Squirting Cucumber, Ecballium elaterium (L.) A. Ritch: An Update of Its Chemical and Pharmacological Profile
Abstract
1. Introduction
2. Chemical Composition of the Plant Leaves, Fruits and Seeds
| Compounds | Formula | Chemical Structure | Quantity | Ref. | ||
|---|---|---|---|---|---|---|
| Leaves | Fruits | Seeds | ||||
| Benzaldehyde (1) | C7H6O |  | 12.3% of total area 1,2 | - | - | [2] |
| Benzeneacetaldehyde (2) | C8H8O | 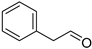 | 0.9% of total area 1,2 | - | - | [2] |
| β-Bisabolol (3) | C15H26O | 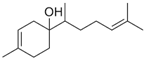 | 0.7% of total area 1,2 | - | - | [2] |
| Butyl cyclohexyl phthalate (4) | C18H24O4 | 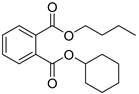 | 1.3% of total area 1,2 | - | - | [2] |
| Cubitene (5) | C20H32 | 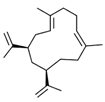 | 1.2% of total area 1,2 | - | - | [2] |
| β-Cyclocitral (6) | C10H16O | 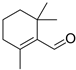 | 0.8% of total area 1,2 | - | - | [2] |
| β-Damascone (7) | C13H20O | 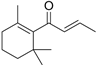 | 1.1% of total area 1,2 | - | - | [2] |
| n-Decanal (8) | C10H20O | 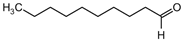 | 1.5% of total area 1,2 | - | - | [2] |
| 4,6-Dimethyl-3,5,7- trioxatetracyclo [7.2.1.0(4,11).0(6,10)] dodecane (9) | C11H16O4 | 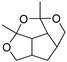 | 1.1% of total area 1,2 | - | - | [2] |
| 6-Ethyl-3-octyl isobutylester phthalic acid (10) | C22H34O4 | 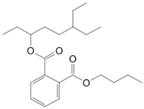 | 0.8% of total area 1,2 | - | - | [2] |
| Ethylsorbate (11) | C8H12O2 | 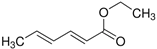 | 0.8% of total area 1,2 | - | - | [2] |
| cis-Eudesma,6,11 diene (12) | C15H24 | 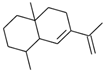 | 0.9% of total area 1,2 | - | - | [2] |
| Eudesmol (13) | C15H26O |  | 1.0% of total area 1,2 | - | - | [2] |
| 10-epi-γ-Eudesmol (14) | C15H26O |  | 2.1% of total area 1,2 | - | - | [2] |
| α-Fenchocamphorone (15) | C9H14O |  | 2.0% of total area 1,2 | - | - | [2] |
| Germacrene A (16) | C15H24 | 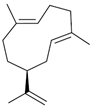 | 0.7% of total area 1,2 | - | - | [2] |
| Hexadecanoicacid, methyl ester (17) | C17H34O2 | 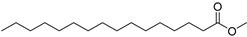 | 2.8% of total area 1,2 | - | - | [2] |
| Hinesol (18) | C15H26O | 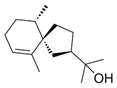 | 17.2% of total area 1,2 | - | - | [2] |
| (E)-β-Ionone (19) | C13H20O | 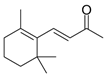 | 7.8% of total area 1,2 | - | - | [2] |
| 2-Isobutylthiazole (20) | C7H11NS |  | 1.6% of total area 1,2 | - | - | [2] |
| Isolongifolene (21) | C15H24 | 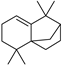 | 1.9% of total area 1,2 | - | - | [2] |
| epi-Laurenene (22) | C20H32 | 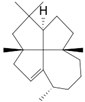 | 1.3% of total area 1,2 | - | - | [2] |
| Longifolol (23) | C15H26O | 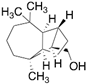 | 0.7% of total area 1,2 | - | - | [2] |
| 2-Methyl-7-octadecyne (24) | C19H36 |  | 2.6% of total area 1,2 | - | - | [2] |
| E-Nerolidol (25) | C15H26O |  | 1.2% of total area 1,2 | - | - | [2] |
| Neryl acetone (26) | C13H22O | 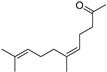 | 2.5% of total area 1,2 | - | - | [2] |
| Nonadecane (27) | C19H40 |  | 1.5% of total area 1,2, 0.9% of total area 1 | 1.4% of total area 1 | - | [2] |
| Safranal (28) | C10H14O | 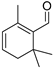 | 1.4% of total area 1,2 | - | - | [2] |
| o-Tolualdehyde (29) | C8H8O |  | 0.8% of total area 1,2 | - | - | [2] |
| Vestitenone (30) | C12H18O | 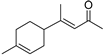 | 1.4% of total area 1,2 | - | - | [2] |
| p-Vinylguaiacol (31) | C9H10O2 | 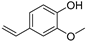 | 2.1% of total area 1,2 | - | - | [2] |
| (24S)-Ethyl-5α-cholesta-7,22,25-trien-3β-ol (32) |  | 0.4mg/g of dry leaves | - | - | [14] | |
| 1-Allyl-1-but-3-enyl-1-silacyclobutane (33) | C10H18Si | 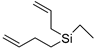 | 1.51% of total area 2 | - | - | [15] |
| Carvacrol (34) | C10H14O |  | 6.09% of total area 2 | - | - | [15] |
| 3,4-Dimethylheptane (35) | C9H20 | 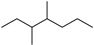 | 3.25% of total area 2 | - | - | [15] |
| (E)-5-Eicosene (36) | C20H40 |  | 6.51% of total area 2 | - | - | [15] |
| n-Hentriacontane (37) | C31H64 |  | 73,97% of total area 2 | - | - | [15] |
| Limonene dioxide (38) | C10H16O2 |  | 7.61% of total area 2 | - | - | [15] |
| Linolenic acid methyl ester (39) | C19H32O2 | 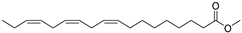 | 1.04% of total area 2 | - | - | [15] |
| Loliolide (40) | C11H16O3 |  | 1.66% of total area 2 | - | - | [15] |
| Neophytadiene (41) | C20H38 |  | 4.89% of total area (Hexane extract) 2 | - | - | [15] |
| n-Pentacosane (42) | C25H52 |  | 1.75% of total area 2 | - | - | [15] |
| 7,10-Pentadecadiynoic acid (43) | C15H22O2 | 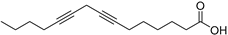 | 4.00% of total area 2 | - | - | [15] |
| Phytol (44) | C20H40O |  | 3.59% of total area 2 | - | - | [15] |
| Propylhexedrine (45) | C10H21N | 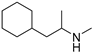 | 1.29% of total area 2 | - | - | [15] |
| l-2-Tetramethyhexadecen-1-ol 3,7,11,15- (46) | C20H40O | 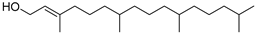 | 1.16% of total area 2 | - | - | [15] |
| Thymol (47) | C10H14O | 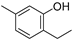 | 12.05% of total area 2 | - | - | [15] |
| (E)-Anethol (48) | C10H12O | 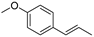 | - | 31.6% of total area 1 | - | [16] |
| Dibuthylphtalate (49) | C16H22O4 | 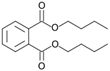 | 0.5% of total area 1 | 3.2% of total area 1 | - | [16] |
| 3-(6,6-Dimethyl-5-oxohept-2-enyl)-cyclohexanone (50) | C15H24O2 |  | 20.4% of total area 1 | 8.8% of total area 1 | - | [16] |
| Eicosane (51) | C20H42 |  | 1.9% of total area 1 | 2.7% of total area 1 | - | [16] |
| Estragol (52) | C10H12O | 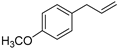 | - | 0.7% of total area 1 | - | [16] |
| Henicosane (53) | C21H44 |  | 1.2% of total area 1 | 2.8% of total area 1 | - | [16] |
| Heptadecane (54) | C17H36 |  | 1.3% of total area 1 | 2.1% of total area 1 | - | [16] |
| Hexadecane (55) | C16H34 |  | 2.5% of total area 1 | 5.2% of total area 1 | - | [16] |
| Hexahydrofarnesyl acetone (56) | C18H36O | 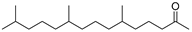 | 19.1% of total area 1 | 2.1% of total area 1 | - | [16] |
| Isobutylphthalate (57) | C12H14O4 | 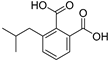 | 1.0% of total area 1 | 1.7% of total area 1 | - | [16] |
| Limonene (58) | C10H16 | 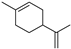 | - | 0.8% of total area 1 | - | [16] |
| Methylheptadecane (59) | C18H38 |  | 0.3% of total area 1 | - | - | [16] |
| Nor-pristane (60) | C18H38 |  | 0.4% of total area 1 | - | [16] | |
| Octadecane (61) | C18H38 |  | 2.9% of total area 1 | 5.7% of total area 1 | - | [16] |
| Octylhexanoate (62) | C14H28O2 |  | 0.8% of total area 1 | - | - | [16] |
| Octyloctanoate (63) | C16H32O2 | 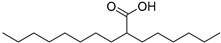 | 30.0% of total area 1 | 3.5% of total area 1 | - | [16] |
| Pentadecane (64) | C15H32 |  | 0.3% of total area 1 | - | - | [16] |
| Phytene (65) | C20H40 | 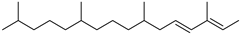 | 1.3% of total area 1 | - | - | [16] |
| Pristane (66) | C19H40 |  | 0.8% of total area 1 | 1.5 % of total area 1 | - | [16] |
| p-Propylanisole (67) | C10H14O |  | - | 8.2% of total area 1 | - | [16] |
| Tetracosane (68) | C24H50 |  | 3.7% of total area 1 | 7.4% of total area 1 | - | [16] |
| Tetradecane (69) | C14H30 |  | 0.3% of total area 1 | 0.8% of total area 1 | - | [16] |
| β-Thujone (70) | C10H16O | 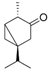 | - | 3.0% of total area 1 | - | [16] |
| Tricosane (71) | C23H48 |  | 2.0% of total area 1 | 4.9% of total area 1 | - | [16] |
| Caffeoylglucaric acid (72) | C15H16O11 | 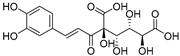 | - | - | - | [17] |
| Catechin (73) | C15H14O6 |  | - | - | - | [17] |
| Catechin-3-O-rutinoside (74) | C27H34O15 | 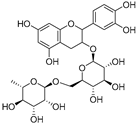 | - | - | - | [17] |
| Colocynthoside B (75) | C42H62O15 | 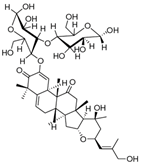 | - | - | - | [17] |
| 4-Feruloylquinic acid (76) | C17H20O9 | 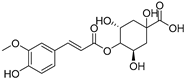 | - | - | - | [17] |
| 9-/ or 13-Hydroxy-9Z,11E-octadecadienoic acid (77) | C18H32O3 | 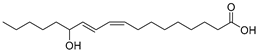 | - | - | - | [17] |
| Linolenic acid (78) | C18H30O2 |  | - | - | - | [17] |
| Procyanidin dimer (79) | C30H26O12 | 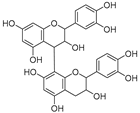 | - | - | - | [17] |
| Shikimic acid hexoside isomer I (80) | C13H20O10 | 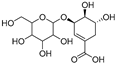 | - | - | - | [17] |
| Shikimic acid hexoside isomer II (81) | C13H20O10 | 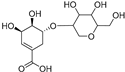 | - | - | - | [17] |
| 3,7,3′,4″-Tetrahydroxyflavanone (82) | C15H12O6 | 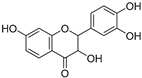 | - | - | - | [17] |
| 7,3′,4′-Trihydroxyflavanone (83) | C15H10O5 | 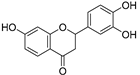 | - | - | - | [17] |
| Trihydroxyflavanone-O-deoxyhexosyl-O-hexoside (84) | C27H34O15 | n.d. | - | - | - | [17] |
| Trihydroxy-octadecadienoicacid (85) | C18H32O5 | 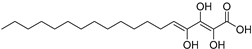 | - | - | - | [17] |
| Trihydroxy-octadecenoicacid (86) | C18H34O5 | 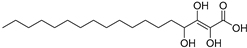 | - | - | - | [17] |
| Elateroside A (87) | C42H66O14 |  | - | 0.3 mg/kg fresh fruit | - | [18] |
| Elateroside B (88) | C48H78O18 | 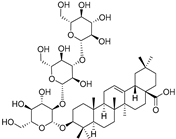 | - | 2 mg/kg fresh fruit | - | [18] |
| 3-O-β-d-Glucopyranosyl-3β-hydroxyolean-12-en-28-oic acid 28-O-β-d-glucopyraonoside (89) | C42H67O13 | 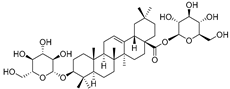 | - | 3 mg/kg fresh fruit | - | [18] |
| Oleanolic acid 3-O-β-d-glucopyranoside (90) | C36H58O8 | 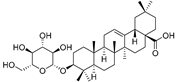 | - | 5 mg/kg fresh fruit | - | [18] |
| Oleanolic acid 3-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside (91) | C42H68O13 | 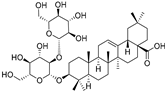 | - | 2 mg/kg fresh fruit | - | [18] |
| Arachidic acid (92) | C20H40O2 | 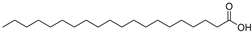 | - | - | 0.84% of total fatty acid | [19] |
| δ-5-Avenasterol (93) | C29H48O | 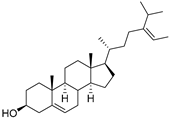 | - | - | 16.44 mg/100 g | [19] |
| δ-7-Avenasterol (94) | C29H48O | 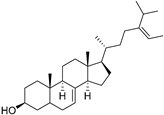 | - | - | 93.81 mg/100 g | [19] |
| Campesterol (95) | C28H48O |  | - | - | 139.63 mg/100 g | [19] |
| δ-7-Campesterol (96) | C28H48O | 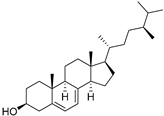 | - | - | 19.18 mg/100 g | [19] |
| Desmosterol (97) | C27H44O | 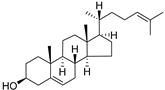 | - | - | 8.89 mg/100 g | [19] |
| Linoleic acid (98) | C18H32O2 | 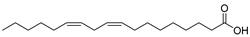 | - | - | 48.64% of total fatty acid | [19] |
| Myristic acid (99) | C14H28O2 | 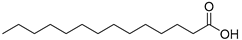 | - | - | 0.09% of total fatty acid | [19] |
| Oleic acid (100) | C18H34O2 | 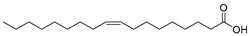 | - | - | 15.58% of total fatty acid | [19] |
| Palmitic acid (101) | C16H32O2 |  | 3.36% of total area2 | - | 4.09% of total fatty acid | [19] |
| Puninic acid (102) | C18H30O2 |  | - | - | 22.38% of total fatty acid | [19] |
| Sitostanol (103) | C29H52O | 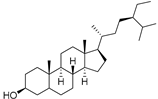 | - | - | 21.29 mg/100 g | [19] |
| β-Sitosterol (104) | C29H50O | 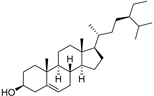 | - | - | 396.25 mg/100 g | [19] |
| Stearic acid (105) | C18H36O2 |  | - | - | 4.93% of total fatty acid | [19] |
| δ-7-Stigmastenol (106) | C29H50O | 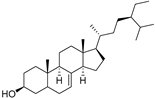 | - | - | 86.68 mg/100 g | [19] |
| Stigmasterol (107) | C29H48O | 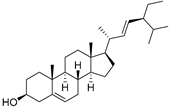 | - | - | 6.29 mg/100 g | [19] |
| α-Tocopherol (108) | C29H50O2 |  | 3.51% of total area 2 | - | 3.62 mg/100 g | [19] |
| β-Tocopherol (109) | C28H48O2 |  | - | - | 1.82 mg/100 g | [19] |
| γ-Tocopherol (110) | C28H48O2 | 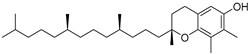 | - | - | 44.23 mg/100 g | [19] |
| δ-Tocopherol (111) | C27H46O2 | 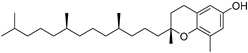 | - | - | 12.44 mg/100 g | [19] |
| Cycloeucalenol acetate (112) | C32H52O2 | 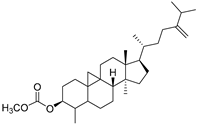 | - | - | - | [20] |
| Fructose (113) | C6H12O6 | 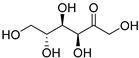 | 32% of total sugars | - | - | [21] |
| Glucose (114) | C6H12O6 |  | 34% of total sugars | - | - | [21] |
| Inositol (115) | C6H12O6 | 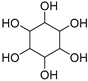 | 13% of total sugars | - | - | [21] |
| Raffinose (116) | C18H32O16 | 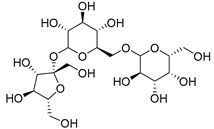 | 7% of total sugars | - | - | [21] |
| Sucrose (117) | C12H22O11 | 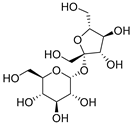 | 14% of total sugars | - | - | [21] |
| N-Ethyl-l-asparagine (118) | C6H12N2O3 | 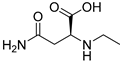 | 66.7mg/kg fresh areal parts | - | - | [22] |
| Phytomelin (119) | C27H30O16 |  | 8.54 mg/g of dry leaves | 1.84 mg/g of dry fruit | - | [23] |
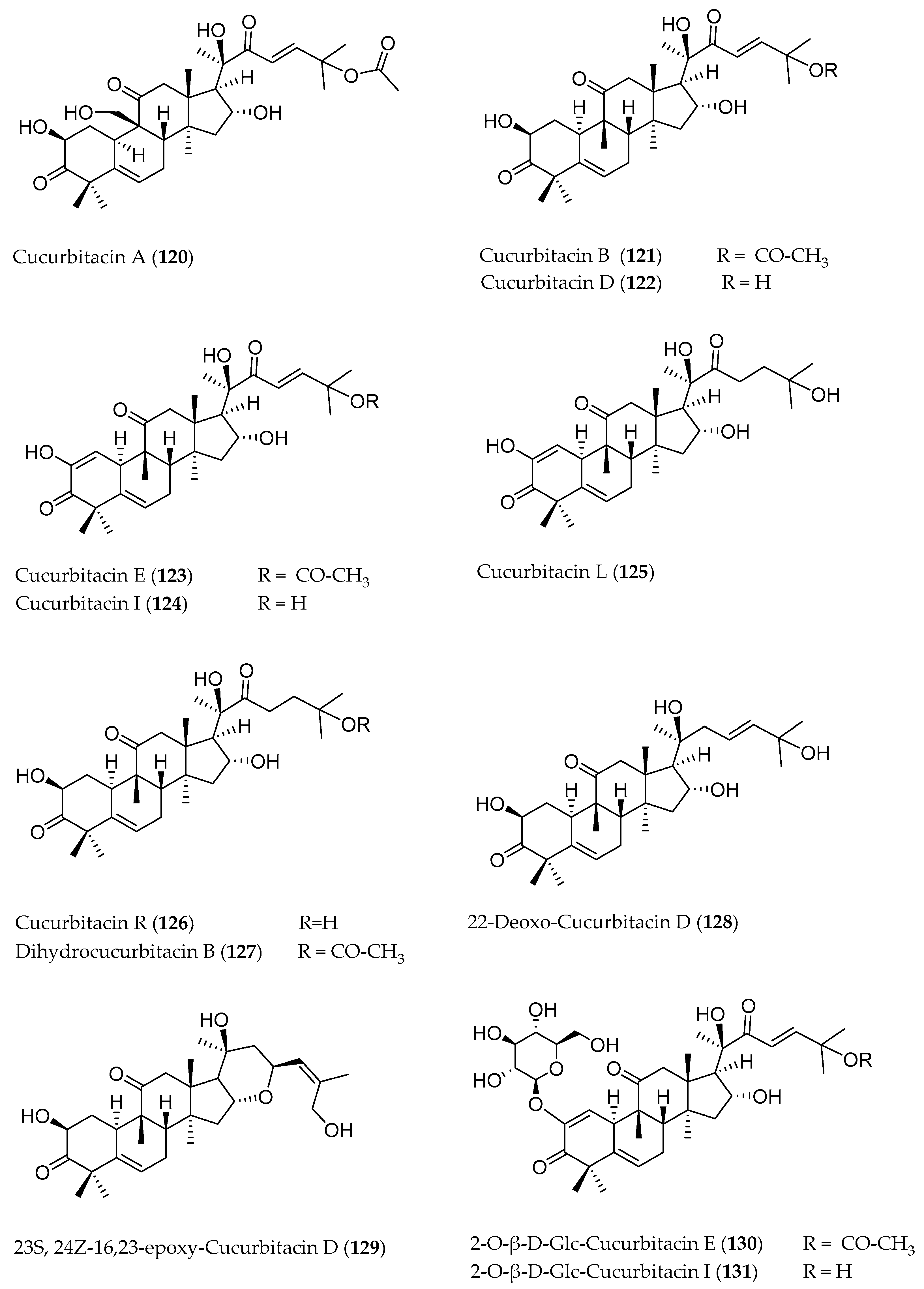
3. Cucurbitacins: Chemical Structure
| Name | Chemical Formula | Molecular Mass (g/mol) | Plant Part | Extraction Solvent | Method | Quantity | Biolological Activity | Ref |
|---|---|---|---|---|---|---|---|---|
| Cucurbitacin A (120) | C32H46O9 | 540.70 | Fruit | Hexane | HPLC-DAD-ESI-MS | - | - | [17] |
| Cucurbitacin B (121) | C32H46O8 | 558.70 | Fruit | Petroleum ether | Silica gel chromatography,IR, 1H-NMR, 13C-NMR | 2.56% of the fruit juice | Anti-hepatotoxic effect in mice | [25] |
| - | - | - | n.a. | Antimicrobial activity against S. aureus, antiviral activity against HSV-1 | [8] | |||
| Fruit | Dichloromethane | HPLC-MS, 1H-NMR, 13C-NMR | n.a. | - | [28] | |||
| Leaves | Methanol | LC-ESI-MS | n.a. | Cytotoxic effect, inhibition of human glioma cell adhesion | [29] | |||
| Fruit | Fruit juice | Preparative TLC 1H-NMR | 2.57 mg crude/g dry juice | Anti-inflammatory activity induced by cucurbitacin B in mice | [26] | |||
| Dihydro-Cucurbitacin B (127) | C32H48O8 | 560.72 | Fruit | Hexane | HPLC-DAD-ESI-MS | - | - | [29] |
| Cucurbitacin D (122) | C30H44O7 | 516.67 | Fruit | Methanol, ethanol | HPLC-ESI-MS | n.a. | Decrease bilirubin level in human plasma | [5] |
| - | - | - | n.a. | Inhibition of lung cancer cell proliferation | [30] | |||
| Fruit | Methanol | Column chromatography1H-NMR | n.a. | Cytotoxic effect on gastric cancer cells | [31] | |||
| Fruit | Dichloromethane | HPLC-DAD-MS, 1H-NMR, 13C-NMR | n.a. | - | [32] | |||
| Fruit | Dichloromethane | HPLC-MS, 1H-NMR, 13C-NMR | 6 mg | - | [28] | |||
| Fruit | Water | HPLC-UV, 1H-NMR, LC-ESI-MS | 86.4 µg/g of dried residue | - | [27] | |||
| Fruit | Hexane | HPLC | n.a. | Cytotoxic, apoptotic, and anti-migration effects against hepatocellular carcinoma | [33] | |||
| 22-Deoxo-Cucurbitacin D (128) | C30H46O6 | 502.33 | Fruit | Dichloromethane | HPLC-MS, 1H-NMR, 13C-NMR | 37 mg (mixture with Cuc. R) | - | [28] |
| Fruit | Dichloromethane | HPLC-DAD-MS, 1H-NMR, 13C-NMR | n.a. | - | [32] | |||
| (23S, 24Z)-16,23-Epoxy Cucurbitacin D (129) | C30H44O6 | 500.67 | Fruit | Dichloromethane | HPLC-MS, 1H-NMR, 13C-NMR | 3 mg | - | [28] |
| Cucurbitacin E (123) | C32H44O8 | 556.69 | Fruit | Chloroform | HPLC-DAD-UV | n.a. | Cytotoxic effect on Parkinson’s disease cells | [34] |
| - | - | - | n.a. | Cytotoxic effect, immunostimulant effect | [35] | |||
| - | - | - | n.a. | Cytotoxic effect on breast carcinoma cancer cells, melanoma and prostate adenocarcinoma | [36] | |||
| Fruit | - | - | n.a. | Cytotoxic effect on ovarian cancer cells | [4] | |||
| Fruit | Methanol | HPLC-UV | n.a. | - | [37] | |||
| Fruit | Methanol | Column chromatography1H-NMR | n.a. | Cytotoxic effect on gastric cancer cells | [31] | |||
| Fruit | Dichloromethane | HPLC-MS, 1H-NMR, 13C-NMR | n.a. | - | [28] | |||
| Fruit | Dichloromethane | HPLC-DAD-MS, 1H-NMR, 13C-NMR | n.a. | - | [32] | |||
| Fruit | Hexane | HPLC | n.a. | Cytotoxic, apoptotic, and anti-migration effects against hepatocellular carcinoma | [33] | |||
| 2-O-β-d-Glucopyranosyl Cucurbitacin E (130) | C38H54O13 | 718.36 | Whole plant | 90% Ethyl alcohol | Silica gel chromatography,1H-NMR | 46.67 mg/kg fresh plant | - | [6] |
| Cucurbitacin I (124) | C30H42O7 | 514.65 | Fruit | Methanol, ethanol | HPLC-ESI-MS | n.a. | - | [5] |
| Fruit | Chloroform | Column chromatography1H-NMR | n.a. | Cytotoxic effect on gastric cancer cells | [31] | |||
| Fruit | Dichloromethane | HPLC-MS, 1H-NMR, 13C-NMR | n.a. | - | [28] | |||
| Fruit | Dichloromethane | HPLC-DAD-MS, 1H-NMR, 13C-NMR | n.a. | - | [32] | |||
| Fruit | Water | HPLC-UV, 1H-NMR, LC-ESI-MS | 61 µg/g of dried residue | - | [27] | |||
| Fruit | Hexane | HPLC-UV, 1H-NMR, 13C-NMR | n.a. | Anti-cancer effect against hepatocellular cancer cells | [38] | |||
| Fruit | Hexane | HPLC | n.a. | Cytotoxic, apoptotic, and anti-migration effects against hepatocellular cancer cells | [33] | |||
| Fruit | Chloroform | HPLC-UV | n.a. | Anti-cancer effects on breast cancer cells | [39] | |||
| 2-O-β-d-Glucopyranosyl Cucurbitacin I (131) | C36H52O12 | 676.35 | Whole plant | 90 % Ethyl alcohol | Silica gel chromatography,1H-NMR | 34.67 mg/kg fresh plant | - | [6] |
| Cucurbitacin L (125) | C30H44O7 | 516.67 | Fruit | Dichloromethane | HPLC-MS, 1H-NMR, 13C-NMR | n.a. | - | [28] |
| Cucurbitacin R (126) | C30H46O7 | 518.68 | Fruit | Dichloromethane | HPLC-MS, 1H-NMR, 13C-NMR | 37 mg (mixture with 22-Deoxo Cuc. D) | - | [28] |
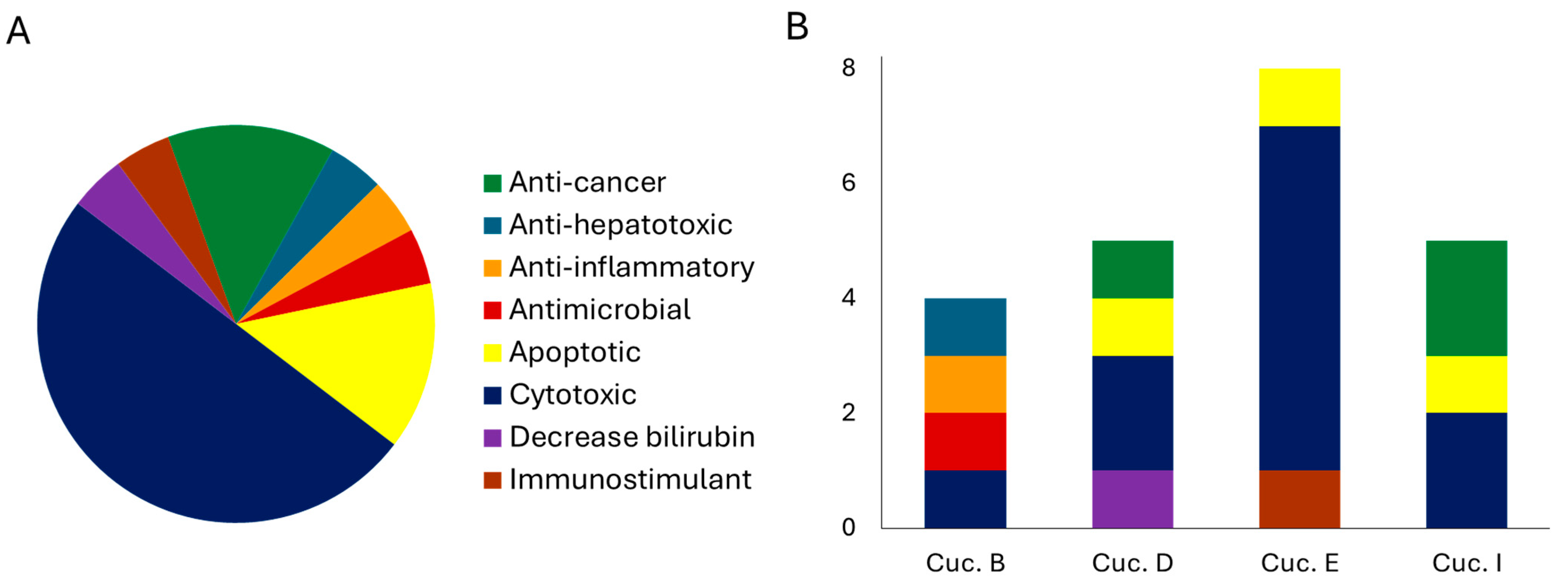
4. Cucurbitacins: Biological Activity
5. Biological Activity of Different Plant Tissues
| Activity | Material Tested | Plant Part | Disease | Observed Effect and Target Organism | Quantity | Ref. |
|---|---|---|---|---|---|---|
| Antibacterial | Hexane, chloroform, ethyl acetate, butanol, ethanol extracts | Fruit | - | Antibacterial effect against 10 K. pneumonia strains | MIC: 32–64 µg/mL | [42] |
| Anti-fibrotic | 80% ethanol extract | Fruit | Fibrosis | Reduced fibrosis, wound healing in rats | 5 mg/kg of body weight | [50] |
| Anti-inflammatory | Aqueous extract | Fruit | Rhinosinusitis | Anti-inflammatory activity in white rabbits | - | [51] |
| 80% ethanolic extract | Fruit | Sepsis-induced lung injury | Anti-inflammatory effect against sepsis-induced lung injury in rats | 2.5 mg/kg of body weight | [41] | |
| Fruit juice | Fruit | Neuroinflammation | Anti-inflammatory activity in rats | 10.9 µg/kg body weight 21.8 µg/kg body weight | [46] | |
| Fruit juice, dichloromethane extract | Fruit | Increased vascular permeability induced by HOAc | Anti-inflammatory activity induced by cucurbitacin B in mice | 50 mg/kg of body weight | [26] | |
| Fruit juice, dichloromethane extract | Fruit | Increased vascular permeability induced by HOAc | Anti-inflammatory activity induced by cucurbitacin B in mice | 50 mg/kg of body weight | [26] | |
| Fruit juice, ethanol extract | Fruit | Inflammation of the nasal mucosa | Anti-inflammatory activity in rats | 100 mg/mL, 200 mg/mL and pure juice | [47] | |
| Methanol extract | Fruit | Carrageenan-induced hind-paw edema | Anti-inflammatory activity in rats | 75 mg/kg | [45] | |
| 80% ethanol extract | Fruit | Sepsis-associated encephalopathy | Anti-inflammatory activity in rats | 2.5 mg/kg | [48] | |
| Fruit juice | Fruit | Liver fibrosis | Anti-inflammatory activity and hepatoprotective effect in mice | 100 mg/kg of body weight | [49] | |
| Antimalarial | Hexane extract | Root | - | Antimalarial effect determined by beta-hematin test | MIC: 4.645 mg/mL | [52] |
| Dichloromethane extract | - | MIC: 2.637 mg/mL | ||||
| Methanol extract | - | MIC: 0.124 mg/mL | ||||
| Dichloromethane extract | Seed | - | MIC: 1.338 mg/mL | |||
| Methanol extract | - | MIC: 0.458 mg/mL | ||||
| Hexane extract | Fruit | - | MIC: 3.518 mg/mL | |||
| Dicloromethane extract | - | MIC: 3.641 mg/mL | ||||
| Antimicrobial | Aqueous extract | Fruit | - | Antimicrobial activity against S. typhi | MIC: 10 µg/mL | [43] |
| 80% ethanol extract | Fruit | - | Antimicrobial effect against S. aureus and C. albicans | MIC between 0.195 and 1.563 mg/mL for S. aureus strains and between 0.097 and 6.250 mg/mL for C. albicans | [44] | |
| Methanol extract | Fruit | - | Antimicrobial activity in S. aureus, B. subtilis, K. pneumonia, S. enteritidis | MIC: 6 mg/mL (B. aureus, B. subtilis), 12 mg/mL (K. pneumonia, S. enteritidis) | [45] | |
| Antioxidant | 90% ethanol extract | Fruit | - | Antioxidant activity | IC50: 3.57 mg/mL | [53] |
| Methanol extract | Fruit | - | Antioxidant activity | 156 μg/mL | [45] | |
| Anti-tumours | Seed oil | Seed | Glioma | Reduced adhesion of glioma cell lines (U87 cell lines) to fibrinogen (IC50 = 9.2 µg /mL), fibronectin (IC50 = 34.1 µg /mL). | Adhesion to fibrinogen (IC50 = 9.2 µg/mL), to fibronectin (IC50 = 34.1 µg/mL) | [29] |
| Antiviral | Fruit juice | Fruit | - | Antiviral activity against BRV/ERU_2018 strain | - | [3] |
| Cytotoxic | Aqueous extract | Fruit | - | Cytotoxic actiovity against AGS and KYSE30 cancer cell lines | IC50: 2.5, 0.7, 0.7 μg/mLfor AGS cells after 24, 48, 72 h. IC50: 500, 150, 125 μg/mL after 24, 48, 72 h for KYSE30 cells | [54] |
| 80% ethanol extract | Seed | - | Cytotoxic activity against MA-104 cell line | LogIC50: 6.03 | [3] | |
| 90% ethanol extract | Fruit | - | Cytotoxic activity against cancerous cell lines | IC50: between 1.953 and 702 μg/mL | [53] | |
| Fruit juice | Fruit | - | Cytotoxic activity against MA-104 cell line | LogIC50: 4.8 | [3] | |
| Fruit juice | Fruit | - | Cytotoxic and mutagenic effect against human blood cells | 72 µL/L for 48 h | [55] | |
| Fruit juice | Fruit | - | Cytotoxic and genotoxic effect against A. cepa meristematic cells | 10, 20 and 50 mL/L and pure juice | [56] | |
| Intraperitoneal adhesion reduction | 80% ethanol extract | Fruit | Postoperative peritoneal adhesion | Reduction of postoperative intraperitoneal adhesions in rats | 2.5 mg/kg of body weight | [57] |
| Pro-apoptotic | Fruit juice | Fruit | CCl4-induced hepatotoxicity | Pro-apoptotic effect in rats | 0.7 mg/kg of body weight | [58] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motti, R.; Marotta, M.; Bonanomi, G.; Cozzolino, S.; Di Palma, A. Ethnobotanical Documentation of the Uses of Wild and Cultivated Plants in the Ansanto Valley (Avellino Province, Southern Italy). Plants 2023, 12, 3690. [Google Scholar] [CrossRef] [PubMed]
- Jebara, R.R.; Hudaib, M.M.D.; Bustanji, Y.K.; Alhourani, N.T.; AlAbbassi, R.; Kasabri, V.N. Essential Oil Composition and Antiproliferative Activity of Ecballium elaterium (L). Aerial Parts: A Medicinal Essential Oil Bearing Plant from Jordan. Jordan J. Pharm. Sci. 2021, 14, 279–292. [Google Scholar]
- Aksoy, E.; Güler, N.; Sözdutmaz, İ.; Kökkaya, S.; Berber, E.; Göksu, A.G. In vitro Antiviral Potential of Cucurbitaceae Ecballium elaterium and its Extract Containing Protease Inhibitors against Bovine Rotavirus. Microbiol. Res. 2023, 14, 2079–2089. [Google Scholar] [CrossRef]
- Attard, E.; Brincat, M.P.; Cuschieri, A. Immunomodulatory Activity of Cucurbitacin E Isolated from Ecballium elaterium. Fitoterapia 2005, 76, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Greige-Gerges, H.; Khalil, R.A.; Mansour, E.A.; Magdalou, J.; Chahine, R.; Ouaini, N. Cucurbitacins from Ecballium elaterium Juice Increase the Binding of Bilirubin and Ibuprofen to Albumin in Human Plasma. Chem. Biol. Interact. 2007, 169, 53–62. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Z.I.; Badr, W.H. Cucurbitacin Glucosides and Biological Activities of the Ethyl Acetate Fraction from Ethanolic Extract of Egyptian Ecballium elaterium. J. Appl. Sci. Res. 2012, 8, 1252–1258. [Google Scholar]
- Souilah, N.; Amrouni, R.; Bendif, H.; Daoud, N.; Lared, H. Ethnobotanical study of the toxicity of Ecballium elaterium (L.) A. Rich. in the Northest of Algeria. J. Med. Bot. 2020, 4, 9–13. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J.; Hassan, K.T.S. Cucurbitacin B Interacts Synergistically with Antibiotics against Staphylococcus Aureus Clinical Isolates and Exhibits Antiviral Activity against HSV-1. S. Afr. J. Bot. 2017, 108, 90–94. [Google Scholar] [CrossRef]
- Gry, J.; Søborg, I.; Andersson, H.C. Cucurbitacins in Plant Food. In TemaNord; Nordic Council of Ministers: Copenhagen, Denmark, 2006. [Google Scholar]
- Salhab, A.S. Human Exposure to Ecballium elaterium Fruit Juice: Fatal Toxicity and Possible Remedy. Pharmacol. Pharm. 2013, 4, 447–450. [Google Scholar] [CrossRef]
- Attard, E.; Scicluna-Spiteri, M. The cultivation and cucurbitacin content of Ecballium elaterum (L.) A. Rich. CGC Reports 2003, 26, 66–69. [Google Scholar]
- Ujváry, I. Pest Control Agents from Natural Products. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Krieger, R., Ed.; Academic Press: San Diego, CA, USA, 2010; Volume 1, pp. 119–229. [Google Scholar]
- Ielciu, I.; Frédérich, M.; Tits, M.; Angenot, L.; Pàltinean, R.; Cieckiewicz, E.; Crisan, G.; Vlase, L. Bryonia Alba, L. and Ecballium elaterium (L.) A. Rich.—Two Related Species of the Cucurbitaceae Family with Important Pharmaceutical Potential. Farmacia 2016, 64, 322–323. [Google Scholar]
- Hylands, P.J.; Oskoui, M.T. The Structure of Elasterol from Ecballium elaterium. Phytochemistry 1979, 18, 1543–1545. [Google Scholar] [CrossRef]
- Molavi, O.; Torkzaban, F.; Jafari, S.; Asnaashari, S.; Asgharian, P. Chemical Compositions and Anti-Proliferative Activity of the Aerial Parts and Rhizomes of Squirting Cucumber, Cucurbitaceae. Jundishapur. J. Nat. Pharm. Prod. 2020, 15. [Google Scholar] [CrossRef]
- Razavi, S.M.; Nejad-Ebrahimi, S. Phytochemical Analysis and Allelopathic Activity of Essential Oils of Ecballium elaterium A. Richard Growing in Iran. Nat. Prod. Res. 2010, 24, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Touihri-Barakati, I.; Kallech-Ziri, O.; Morjen, M.; Marrakchi, N.; Luis, J.; Hosni, K. Inhibitory Effect of Phenolic Extract from Squirting Cucumber (Ecballium elaterium (L.) A. Rich) Seed Oil on Integrin-Mediated Cell Adhesion, Migration and Angiogenesis. RSC Adv. 2022, 12, 31747–31756. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, K.A.; Sarkar, T.; Das, B.; Masudab, K.; Shiojima, K. Triterpenoid Glycosides from Fruits of Ecballium elaterium. J. lndian Chem. Soc. 1997, 74, 858–863. [Google Scholar]
- Touihri, I.; Kallech-Ziri, O.; Boulila, A.; Fatnassi, S.; Marrakchi, N.; Luis, J.; Hanchi, B. Ecballium elaterium (L.) A. Rich. Seed Oil: Chemical Composition and Antiproliferative Effect on Human Colonic Adenocarcinoma and Fibrosarcoma Cancer Cell Lines. Arab J. Chem. 2019, 12, 2347–2355. [Google Scholar] [CrossRef]
- Oskoui, M.T. Cycloeucalenol from the Leaves of Ecballium elaterium. Planta Med. 1986, 52, 159. [Google Scholar] [CrossRef]
- Akinci, S.; Losel, D.M. The Soluble Sugars Determination in Cucurbitaceae Species under Water Stress and Recovery Periods. Adv. Environ. Biol. 2009, 3, 175–183. [Google Scholar]
- Gray, D.O.; Fowden, L. N-Ethyl-L-Asparagine: A New Amino-Acid Amide from Ecballium. Nature 1961, 189, 401–402. [Google Scholar] [CrossRef]
- Jaradat, N.; Rinno, B.T.; Kharoof, C.M.; Naser Zaid, A.; Hannon, M. Determination the Presence of Phytomelin in Ecballium elaterium to Approve its Folk Uses. Int. J. Pharm. Sci. 2012, 4, 233. [Google Scholar]
- Sturm, S.; Stuppner, H. Analysis of Cucurbitacins in Medicinal Plants by High-Pressure Liquid Chromatography-Mass Spectrometry. Phytochem. Anal. 2000, 11, 121–127. [Google Scholar] [CrossRef]
- Agil, A.; Mirò, M.; Jimenez, J.; Aneiros, J.; Caracuel, M.D.; Garcia-Granados, A.; Navarro, M.C. Isolation of an Anti-Hepatotoxic Principle from the Juice of Ecballium elaterium. Planta Med. 1999, 65, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Yesilada, E.; Tanaka, S.; Sezik, E.; Tabata, M. Isolation of an Anti-Inflammatory principle from the fruit juice of Ecballium elaterium. J. Nat. Prod. 1988, 51, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Tosun, E.; Baysar, A. Isolation and Purification of Cucurbitacin D and I from Ecballium elaterium (L.) A. Rich Fruit Juice. Maced. J. Chem. Chem. En. 2019, 38, 171–182. [Google Scholar] [CrossRef]
- Seger, C.; Sturm, S.; Haslinger, E.; Stuppner, H. A New Cucurbitacin D Related 16,23-Epoxy Derivative and Its Isomerization Products. Org. Lett. 2004, 6, 633–636. [Google Scholar] [CrossRef]
- Touihri-Barakati, I.; Kallech-Ziri, O.; Ayadi, W.; Kovacic, H.; Hanchi, B.; Hosni, K.; Luis, J. Cucurbitacin B Purified from Ecballium elaterium (L.) A. Rich from Tunisia Inhibits A5β1 Integrin-Mediated Adhesion, Migration, Proliferation of Human Glioblastoma Cell Line and Angiogenesis. Eur. J. Pharmacol. 2017, 797, 153–161. [Google Scholar] [CrossRef]
- Jacquot, C.; Rousseau, B.; Carbonnelle, D.; Chinou, I.; Malleter, M.; Tomasoni, C.; Roussakis, C. Cucurbitacin-D-Induced CDK1 MRNA Up Regulation Causes Proliferation Arrest of a Non-Small Cell Lung Carcinoma Cell Line (NSCLC-N6). Anticancer Res. 2014, 34, 4797–4806. [Google Scholar]
- Jafargholizadeh, N.; Zargar, S.J.; Aftabi, Y. The Cucurbitacins D, E, and I from Ecballium elaterium (L.) Upregulate the LC3 Gene and Induce Cell-Cycle Arrest in Human Gastric Cancer Cell Line AGS. Iran J. Basic Med. Sci. 2018, 21, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Seger, C.; Sturm, S.; Haslinger, E.; Stuppner, H. NMR Signal Assignment of 22-Deoxocucurbitacin D and Cucurbitacin D from Ecballium elaterium L. (Cucurbitaceae). Monatshefte Chem. 2005, 136, 1645–1649. [Google Scholar] [CrossRef]
- Üremiş, M.M.; Türköz, Y.; Üremiş, N. Investigation of Apoptotic Effects of Cucurbitacin D, I, and E Mediated by Bax/Bcl-XL, Caspase-3/9, and Oxidative Stress Modulators in HepG2 Cell Line. Drug Dev. Res. 2024, 85, e22174. [Google Scholar] [CrossRef]
- Arel-Dubeau, A.M.; Longpré, F.; Bournival, J.; Tremblay, C.; Demers-Lamarche, J.; Haskova, P.; Attard, E.; Germain, M.; Martinoli, M.G. Cucurbitacin E has Neuroprotective Properties and Autophagic Modulating Activities on Dopaminergic Neurons. Oxid. Med. Cell. Longev. 2014, 2014, 425496. [Google Scholar] [CrossRef]
- Attard, E.; Cuschieri, A.; Scicluna-Spiteri, A.; Brincat, M.P. The Effects of Cucurbitacin E on Two Lymphocyte Models. Pharm. Biol. 2004, 42, 170–175. [Google Scholar] [CrossRef]
- Attard, E.; Cuschieri, A. Cytotoxicity of Cucurbitacin E Extracted from Ecballium elaterium in vitro. J. Nat. Rem. 2004, 4, 137–144. [Google Scholar]
- Darwish, M.; Agha, M.I.H.; Barkil, S.; Mansour, O. Separating and Determining the Effective Compounds Percentage in Ecballium elaterium L. Cucurbitacins Syria. Res. J. Pharm. Technol. 2015, 8, 829. [Google Scholar] [CrossRef]
- Üremiş, N.; Üremiş, M.M.; Çiğremiş, Y.; Tosun, E.; Baysar, A.; Türköz, Y. Cucurbitacin I Exhibits Anticancer Efficacy through Induction of Apoptosis and Modulation of JAK/STAT3, MAPK/ERK, and AKT/MTOR Signaling Pathways in HepG2 Cell Line. J. Food Biochem. 2022, 46, e14333. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, K.; Karakuş, F.; Eyol, E.; Yılmaz, İ.; Ünüvar, S. Cytotoxic Effects of Cucurbitacin I and Ecballium elaterium on Breast Cancer Cells. Nat. Prod. Commun. 2018, 13, 1445–1448. [Google Scholar] [CrossRef]
- Yılmaz, B.; Deniz, İ. The Effects of Cultivation Area and Altitude Variation on the Composition of Essential Oil of Laurus nobilis l. Grown in Eastern, Western and Central Karadeniz Region. Int. J. Second Metab. 2017, 4, 187–195. [Google Scholar] [CrossRef][Green Version]
- Demir, M.; Taylan, M.; Kaya, H.; Ekinci, A.; Arslan, D.; Aslan, E.; Keles, A.; Yılmaz, S.; Sezgi, C. Histopathological and Biochemical Effects of Ecballium elaterium on Sepsis-Induced Lung Injury. J. Investig. Surg. 2016, 29, 302–308. [Google Scholar] [CrossRef]
- Koca, U.; Özçelik, B.; Özgen, S. Comparative in vitro Activity of Medicinal Plants Arnebia densiflora and Ecballium elaterium Against Isolated Strains of Klebsiella pneumoniae. Turk. J. Pharm. Sci. 2010, 7, 197–204. [Google Scholar]
- Elkhateeb, H.A.; Mabrouk, M.I.; Othman, A.S.; Ibrahim, M.K. Macro and Nano Ecballium elaterium in vitro Activity against Multidrug-Resistant Salmonella typhi with Genetic Detection and Sequencing. J. Microbiol. Biotechnol. Food Sci. 2024, 13, e10067. [Google Scholar] [CrossRef]
- Adwan, G.; Salameh, Y.; Adwan, K. Effect of Ethanolic Extract of Ecballium elaterium against Staphylococcus aureus and Candida albicans. Asian Pac. J. Trop. Biomed. 2011, 1, 456–460. [Google Scholar] [CrossRef]
- Felhi, S.; Saoudi, M.; Daoud, A.; Hajlaoui, H.; Ncir, M.; Chaabane, R.; El Feki, A.; Gharsallah, N.; Kadri, A. Investigation of Phytochemical Contents, in vitro Antioxidant and Antibacterial Behavior and in vivo Anti-Inflammatory Potential of Ecballium elaterium Methanol Fruits Extract. Food Sci. Tech-Brazinol. 2017, 37, 558–563. [Google Scholar] [CrossRef]
- Heysieattalab, S.; Sadeghi, L. Ecballium elaterium Attenuates Neuroinflammation in an Animal Model of Alzheimer’s Disease through Modulation of Nuclear Factor ΚB Pathway. Avicenna J. Phytomed. 2022, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Eti, C.M.; Vayisoglu, Y.; Kardas, B.; Arpaci, R.B.; Horasan, E.S.; Kanik, A.; Eti, N.; Yalin, S.; Talas, D.U. Histopathologic evaluation of Ecballium elaterium applied nasal mucosa in rat rhinosinusitis model. Ear Nose Throat J. 2018, 97, 14–17. [Google Scholar] [CrossRef]
- Arslan, D.; Ekinci, A.; Arici, A.; Bozdemir, E.; Akil, E.; Ozdemir, H.H. Effects of Ecballium elaterium on Brain in a Rat Model of Sepsis-Associated Encephalopathy. Libyan J. Med. Sci. 2017, 12. [Google Scholar] [CrossRef]
- Ghanim, M.; Amer, J.; Salhab, A.; Jaradat, N. Ecballium elaterium Improved Stimulatory Effects of Tissue-Resident NK Cells and Ameliorated Liver Fibrosis in a Thioacetamide Mice Model. Biomed. Pharmacother. 2022, 150, 112942. [Google Scholar] [CrossRef]
- İbiloglu, İ.; Alabalik, U.; Keles, A.N.; Aydogdu, G.; Basuguy, E.; Buyukbayram, H. Ecballium elaterium Extract Reduces Fibrosis during Wound Healing in Rats. Biotechnol. Biotechnol. Equip. 2021, 35, 696–703. [Google Scholar] [CrossRef]
- Uslu, C.; Karasen, R.M.; Sahin, F.; Taysi, S.; Akcay, F. Effect of Aqueous Extracts of Ecballium elaterium Rich, in the Rabbit Model of Rhinosinusitis. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, P.; Ghalbi, Z.; Sarvari, Y.; Delazar, A.; Bamdad, S.; Asnaashari, S. Phytochemical and Antimalarial Effects of Ecballium elaterium (L.) Rich. Growing in Iran. Jundishapur J. Nat. Pharm. Prod. 2021, 16. [Google Scholar] [CrossRef]
- Kianbakht, S.; Ziaee, M.; Momtaz, M.; Hajiaghaee, R. A Toxicological and Phytochemical Study on the Iran’s Ecballium elaterium (L.) A. Rich. J. Med. Plants 2019, 18, 67–76. [Google Scholar] [CrossRef]
- Bohlooli, S.; Jafari, N.; Jahed, S. Cytotoxic Effect of Freeze-Dried Extract of Ecballium elaterium Fruit on Gastric Adenocarcinoma (AGS) and Esophageal Squamous Cell Carcinoma (KYSE30) Cell Lines. J. Gastrointest. Cancer 2012, 43, 579–583. [Google Scholar] [CrossRef]
- Rencüzoğullari, E.; İla, H.B.; Kayraldiz, A.; Diler, S.B.; Yavuz, A.; Arslan, M.; Funda Kaya, F.; Topaktas, M. The Mutagenic and Antimutagenic Effects of Ecballium elaterium Fruit Juice in Human Peripheral Lymphocytes. Russ. J. Genet. 2006, 42, 623–627. [Google Scholar] [CrossRef]
- Çelik, T.A.; Aslantürk, Ö.S. Investigation of Cytotoxic and Genotoxic Effects of Ecballium elaterium Juice Based on Allium Test. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.H.; Aydogdu, B.; Arslan, M.S.; Alabalik, U.; Arslan, S.; Kara, İ.; Canpolat, F.; Şahin, A.; Otcu, S. Intra-Peritoneal Administration of Ecballium elaterium Diminishes Postoperative Adhesions. Acta Cir. Bras. 2014, 29, 639–643. [Google Scholar] [CrossRef] [PubMed]
- El Naggar, E.M.B.; Chalupová, M.; Pražanová, G.; Parák, T.; Švajdlenka, E.; Žemlička, M.; Suchý, P. Hepatoprotective and Proapoptotic Effect of Ecballium elaterium on CCl4-Induced Hepatotoxicity in Rats. Asian Pac. J. Trop. Med. 2015, 8, 526–531. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anzano, A.; Falco, B.d.; Grauso, L.; Lanzotti, V. Squirting Cucumber, Ecballium elaterium (L.) A. Ritch: An Update of Its Chemical and Pharmacological Profile. Molecules 2024, 29, 4377. https://doi.org/10.3390/molecules29184377
Anzano A, Falco Bd, Grauso L, Lanzotti V. Squirting Cucumber, Ecballium elaterium (L.) A. Ritch: An Update of Its Chemical and Pharmacological Profile. Molecules. 2024; 29(18):4377. https://doi.org/10.3390/molecules29184377
Chicago/Turabian StyleAnzano, Attilio, Bruna de Falco, Laura Grauso, and Virginia Lanzotti. 2024. "Squirting Cucumber, Ecballium elaterium (L.) A. Ritch: An Update of Its Chemical and Pharmacological Profile" Molecules 29, no. 18: 4377. https://doi.org/10.3390/molecules29184377
APA StyleAnzano, A., Falco, B. d., Grauso, L., & Lanzotti, V. (2024). Squirting Cucumber, Ecballium elaterium (L.) A. Ritch: An Update of Its Chemical and Pharmacological Profile. Molecules, 29(18), 4377. https://doi.org/10.3390/molecules29184377






