Two-Dimensional Polarized Blue P/SiS Heterostructures as Promising Photocatalysts for Water Splitting
Abstract
1. Introduction
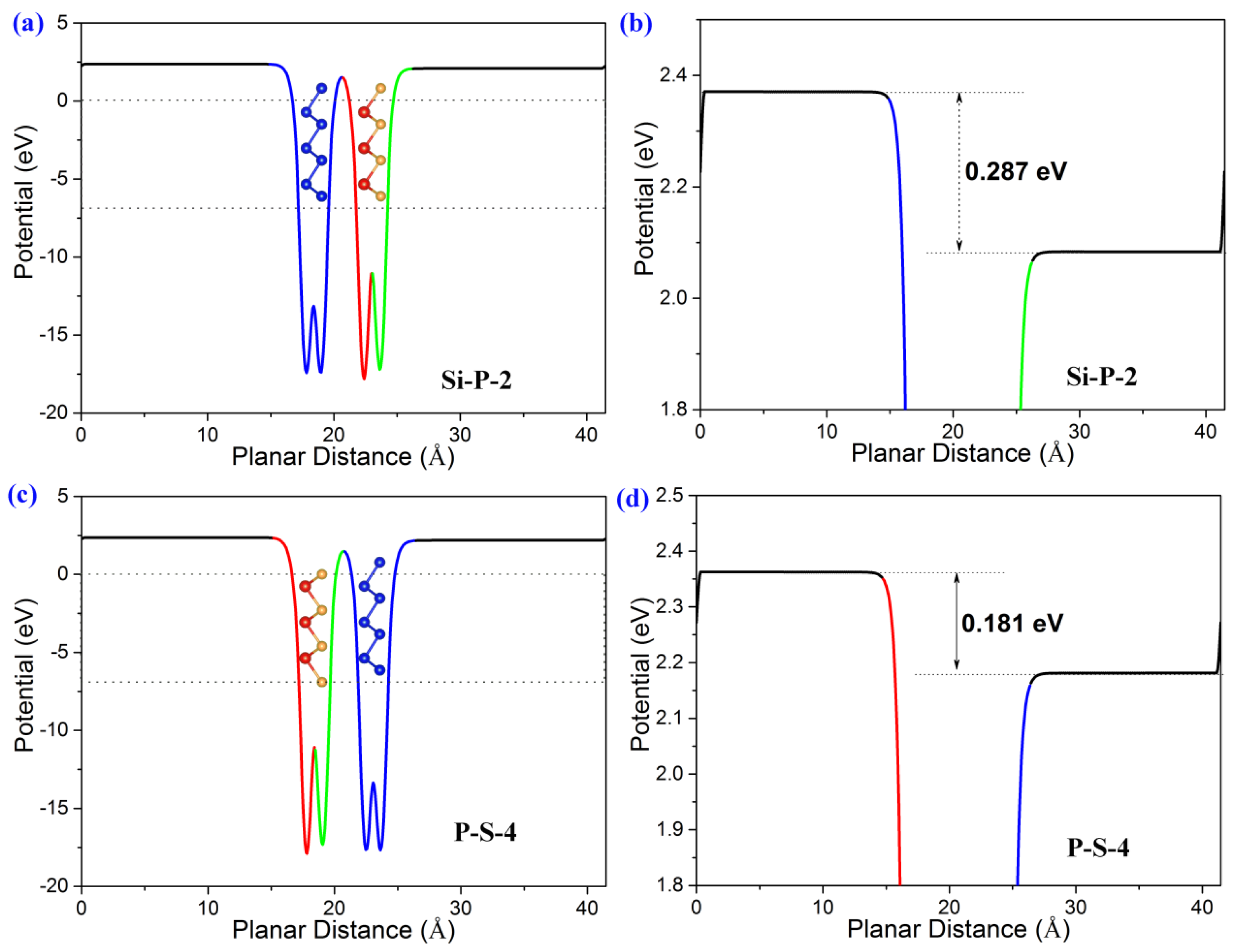
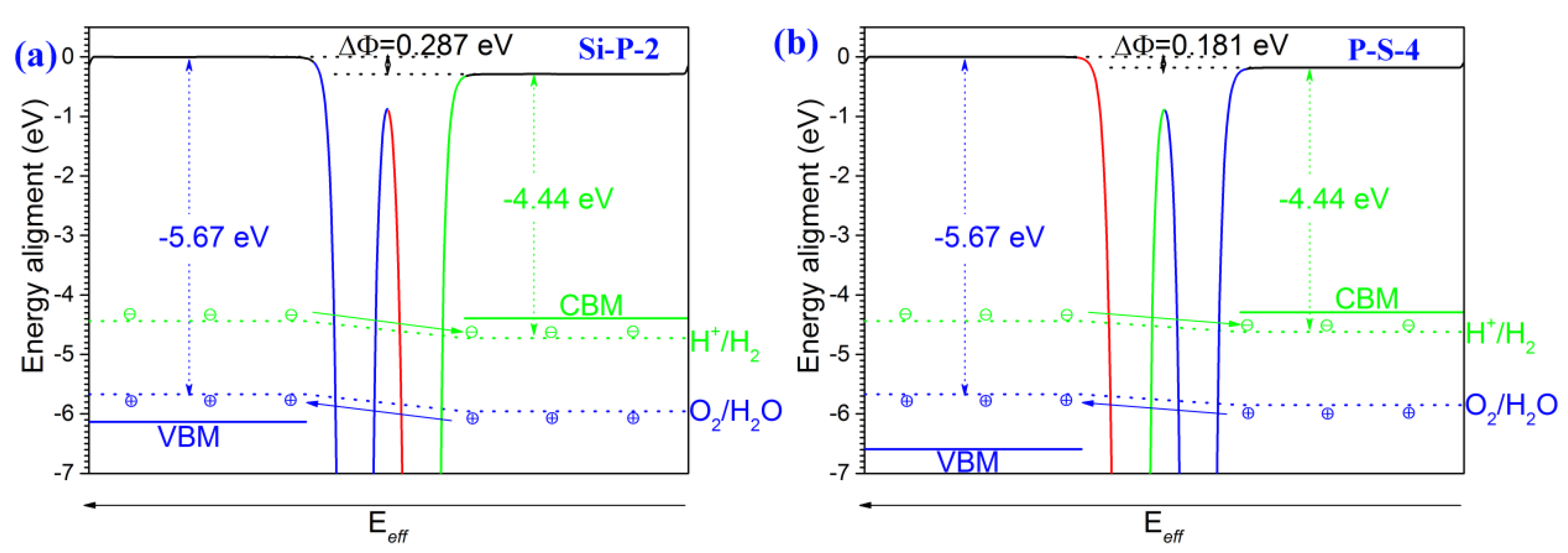
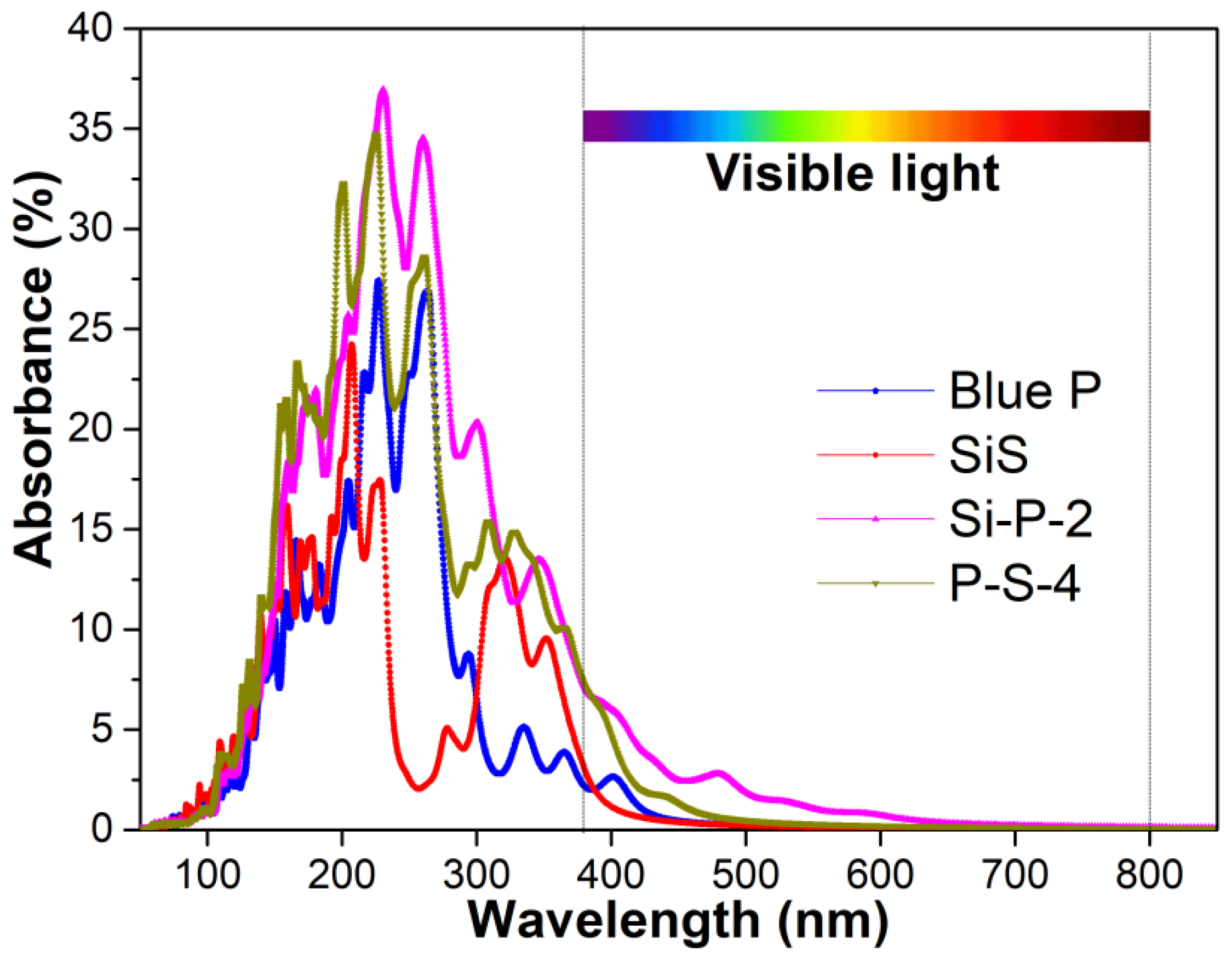
2. Results and Discussion
3. Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esswein, A.J.; Nocera, D.G. Hydrogen production by molecular photocatalysis. Chem. Rev. 2007, 107, 4022. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m(2) scale. Nature 2021, 598, 304. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Navid, A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, H. Ferroelectrics in Photocatalysis. Chemistry 2022, 28, e202103975. [Google Scholar] [CrossRef]
- Wang, G.; Chang, J.; Tang, W.; Xie, W.; Ang, Y.S. 2D materials and heterostructures for photocatalytic water-splitting: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 293002. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69. [Google Scholar] [CrossRef]
- Wang, G.; Huang, B.; Li, Z.; Lou, Z.; Wang, Z.; Dai, Y.; Whangbo, M.-H. Synthesis and characterization of ZnS with controlled amount of S vacancies for photocatalytic H2 production under visible light. Sci. Rep. 2015, 5, 8544. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Torres-Martínez, L.M. Earth-abundant ZnS/ZnO/CuFeS2 films for air purification and solar fuels production. Mater. Sci. Semicond. Process. 2021, 134, 106029. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.Z.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1. Nat. Mater. 2016, 15, 611. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Yang, J. Proposed photosynthesis method for producing hydrogen from dissociated water molecules using incident near-infrared light. Phys. Rev. Lett. 2014, 112, 018301. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269. [Google Scholar] [CrossRef] [PubMed]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53. [Google Scholar] [CrossRef]
- Yang, H.; Sun, C.; Qiao, S.; Zou, J.; Liu, G.; Smith, S.; Cheng, H.; Lu, G. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428. [Google Scholar] [CrossRef]
- Ying, Y.; Lin, Z.; Huang, H. “Edge/Basal Plane Half-Reaction Separation” Mechanism of Two-Dimensional Materials for Photocatalytic Water Splitting. ACS Energy Lett. 2023, 8, 1416. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Q.; Hu, W.; Yang, J. First-Principles Computational Screening of Two-Dimensional Polar Materials for Photocatalytic Water Splitting. ACS Nano 2024, 18, 19381. [Google Scholar] [CrossRef]
- Fu, C.; Sun, J.; Luo, Q.; Li, X.; Hu, W.; Yang, J. Intrinsic Electric Fields in Two-dimensional Materials Boost the Solar-to-Hydrogen Efficiency for Photocatalytic Water Splitting. Nano Lett. 2018, 18, 6312. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, J.; Wang, J.; Li, Q.; Yang, J. beta-SnS/GaSe heterostructure: A promising solar-driven photocatalyst with low carrier recombination for overall water splitting. J. Mater. Chem. A 2022, 10, 3443. [Google Scholar] [CrossRef]
- Jamdagni, P.; Pandey, R.; Tankeshwar, K. First principles study of Janus WSeTe monolayer and its application in photocatalytic water splitting. Nanotechnology 2022, 33, 025703. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wen, B.; Ge, Q.; Zou, D.; Xu, Y.; Liu, M.; Wei, X.; Chen, M.; Fan, X. Role of intrinsic dipole on photocatalytic water splitting for Janus MoSSe/nitrides heterostructure: A first-principles study. Prog. Nat. Sci. Mater. Int. 2019, 29, 335. [Google Scholar] [CrossRef]
- Chen, F.; Huang, H.; Guo, L.; Zhang, Y.; Ma, T. The Role of Polarization in Photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 10061. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Shang, J.; Tang, X.; Kou, L. Tunable Photocatalytic Water Splitting by the Ferroelectric Switch in a 2D AgBiP2Se6 Monolayer. J. Am. Chem. Soc. 2020, 142, 1492. [Google Scholar] [CrossRef]
- Gao, X.; Shen, Y.; Liu, J.; Lv, L.; Zhou, M.; Zhou, Z.; Feng, Y.P.; Shen, L. Developing Dipole-scheme heterojunction photocatalysts. Appl. Surf. Sci. 2022, 599, 153942. [Google Scholar] [CrossRef]
- Liang, Y.; Li, J.; Jin, H.; Huang, B.; Dai, Y. Photoexcitation Dynamics in Janus-MoSSe/WSe2 Heterobilayers: Ab Initio Time-Domain Study. J. Phys. Chem. Lett. 2018, 9, 2797. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, P.; Li, S.; Liu, W. Designing a Dipole-Scheme Heterostructure Based on Janus TMDCs for Highly Efficient Photocatalytic Overall Water Splitting. Adv. Mater. Interfaces 2023, 10, 2300170. [Google Scholar] [CrossRef]
- Wang, G.; Chen, C.; Teketel, B.S.; Xu, B.; Lin, B. Constructing a new 2D Janus black phosphorus/SMoSe heterostructure for spontaneous wide-spectral-responsive photocatalytic overall water splitting. Int. J. Hydrogen Energy 2021, 46, 39183. [Google Scholar] [CrossRef]
- Ma, X.; Wu, X.; Wang, H.; Wang, Y. A Janus MoSSe monolayer: A potential wide solar-spectrum water-splitting photocatalyst with a low carrier recombination rate. J. Mater. Chem. A 2018, 6, 2295. [Google Scholar] [CrossRef]
- Peng, R.; Ma, Y.; Huang, B.; Dai, Y. Two-dimensional Janus PtSSe for photocatalytic water splitting under the visible or infrared light. J. Mater. Chem. A 2019, 7, 603. [Google Scholar] [CrossRef]
- Ju, L.; Bie, M.; Tang, X.; Shang, J.; Kou, L. Janus WSSe Monolayer: An Excellent Photocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 29335. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, J.; Zhao, X.; Hu, G.; Yuan, X.; Ren, J. Two-dimensional Janus AsXY (X = Se, Te; Y = Br, I) monolayers for photocatalytic water splitting. Eur. Phys. J. B 2023, 96, 17. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Lin, J. Janus monolayer SiXY (X = P, as and Sb, Y = N, P, As) for photocatalytic water splitting. Appl. Surf. Sci. 2023, 621, 156883. [Google Scholar] [CrossRef]
- Guan, Y.; Wen, L.; Dong, M.; Zhang, X.; Tan, B.; Song, Q.; Wang, J. First-principles study on MoSSe/ GaTe van der Waals heterostructures: A promising water-splitting photocatalyst. Results Phys. 2024, 58, 107520. [Google Scholar] [CrossRef]
- Yang, F.; Boulet, P.; Record, M.-C. Electronic structure and photocatalytic performance of Janus MoSSe/Ga2SSe van der Waals heterostructures. Int. J. Hydrogen Energy 2024, 73, 536. [Google Scholar] [CrossRef]
- He, Q.W.; Wu, Y.; Yang, C.H.; Zhang, H.N.; Tang, D.S.; Shang, X.; Wang, X.C. Switch effect on controlled water splitting by biaxial strain regulating the promising two-dimensional Janus X(2)PAs (X = Si, Ge and Sn) photocatalyst. Nanoscale 2023, 15, 10458. [Google Scholar] [CrossRef]
- Qi, C.; Yan, C.; Li, Q.; Yang, T.; Qiu, S.; Cai, J. Two-dimensional Janus monolayers Al2XYZ (X/Y/Z = S, Se, Te, X ≠ Y ≠ Z): First-principles insight into the photocatalytic and highly adjustable piezoelectric properties. J. Mater. Chem. C 2023, 11, 3262. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Liu, J.; Lv, L.; Zhou, M.; Yang, X.; Meng, X.; Zhang, B.; Zhou, Z. Symmetry-breaking-induced ferroelectric HfSnX(3) monolayers and their tunable Janus structures: Promising candidates for photocatalysts and nanoelectronics. Phys. Chem. Chem. Phys. PCCP 2023, 25, 22889. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, M.; Dong, H.; Hou, T.; Wang, L.; Li, Y. Two-dimensional germanium monochalcogenide photocatalyst for water splitting under ultraviolet, visible to near-infrared light. Nanoscale 2017, 9, 8608. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Tao, X.; Chen, H.; Zhu, W.; Ouyang, Y.; Peng, Q. Enhanced photocatalytic activity for water splitting of blue phase GeS and GeSe monolayers via biaxial straining. Nanoscale 2019, 11, 2335. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, M.; Yu, J.; Li, S.; Lei, S.; Lin, L.; Wang, J. Two-dimensional blue-phase CX (X = S, Se) monolayers with high carrier mobility and tunable photocatalytic water splitting capability. Chin. Chem. Lett. 2021, 32, 1977. [Google Scholar] [CrossRef]
- Gu, D.; Chen, X.; Xu, X.; Qin, W.; Tao, X.; Ouyang, Y.; Zhu, W. Polarization Electric Field in 2D Polar Monolayer Silicon Monochalcogenides SiX (X = S, Se) as Potential Photocatalysts for Water Splitting. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2022, 17, 2200179. [Google Scholar] [CrossRef]
- Qiao, N.; Yang, C.; Wang, M.; Ma, X. Two-dimensional TeX(X = C, Si, Ge) monolayers with strong intrinsic electric field for efficiency hydrogen evolution reaction. Surf. Interfaces 2022, 31, 102011. [Google Scholar] [CrossRef]
- Zaharo, A.; Purqon, A.; Winata, T.; Saito, M. Electronic structure of puckered group IV–VI two-dimensional monolayer materials. Jpn. J. Appl. Phys. 2020, 59, 071006. [Google Scholar] [CrossRef]
- Ren, K.; Ma, X.; Liu, X.; Xu, Y.; Huo, W.; Li, W.; Zhang, G. Prediction of 2D IV-VI semiconductors: Auxetic materials with direct bandgap and strong optical absorption. Nanoscale 2022, 14, 8463. [Google Scholar] [CrossRef]
- Behzad, S.; Chegel, R. Engineering the light absorption spectrum and electronic properties of black and blue phases of a SiSe monolayer via biaxial straining. J. Comput. Electron. 2023, 22, 971. [Google Scholar] [CrossRef]
- Gu, D.; Qin, W.; Hu, S.; Li, R.; Chen, X.; Tao, X.; Ouyang, Y.; Zhu, W. Enhanced Photocatalytic Activity of Two-Dimensional Polar Monolayer SiTe for Water-Splitting via Strain Engineering. Molecules 2023, 28, 2971. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, J.; Song, Y.; Wang, S.; Xue, K.; Xu, M.; Cheng, X.; Miao, X. Two-dimensional silicon chalcogenides with high carrier mobility for photocatalytic water splitting. J. Mater. Sci. 2019, 54, 11485. [Google Scholar] [CrossRef]
- Gu, D.; Tao, X.; Chen, H.; Ouyang, Y.; Zhu, W.; Peng, Q.; Du, Y. Strain Enhanced Visible–Ultraviolet Absorption of Blue Phosphorene/MoX2(X = S,Se) Heterolayers. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2019, 13, 1800659. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Long, M.; Fang, G.; Liu, M. Stacking order effects on the electronic and optical properties of GaS/XMoY (X/Y = S, Se, Te) Van der Waals heterostructures: A first-principles study. J. Phys. D Appl. Phys. 2022, 56, 035301. [Google Scholar] [CrossRef]
- Lin, H.F.; Liu, H.Y.; Wang, M.; Wang, S.S.; Hou, T.P.; Wu, K.M. Janus Ga(2)SeTe/In(2)SSe heterostructures: Tunable electronic, optical, and photocatalytic properties. Phys. Chem. Chem. Phys. PCCP 2022, 24, 4425. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zeng, H.; Wang, G.; Cheng, X.; Yang, S.A.; Zhang, H. Out-of-plane dipole-modulated photogenerated carrier separation and recombination at Janus-MoSSe/MoS(2) van der Waals heterostructure interfaces: An ab initio time-domain study. Phys. Chem. Chem. Phys. PCCP 2022, 24, 11743. [Google Scholar] [CrossRef]
- Jiang, Y.; Lei, S.; Wang, M. S-Scheme Boron Phosphide/MoS(2) Heterostructure with Excellent Light Conversion Ability for Solar Cells and Water Splitting Photocatalysts. ACS Appl. Mater. Interfaces 2024, 16, 30521. [Google Scholar] [CrossRef]
- Li, R.; Zhu, S.; Ding, J. Direct Z-scheme GeH/InSe heterostructure with high solar-to-hydrogen efficiency for photocatalytic water splitting. Chem. Phys. Lett. 2024, 840, 141160. [Google Scholar] [CrossRef]
- Ren, K.; Yu, J.; Tang, W. Two-dimensional ZnO/BSe van der waals heterostructure used as a promising photocatalyst for water splitting: A DFT study. J. Alloys Compd. 2020, 812, 152049. [Google Scholar] [CrossRef]
- Peterson, E.A.; Debela, T.T.; Gomoro, G.M.; Neaton, J.B.; Asres, G.A. Electronic structure of strain-tunable Janus WSSe-ZnO heterostructures from first-principles. RSC Adv. 2022, 12, 31303. [Google Scholar] [CrossRef]
- Wang, G.; Tang, W.; Xie, W.; Tang, Q.; Wang, Y.; Guo, H.; Gao, P.; Dang, S.; Chang, J. Type-II CdS/PtSSe heterostructures used as highly efficient water-splitting photocatalysts. Appl. Surf. Sci. 2022, 589, 152931. [Google Scholar] [CrossRef]
- Wang, G.; Tang, W.; Xu, C.; He, J.; Zeng, Q.; Xie, W.; Gao, P.; Chang, J. Two-dimensional CdO/PtSSe heterojunctions used for Z-scheme photocatalytic water-splitting. Appl. Surf. Sci. 2022, 599, 153960. [Google Scholar] [CrossRef]
- Wang, G.; Xie, W.; Guo, S.; Chang, J.; Chen, Y.; Long, X.; Zhou, L.; Ang, Y.S.; Yuan, H. Two-Dimensional GeC/MXY (M = Zr, Hf; X, Y = S, Se) Heterojunctions Used as Highly Efficient Overall Water-Splitting Photocatalysts. Molecules 2024, 29, 2793. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, Q.; Cao, L.; Fan, L.; Gu, F.; Zhang, Y.; Zheng, C.; Xiong, S.; Xu, L. Two-Dimensional SiH/g-C3N4 van der Waals Type-II Heterojunction Photocatalyst: A New Effective and Promising Photocatalytic Material. Coatings 2024, 14, 263. [Google Scholar] [CrossRef]
- Guo, Z.; Miao, N.; Zhou, J.; Sa, B.; Sun, Z. Strain-mediated type-I/type-II transition in MXene/Blue phosphorene van der Waals heterostructures for flexible optical/electronic devices. J. Mater. Chem. C 2017, 5, 978. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, B.; Liu, Y.; Liu, L.; Xia, T.; Zhang, X.; Ye, C.; Yu, Y.; Wang, B. Two-Dimensional As/BlueP van der Waals Hetero-Structure as a Promising Photocatalyst for Water Splitting: A DFT Study. Coatings 2020, 10, 1160. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Yin, H.; Yang, C. Two-dimensional BP/β-AsP van der Waals heterostructures as promising photocatalyst for water splitting. Appl. Phys. Lett. 2020, 117, 063901. [Google Scholar] [CrossRef]
- Tu, J.; Lei, X.; Li, P. Strain-induced ultrahigh power conversion efficiency in BP-MoSe(2)vdW heterostructure. Nanotechnology 2022, 34, 085403. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Zhao, R.; Cai, X.; Yu, W.; Li, W.; Liu, Z.; Zhang, L.; Ke, S. Electronic structures and enhanced photocatalytic properties of blue phosphorene/BSe van der Waals heterostructures. J. Mater. Chem. A 2018, 6, 8923. [Google Scholar] [CrossRef]
- Behzad, S.; Chegel, R. First principles study of biaxially deformed hexagonal buckled XS (X = Ge and Si) monolayers with light absorption in the visible region. Thin Solid Film. 2022, 759, 139457. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes forab initiototal-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787. [Google Scholar] [CrossRef] [PubMed]
- Klimeš, J.; Bowler, D.R.; Michaelides, A. Chemical accuracy for the van der Waals density functional. J. Physics. Condens. Matter Inst. Phys. J. 2009, 22, 022201. [Google Scholar] [CrossRef] [PubMed]
- Klimeš, J.; Bowler, D.R.; Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 2011, 83, 195131. [Google Scholar] [CrossRef]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé–Hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Paier, J.; Marsman, M.; Hummer, K.; Kresse, G.; Gerber, I.C.; Angyan, J.G. Screened hybrid density functionals applied to solids. J. Chem. Phys. 2006, 124, 154709. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.; Tang, G.; Geng, W. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]


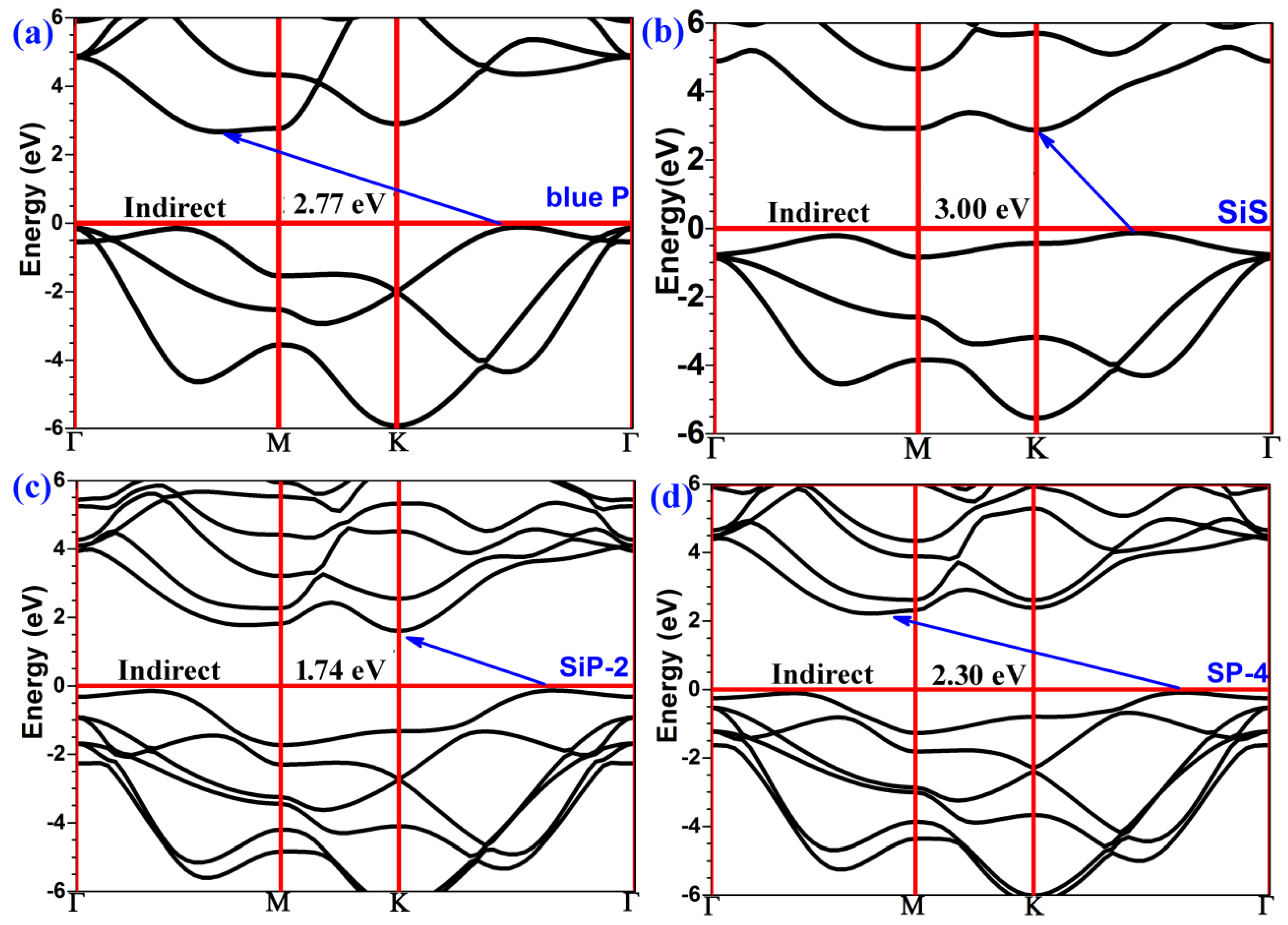
| Structure | a | dP-P | dSi-S | dT | dI | Eg | EgHSE06 | Ref. |
|---|---|---|---|---|---|---|---|---|
| (Å) | (Å) | (Å) | (Å) | (Å) | (eV) | (eV) | ||
| Blue P | 3.27 | 2.26 | 1.24 | 1.95 | 2.77 | This work | ||
| Blue P | 3.27 | 2.26 | 2.78 | [66] | ||||
| Blue P | 3.27 | 2.26 | 1.97 | 2.74 | [67] | |||
| SiS | 3.30 | 2.32 | 1.33 | 2.20 | 3.00 | This work | ||
| SiS | 3.31 | 2.33 | 2.19 | 3.03 | [68] | |||
| SiS | 3.30 | 2.32 | 1.33 | 2.20 | 3.00 | [50] | ||
| Si-P-2 heterostructure | 3.29 | 2.27 | 2.32 | 5.91 | 3.35 | 1.07 | 1.74 | This work |
| P-S-4 heterostructure | 3.29 | 2.26 | 2.32 | 5.91 | 3.35 | 1.52 | 2.30 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Gu, D.; Tao, X.; Ouyang, Y.; Duan, C.; Liang, G. Two-Dimensional Polarized Blue P/SiS Heterostructures as Promising Photocatalysts for Water Splitting. Molecules 2024, 29, 4355. https://doi.org/10.3390/molecules29184355
Liu Y, Gu D, Tao X, Ouyang Y, Duan C, Liang G. Two-Dimensional Polarized Blue P/SiS Heterostructures as Promising Photocatalysts for Water Splitting. Molecules. 2024; 29(18):4355. https://doi.org/10.3390/molecules29184355
Chicago/Turabian StyleLiu, Yin, Di Gu, Xiaoma Tao, Yifang Ouyang, Chunyan Duan, and Guangxing Liang. 2024. "Two-Dimensional Polarized Blue P/SiS Heterostructures as Promising Photocatalysts for Water Splitting" Molecules 29, no. 18: 4355. https://doi.org/10.3390/molecules29184355
APA StyleLiu, Y., Gu, D., Tao, X., Ouyang, Y., Duan, C., & Liang, G. (2024). Two-Dimensional Polarized Blue P/SiS Heterostructures as Promising Photocatalysts for Water Splitting. Molecules, 29(18), 4355. https://doi.org/10.3390/molecules29184355







