Bioactivity-Guided Isolation of Antistroke Compounds from Gymnadenia conopsea (L.) R. Br.
Abstract
1. Introduction
2. Results
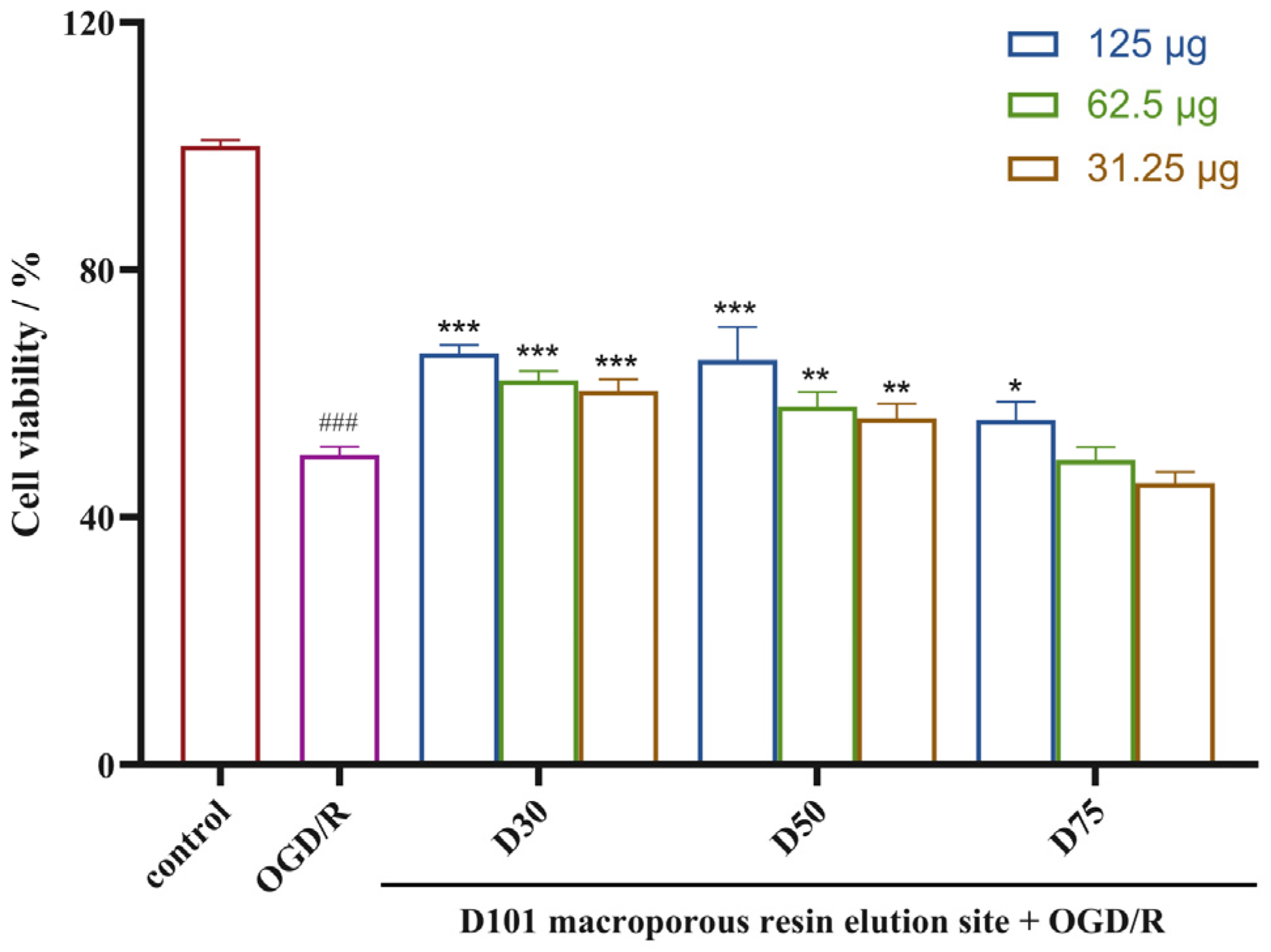
2.1. Identification of the Active Fractions
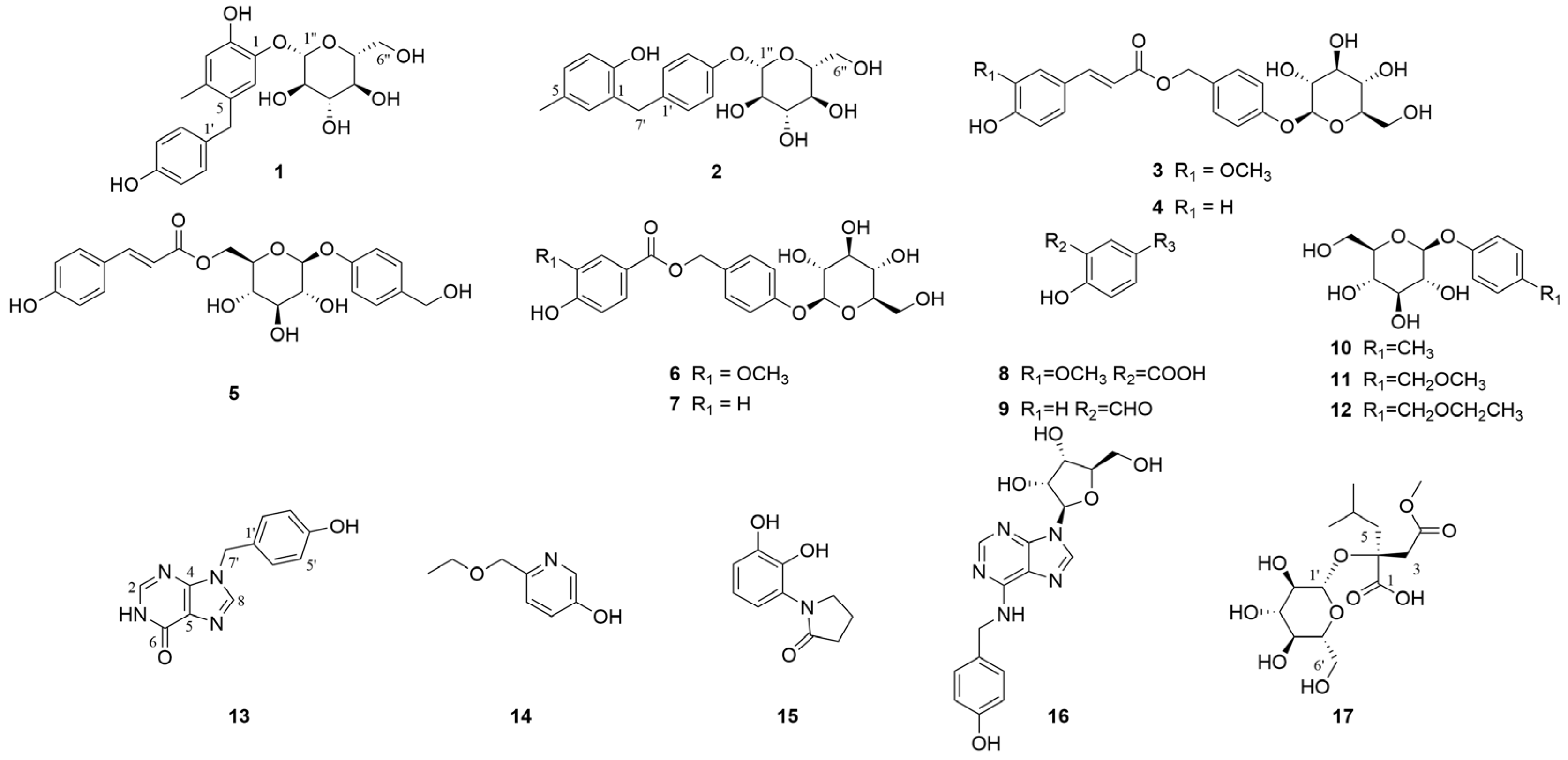
2.2. Identification of the Structures
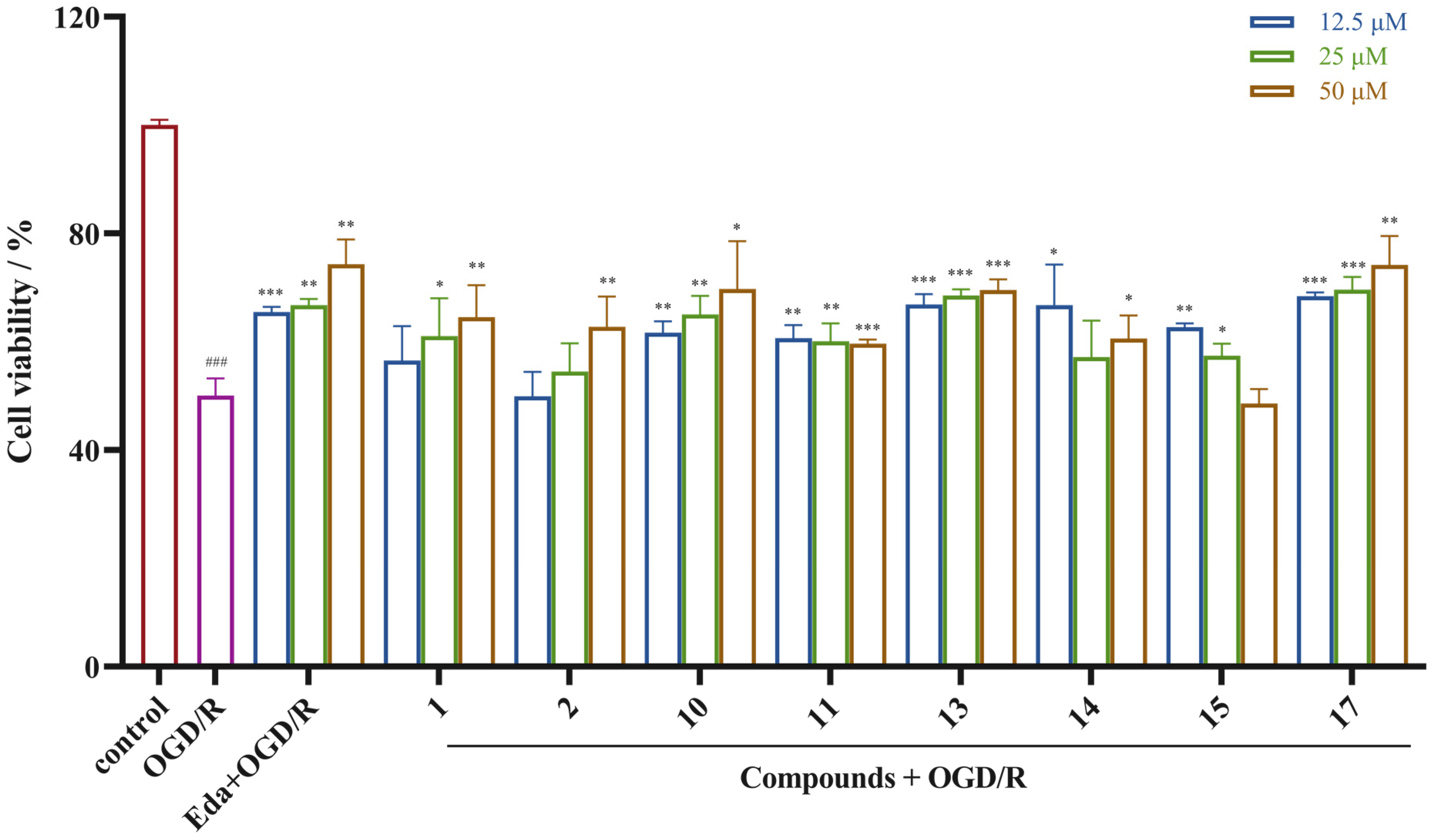
2.3. Neuroprotective Activity Results
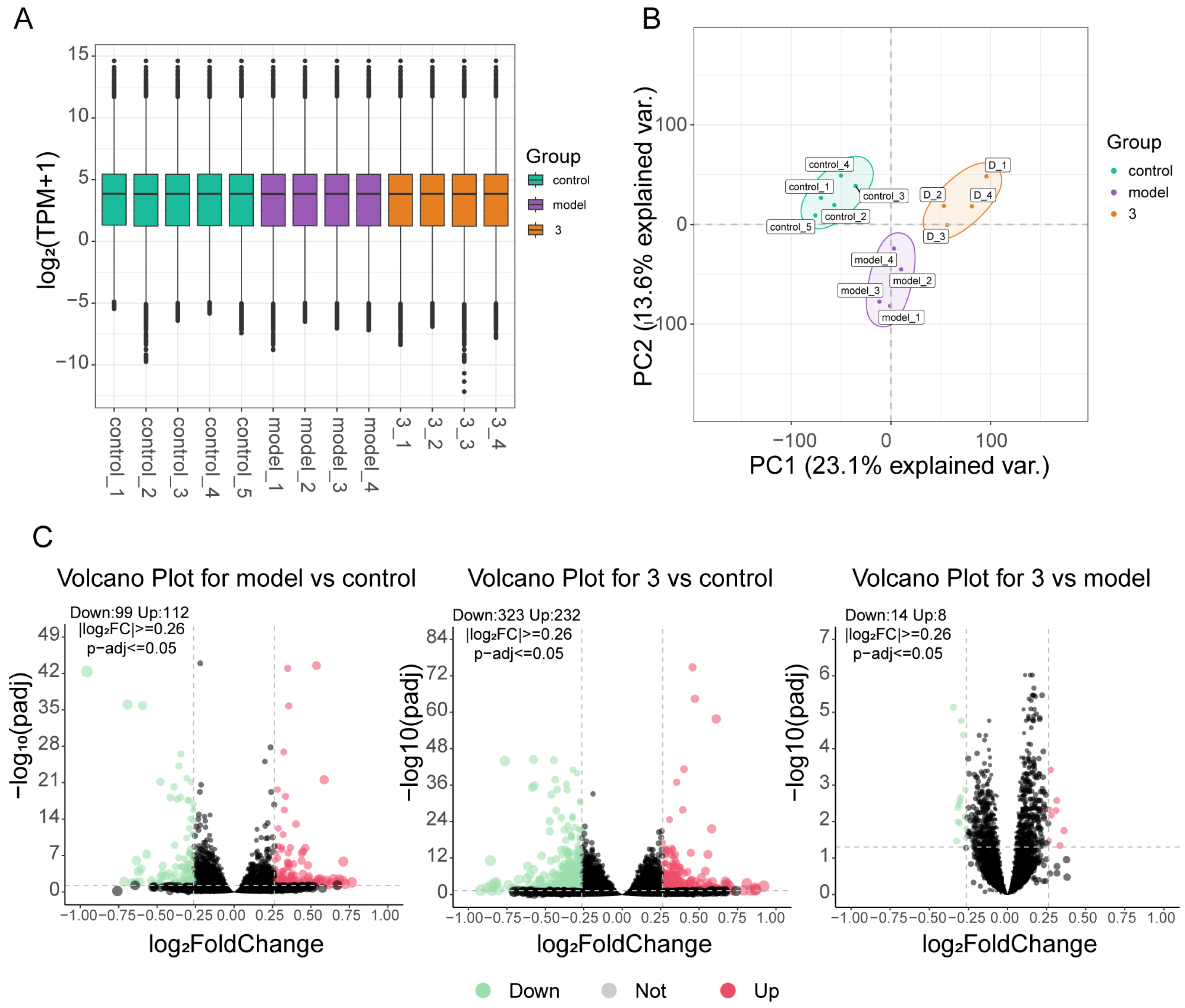
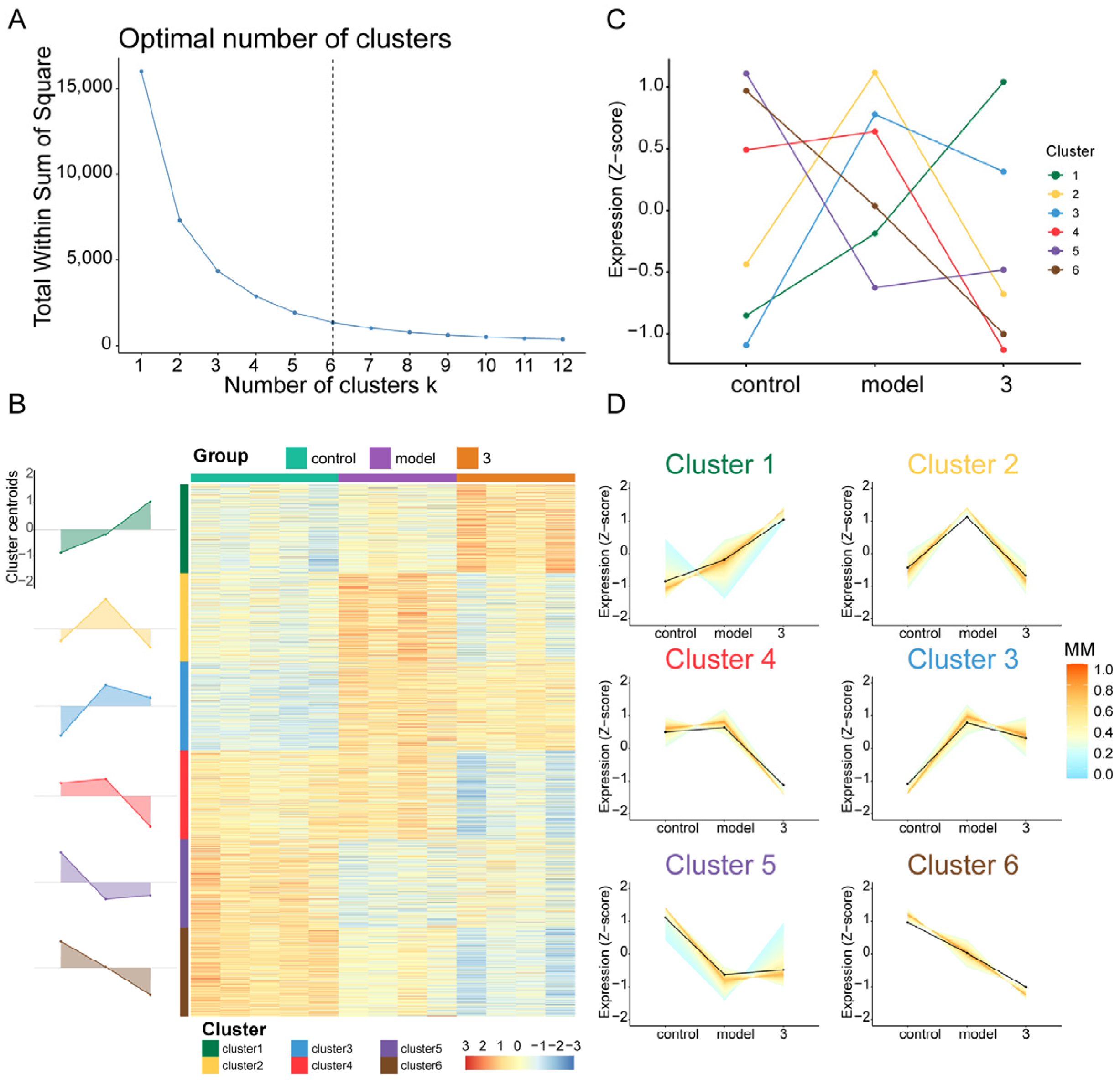
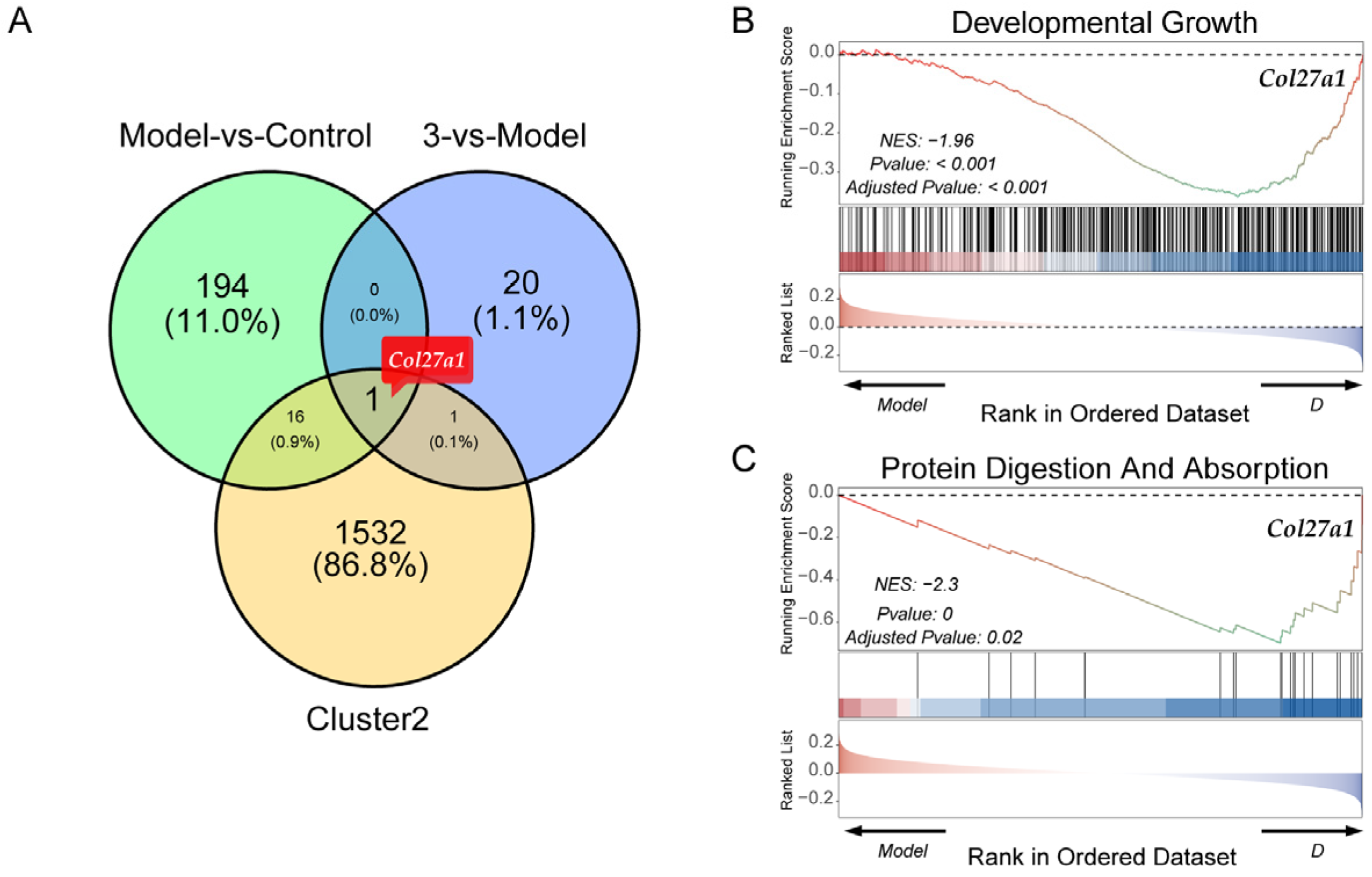
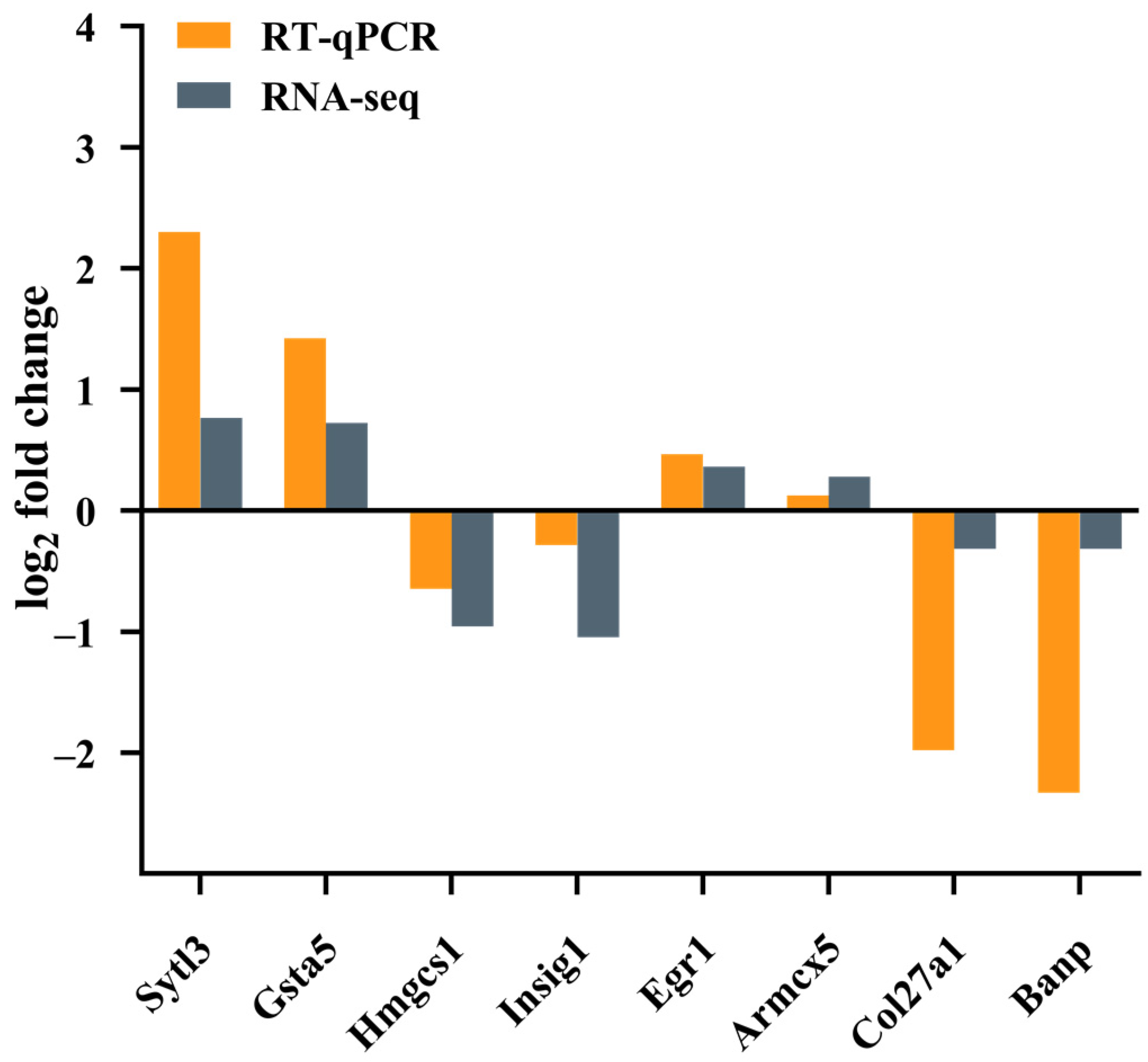
2.4. Transcriptomic and Bioinformatic Analyses and qPCR Experiments Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis
3.5. Computer Simulations
3.6. Neuroprotective Effect Evaluation
3.7. RNA Extraction, Library Preparation and Sequencing
3.8. Differential Expression Analysis
3.9. Soft Clustering Analysis
3.10. qPCR Assay
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shang, X.; Guo, X.; Liu, Y.; Pan, H.; Miao, X.; Zhang, J. Gymnadenia conopsea (L.) R. Br.: A systemic review of the ethnobotany, phytochemistry, and pharmacology of an important asian folk medicine. Front. Pharmacol. 2017, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, C.; Guo, S.; Yang, J.; Xiao, P. Advances in studies on chemical constituents and pharmacological activities for plants of Gymnadenia R. Br. Chin. Trad. Herb. Drugs. 2006, 8, 1264–1268. [Google Scholar]
- Wang, X.; Zhong, X.-J.; Zhou, N.; Cai, N.; Xu, J.-H.; Wang, Q.-B.; Li, J.-J.; Liu, Q.; Lin, P.-C.; Shang, X.-Y. Rapid characterizaiton of chemical constituents of the tubers of Gymnadenia conopsea by UPLC–Orbitrap–MS/MS analysis. Molecules 2020, 25, 898. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, S.; Wang, C.; Xiao, P. Studies on chemical constituents of tubers of Gymnadenia conopsea. Chin. Pharm. J. 2007, 22, 1696–1698. [Google Scholar]
- Li, M.; Guo, S.; Wang, C.; Yang, J.; Xiao, P. Chemical constituents of the tubers of Gymnadenia conopsea. Chin. Pharm. J. 2008, 6, 409–412. [Google Scholar]
- Morikawa, T.; Xie, H.; Matsuda, H.; Yoshikawa, M. Glucosyloxybenzyl 2-isobutylmalates from the tubers of Gymnadenia conopsea. J. Nat. Prod. 2006, 69, 881–886. [Google Scholar] [CrossRef]
- Yue, Z.; Zi, J.; Zhu, C.; Lin, S.; Yang, Y.; Shi, J. Constituents of Gymnadenia conopsea. China J. Chin. Mater. Med. 2010, 35, 2852–2861. [Google Scholar]
- Matsuda, H.; Morikawa, T.; Xie, H.; Yoshikawa, M. Antiallergic phenanthrenes and stilbenes from the tubers of Gymnadenia conopsea. Planta Med. 2004, 70, 847–855. [Google Scholar] [CrossRef]
- Lin, P.-C.; Yao, J.; Wu, J.; Tian, J.; Bao, Y.; Lin, S. A new ureido-substituted amino acid from the tubers of Gymnadenia conopsea. Chin. Chem. Lett. 2017, 28, 257–259. [Google Scholar] [CrossRef]
- Zi, J.-C.; Lin, S.; Zhu, C.-G.; Yang, Y.-C.; Shi, J.-G. Minor constituents from the tubers of Gymnadenia Conopsea. J. Asian Nat. Prod. Res. 2010, 12, 477–484. [Google Scholar] [CrossRef]
- Meng, X.-H.; Wang, M.; Lin, P.-C. Gymnadenia conopsea (L.) R. Br.: Comprehensive review of propagation and breeding, traditional uses, chemical composition, pharmacology, quality control, and processing. J. Ethnopharmacol. 2023, 306, 116205. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yang, Y.; Chen, S.; Tang, Z.; Xu, H. The processing methods, phytochemistry and pharmacology of Gastrodia elata Bl.: A comprehensive review. J. Ethnopharmacol. 2023, 314, 116467. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, A.; Lounkine, E.; Glick, M. Bioturbo similarity searching: Combining chemical and biological similarity to discover structurally diverse bioactive molecules. J. Chem. Inf. Model. 2013, 53, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Kozai, S.; Uchida, M. Synthesis of N-aryl uracils and hypoxanthines and their biological properties. Nucleos. Nucleot. 1999, 18, 661–671. [Google Scholar] [CrossRef]
- Kizu, H.; Kaneko, E.; Tomimori, T. Studies on nepalese crude drugs. XXVI. chemical constituents of Panch Aunle, the roots of Dactylorhiza hatagirea D. DON. Chem. Pharm. Bull. 1999, 47, 1618–1625. [Google Scholar] [CrossRef]
- Diao, W.; Chen, H.; Jiang, Z.; Li, X.; Yu, X.; Liu, T. One kind of gastrodin ferulate derivative and its preparation method and application. Chinese Patent CN104231013, 26 August 2014. [Google Scholar]
- Zidorn, C.; Ellmerer-Muller, E.P.; Stuppner, H. A germacranolide and three hydroxybenzyl alcohol derivatives from Hieracium murorum and Crepis bocconi. Phytochem. Anal. 2001, 12, 281–285. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Yuan, W.; Yuan, Q.-Y.; Li, X.-P.; Qin, B. A new biphenanthrene glucoside with cytotoxic activity from Cremastra appendiculata. Chem. Nat. Compd. 2017, 53, 211–214. [Google Scholar] [CrossRef]
- Ge, L.; Xie, Q.; Wei, X.; Li, Y.; Shen, W.; Hu, Y.; Yao, J.; Wang, S.; Du, X.; Zeng, X. Five undescribed plant-derived bisphenols from Artemisia capillaris aerial parts: Structure elucidation, anti-hepatoma activities and plausible biogenetic pathway. Arabian J. Chem. 2023, 16, 104580. [Google Scholar] [CrossRef]
- Yaguchi, Y.; Sakurai, N.; Nagai, M.; Inoue, T. Constituents of Myrica rubra. III. structures of two glycosides of myricanol. Chem. Pharm. Bull. 1988, 36, 1419–1424. [Google Scholar] [CrossRef]
- Zhou, Z.-Q.; Xiao, J.; Fan, H.-X.; Yu, Y.; He, R.-R.; Feng, X.-L.; Kurihara, H.; So, K.-F.; Yao, X.-S.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2017, 214, 644–654. [Google Scholar] [CrossRef]
- Do, K.M.; Nakashima, Y.; Kodama, T.; Lee, Y.; Nguyen, H.M.; Ikumi, N.; Morita, H. Phenolic derivatives with anti-acetylcholinesterase inhibitory activities from Galeola nudifolia in Vietnam. Chem. Biodivers. 2023, 20, e202301482. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, H.; Li, T.; Chen, X.; Wang, Z.; Yao, X.; Xiao, W.; Yu, Y. One undescribed glycoside benzofuran derivative and a new p-hydroxybenzoate glycoside from the leaves of Illicium dunnianum tutcher. Nat. Prod. Res. 2023, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-B.; Wu, Z.; Chen, F.-K.; Zou, L.-B. The protective effects of phenolic constituents from Gastrodia elata on the cytotoxicity induced by KCl and glutamate. Arch. Pharmacal Res. 2006, 29, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Wang, M.; Sun, C.; Li, X. Five new compounds from Hosta plantaginea flowers and their anti-inflammatory activities. Bioorganic Chem. 2020, 95, 103494. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, H.; Dong, C.; Lai, S.; Liu, J.; Chen, R.; Kang, J. Benzobicyclic ketones, cycloheptenone oxide derivatives, guaiane-type sesquiterpenes, and alkaloids isolated from Taraxacum mongolicum Hand.-Mazz. Phytochemistry 2022, 201, 113277. [Google Scholar] [CrossRef]
- Chen, M.; Lu, Y.; Zhou, M.; Wang, W.; Zheng, M.; Liu, C. The protection impact of tectoridin on PC12 cell preventing OGD/R-caused damage through PI3K/AKT signaling channel. Eur. J. Pharmacol. 2023, 941, 175491. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, X.; Wang, R.; Pang, H.; Wang, J.; Liu, L. Determining the chemical profile of Caragana jubata (Pall.) Poir. by UPLC–QTOF–MS analysis and evaluating its anti-ischemic stroke effects. J. Ethnopharmacol. 2023, 309, 116275. [Google Scholar] [CrossRef]
- Jenkins, E.; Moss, J.-B.; Pace, J.-M.; Bridgewater, L.-C. The new collagen gene COL27A1 contains SOX9-responsive enhancer ele-ments. Matrix Biol. 2005, 24, 177–184. [Google Scholar] [CrossRef]
- Sun, W.; Cornwell, A.; Li, J.; Peng, S.; Osorio, M.-J.; Aalling, N.; Wang, S.; Benraiss, A.; Lou, N.; Goldman, S.-A.; et al. SOX9 is an as-trocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J. Neurosci. 2017, 37, 4493–4507. [Google Scholar] [CrossRef]
- Chai, X.Y.; Song, Y.L.; Xu, Z.R.; Shi, H.M.; Bai, C.C.; Bi, D.; Wen, J.; Li, F.F.; Tu, P.F. Itosides J–N from Itoa orientalis and structure–anti-COX-2 activity relationship of phenolic glycosides. J. Nat. Prod. 2008, 71, 814–819. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Futschik, M.E. Mfuzz: A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5–7. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Xue, S.; Xu, C.; Jin, J.; Wang, J.; Yuan, H.; Liu, L. Bioactivity-Guided Isolation of Antistroke Compounds from Gymnadenia conopsea (L.) R. Br. Molecules 2024, 29, 4389. https://doi.org/10.3390/molecules29184389
Qin J, Xue S, Xu C, Jin J, Wang J, Yuan H, Liu L. Bioactivity-Guided Isolation of Antistroke Compounds from Gymnadenia conopsea (L.) R. Br. Molecules. 2024; 29(18):4389. https://doi.org/10.3390/molecules29184389
Chicago/Turabian StyleQin, Juan, Shiyi Xue, Chao Xu, Jian Jin, Jianbin Wang, Hailian Yuan, and Liang Liu. 2024. "Bioactivity-Guided Isolation of Antistroke Compounds from Gymnadenia conopsea (L.) R. Br." Molecules 29, no. 18: 4389. https://doi.org/10.3390/molecules29184389
APA StyleQin, J., Xue, S., Xu, C., Jin, J., Wang, J., Yuan, H., & Liu, L. (2024). Bioactivity-Guided Isolation of Antistroke Compounds from Gymnadenia conopsea (L.) R. Br. Molecules, 29(18), 4389. https://doi.org/10.3390/molecules29184389





