A Silver Modified Nanosheet Self-Assembled Hollow Microsphere with Enhanced Conductivity and Permeability
Abstract
1. Introduction
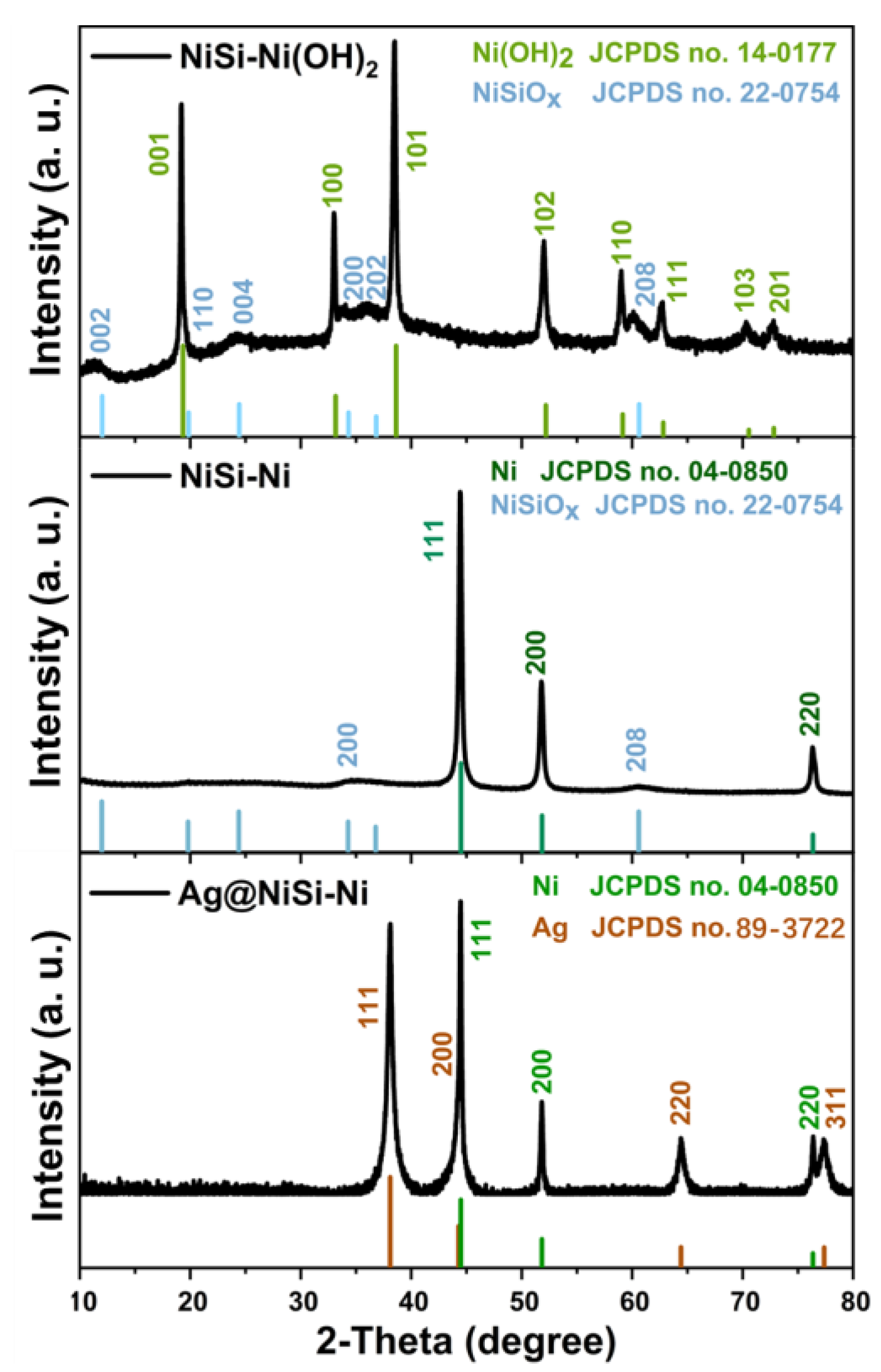
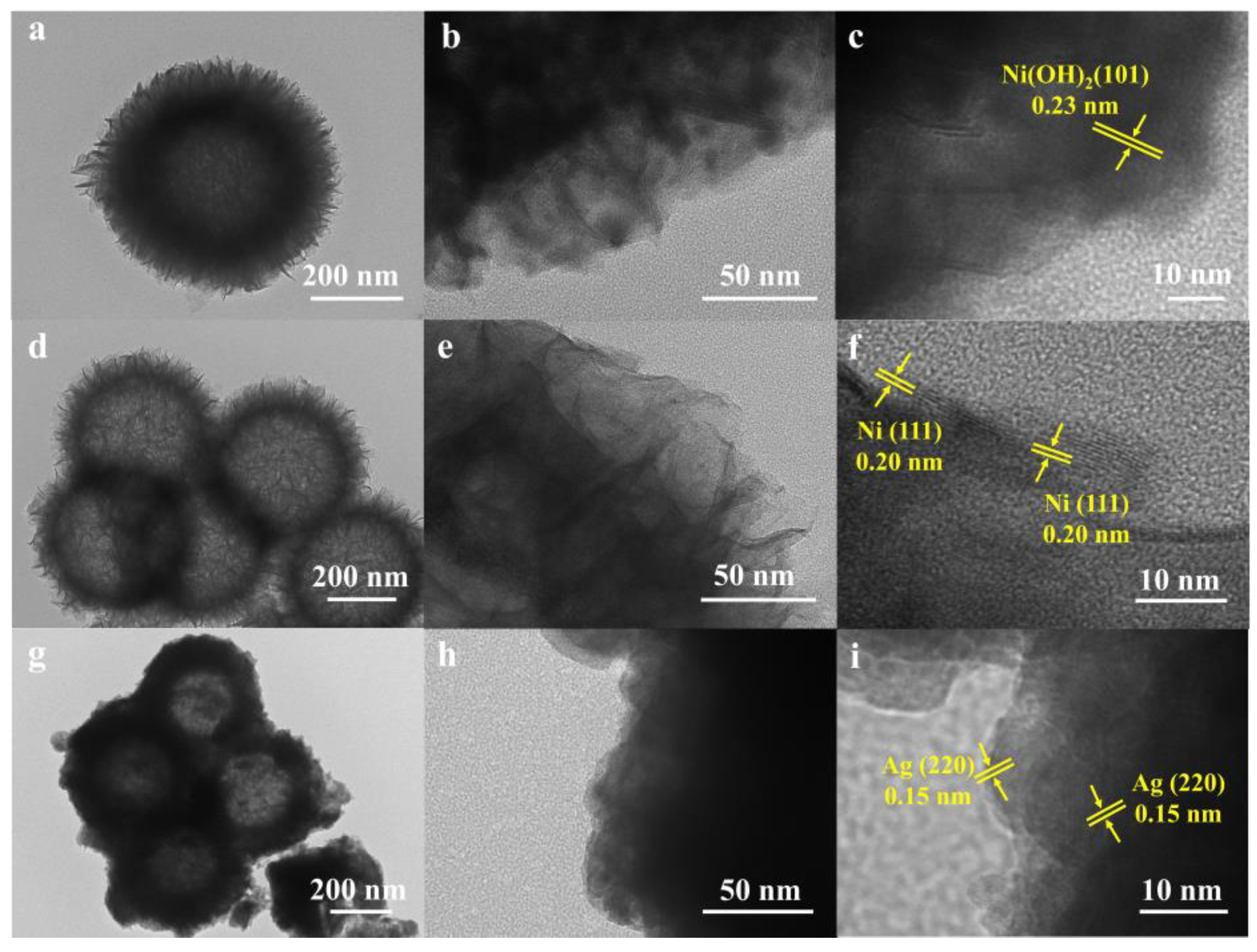
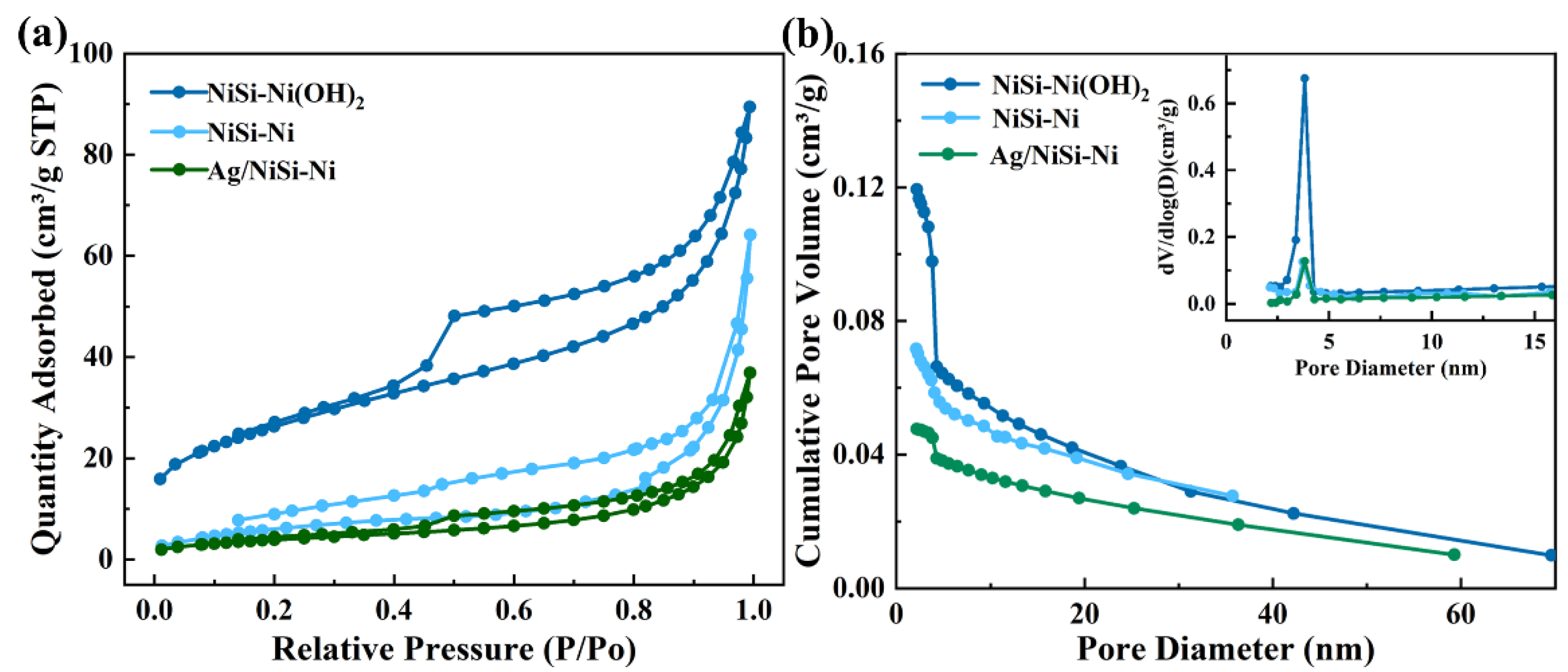
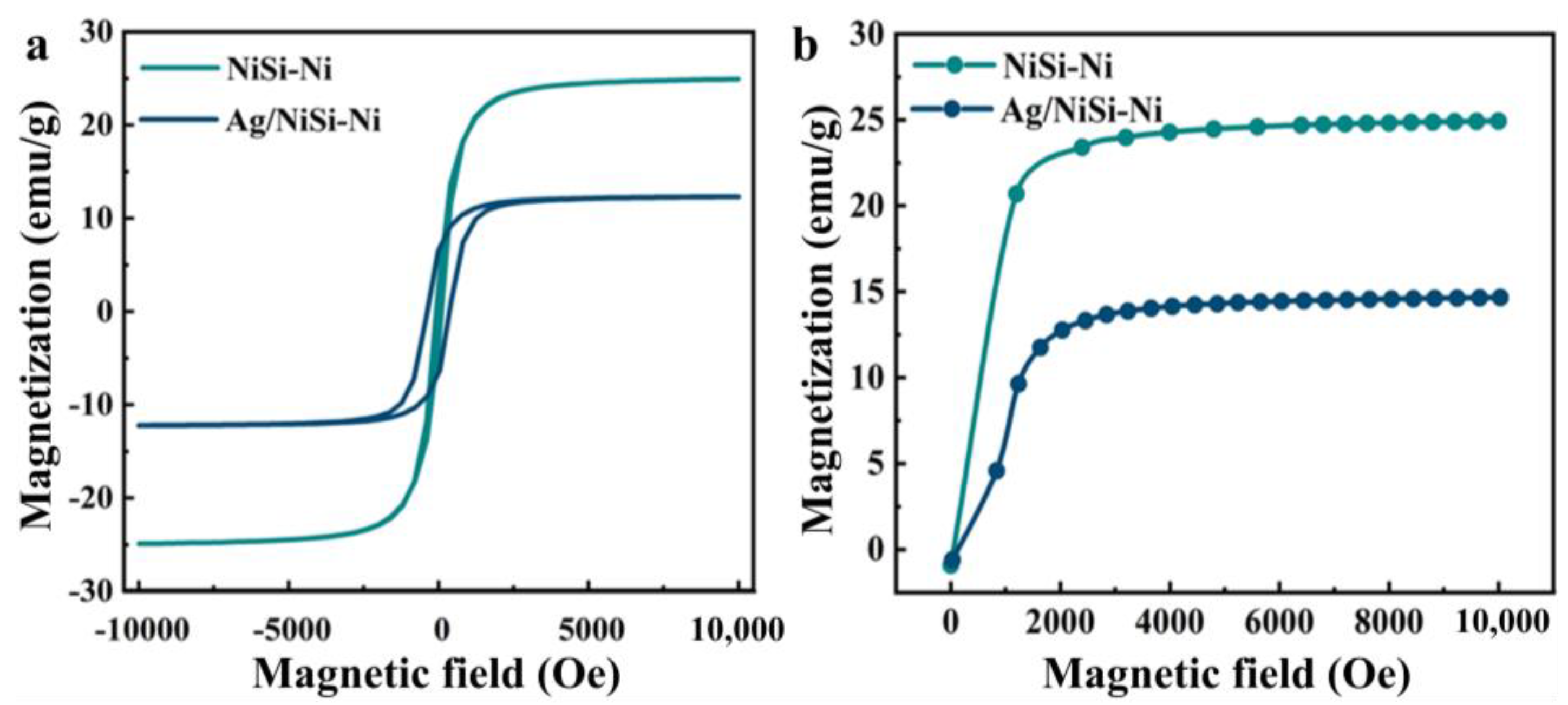
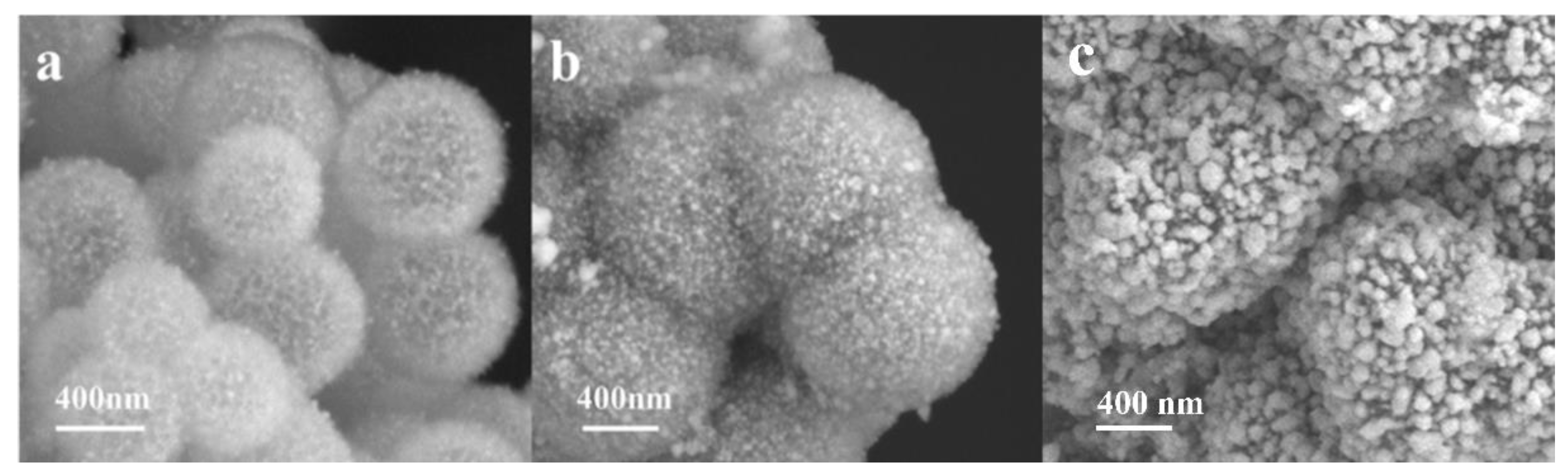
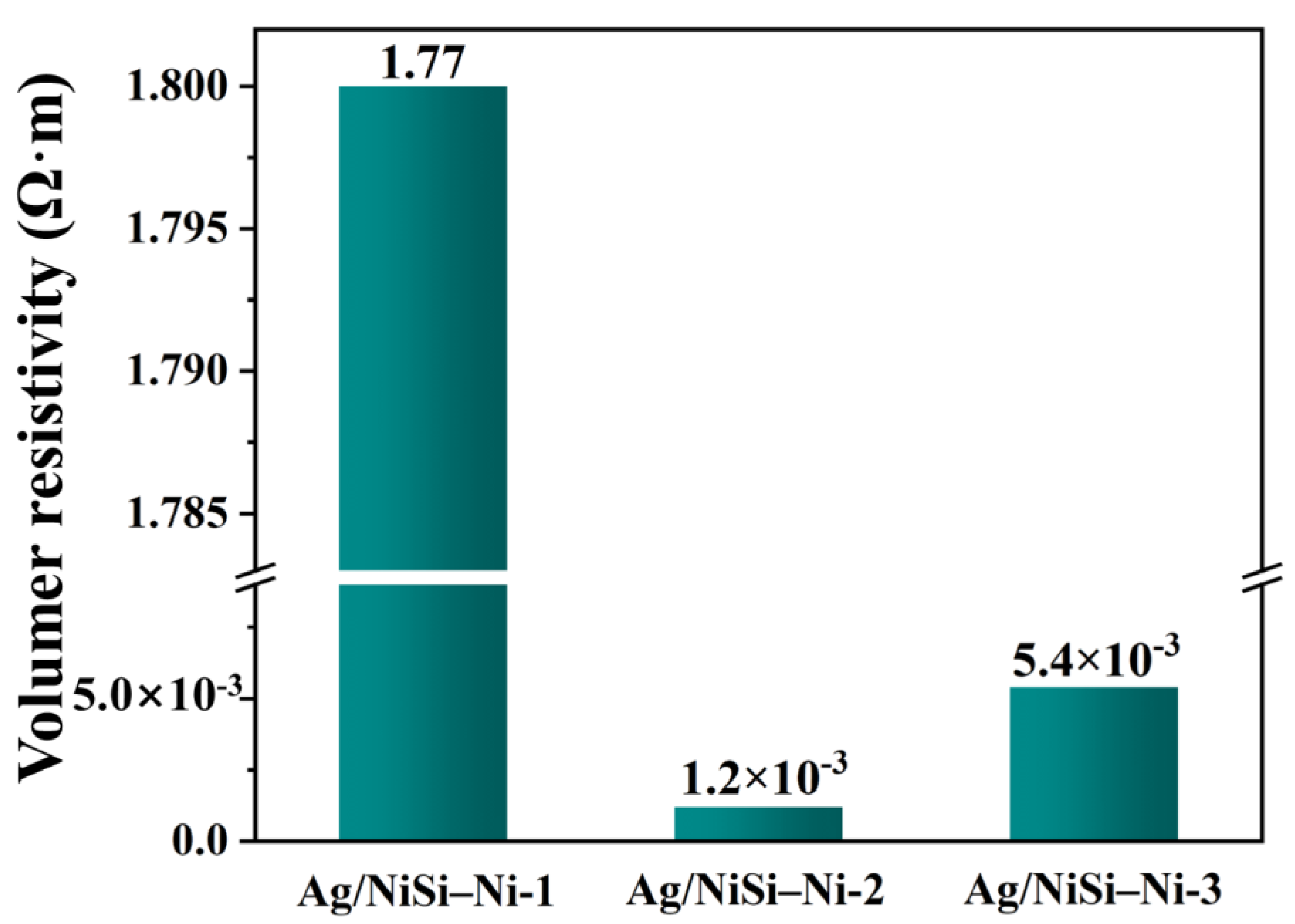
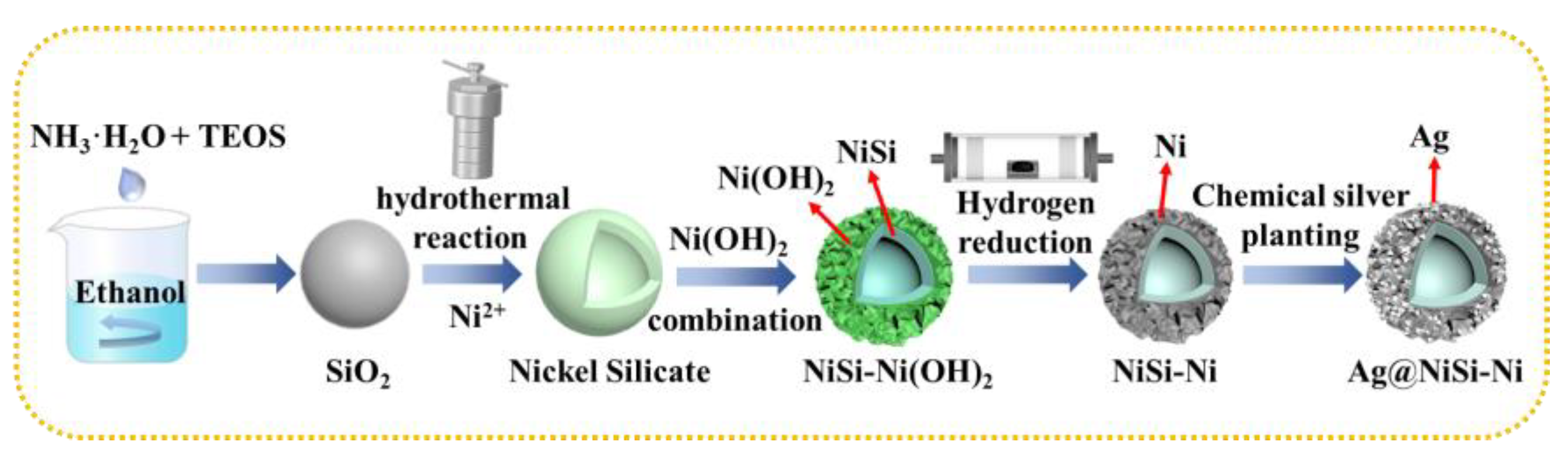
2. Results and Discussion
3. Materials and Methods
3.1. Material Preparation
3.1.1. Synthesis of NiSi-Ni
3.1.2. Synthesis of Ag/NiSi-Ni
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, M.K.; Chiu, R.; He, E.R.; Chen, J.Y.; Chu, F.; Tsai, J.; Wang, Y.P.; Jian, S.Y.; Chen, S.M. Innovative EMI Shielding Solutions on Advanced SiP Module for 5G Application. In Proceedings of the Electronics Packaging Technology Conference, Singapore, 4–6 December 2019; pp. 601–607. [Google Scholar]
- Zeng, S.; Huang, Z.X.; Jiang, H.; Li, Y. From Waste to Wealth: A Lightweight and Flexible Leather Solid Waste/Polyvinyl Alcohol/Silver Paper for Highly Efficient Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2020, 12, 52038–52049. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tsai, M.; Chiu, R.; He, E.; Hsieh, A.; Tsai, M.F.; Chu, F.; Chen, J.Y.; Jian, S.; Chen, S.; et al. EMI Shielding Technology in 5G RF System in Package Module. In Proceedings of the 2020 IEEE 70th Electronic Components and Technology Conference, Orlando, FL, USA, 3–30 June 2020; pp. 931–937. [Google Scholar]
- Pande, S.; Singh, B.; Mathur, R.; Dhami, T.; Saini, P.; Dhawan, S. Improved Electromagnetic Interference Shielding Properties of MWCNT-PMMA Composites Using Layered Structures. Nanoscale Res. Lett. 2009, 4, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, N.; Ma, Y.; Du, F.; Li, F.; He, X.; Lin, X.; Gao, H.; Chen, Y. The influence of single-walled carbon nanotube structure on the electromagnetic interference shielding efficiency of its epoxy composites. Carbon 2007, 45, 1614–1621. [Google Scholar] [CrossRef]
- Wang, C.; Murugadoss, V.; Kong, J.; He, Z.; Mai, X.; Shao, Q.; Chen, Y.; Guo, L.; Liu, C.; Angaiah, S.; et al. Overview of carbon nanostructures and nanocomposites for electromagnetic wave shielding. Carbon 2018, 140, 696–733. [Google Scholar] [CrossRef]
- Wen, B.; Cao, M.S.; Lu, M.M.; Cao, W.Q.; Shi, H.L.; Liu, J.; Wang, X.X.; Jin, H.B.; Fang, X.Y.; Wang, W.Z.; et al. Reduced Graphene Oxides: Light-Weight and High-Efficiency Electromagnetic Interference Shielding at Elevated Temperatures. Adv. Mater. 2014, 26, 3484–3489. [Google Scholar] [CrossRef]
- Cao, M.S.; Wang, X.X.; Cao, W.Q.; Yuan, J. Ultrathin graphene: Electrical properties and highly efficient electromagnetic interference shielding. J. Mater. Chem. C 2015, 3, 6589–6599. [Google Scholar] [CrossRef]
- Xu, H.L.; Yin, X.W.; Li, X.L.; Li, M.H.; Liang, S.; Zhang, L.T.; Cheng, L.F. Lightweight Ti2CTx MXene/Poly(vinyl alcohol) Composite Foams for Electromagnetic Wave Shielding with Absorption-Dominated Feature. ACS Appl. Mater. Interfaces 2019, 11, 10198–10207. [Google Scholar] [CrossRef]
- Ji, K.; Zhao, H.; Zhang, J.; Chen, J.; Dai, Z. Fabrication and electromagnetic interference shielding performance of open-cell foam of a Cu–Ni alloy integrated with CNTs. Appl. Surf. Sci. 2014, 311, 351–356. [Google Scholar] [CrossRef]
- Li, X.F.; Chang, Z.Z.; Zhang, Y.S.; Gao, G.X. Nickel and silver coated nano-SiO2 with excellent conductivity and permeability. Surf. Eng. 2014, 31, 427–432. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Manna, K. Recent advancements in the electromagnetic interference shielding performance of nanostructured materials and their nanocomposites: A review. J. Mater. Chem. A 2022, 10, 7431–7496. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; Jiang, Y.; Song, P.A.; Wang, H. Lightweight high performance carbon-polymer nanocomposites for electromagnetic interference shielding. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106376. [Google Scholar] [CrossRef]
- Yang, W.Q.; Yang, D.; Mei, H.; Yao, L.; Xiao, S.S.; Yao, Y.T.; Chen, C.; Cheng, L.F. 3D printing of PDC-SiOC@SiC twins with high permittivity and electromagnetic interference shielding effectiveness. J. Eur. Ceram. Soc. 2021, 41, 5437–5444. [Google Scholar] [CrossRef]
- Panigrahi, R.; Srivastava, S.K. Tollen’s reagent assisted synthesis of hollow polyaniline microsphere/Ag nanocomposite and its applications in sugar sensing and electromagnetic shielding. Mater. Res. Bull. 2015, 64, 33–41. [Google Scholar] [CrossRef]
- Goyal, R.K.; Sulakhe, R. Study on poly (vinylidene fluoride)/nickel composites with low percolation. Adv. Mater. Lett. 2015, 6, 309–317. [Google Scholar] [CrossRef]
- Wang, G.L.; Wang, L.; Mark, L.H.; Shaayegan, V.; Wang, G.Z.; Li, H.P.; Zhao, G.Q.; Park, C.B. Ultralow threshold and lightweight biodegradable porous PLA/MWCNT with segregated conductive networks for high performance thermal insulation and electromagnetic interference shielding applications. ACS Appl. Mater. Interfaces 2018, 10, 1195–1203. [Google Scholar] [CrossRef]
- Feng, D.; Wang, Q.Q.; Xu, D.W.; Liu, P.J. Microwave assisted sinter molding of polyetherimide/carbon nanotubes composites with segregated structure for high-performance EMI shielding applications. Compos. Sci. Technol. 2019, 182, 107753. [Google Scholar] [CrossRef]
- Shahzad, F.; Lee, S.H.; Hong, S.M.; Koo, C.M. Segregated reduced graphene oxide polymer composite as a high performance electromagnetic interference shield. Res. Chem. Intermed. 2018, 44, 4707–4719. [Google Scholar] [CrossRef]
- Prashantha, K.; Roger, F. Multifunctional properties of 3D printed poly (lactic acid)/graphene nanocomposites by fused deposition modeling. J. Macromol. Sci. Part A 2017, 54, 24–29. [Google Scholar] [CrossRef]
- Mai, T.; Chen, L.; Wang, P.L.; Liu, Q.; Ma, M.G. Hollow Metal-Organic Framework/MXene/Nanocellulose Composite Films for Giga/Terahertz Electromagnetic Shielding and Photothermal Conversion. Nano-Micro Lett. 2024, 16, 169. [Google Scholar] [CrossRef]
- Chen, Y.M.; Pang, L.; Li, Y.; Luo, H.; Duan, G.G.; Mei, C.T.; Xu, W.H.; Zhou, W.; Liu, K.M.; Jiang, S.H. Ultra-thin and highly flexible cellulose nanofiber/silver nanowire conductive paper for effective electromagnetic interference shielding. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105960. [Google Scholar] [CrossRef]
- Wong, Y.J.; Zhu, L.F.; Teo, W.S.; Tan, Y.W.; Yang, Y.H.; Wang, C.; Chen, H.Y. Revisiting the Stober Method: In homogeneity in Silica Shells. J. Am. Chem. Soc. 2011, 133, 11422–11425. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Mou, C.Y.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Xiao, J.; Jiang, H.; Li, X.; Meng, C. A novel ordered hollow spherical nickel silicate–nickel hydroxide composite with two types of morphologies for enhanced electrochemical storage performance. Mater. Chem. Front. 2019, 3, 2090–2101. [Google Scholar] [CrossRef]
- Kumar, P.; Shahzad, F.; Yu, S.; Hong, S.M.; Kim, Y.H.; Koo, C.M. Large-area reduced graphene oxide thin film with excellent thermal conductivity and electromagnetic interference shielding effectiveness. Carbon 2015, 94, 494–500. [Google Scholar] [CrossRef]
- Hu, P.Y.; Lyu, J.; Fu, C.; Gong, W.B.; Liao, J.H.; Lu, W.B.; Chen, Y.P.; Zhang, X.T. Multifunctional Aramid Nanofiber/Carbon Nanotube Hybrid Aerogel Films. ACS Nano 2020, 14, 688–697. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.K.; Imakita, K.; Fujii, M. Evidence of oxygen vacancy induced room temperature ferromagnetism in solvothermally synthesized undoped TiO2 nanoribbons. Nanoscale 2013, 5, 5476–5488. [Google Scholar] [CrossRef]
- Miao, B.F.; Huang, S.Y.; Qu, D.; Chien, C.L. Inverse Spin Hall Effect in a Ferromagnetic Metal. Phys. Rev. Lett. 2013, 111, 066602. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Treutmann, W.; Untersteller, E. Anisotropic spin glass pseudobrookite: Evidence for transverse freezing and possible implications. Phys. Rev. B 2003, 68, 144404. [Google Scholar] [CrossRef]
- Li, J.; Lu, J.; Wang, Y.; Wang, T.; Xiao, J. Research development of conductive inks and nanoparticles applied in conductive inks. Electron. Compon. Mater. 2014, 33, 12–16. [Google Scholar]
- Rasouli, T.; Pourabdoli, M.; Lashgari, V.A.; Hamidi, A.G. Characterization of silver-coated copper particles synthesized by mechanical activation and electroless plating. Transit. Met. Chem. 2024, 6, 1–12. [Google Scholar] [CrossRef]
- Manikam, V.R.; Razak, K.A.; Cheong, K.Y. Physical and electrical attributes of sintered Ag80-Al20 high temperature die attach material with different organic additives content. J. Mater. Sci. Mater. Electron. 2013, 24, 720–733. [Google Scholar] [CrossRef]
- Hao, T.R.; Xu, H.L.; Sun, S.P.; Yu, H.; Qin, Q.Q.; Song, B.; Li, M.L.; Shao, G.; Fan, B.B.; Wang, H.L.; et al. Assembling flower-like MgAl-LDH nanospheres and g-C3N4 nanosheets for high efficiency removal of methyl orange. Ceram. Int. 2024, 50, 10724–10734. [Google Scholar] [CrossRef]
- Han, Y.; Chao, M.; Luo, C.J.; Yan, L. Self-assembled B-doped flower-like graphitic carbon nitride with high specific surface area for enhanced photocatalytic performance. J. Colloid Interface Sci. 2024, 657, 309–319. [Google Scholar] [CrossRef]
- Sharma, V.; Manna, K.; Srivastava, S.K.; Chandra, A. Hollow nanostructures of metal oxides as efficient absorbers for electromagnetic interference shielding. J. Phys. D Appl. Phys. 2019, 52, 015301. [Google Scholar] [CrossRef]
- Petryshynets, I.; Kovac, F.; Stoyka, V.; Boruta, J. Influence of microstructure evolution on the coercive forces in low silicon non-oriented steels. Acta Phys. Pol. A 2010, 118, 1013–1014. [Google Scholar] [CrossRef]
- Gopkalo, O.; Bezlyudko, G.; Nekhotiashchiy, V.; Gopkalo, O.; Kurash, Y. Damage Evaluation for AISI 304 Steel Under Cyclic Loading Based On Co-ercive Force Measurements. Int. J. Fatigue 2020, 139, 105752. [Google Scholar] [CrossRef]
- Chang, C.H.T.; Kuo, W.H.; Chang, Y.C.; Tsay, J.S.; Yau, S.L. Tuning coercive force by adjusting electric potential in solution processed Co/Pt(111) and the mechanism involved. Sci. Rep. 2017, 7, 43700. [Google Scholar] [CrossRef]
- Kato, T.; Limsuwan, N.; Yu, C.Y.; Akatsu, K.; Lorenz, R.D. Rare earth reduction using a novel variable magnetomotive force flux-Intensified IPM machine. IEEE Trans. Ind. Appl. 2014, 50, 1748–1756. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.Y.; Chen, Y.J.; Xiong, C.Y.; He, G.W.; Cao, Y.; Wu, H.; Guiver, M.D.; Jiang, Z.Y. Constructing efficient ion nanochannels in alkaline anion exchange membranes by in-situ assembly of poly (ionic liquid) in metal-organic frameworks. J. Mater. Chem. A 2016, 4, 2340–2348. [Google Scholar] [CrossRef]
- He, X.Y.; Gang, M.Y.; Li, Z.; He, G.W.; Yin, Y.H.; Cao, L.; Zhang, B.; Wu, H.; Jiang, Z.Y. Highly conductive and robust composite anion exchange membranes by incorporating quaternized MIL-101(Cr). Sci. Bull. 2017, 62, 266–276. [Google Scholar] [CrossRef]
- Yang, F.; Xu, G.; Dou, Y.B.; Wang, B.; Zhang, H.; Wu, H.; Zhou, W.; Li, J.R.; Chen, B.L. A flexible metal-organic framework with a high density of sulfonic acid sites for proton conduction. Nat. Energy 2017, 2, 877–883. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, Z.Q.; Ying, W.; Chen, L.P.; Liu, Y.Z.; Wang, X.B.; Jiang, Z.J.; Chen, B.L.; Peng, X.S. DNA-Threaded ZIF-8 Membrane with High Proton Conductivity and Low Methanol Permeability. Adv. Mater. 2018, 30, 1705155. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Dong, X.; Zhao, Y.; He, Z.; Song, W.; Li, C.; Li, J.; Huang, J.; Miao, Z. A Silver Modified Nanosheet Self-Assembled Hollow Microsphere with Enhanced Conductivity and Permeability. Molecules 2024, 29, 4384. https://doi.org/10.3390/molecules29184384
Wang F, Dong X, Zhao Y, He Z, Song W, Li C, Li J, Huang J, Miao Z. A Silver Modified Nanosheet Self-Assembled Hollow Microsphere with Enhanced Conductivity and Permeability. Molecules. 2024; 29(18):4384. https://doi.org/10.3390/molecules29184384
Chicago/Turabian StyleWang, Fangmin, Xue Dong, Yuzhen Zhao, Zemin He, Wenqi Song, Chunsheng Li, Jiayin Li, Jianfeng Huang, and Zongcheng Miao. 2024. "A Silver Modified Nanosheet Self-Assembled Hollow Microsphere with Enhanced Conductivity and Permeability" Molecules 29, no. 18: 4384. https://doi.org/10.3390/molecules29184384
APA StyleWang, F., Dong, X., Zhao, Y., He, Z., Song, W., Li, C., Li, J., Huang, J., & Miao, Z. (2024). A Silver Modified Nanosheet Self-Assembled Hollow Microsphere with Enhanced Conductivity and Permeability. Molecules, 29(18), 4384. https://doi.org/10.3390/molecules29184384





