.; Nielsen, S.S.; Bressan, R.A.; Hasegawa, P.M. Two Wound-Inducible Soybean Cysteine Proteinase Inhibitors Have Greater Insect Digestive Proteinase Inhibitory Activities than a Constitutive Homolog. Plant Physiol. 1996, 111, 1299–1306. [Google Scholar] [CrossRef]
- Bolter, C.J.; Jongsma, M.A. Colorado Potato Beetles (Leptinotarsa decemlineata) Adapt to Proteinase Inhibitors Induced in Potato Leaves by Methyl Jasmonate. J. Insect Physiol. 1995, 41, 1071–1078. [Google Scholar] [CrossRef]
- Cloutier, C.; Jean, C.; Fournier, M.; Yelle, S.; Michaud, D. Adult Colorado Potato Beetles, Leptinotarsa Decemlineata Compensate for Nutritional Stress on Oryzacystatin I-transgenic Potato Plants by Hypertrophic Behavior and Over-production of Insensitive Proteases. Arch. Insect Biochem. Physiol. 2000, 44, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar-Leighton, S.; Broadway, R.M. Identification of Six Chymotrypsin cDNAs from Larval Midguts of Helicoverpa Zea and Agrotis Ipsilon Feeding on the Soybean (Kunitz) Trypsin Inhibitor. Insect Biochem. Mol. Biol. 2001, 31, 633–644. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Simmons, K.M.; Michels, A.W. Type 1 Diabetes: A Predictable Disease. World J. Diabetes 2015, 6, 380. [Google Scholar] [CrossRef]
- Banu, S.; Bhowmick, A. Therapeutic Targets of Type 2 Diabetes: An Overview. MOJ Drug Des. Dev. Ther 2017, 1, 2–7. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A Review of Alpha-Glucosidase Inhibitors from Plants as Potential Candidates for the Treatment of Type-2 Diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- Mandel, A.L.; Breslin, P.A. High Endogenous Salivary Amylase Activity Is Associated with Improved Glycemic Homeostasis Following Starch Ingestion in Adults. J. Nutr. 2012, 142, 853–858. [Google Scholar] [CrossRef]
- Peyrot des Gachons, C.; Breslin, P.A. Salivary Amylase: Digestion and Metabolic Syndrome. Curr. Diabetes Rep. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Jongkees, S.A.; Withers, S.G. Unusual Enzymatic Glycoside Cleavage Mechanisms. Acc. Chem. Res. 2014, 47, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.A.Z.; Mokhtar, R.A.M.; Iqbal, M.; Abdullah, A.; Azizah, R.; Sulistyorini, L.; Mahfudh, N.; Zakaria, Z.A. Medicinal Plants of Southeast Asia with Anti-α-Glucosidase Activity as Potential Source for Type-2 Diabetes Mellitus Treatment. J. Ethnopharmacol. 2024, 330, 118239. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.; Wood, I.; Palejwala, A.; Ellis, A.; Shirazi-Beechey, S. Expression of Monosaccharide Transporters in Intestine of Diabetic Humans. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 282, G241–G248. [Google Scholar] [CrossRef] [PubMed]
- Surya, S.; Salam, A.D.; Tomy, D.V.; Carla, B.; Kumar, R.A.; Sunil, C. Diabetes Mellitus and Medicinal Plants-a Review. Asian Pac. J. Trop. Dis. 2014, 4, 337–347. [Google Scholar] [CrossRef]
- Some, S.; Das, S.; Mondal, R.; Gangopadhyay, M.; Basak, G.K. Medicinal Plant Extract Mediated Green Synthesis of Metallic Nanoparticles: A Review. Int. J. Plant Environ. 2021, 7, 119–132. [Google Scholar] [CrossRef]
- Thant, T.M.; Aminah, N.S.; Kristanti, A.N.; Ramadhan, R.; Aung, H.; Takaya, Y. Antidiabetes and Antioxidant Agents from Clausena Excavata Root as Medicinal Plant of Myanmar. Open Chem. 2019, 17, 1339–1344. [Google Scholar] [CrossRef]
- Hussain, F.; Hafeez, J.; Khalifa, A.S.; Naeem, M.; Ali, T.; Eed, E.M. In Vitro and in Vivo Study of Inhibitory Potentials of α-Glucosidase and Acetylcholinesterase and Biochemical Profiling of M. Charantia in Alloxan-Induced Diabetic Rat Models. Am. J. Transl. Res. 2022, 14, 3824. [Google Scholar] [PubMed]
- Kim, H.-R.; Antonisamy, P.; Kim, Y.-S.; Lee, G.; Ham, H.-D.; Kwon, K.-B. Inhibitory Effect of Amomum Villosum Water Extracts on α-Glucosidase Activity. Physiol. Mol. Plant Pathol. 2022, 117, 101779. [Google Scholar] [CrossRef]
- Barber, E.; Houghton, M.J.; Williamson, G. Flavonoids as Human Intestinal α-Glucosidase Inhibitors. Foods 2021, 10, 1939. [Google Scholar] [CrossRef]
- Lindskog, S. Structure and Mechanism of Carbonic Anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- Akocak, S.; Supuran, C.T. Activation of α-, β-, γ-δ-, ζ-and η-Class of Carbonic Anhydrases with Amines and Amino Acids: A Review. J. Enzym. Inhib. Med. Chem. 2019, 34, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An Overview of the Alpha-, Beta-and Gamma-Carbonic Anhydrases from Bacteria: Can Bacterial Carbonic Anhydrases Shed New Light on Evolution of Bacteria? J. Enzym. Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Boone, C.D.; Kondeti, B.; McKenna, R. Structural Annotation of Human Carbonic Anhydrases. J. Enzym. Inhib. Med. Chem. 2013, 28, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Mitterberger, M.C.; Kim, G.; Rostek, U.; Levine, R.L.; Zwerschke, W. Carbonic Anhydrase III Regulates Peroxisome Proliferator-Activated Receptor-Γ2. Exp. Cell Res. 2012, 318, 877–886. [Google Scholar] [CrossRef]
- Arechederra, R.L.; Waheed, A.; Sly, W.S.; Supuran, C.T.; Minteer, S.D. Effect of Sulfonamides as Carbonic Anhydrase VA and VB Inhibitors on Mitochondrial Metabolic Energy Conversion. Bioorg. Med. Chem. 2013, 21, 1544–1548. [Google Scholar] [CrossRef]
- van Karnebeek, C.D.; Sly, W.S.; Ross, C.J.; Salvarinova, R.; Yaplito-Lee, J.; Santra, S.; Shyr, C.; Horvath, G.A.; Eydoux, P.; Lehman, A.M. Mitochondrial Carbonic Anhydrase VA Deficiency Resulting from CA5A Alterations Presents with Hyperammonemia in Early Childhood. Am. J. Hum. Genet. 2014, 94, 453–461. [Google Scholar] [CrossRef]
- Thatcher, B.; Doherty, A.; Orvisky, E.; Martin, B.; Henkin, R. Gustin from Human Parotid Saliva Is Carbonic Anhydrase VI. Biochem. Biophys. Res. Commun. 1998, 250, 635–641. [Google Scholar] [CrossRef]
- Bootorabi, F.; Jänis, J.; Smith, E.; Waheed, A.; Kukkurainen, S.; Hytönen, V.; Valjakka, J.; Supuran, C.T.; Vullo, D.; Sly, W.S. Analysis of a Shortened Form of Human Carbonic Anhydrase VII Expressed in Vitro Compared to the Full-Length Enzyme. Biochimie 2010, 92, 1072–1080. [Google Scholar] [CrossRef]
- Dorai, T.; Sawczuk, I.S.; Pastorek, J.; Wiernik, P.H.; Dutcher, J.P. The Role of Carbonic Anhydrase IX Overexpression in Kidney Cancer. Eur. J. Cancer 2005, 41, 2935–2947. [Google Scholar] [CrossRef]
- Waheed, A.; Sly, W.S. Carbonic Anhydrase XII Functions in Health and Disease. Gene 2017, 623, 33–40. [Google Scholar] [CrossRef]
- Hilvo, M.; Supuran, C.T.; Parkkila, S. Characterization and Inhibition of the Recently Discovered Carbonic Anhydrase Isoforms CA XIII, XIV and XV. Curr. Top. Med. Chem. 2007, 7, 893–899. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A. Carbonic Anhydrase Inhibitors and Their Therapeutic Potential. Expert Opin. Ther. Pat. 2000, 10, 575–600. [Google Scholar] [CrossRef]
- Ahlskog, J.K.; Dumelin, C.E.; Trüssel, S.; Mårlind, J.; Neri, D. In Vivo Targeting of Tumor-Associated Carbonic Anhydrases Using Acetazolamide Derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 4851–4856. [Google Scholar] [CrossRef] [PubMed]
- Rucquoy, M.; Sorel, L. Diclofenamide in the Treatment of Therapy-Resistant Epilepsy. Acta Neurol Belg 1978, 78, 174–182. [Google Scholar] [PubMed]
- Oie, S.; Ishida, K.; Yamamoto, T. Impact of Intraocular Pressure Reduction on Visual Field Progression in Normal-Tension Glaucoma Followed up over 15 Years. Jpn. J. Ophthalmol. 2017, 61, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Ha, Q. The Number of People with Glaucoma Worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar]
- Burki, T. European Commission Classifies Obesity as a Chronic Disease. Lancet Diabetes Endocrinol. 2021, 9, 418. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure-Based Drug Discovery of Carbonic Anhydrase Inhibitors. J. Enzym. Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef]
- Aronne, L.J.; Wadden, T.A.; Peterson, C.; Winslow, D.; Odeh, S.; Gadde, K.M. Evaluation of Phentermine and Topiramate versus Phentermine/Topiramate Extended-release in Obese Adults. Obesity 2013, 21, 2163–2171. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Grima, M.T.; Sari, C.I.; Karapanagiotidis, S.; Wong, C.; Eikelis, N.; Richards, K.L.; Lee, G.; Nestel, P.J.; Dixon, J.B. The Relation of Glucose Metabolism to Left Ventricular Mass and Function and Sympathetic Nervous System Activity in Obese Subjects with Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E227–E237. [Google Scholar] [CrossRef]
- De Simone, G.; Di Fiore, A.; Menchise, V.; Pedone, C.; Antel, J.; Casini, A.; Scozzafava, A.; Wurl, M.; Supuran, C.T. Carbonic Anhydrase Inhibitors. Zonisamide Is an Effective Inhibitor of the Cytosolic Isozyme II and Mitochondrial Isozyme V: Solution and X-Ray Crystallographic Studies. Bioorg. Med. Chem. Lett. 2005, 15, 2315–2320. [Google Scholar] [CrossRef] [PubMed]
| Protease Inhibitor | Type | Effect | Bibliography |
|---|---|---|---|
| Phytocystatins | Cysteine protease inhibitor | Inhibits papain-like cysteine proteases, regulates developmental processes, and responds to stresses. | U.Schlüter (https://doi.org/10.1016/j.sajb.2009.02.101) [26] |
| Actinonin | Metalloprotease inhibitor | Chelates metal ions in the enzyme’s active site. | Leipoldt et al. (https://doi.org/10.1038/s41467-017-01975-6) [31] |
| Matlystatins | Metalloprotease inhibitor | Chelates metal ions in the enzyme’s active site. | Leipoldt et al. (https://doi.org/10.1038/s41467-017-01975-6) [31] |
| Aspartic protease inhibitors (APIs) | Aspartic protease inhibitor | Blocks the catalytic function of aspartyl proteases, essential for physiological processes and life cycles. | Osmani et al., 2022; Stewart K et al., 1999; Cater et al., 2002 [32,33,34] |
| Tryptase inhibitors | Serine protease inhibitor | Flax (Linum usitatissimum) is noted for tryptase inhibitors and various health benefits. | Rosu et al., 2010 [35] |
| Type of Inhibitor | Effect | Bibliography |
|---|---|---|
| Bowman–Birk Serine Protease Inhibitors | Suppress inflammation and protease activity; show potential in cancer chemoprevention and multiple sclerosis treatment. | https://doi.org/10.3390/ph13120421 [52] |
| Cereal Trypsin/α-Amylase Inhibitors | Suppress the activities of enzymes that degrade nutrients: α-amylase (which breaks down starch) and trypsin (which breaks down proteins), and are among the various groups of wheat seed proteins that contribute to natural pest and pathogen defense. | https://doi.org/10.1007/s00394-022-02841-y [53] |
| Cysteine Protease Inhibitors | Cysteine proteases have been identified as crucial enzymes in the regulation of programmed cell death in animals. | https://doi.org/10.1105/tpc.11.3.431 [54] |
| Metallocarboxypeptidase Inhibitors | Metallocarboxypeptidase inhibitors are produced in response to stress and play a role in defending against pests. | https://doi.org/10.3390/molecules25030700 [55] |
| Mustard Trypsin Inhibitors | They contribute to plant protection. | https://doi.org/10.1016/s0965-1748(00)00164-8 [56] |
| Potato Type I Protease Inhibitors | Modulating plant physiology (e.g., dehydration response, programmed cell death, plant growth, trichome density, and branching) and host resistance. | https://doi.org/10.2174/138920311796391151 [57] |
| Potato Type II Protease Inhibitors | Modulating plant physiology (e.g., dehydration response, programmed cell death, plant growth, trichome density, and branching) and host resistance. | https://doi.org/10.2174/138920311796391151 [57] |
| Serpins | They can be responsible for cell death and pathogen-associated stress. | https://doi.org/10.3390/ijms20061345 [38] |
| Soybean Trypsin (Kunitz) Inhibitors | They are known to play a protective role against herbivores. | https://doi.org/10.3389/fpls.2023.1129454 [58] |
| Squash Inhibitors | Potent canonical serine proteinase inhibitors isolated from Cucurbitaceae | https://doi.org/10.2174/1389203043379477 [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Prete, S.; Pagano, M. Enzyme Inhibitors as Multifaceted Tools in Medicine and Agriculture. Molecules 2024, 29, 4314. https://doi.org/10.3390/molecules29184314
Del Prete S, Pagano M. Enzyme Inhibitors as Multifaceted Tools in Medicine and Agriculture. Molecules. 2024; 29(18):4314. https://doi.org/10.3390/molecules29184314
Chicago/Turabian StyleDel Prete, Sonia, and Mario Pagano. 2024. "Enzyme Inhibitors as Multifaceted Tools in Medicine and Agriculture" Molecules 29, no. 18: 4314. https://doi.org/10.3390/molecules29184314
APA StyleDel Prete, S., & Pagano, M. (2024). Enzyme Inhibitors as Multifaceted Tools in Medicine and Agriculture. Molecules, 29(18), 4314. https://doi.org/10.3390/molecules29184314







