Application of Electrospun Nanofiber-Based Electrochemical Sensors in Food Safety
Abstract
1. Introduction
2. Electrochemical Sensors
3. Electrospinning
4. Application of Electrospun Nanofiber-Based Electrochemical Sensors in Food Safety
4.1. Heavy Metals
4.2. Pesticide Residues
4.3. Antibiotics
4.4. Food Additives
4.5. Microorganisms and Toxins
4.6. Food Freshness
4.7. Others
5. Conclusions and Outlook
5.1. Outlook
- Development of Advanced Nanofiber Materials: To enhance sensitivity, selectivity, and stability, novel nanofiber materials with improved performance should be developed. The synthesis of high-performance nanomaterials typically requires expertise, and the electrode surface preparation process can be complex and time-consuming. Additionally, the long-term stability of nanomaterials and receptors needs improvement, and there is a limited number of alternative receptors for sensitive and selective detection of specific analytes. Beyond new material development, advanced characterization techniques are crucial for understanding and enhancing the performance of nanofiber-based sensors. Addressing these issues is key to advancing the application of electrochemical sensors.
- Complexity of Food Matrices: Food matrices are complex, with food safety hazards often present at low concentrations compared to background substances such as proteins and cells. Freshness detection methods based on volatile spoilage markers are less applicable. The nutritional components and microbial contaminants differ among various types of meat and seafood, resulting in varied levels and compositions of spoilage markers. Moreover, non-specific adsorption of biofouling from sample matrices on the electrode surface reduces the signal-to-noise ratio, severely affecting sensor reliability and accuracy. Research into antifouling materials and antifouling electrode surfaces can mitigate biofouling issues and simplify sample pretreatment, thereby promoting the practical application of electrochemical sensors in real-world testing.
- On-Site Food Safety Hazard Detection: Rapid data processing and decision-making are necessary for on-site detection of food safety hazards. The application of nanofiber sensors in portable and field-deployable devices faces challenges, including device miniaturization, power management, and data processing. These sensors need to be integrated into micro platforms to enable real-time monitoring of contaminants. Simplifying detector operations to make systems user-friendly and suitable for portable devices is essential. Combining these sensors with big data platforms and machine learning can enhance data collection and processing capabilities. Machine learning algorithms can improve the detection capabilities of nanofiber sensors, enabling rapid and accurate identification of specific pollutants in environmental samples. Research should focus on developing microfluidic-based systems, energy-efficient power technologies, and data transmission solutions to promote their widespread use in environmental monitoring and toxin detection.
5.2. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, T.; Zheng, N.; Ma, Y.; Zhang, Y.; Lei, H.; Zhen, X.; Wang, Y.; Gou, D.; Zhao, J. Recent advancements in chitosan-based intelligent food freshness indicators: Categorization, advantages, and applications. Int. J. Biol. Macromol. 2024, 275, 133554. [Google Scholar]
- Yu, Z.; Boyarkina, V.; Liao, Z.; Lin, M.; Zeng, W.; Lu, X. Boosting food system sustainability through intelligent packaging: Application of biodegradable freshness indicators. ACS Food Sci. Technol. 2023, 3, 199–212. [Google Scholar]
- Mukherjee, A.G.; Renu, K.; Gopalakrishnan, A.V.; Veeraraghavan, V.P.; Vinayagam, S.; Paz-Montelongo, S.; Dey, A.; Vellingirl, B.; George, A.; Madhyastha, H.; et al. Heavy metal and metalloid contamination in food and emerging technologies for its detection. Sustainability 2023, 15, 1195. [Google Scholar] [CrossRef]
- Vonnie, J.M.; Rovina, K.; Mariah, A.M.A.; Erna, K.H.; Felicia, W.X.L.; ‘Aqilah, M.N.N. Trends in nanotechnology techniques for detecting heavy metals in food and contaminated water: A review. Int. J. Environ. Sci. Technol. 2023, 20, 8041–8072. [Google Scholar]
- Elafify, M.; Marwa, E.L.T.; Sallam, K.I.; Sadoma, N.M.; Abd-Elghany, S.M.; Abdelkhalek, A.; El-Baz, A.H. Heavy metal residues in milk and some dairy products with insight into their health risk assessment and the role of Lactobacillus rhamnosus in reducing the lead and cadmium load in cheese. Food Chem. Adv. 2023, 2, 100261. [Google Scholar]
- World Health Organization. Achieving Well-Being: A Global Framework for Integrating Well-Being into Public Health Utilizing a Health Promotion Approach; World Health Organization: Geneva, Switzerland, 2024.
- Savoie-Roskos, M.R.; Hood, L.B.; Hagedorn-Hatfield, R.L.; Landry, M.J.; Patton-López, M.M.; Richards, R.; Vogelzang, J.L.; Qamar, Z.; OoNorasak, K.; Mann, G. Creating a culture that supports food security and health equity at higher education institutions. Public Health Nutr. 2023, 26, 503–509. [Google Scholar]
- Rahmah, F.A.; Nurlaela, N.; Anugrah, R.; Putri, Y. Safe food treatment technology: The key to realizing the sustainable development goals (SDGs) zero hunger and optimal health. ASEAN J. Agric. Food Eng. 2024, 3, 57–66. [Google Scholar]
- Salam, M.A.; Al-Amin, M.Y.; Pawar, J.S.; Akhter, N.; Lucy, I.B. Conventional methods and future trends in antimicrobial susceptibility testing. Saudi J. Biol. Sci. 2023, 30, 103582. [Google Scholar]
- Hassoun, A.; Jagtap, S.; Garcia-Garcia, G.; Trollman, H.; Pateiro, M.; Lorenzo, J.M.; Trif, M.; Rusu, A.V.; Aadil, R.M.; Šimat, V.; et al. Food quality 4.0: From traditional approaches to digitalized automated analysis. J. Food Eng. 2023, 337, 111216. [Google Scholar]
- Mattarozzi, M.; Laski, E.; Bertucci, A.; Giannetto, M.; Bianchi, F.; Zoani, C.; Careri, M. Metrological traceability in process analytical technologies and point-of-need technologies for food safety and quality control: Not a straightforward issue. Anal. Bioanal. Chem. 2023, 415, 119–135. [Google Scholar]
- Ling, Z.; Yang, J.; Zhang, Y.; Tang, Y.; Wang, S.; Liu, H.; Zhao, G.; Zhang, Y.; Wu, Q. Applications of advanced materials in the pretreatment and rapid detection of small molecules in foods: A review. Trends Food Sci. Technol. 2023, 141, 104175. [Google Scholar]
- Du, R.; Zhang, Y.; Bian, Y.; Yu, Z.; Liu, W.; Wang, Q. Rhodamine and related substances in food: Recent updates on pretreatment and analysis methods. Food Chem. 2024, 459, 140384. [Google Scholar]
- Zhang, L.; Guo, W.; Lv, C.; Yu, Z.; Wang, X.; Li, H.; Chen, J.; Liu, M. Electrochemical biosensors represent promising detection tools in medical field. Adv. Sens. Energy Mater. 2023, 2, 100081. [Google Scholar]
- Wang, B.; Wang, H.; Lu, X.; Zheng, X.; Yang, Z. Recent advances in electrochemical biosensors for the detection of foodborne pathogens: Current perspective and challenges. Foods 2023, 12, 2795. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lin, X.; Ee, L.Y.; Li, S.F.Y.; Huang, M. A review on electrospinning as versatile supports for diverse nanofibers and their applications in environmental sensing. Adv. Fiber Mater. 2023, 5, 429–460. [Google Scholar]
- Peng, R.; Zhang, S.; Yao, Y.; Wang, J.; Zhu, X.; Jiang, R.; Zhang, J.; Zhang, W.; Wang, C. MOFs meet electrospinning: New opportunities for water treatment. Chem. Eng. J. 2023, 453, 139669. [Google Scholar]
- Leote, R.J.B.; Beregoi, M.; Enculescu, I.; Diculescu, V.C. Metallized electrospun polymeric fibers for electrochemical sensors and actuators. Curr. Opin. Electrochem. 2022, 34, 101024. [Google Scholar]
- Si, Y.; Shi, S.; Hu, J. Applications of electrospinning in human health: From detection, protection, regulation to reconstruction. Nano Today 2023, 48, 101723. [Google Scholar]
- Kanjwal, M.A.; Ghaferi, A.A. Graphene incorporated electrospun nanofiber for electrochemical sensing and biomedical applications: A critical review. Sensors 2022, 22, 8661. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Zhao, X.; Tian, Y.; Duan, Y. Electrospun nanofiber-based indicator paper sensing platform for fluorescence and visualization detection of norfloxacin. Biosens. Bioelectron. 2023, 238, 115562. [Google Scholar]
- Vanaraj, R.; Arumugam, B.; Mayakrishnan, G.; Kim, I.S.; Kim, S.C. A review on electrospun nanofiber composites for efficient electrochemical sensor applications. Sensors 2023, 23, 6705. [Google Scholar] [CrossRef] [PubMed]
- Maleki, F.; Razmi, H.; Rashidi, M.R.; Yousefi, M.; Ghorbani, M. Recent advances in developing electrochemical (bio) sensing assays by applying natural polymer-based electrospun nanofibers: A comprehensive review. Microchem. J. 2023, 197, 109799. [Google Scholar]
- Ye, C.; Lukas, H.; Wang, M.; Lee, Y.; Gao, W. Nucleic acid-based wearable and implantable electrochemical sensors. Chem. Soc. Rev. 2024, 53, 7960–7982. [Google Scholar] [PubMed]
- Xue, R.; Liu, Y.S.; Huang, S.L.; Yang, G.Y. Recent progress of covalent organic frameworks applied in electrochemical sensors. ACS Sens. 2023, 8, 2124–2148. [Google Scholar] [PubMed]
- Jiang, Y.; Sima, Y.; Liu, L.; Zhou, C.; Shi, S.; Wan, K.; Chen, A.; Tang, N.; He, Q.; Liu, J. Research progress on portable electrochemical sensors for detection of mycotoxins in food and environmental samples. Chem. Eng. J. 2024, 485, 149860. [Google Scholar]
- Barhoum, A.; Hamimed, S.; Slimi, H.; Othmani, A.; Abdel-Haleem, F.M.; Bechelany, M. Modern designs of electrochemical sensor platforms for environmental analyses: Principles, nanofabrication opportunities, and challenges. Trends Environ. Anal. Chem. 2023, 38, e00199. [Google Scholar]
- Sow, W.T.; Ye, F.; Zhang, C.; Li, J.; Li, H. Smart materials for point-of-care testing: From sample extraction to analyte sensing and readout signal generator. Biosens. Bioelectron. 2020, 170, 112682. [Google Scholar]
- Lv, L.; Wang, X. Recent advances in ochratoxin A electrochemical biosensors: Recognition elements, sensitization technologies, and their applications. J. Agric. Food Chem. 2020, 68, 4769–4787. [Google Scholar]
- He, Q.; Wang, B.; Liang, J.; Liu, J.; Bo, L.; Li, G.; Long, Y.; Zhang, G.; Liu, H. Research on the construction of portable electrochemical sensors for environmental compounds quality monitoring. Mater. Today Adv. 2023, 17, 100340. [Google Scholar]
- Chu, N.; Liang, Q.; Hao, W.; Jiang, Y.; Liang, P.; Zeng, R.J.X. Microbial electrochemical sensor for water biotoxicity monitoring. Chem. Eng. J. 2021, 404, 127053. [Google Scholar]
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in electrochemical sensors: A review. Electroanalysis 2021, 33, 1827–1851. [Google Scholar]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Recent advances in microfluidics-based electrochemical sensors for foodborne pathogen detection. Biosensors 2023, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Jadon, N.; Tomar, P.; Shrivastava, S.; Hosseinzadeh, B.; Kaya, S.I.; Ozkan, S.A. Monitoring of specific phytoestrogens by dedicated electrochemical sensors: A review. Food Chem. 2024, 460, 140404. [Google Scholar] [PubMed]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal nanoparticles and carbon-based nanomaterials for improved performances of electrochemical (bio)sensors with biomedical applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef]
- Tehrani, M.A.; Ahmadi, S.H.; Alimohammadi, S.; Sasanpour, P.; Batvani, N.; Kazemi, S.H.; Kiani, M.A. Continuous glucose monitoring using wearable non-enzymatic sensors in a physiological environment. Biosens. Bioelectron. X 2024, 18, 100482. [Google Scholar]
- Kalambate, R.P.; Kalambate, P.K.; Khosropour, H.; Thummarati, P.; Chiabchalard, A.; Boonlue, W.; Laiwattanapaisal, W. Exploring advanced functional nanomaterial-based electrochemical sensors for the detection of mycotoxins in food matrices: A comprehensive review. Chem. Inorg. Mater. 2024, 3, 100044. [Google Scholar]
- Loguercio, L.F.; Thesing, A.; Demingos, P.; de Albuquerque, C.D.; Rodrigues, R.S.; Brolo, A.G.; Santos, J.F. Efficient acetylcholinesterase immobilization for improved electrochemical performance in polypyrrole nanocomposite-based biosensors for carbaryl pesticide. Sens. Actuators B Chem. 2021, 339, 129875. [Google Scholar]
- Hernández-Saravia, L.P.; Núñez, C.; Castroagudín, M.; Bertotti, M.; Vizcarra, A.; Arriaza, B.; Nelson, R. Development of a fast and simple electrochemical sensor for trace determination of sulfite using copper (II) triazole complexes. Microchem. J. 2024, 201, 110560. [Google Scholar]
- Tu, J.; Torrente-Rodríguez, R.M.; Wang, M.; Gao, W. The era of digital health: A review of portable and wearable affinity biosensors. Adv. Funct. Mater. 2020, 30, 1906713. [Google Scholar]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The history of electrospinning: Past, present, and future developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar]
- Al-Dhahebi, A.M.; Ling, J.K.; Krishnan, S.G.; Yousefzadeh, M.; Elumalai, N.K.; Mohamed Saheed, M.S.; Ramakrishna, S.; Jose, R. Electrospinning research and products: The road and the way forward. Appl. Phys. Rev. 2022, 9, 011319. [Google Scholar]
- Yang, L.; Niu, C.; Cao, X.; Wang, Y.; Zhu, Z.; Sun, H.; Liang, W.; Li, J.; Li, A. Mechanically robust conjugated microporous polymer membranes prepared using polyvinylpyrrolidone (PVP) electrospun nanofibers as a template for efficient PM capture. J. Colloid Interface Sci. 2023, 637, 305–316. [Google Scholar] [PubMed]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar]
- Liu, Q.; Yan, K.; Chen, J.; Xia, M.; Li, M.; Liu, K.; Wang, D.; Wu, C.; Xie, Y. Recent advances in novel aerogels through the hybrid aggregation of inorganic nanomaterials and polymeric fibers for thermal insulation. Aggregate 2021, 2, e30. [Google Scholar]
- Guo, Y.; Wang, X.; Shen, Y.; Dong, K.; Shen, L.; Alzalab, A.A.A. Research progress, models and simulation of electrospinning technology: A review. J. Mater. Sci. 2022, 57, 58–104. [Google Scholar]
- Ahmadian, A.; Shafiee, A.; Aliahmad, N.; Agarwal, M. Overview of nano-fiber mats fabrication via electrospinning and morphology analysis. Textiles 2021, 1, 206–226. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Stachewicz, U. The impact of relative humidity on electrospun polymer fibers: From structural changes to fiber morphology. Adv. Colloid Interface Sci. 2020, 286, 102315. [Google Scholar]
- Abir, S.S.H.; Hasan, M.T.; Alcoutlabi, M.; Lozano, K. The effect of solvent and molecular weight on the morphology of centrifugally spun poly (vinylpyrrolidone) nanofibers. Fibers Polym. 2021, 22, 2394–2403. [Google Scholar]
- Sánchez-Cid, P.; Rubio-Valle, J.F.; Jiménez-Rosado, M.; Pérez-Puyana, V.; Romero, A. Effect of solution properties in the development of cellulose derivative nanostructures processed via electrospinning. Polymers 2022, 14, 665. [Google Scholar] [CrossRef]
- Behroozi, A.H.; Al-Shaeli, M.; Vatanpour, V. Fabrication and modification of nanofiltration membranes by solution electrospinning technique: A review of influential factors and applications in water treatment. Desalination 2023, 558, 116638. [Google Scholar]
- Sanaeepur, H.; Amooghin, A.E.; Shirazi, M.M.A.; Pishnamazi, M.; Shirazian, S. Water desalination and ion removal using mixed matrix electrospun nanofibrous membranes: A critical review. Desalination 2022, 521, 115350. [Google Scholar]
- Yan, S.; Qian, Y.; Haghayegh, M.; Xia, Y.; Yang, S.; Cao, R.; Zhu, M. Electrospun organic/inorganic hybrid nanofibers for accelerating wound healing: A review. J. Mater. Chem. B 2024, 12, 3171–3190. [Google Scholar]
- Xu, X.; Si, Y.; Zhao, Y.; Ke, Q.; Hu, J. Electrospun textile strategies in tendon to bone junction reconstruction. Adv. Fiber Mater. 2023, 5, 764–790. [Google Scholar]
- Shirvan, A.R.; Nouri, A.; Sutti, A.A. perspective on the wet spinning process and its advancements in biomedical sciences. Eur. Polym. J. 2022, 181, 111681. [Google Scholar]
- Khan, J.; Khan, A.; Khan, M.Q.; Khan, H. Applications of co-axial electrospinning in the biomedical field. Next Mater. 2024, 3, 100138. [Google Scholar]
- Wu, J.; Liu, S.; Zhang, M.; Wu, G.; Yu, H.; Li, H.; Li, F.; Jia, L. Coaxial electrospinning preparation and antibacterial property of polylactic acid/tea polyphenol nanofiber membrane. J. Ind. Text. 2022, 51 (Suppl. S2), 1778S–1792S. [Google Scholar]
- Liu, Y.; Chen, X.; Lin, X.; Yan, J.; Yu, D.G.; Liu, P.; Yang, H. Electrospun multi-chamber core-shell nanofibers and their controlled release behaviors: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1954. [Google Scholar]
- Kailasa, S.; Reddy, M.S.B.; Maurya, M.R.; Rani, B.G.; Rao, K.V.; Sadasivuni, K.K. Electrospun nanofibers: Materials, synthesis parameters, and their role in sensing applications. Macromol. Mater. Eng. 2021, 306, 2100410. [Google Scholar]
- Kang, S.; Zhao, K.; Yu, D.G.; Zheng, X.; Huang, C. Advances in biosensing and environmental monitoring based on electrospun nanofibers. Adv. Fiber Mater. 2022, 4, 404–435. [Google Scholar]
- Wang, Y.; Yokota, T.; Someya, T. Electrospun nanofiber-based soft electronics. NPG Asia Mater. 2021, 13, 22. [Google Scholar]
- Ayerdurai, V.; Cieplak, M.; Kutner, W. Molecularly imprinted polymer-based electrochemical sensors for food contaminants determination. TrAC Trends Anal. Chem. 2023, 158, 116830. [Google Scholar]
- Muhammad, F.; Dik, G.; Kolak, S.; Karadaş Gedik, K.; Bakar, B.; Ulu, A.; Ateş, B. Design of highly selective, and sensitive screen-printed electrochemical sensor for detection of uric acid with uricase immobilized polycaprolactone/polyethylene imine electrospun nanofiber. Electrochim. Acta 2023, 439, 141675. [Google Scholar]
- Vanisree, C.R.; Sankhla, M.S.; Singh, P.; Jadhav, E.B.; Verma, R.K.; Awasthi, K.K.; Awasthi, G.; Nagar, V. Heavy metal contamination of food crops: Transportation via food chain, human consumption, toxicity and management strategies. In Environmental Impact and Remediation of Heavy Metals; Springer International Publishing: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Núñez, C.; Triviño, J.J.; Arancibia, V. An electrochemical biosensor for As (III) detection based on the catalytic activity of Alcaligenes faecalis immobilized on a gold nanoparticle–modified screen–printed carbon electrode. Talanta 2021, 223, 121702. [Google Scholar] [PubMed]
- Anderson, A.; Anbarasu, A.; Pasupuleti, R.R.; Manigandan, S.; Praveenkumar, T.R.; Kumar, J.A. Treatment of heavy metals containing wastewater using biodegradable adsorbents: A review of mechanism and future trends. Chemosphere 2022, 295, 133724. [Google Scholar]
- Pachaiappan, R.; Cornejo-Ponce, L.; Rajendran, R.; Manavalan, K.; Rajan, V.F.; Awad, F. A review on biofiltration techniques: Recent advancements in the removal of volatile organic compounds and heavy metals in the treatment of polluted water. Bioengineered 2022, 13, 8432–8477. [Google Scholar]
- Liu, S.; Zhang, X. Preparation of nitrogen doped carbon nanofibers for electrochemical determination of Cd (II) and Pb (II) ions. Int. J. Electrochem. Sci. 2020, 15, 9838–9848. [Google Scholar]
- Machate, D.J. Anthropogenic hyperactivity for natural resources increases heavy metals concentrations in the environment: Toxicity of healthy food and cancer risks estimated. J. Trace Elem. Min. 2023, 4, 100057. [Google Scholar]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Li, B.; Xie, X.; Meng, T.; Guo, X.; Li, Q.; Yang, Y.; Jin, H.; Jin, C.; Meng, X.; Pang, H. Recent advance of nanomaterials modified electrochemical sensors in the detection of heavy metal ions in food and water. Food Chem. 2023, 440, 138213. [Google Scholar]
- Pichún, B.; Núñez, C.; Arancibia, V.; Martí, A.A.; Aguirre, M.J.; Pizarro, J.; Segura, R.; Flores, E. Enhanced voltammetric sensing platform based on gold nanorods and electrochemically reduced graphene oxide for As (III) determination in seafood samples. J. Appl. Electrochem. 2024, 54, 1595–1606. [Google Scholar]
- Tang, Q.; Zhu, G.; Ge, Y.; Yang, J.; Huang, M.; Liu, J. AuNPs-polyaniline nanosheet array on carbon nanofiber for the determination of As (III). J. Electroanal. Chem. 2020, 873, 114381. [Google Scholar]
- Raina, J.; Kaur, G.; Singh, I. Recent progress in nanomaterial-based aptamers as biosensors for point of care detection of Hg2+ ions and its environmental applications. Talanta 2024, 277, 126372. [Google Scholar] [PubMed]
- Teodoro, K.B.R.; Migliorini, F.L.; Facure, M.H.M.; Correa, D.S. Conductive electrospun nanofibers containing cellulose nanowhiskers and reduced graphene oxide for the electrochemical detection of mercury (II). Carbohydr. Polym. 2019, 207, 747–754. [Google Scholar] [PubMed]
- Ehzari, H.; Safari, M.; Shahlaei, M. A new sensing strategy based on thymine bases-Hg2+-methylene blue coordination on the electrospun PES-QDs platform for detection of Hg2+ in fruit juice samples. J. Iran. Chem. Soc. 2019, 16, 2269–2279. [Google Scholar]
- Xie, H.; Niu, Y.; Deng, Y.; Cheng, H.; Ruan, C.; Li, G.; Sun, W. Electrochemical aptamer sensor for highly sensitive detection of mercury ion with Au/Pt@carbon nanofiber-modified electrode. J. Chin. Chem. Soc. 2021, 68, 114–120. [Google Scholar]
- Rotake, D.; Goswami, P.P.; Singh, S.G. Ultraselective, ultrasensitive, point-of-care electrochemical sensor for detection of Hg (II) ions with electrospun-InZnO nanofibers. J. Electroanal. Chem. 2022, 915, 116350. [Google Scholar]
- Vilian, A.T.E.; Ranjith, K.S.; Hwang, S.K.; Bhaskaran, G.; Alhammadi, M.; Park, S.Y.; Huh, Y.S.; Han, Y.-K. Interface engineering of MoS2 nanopetal grown on carbon nanofibers for the electrocatalytic sensing of mercury (II) and efficient hydrogen evolution. Mater. Today Nano 2022, 20, 100262. [Google Scholar]
- Qi, X.; Wang, Z. Graphene quantum dots functionalized Ce-ZnO nanofibers with enriched oxygen vacancy sites morphology to improve the efficiency of selective electrochemical detection of Hg (II). Diam. Relat. Mater. 2023, 139, 110241. [Google Scholar]
- Sadeghi, E.; Rahimi, F.; Azizi, Z.; Kaki, S.; Babakhanian, A. Fabrication of a sensitive electrochemical sensor based on hybrid polyamide/chromotropic acid nanofibers electrospun on glassy carbon electrode for Hg2+ sensing in drinking water and canned fish samples. Food Chem. 2023, 414, 135467. [Google Scholar]
- Jyothilakshmi, V.P.; Manu, R.; Swaminathan, S. The fabrication of TiO2/AgCNF/Nafion/GC electrodes for simultaneous electrochemical detection of Cd (II), Pb (II), Cu (II), and Hg (II) by optimizing the porosity of electrospun TiO2 nanofibers. J. Appl. Electrochem. 2024, 54, 2377–2387. [Google Scholar]
- Zhang, X.; Cai, Q.; Liu, K.; Yan, G.; Chen, J.; Wu, Y.; Li, Z.; Wang, L.; Li, H. Flexible and robust Eu/cellulose acetate nanofibrous membrane for the rapid detection of Hg2+ with ultra-stable recycle capability. Measurement 2023, 222, 113660. [Google Scholar]
- Romaguera-Barcelay, Y.; Gandarilla, A.; Alves, J.F.M.; Tavares, A.P.M.; de Souza, T.C.S.; Segala, K.; Farias, T.; Cunha, J.; Sales, M.G.F.; Brito, W.R. Electrochemical Hg2+ sensor based on electrospun cellulose acetate nanofibers doped with Ag nanoparticles. Microchem. J. 2024, 204, 111069. [Google Scholar]
- Tan, H.W.; Seen, D.L.T.; Xu, Y.-M.; Lau, A.T.Y. Cadmium, Cellular Senescence, and Cancer. Rev. Environ. Contam. Toxicol. 2023, 261, 21. [Google Scholar]
- Parida, L.; Patel, T.N. Systemic impact of heavy metals and their role in cancer development: A review. Environ. Monit. Assess. 2023, 195, 766. [Google Scholar]
- Wang, J.; Li, Z.; Zhang, H.; Wu, W.; Wu, Y.; Liu, M.; Ao, Y.; Li, M. Multistage pore structure legume-like UiO-66-NH2@carbon nanofiber aerogel modified electrode as an electrochemical sensor for high sensitivity detection of HMIs. J. Environ. Chem. Eng. 2023, 11, 111488. [Google Scholar]
- Girija, S.; Sankar, S.S.; Thenrajan, T.; Kundu, S.; Wilson, J. Bi-metallic zeolite imidazole framework nanofibers for the selective determination of Cd2+ ions. J. Mater. Chem. B 2021, 9, 5656–5663. [Google Scholar]
- Ngoensawat, U.; Pisuchpen, T.; Sritana-Anant, Y.; Rodthongkum, N.; Hoven, V.P. Conductive electrospun composite fibers based on solid-state polymerized poly(3,4-ethylenedioxythiophene) for simultaneous electrochemical detection of metal ions. Talanta 2022, 241, 123253. [Google Scholar]
- Fakude, C.T.; Arotiba, O.A.; Arduini, F.; Mabuba, N. Flexible polyester screen-printed electrode modified with carbon nanofibers for the electrochemical aptasensing of cadmium (II). Electroanalysis 2020, 32, 2650–2658. [Google Scholar]
- Yousefi, A.; Aghaie, H.; Giahi, M.; Maleknia, L. Determination of Cd2+ and Pb2+ by polyindole/Mn2O3 nanocomposite and polyindole/Mn2O3/polyaniline nanofibers modified glassy carbon electrode. Chem. Pap. 2023, 77, 733–743. [Google Scholar]
- Dong, J.; Wen, L.; Zhao, D.; Yang, H.; Zhao, J.; Hu, Z.; Ma, Y.; Hou, C.; Huo, D. Flexible carbon fiber cloth supports decorated with cerium metal-organic frameworks and multi-walled carbon nanotubes for simultaneous on-site detection of Cd2+ and Pb2+ in food and water samples. Food Chem. 2023, 418, 135869. [Google Scholar]
- Oliveira, V.H.B.; Rechotnek, F.; da Silva, E.P.; Marques, V.S.; Rubira, A.F.; Silva, R.; Lourenço, S.A.; Muniz, E.C. A sensitive electrochemical sensor for Pb2+ ions based on ZnO nanofibers functionalized by L-cysteine. J. Mol. Liq. 2020, 309, 113041. [Google Scholar]
- Fotia, A.; Frontera, P.; Bonaccorsi, L.; Sciacca, B.; Malara, A. Encapsulation of Disodium-EDTA in Electrospun Polymeric Fibers for the Detection of Heavy Metals. Adv. Sustain. Syst. 2024, 8, 2300463. [Google Scholar]
- Kogularasu, S.; Lee, Y.Y.; Chang-Chien, G.P.; Govindasamy, M.; Sheu, J.K. Nanofibers: Empowering electrochemical sensors for reliable detection of food and environmental toxins. J. Electrochem. Soc. 2023, 170, 077514. [Google Scholar]
- Sindhu, S.; Manickavasagan, A. Nondestructive testing methods for pesticide residue in food commodities: A review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1226–1256. [Google Scholar]
- Kumaravel, A.; Aishwarya, S.; Sathyamoorthi, S. Emerging Technologies for Sensitive Detection of Organophosphate Pesticides: A Review. Curr. Anal. Chem. 2024, 20, 383–409. [Google Scholar]
- Wang, Q.; Wang, M.; Zhang, N.; Huang, X.; Wang, X.; Wang, S. A novel electrochemical sensor based on MoS2 electrospun nanofibers and polyoxometalate composite for the simultaneous detection of ractopamine and clenbuterol. Microchem. J. 2023, 189, 108434. [Google Scholar]
- Shan, X.; Lu, J.; Wu, Q.; Li, C.; Li, H.; Yang, S.; Tian, L. Solid-state electrochemiluminescence sensor of CQDs based on ZIFs electrospun carbon fiber for malathion detection. Microchem. J. 2023, 195, 109496. [Google Scholar]
- Cacciotti, I.; Pallotto, F.; Scognamiglio, V.; Moscone, D.; Arduini, F. Reusable optical multi-plate sensing system for pesticide detection by using electrospun membranes as smart support for acetylcholinesterase immobilisation. Mater. Sci. Eng. C 2020, 111, 110744. [Google Scholar]
- Migliorini, F.L.; Sanfelice, R.C.; Mercante, L.A.; Facure, M.H.M.; Correa, D.S. Electrochemical sensor based on polyamide 6/polypyrrole electrospun nanofibers coated with reduced graphene oxide for malathion pesticide detection. Mater. Res. Express 2020, 7, 015601. [Google Scholar]
- Hu, J.; Wen, P.; Wang, Y.; Yang, J.; Xiao, Z.; Xu, Z.; Shen, Y.; Wang, H.; Hammock, B.D. Fabrication of a label-free electrochemical immunosensor by functionalized nanofiber membrane for the ultrasensitive detection of quinalphos. Food Control 2024, 162, 110423. [Google Scholar]
- Yin, W.; Zhang, J.; Wang, H.; Wang, Y.; Zeng, X.; Xu, Z.; Yang, J.; Xiao, Z.; Hammock, B.D.; Wen, P. A highly sensitive electrochemical immunosensor based on electrospun nanocomposite for the detection of parathion. Food Chem. 2023, 404, 134371. [Google Scholar]
- Topsoy, O.K.; Muhammad, F.; Kolak, S.; Ulu, A.; Güngör, Ö.; Şimşek, M.; Köytepe, S.; Ateş, B. Fabrication of electrospun polycaprolactone/chitosan nanofiber-modified screen-printed electrode for highly sensitive detection of diazinon in food analysis. Measurement 2022, 187, 110250. [Google Scholar]
- Hatamluyi, B.; Sadeghzadeh, S.; Rezayi, M.; Sany, S.B.T. Diazinon electrochemical biosensor mediated by aptamer and nanoscale porous carbon derived from ZIF-8. Sens. Actuators B Chem. 2023, 381, 133424. [Google Scholar]
- Supraja, P.; Tripathy, S.; Vanjari, S.R.K.; Singh, V.; Singh, S.G. Electrospun tin (IV) oxide nanofiber based electrochemical sensor for ultra-sensitive and selective detection of atrazine in water at trace levels. Biosens. Bioelectron. 2019, 141, 111441. [Google Scholar]
- Supraja, P.; Singh, V.; Vanjari, S.R.K.; Singh, S.G. Electrospun CNT embedded ZnO nanofiber based biosensor for electrochemical detection of atrazine: A step closure to single molecule detection. Microsyst. Nanoeng. 2020, 6, 3. [Google Scholar]
- Dey, B.; Ahmad, M.W.; Syed, A.; Bahkali, A.H.; Verma, M.; Choudhury, A. Iron metal organic framework decorated carbon nanofiber-based electrode for electrochemical sensing platform of chlorpyrifos in fruits and vegetables. Mater. Sci. Semicond. Process. 2024, 181, 108669. [Google Scholar]
- Lv, Y.; Yang, T.; Hou, X.; Fang, Z.; Rajan, K.; Di, Y.; Peng, W.; Deng, Y.; Liang, T. Zirconia nanofibers-loaded reduced graphene oxide fabrication for specific electrochemical detection of methyl parathion. J. Alloys Compd. 2022, 904, 163798. [Google Scholar]
- Wang, Z.; Ma, B.; Shen, C.; Cheong, L.Z. Direct, selective and ultrasensitive electrochemical biosensing of methyl parathion in vegetables using Burkholderia cepacia lipase@MOF nanofibers-based biosensor. Talanta 2019, 197, 356–362. [Google Scholar]
- Kokulnathan, T.; Vishnuraj, R.; Chen, S.-M.; Pullithadathil, B.; Ahmed, F.; Hasan, P.M.Z.; Bilgrami, A.L.; Kumar, S. Tailored construction of one-dimensional TiO2/Au nanofibers: Validation of an analytical assay for detection of diphenylamine in food samples. Food Chem. 2022, 380, 132052. [Google Scholar]
- Rayappa, M.K.; Kavya, K.S.; Rattu, G.; Krishna, P.M. Advances and effectiveness of metal-organic framework based bio/chemical sensors for rapid and ultrasensitive probing of antibiotic residues in foods. Sustain. Food Technol. 2023, 1, 152–184. [Google Scholar]
- Kakimova, Z.; Zharykbasova, K.; Kakimov, A.; Mirasheva, G.; Toleubekova, S.; Zharykbasov, Y.; Tulkebayeva, G.; Muratbayev, A.; Utegenova, A. Study on the detection of antibiotics in food based on enzyme-free labelless aptamer sensor. Food Sci. Technol. 2022, 42, e70421. [Google Scholar]
- Huang, S.J.; Gokulkumar, K.; Govindasamy, M.; Albaqami, M.D.; Wabaidur, S.M. Nanoarchitectonics of europium vanadate nanoparticles decorated carbon nanofibers for electrochemical detection of fungicide in fruits. J. Taiwan Inst. Chem. Eng. 2024, 161, 105563. [Google Scholar]
- Song, J.; Huang, M.; Lin, X.; Li, S.F.Y.; Jiang, N.; Liu, Y.; Guo, H.; Li, Y. Novel Fe-based metal-organic framework (MOF) modified carbon nanofiber as a highly selective and sensitive electrochemical sensor for tetracycline detection. Chem. Eng. J. 2022, 427, 130913. [Google Scholar]
- Mohammadi, S.; Pooshang Bagheri, K.; Mousavi Nadushan, R.; Adabi, M. Nanoarchitectonics of electrochemical aptasensor based on electrospun carbon nanofibers and gold nanoparticles for tetracycline detection in chicken ham. Appl. Phys. A 2023, 129, 176. [Google Scholar]
- Sebastian, N.; Yu, W.C.; Balram, D.; Hong, G.T.; Alharthi, S.S.; Al-Saidi, H.M. Ultrasensitive detection and photocatalytic degradation of polyketide drug tetracycline in environment and food samples using dual-functional Ag doped zinc ferrite embedded functionalized carbon nanofibers. Chemosphere 2024, 348, 140692. [Google Scholar]
- Kokulnathan, T.; Vishnuraj, R.; Wang, T.J.; Pullithadathil, B. Multidimensional nanoarchitectures of TiO2/Au nanofibers with O-doped C3N4 nanosheets for electrochemical detection of nitrofurazone. Appl. Surf. Sci. 2022, 604, 154474. [Google Scholar]
- Wei, X.; Sun, Y.; Luo, Y.; Shu, R.; Lin, H.; Xu, D. Electrochemical biosensor Bi2O3@Au@Apta based on Bi2O3 nanofibers for sensitive detection of ampicillin. Biochem. Eng. J. 2023, 200, 109107. [Google Scholar]
- Mariappan, K.; Alagarsamy, S.; Chen, S.-M.; Sakthinathan, S. Electrochemical detection of metronidazole by the fabricated composites of orthorhombic iron tungsten oxide decorated with carbon nanofiber composites electrode. J. Electrochem. Soc. 2023, 170, 037514. [Google Scholar]
- Vilian, A.T.E.; Ranjith, K.S.; Lee, S.J.; Umapathi, R.; Hwang, S.-K.; Oh, C.W.; Huh, Y.S.; Han, Y.-K. Hierarchical dense Ni−Co layered double hydroxide supported carbon nanofibers for the electrochemical determination of metronidazole in biological samples. Electrochim. Acta 2020, 354, 136723. [Google Scholar]
- Muthukutty, B.; Arumugam, B.; Chen, S.-M.; Kannan, S.R. Low potential detection of antiprotozoal drug metronidazole with aid of novel dysprosium vanadate incorporated oxidized carbon nanofiber modified disposable screen-printed electrode. J. Hazard. Mater. 2021, 407, 124745. [Google Scholar]
- Shoba, S.; Mambanda, A.; Booysen, I.N. Electrospun Nanofiber Composite Utilized as an Electrocatalyst for the Detection of Acetaminophen in Multifarious Water Samples. ACS Omega 2024, 9, 5734–5750. [Google Scholar] [PubMed]
- Kumar, S.; Deep, A.; Wangoo, N.; Bhardwaj, N. Recent advancements in nanomaterials based optical detection of food additives: A review. Analyst 2023, 148, 5322–5339. [Google Scholar]
- Kaur, I.; Batra, V.; Bogireddy, N.K.R.; Landa, S.D.T.; Agarwal, V. Detection of organic pollutants, food additives and antibiotics using sustainable carbon dots. Food Chem. 2023, 406, 135029. [Google Scholar] [PubMed]
- Jagirani, M.S.; Zhou, W.; Nazir, A.; Akram, M.Y.; Huo, P.; Yan, Y. A Recent Advancement in Food Quality Assessment: Using MOF-Based Sensors: Challenges and Future Aspects. Crit. Rev. Anal. Chem. 2024; online ahead of print. [Google Scholar]
- Wang, F.; Li, Y.; Yan, C.; Ma, Q.; Yang, X.; Peng, H.; Wang, H.; Du, J.; Zheng, B.; Guo, Y. Bismuth-Decorated Honeycomb-like Carbon Nanofibers: An Active Electrocatalyst for the Construction of a Sensitive Nitrite Sensor. Molecules 2023, 28, 3881. [Google Scholar] [CrossRef]
- Le, H.T.; Tran, D.T.; Kim, N.H.; Lee, J.H. Worm-like gold nanowires assembled carbon nanofibers-CVD graphene hybrid as sensitive and selective sensor for nitrite detection. J. Colloid Interface Sci. 2021, 583, 425–434. [Google Scholar]
- Ranjith, K.S.; Ezhil Vilian, A.T.; Ghoreishian, S.M.; Umapathi, R.; Huh, Y.S.; Han, Y.-K. An ultrasensitive electrochemical sensing platform for rapid detection of rutin with a hybridized 2D-1D MXene-FeWO4 nanocomposite. Sens. Actuators B Chem. 2021, 344, 130202. [Google Scholar]
- Zhang, L.; Qin, D.; Feng, J.; Tang, T.; Cheng, H. Rapid quantitative detection of luteolin using an electrochemical sensor based on electrospinning of carbon nanofibers doped with single-walled carbon nanoangles. Anal. Methods 2023, 15, 3073–3083. [Google Scholar]
- Vinoth, S.; Wang, S.F. Rhombohedral type of LaCoO3 with carbon nanofiber composite as an electrocatalyst enables the amperometry detection of vanillin in food samples. New J. Chem. 2023, 47, 9229–9238. [Google Scholar]
- Nixon, E.J.; Sakthivel, R.; ALOthman, Z.A.; Ganesh, P.S.; Chung, R.J. Lanthanum nickelate spheres embedded acid functionalized carbon nanofiber composite: An efficient electrocatalyst for electrochemical detection of food additive vanillin. Food Chem. 2023, 409, 135324. [Google Scholar]
- Gokulkumar, K.; Huang, S.J.; Kogularasu, S.; Aljuwayid, A.M.; Maheshwaran, S.; Govindasamy, M. Enhanced electrochemical detection of tartrazine in beverages and liquid soap via nickel phosphide-adorned functionalized carbon nanofibers. J. Taiwan Inst. Chem. Eng. 2024, 157, 105420. [Google Scholar]
- Jenisha Daisy Priscillal, I.; Wang, S.F. Designing nano ranged electrode modifier comprised of samarium niobate anchored carbon nanofibers for trace level detection of food colourant: Tartrazine. Food Chem. 2023, 422, 136230. [Google Scholar] [PubMed]
- Radha, A.; Wang, S.F. Neodymium (III) Vanadate-Decorated functionalized carbon nanofiber nanocomposite: An electrochemical tool for tartrazine monitoring. ACS Appl. Nano Mater. 2023, 6, 18036–18044. [Google Scholar]
- Ansari, S.H.; Arvand, M. Electrospun ruthenium oxide nanofibers/poly(sulfosalicylic acid) nanocomposite as an highly sensitive electrochemical platform for determination of sunset yellow in food samples. J. Food Meas. Charact. 2023, 17, 1616–1627. [Google Scholar]
- Atilgan, H.; Unal, B.; Yalcinkaya, E.E.; Evren, G.; Atik, G.; Ozturk Kirbay, F.; Kilic, N.M.; Odaci, D. Development of an enzymatic biosensor using glutamate oxidase on organic-inorganic-structured, electrospun nanofiber-modified electrodes for monosodium glutamate detection. Biosensors 2023, 13, 430. [Google Scholar] [CrossRef]
- Oyedeji, A.B.; Green, E.; Adebiyi, J.A.; Ogundele, O.M.; Gbashi, S.; Adefisoye, M.A.; Oyeyinka, S.A.; Adebo, O.A. Metabolomic approaches for the determination of metabolites from pathogenic microorganisms: A review. Food Res. Int. 2021, 140, 110042. [Google Scholar]
- Ganesan, A.R.; Mohan, K.; Rajan, D.K.; Pillay, A.A.; Palanisami, T.; Sathishkumar, P.; Conterno, L. Distribution, toxicity, interactive effects, and detection of ochratoxin and deoxynivalenol in food: A review. Food Chem. 2022, 378, 131978. [Google Scholar]
- Cui, Q.; Gan, S.; Zhong, Y.; Yang, H.; Wan, Y.; Zuo, Y.; Yang, H.; Li, M.; Zhang, S.; Negahdary, M.; et al. High-throughput and specific detection of microorganisms by intelligent modular fluorescent photoelectric microbe detector. Anal. Chim. Acta 2023, 1265, 341282. [Google Scholar]
- Wang, R.F.; Wang, R. Modification of polyacrylonitrile-derived carbon nanofibers and bacteriophages on screen-printed electrodes: A portable electrochemical biosensor for rapid detection of Escherichia coli. Bioelectrochemistry 2022, 148, 108229. [Google Scholar]
- Shaibani, P.M.; Jiang, K.; Haghighat, G.; Hassanpourfard, M.; Etayash, H.; Naicker, S.; Thundat, T. The detection of Escherichia coli (E. coli) with the pH sensitive hydrogel nanofiber-light addressable potentiometric sensor (NF-LAPS). Sens. Actuators B Chem. 2016, 226, 176–183. [Google Scholar]
- Soares, A.C.; Soares, J.C.; dos Santos, D.M.; Migliorini, F.L.; Popolin-Neto, M.; Pinto, D.S.C.; Carvalho, W.A.; Brandão, H.M.; Paulovich, F.V.; Correa, D.S.; et al. Nanoarchitectonic E-tongue of electrospun zein/curcumin carbon dots for detecting Staphylococcus aureus in milk. ACS Omega 2023, 8, 13721–13732. [Google Scholar]
- Zhang, Z.; Du, M.; Cheng, X.; Dou, X.; Zhou, J.; Wu, J.; Xie, X.; Zhu, M. A disposable paper-based electrochemical biosensor decorated by electrospun cellulose acetate nanofibers for highly sensitive bio-detection. Analyst 2024, 149, 2436–2444. [Google Scholar] [PubMed]
- Moradi, S.; Azizi-Lalabadi, M.; Bagheri, V.; Sadeghi, E. Fabrication of electrospun sensor based on a synthesized component doped into PAN (polyacrylonitrile) nanofibers for electrochemical detection of zearalenone mycotoxin in foods simulant. Sens. Bio-Sens. Res. 2020, 28, 100321. [Google Scholar]
- Huang, S.-J.; Gokulkumar, K.; Mani, G.; Lee, Y.-Y.; Kogularasu, S.; Chang-Chien, G.-P. Synthesis and characterization of Bi2S3-embedded carbon nanofibers as a novel electrochemical biosensor for the detection of mycotoxin zearalenone in food crops. FlatChem 2024, 45, 100652. [Google Scholar]
- Rahmani, H.R.; Adabi, M.; Bagheri, K.P.; Karim, G. Development of electrochemical aptasensor based on gold nanoparticles and electrospun carbon nanofibers for the detection of aflatoxin M1 in milk. J. Food Meas. Charact. 2021, 15, 1826–1833. [Google Scholar]
- Ebrahimi Vafaye, S.; Rahman, A.; Safaeian, S.; Adabi, M. An electrochemical aptasensor based on electrospun carbon nanofiber mat and gold nanoparticles for the sensitive detection of Penicillin in milk. J. Food Meas. Charact. 2021, 15, 876–882. [Google Scholar]
- El-Moghazy, A.Y.; Amaly, N.; Istamboulie, G.; Nitin, N.; Sun, G. A signal-on electrochemical aptasensor based on silanized cellulose nanofibers for rapid point-of-use detection of ochratoxin A. Microchim. Acta 2020, 187, 535. [Google Scholar]
- Al-Dhahebi, A.M.; Jose, R.; Mustapha, M.; Saheed, M.S.M. Ultrasensitive aptasensor using electrospun MXene/polyvinylidene fluoride nanofiber composite for Ochratoxin A detection. Food Chem. 2022, 390, 133105. [Google Scholar]
- Dos Santos, D.M.; Migliorini, F.L.; Soares, A.C.; Mattoso, L.H.C.; Oliveira, O.N., Jr.; Correa, D.S. Electrochemical Immunosensor Made with Zein-based Nanofibers for On-site Detection of Aflatoxin B1. Electroanalysis 2023, 35, e202100672. [Google Scholar]
- Bekhit, A.E.D.A.; Giteru, S.G.; Holman, B.W.B.; Hopkins, D.L. Total volatile basic nitrogen and trimethylamine in muscle foods: Potential formation pathways and effects on human health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3620–3666. [Google Scholar]
- Joshi, N.; Pransu, G.; Adam Conte-Junior, C. Critical review and recent advances of 2D materials-Based gas sensors for food spoilage detection. Crit. Rev. Food Sci. Nutr. 2023, 63, 10536–10559. [Google Scholar]
- Das, J.; Mishra, H.N. A comprehensive review of the spoilage of shrimp and advances in various indicators/sensors for shrimp spoilage monitoring. Food Res. Int. 2023, 173, 113270. [Google Scholar] [PubMed]
- Ashiq, J.; Saeed, U.; Li, Z.; Nawaz, M.H. Advances in Meat Spoilage Detection: A Review of Methods Involving 2D-Based Nanomaterials for Detection of Spoiled Meat. J. Food Compos. Anal. 2024, 132, 106295. [Google Scholar]
- Li, H.; Chu, S.; Ma, Q.; Fang, Y.; Wang, J.; Che, Q.; Wang, G.; Yang, P. Novel construction of morphology-tunable C-N/SnO2/ZnO/Au microspheres with ultrasensitivity and high selectivity for triethylamine under various temperature detections. ACS Appl. Mater. Interfaces 2019, 11, 8601–8611. [Google Scholar] [PubMed]
- Rianjanu, A.; Aflaha, R.; Khamidy, N.I.; Djamal, M.; Triyana, K.; Wasisto, H.S. Room-temperature ppb-level trimethylamine gas sensors functionalized with citric acid-doped polyvinyl acetate nanofibrous mats. Mater. Adv. 2021, 2, 3705–3714. [Google Scholar]
- Ma, S.; Guo, J.; Zhang, H.; Shao, X.; Zhang, D. A Room Temperature Trimethylamine Gas Sensor Based on Electrospun Molybdenum Oxide Nanofibers/Ti3C2Tx MXene Heterojunction. Nanomaterials 2024, 14, 537. [Google Scholar]
- Qu, C.; Zhao, P.; Wu, C.; Zhuang, Y.; Liu, J.; Li, W.; Liu, Z.; Liu, J. Electrospun PAN/PANI fiber film with abundant active sites for ultrasensitive trimethylamine detection. Sens. Actuators B Chem. 2021, 338, 129822. [Google Scholar]
- Li, Y.; Liu, J.; Zhang, J.; Liang, X.; Zhang, X.; Qi, Q. Deposition of In2O3 nanofibers on polyimide substrates to construct high-performance and flexible trimethylamine sensor. Chin. Chem. Lett. 2020, 31, 2142–2144. [Google Scholar]
- Yang, J.; Han, W.; Ma, J.; Wang, C.; Shimanoe, K.; Zhang, S.; Sun, Y.; Cheng, P.; Wang, Y.; Zhang, H.; et al. Sn doping effect on NiO hollow nanofibers based gas sensors about the humidity dependence for triethylamine detection. Sens. Actuators B Chem. 2021, 340, 129971. [Google Scholar]
- Cai, L.X.; Chen, L.; Sun, X.Q.; Geng, J.; Liu, C.C.; Wang, Y.; Guo, Z. Ultra-sensitive triethylamine gas sensors based on polyoxometalate-assisted synthesis of ZnWO4/ZnO hetero-structured nanofibers. Sens. Actuators B Chem. 2022, 370, 132422. [Google Scholar]
- Ma, Q.; Li, H.; Chu, S.; Liu, Y.; Liu, M.; Fu, X.; Li, H.; Guo, J. In2O3 hierarchical structures of one-dimensional electrospun fibers with in situ growth of octahedron-like particles with superior sensitivity for triethylamine at near room temperature. ACS Sustain. Chem. Eng. 2020, 8, 5240–5250. [Google Scholar]
- Xie, Q.; Ding, Y.; Wang, Q.; Song, P. Fabrication of 1D/2D In2O3 nanofibers/Ti3C2Tx MXene composites for high performance detection of trimethylamine at low temperature. Sens. Actuators B Chem. 2024, 405, 135338. [Google Scholar]
- Wang, Y.; Liu, Z.; Li, Y.; Liu, Y.; Liang, X.; Liu, F.; Lu, G. Trace PdO and Co-MOF derivative modified SnO2 nanofibers for rapid triethylamine detection with little humidity disturbance. Sens. Actuators B Chem. 2024, 403, 135239. [Google Scholar]
- Conti, P.P.; dos Santos, D.M.; Goldthorpe, I.A.; Correa, D.S. TiO2 hollow nanofiber/polyaniline nanocomposites for ammonia detection at room temperature. ChemNanoMat 2022, 8, e202200154. [Google Scholar]
- Liu, D.; Shi, Q.; Jin, S.; Shao, Y.; Huang, J. Self-assembled core-shell structured organic nanofibers fabricated by single-nozzle electrospinning for highly sensitive ammonia sensors. InfoMat 2019, 1, 525–532. [Google Scholar]
- Aflaha, R.; Sari, N.L.I.; Katriani, L.; As’ari, A.H.; Kusumaatmaja, A.; Rianjanu, A.; Roto, R.; Wasisto, H.S.; Triyana, K. Maltodextrin-overlaid polyvinyl acetate nanofibers for highly sensitive and selective room-temperature ammonia sensors. Microchem. J. 2023, 193, 109237. [Google Scholar]
- Rianjanu, A.; Rayhan, M.A.A.P.; Aulya, M.; Aflaha, R.; Triyana, K.; Taher, T.; Wasisto, H.S. Early spoilage detection of cultured Pacific white shrimp using quartz crystal microbalance gas sensors. ChemistrySelect 2024, 9, e202304694. [Google Scholar]
- Andre, R.S.; Facure, M.H.M.; Mercante, L.A.; Correa, D.S. Electronic nose based on hybrid free-standing nanofibrous mats for meat spoilage monitoring. Sens. Actuators B Chem. 2022, 353, 131114. [Google Scholar]
- Dey, B.; Ahmad, M.W.; Kim, B.H.; Kamal, T.; Yang, D.J.; Patra, C.N.; Hossain, S.K.S.; Choudhury, A. Manganese cobalt-MOF@ carbon nanofiber-based non-enzymatic histamine sensor for the determination of food freshness. Anal. Bioanal. Chem. 2023, 415, 3487–3501. [Google Scholar]
- Wang, J.; Zhang, D.; Gao, Y.; Chen, F.; Wang, T.; Xia, H.; Sui, X.; Wang, Z. Fast-response hydrogen sulfide gas sensor based on electrospinning Co3O4 nano-fibers-modified CuO nanoflowers: Experimental and DFT calculation. Sens. Actuators B Chem. 2023, 396, 134579. [Google Scholar]
- Huo, Y.; Qiu, L.; Wang, T.; Yu, H.; Yang, W.; Dong, X.; Yang, Y. P-N Heterojunction formation: Metal Sulfide@ Metal Oxide Chemiresistor for ppb H2S Detection from Exhaled Breath and Food Spoilage at Flexible Room Temperature. ACS Sens. 2024, 9, 3433–3443. [Google Scholar]
- Lei, Y.; Zhang, Y.; Wang, B.; Zhang, Z.; Yuan, L.; Li, J. A lab-on-injector device with Au nanodots confined in carbon nanofibers for in situ electrochemical BPA sensing in beverages. Food Control 2022, 134, 108747. [Google Scholar]
- Shoukat, N.; Anzar, S.; Asad, M.; Al-Sulami, A.I.; Khalid, H.; Choudhary, A.A.; Sherin, L.; Akhtar, N. Fabrication of CuO-NiO wrapped cellulose acetate/polyaniline electrospun nanofibers for sensitive monitoring of bisphenol-A. ACS Sustain. Chem. Eng. 2023, 11, 4299–4307. [Google Scholar]
- Dey, B.; Ahmad, M.W.; Al-Shannaq, R.; Al-Humaidi, J.Y.; Hossain, S.K.S.; Patra, C.N.; Althomali, R.H.; Rahman, M.M.; Choudhury, A. Non-Enzymatic Electrochemical Sensing of Bisphenol A in Drinking Water and Milk Using Bimetallic Nickel-Copper Metal-Organic Framework. J. Anal. Test. 2024, 1–15. [Google Scholar] [CrossRef]
- Quezada, V.; Martinez, T.; Nelson, R.; Pérez-Fehrmann, M.; Zaragoza, G.; Vizcarra, A.; Kesternich, V.; Hernández-Saravia, L.P. A novel platform of using copper (II) complex with triazole-carboxilated modified as bidentated ligand SPCE for the detection of hydrogen peroxide in milk. J. Electroanal. Chem. 2020, 879, 114763. [Google Scholar]
- Liu, T.; Guo, Y.; Zhang, Z.; Miao, Z.; Zhang, X.; Su, Z. Fabrication of hollow CuO/PANI hybrid nanofibers for non-enzymatic electrochemical detection of H2O2 and glucose. Sens. Actuators B Chem. 2019, 286, 370–376. [Google Scholar]
- Zhu, Y.; Miao, Q.; Han, B. Electrospinning Synthesis of Ag Nanoparticles-doped Carbon Nanofibers for Voltammetric Determination of H2O2. Int. J. Electrochem. Sci. 2021, 16, 210226. [Google Scholar]




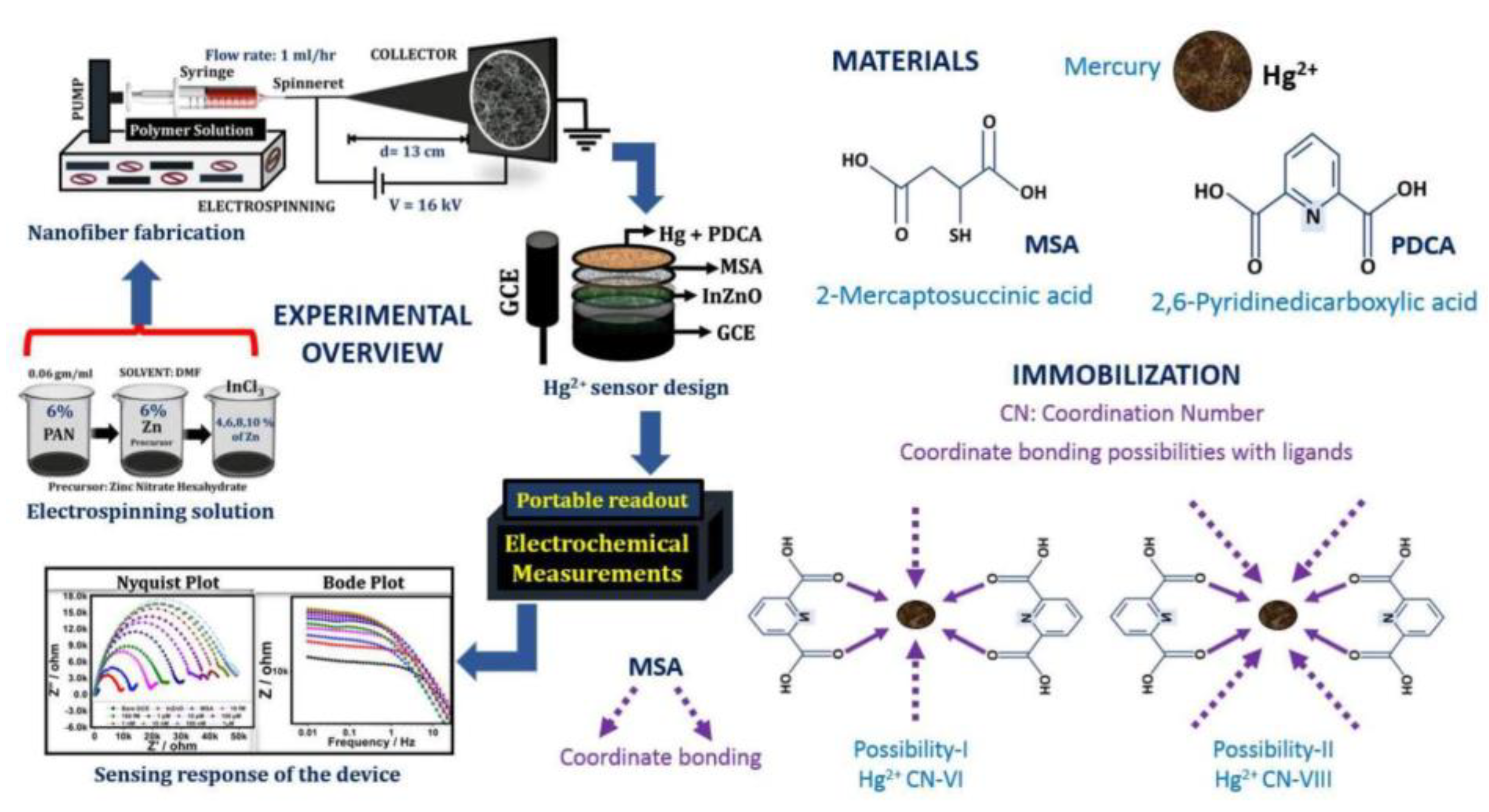

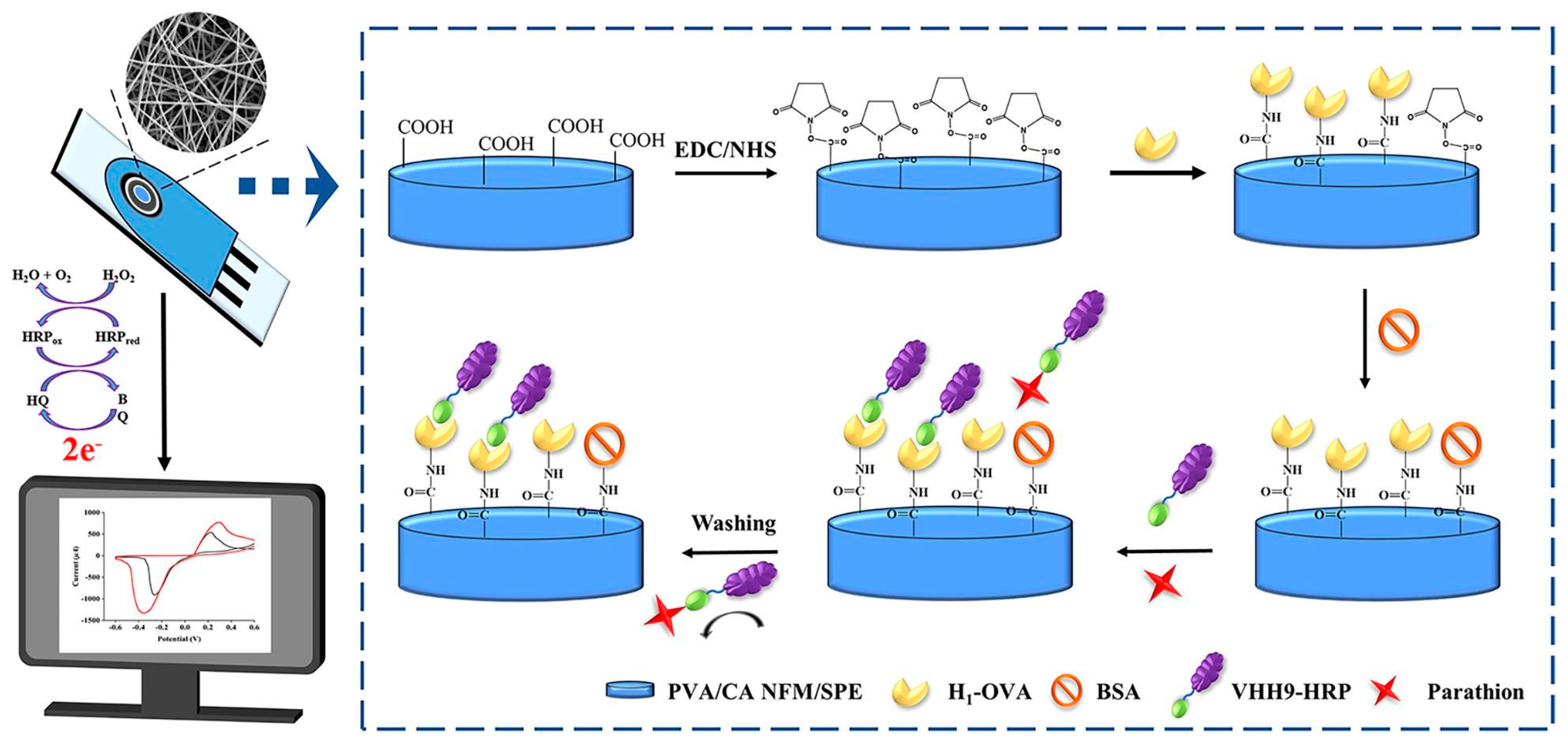



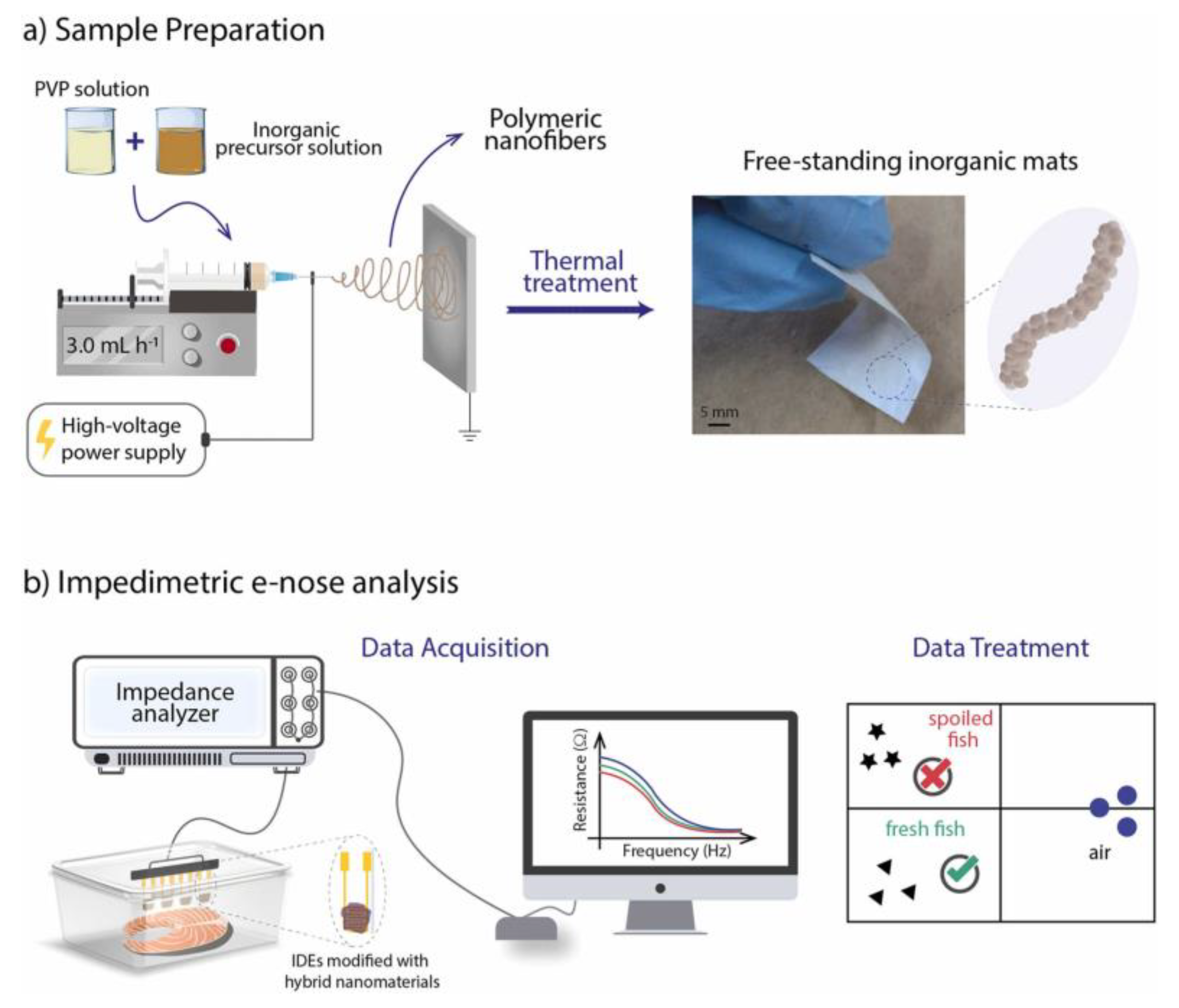
| Analyte | Material | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|
| Hg2+ | PA6/CNW:rGO nanofiber | 2.5–200 μM | 5.2 nM | [75] |
| Hg2+ | Aptamer/Au/Pt@CNF | 1.0 × 10−15–1.0 × 10−6 M | 3.33 × 10−16 M | [77] |
| Hg2+ | InZnO nanofiber | 10 fM–1 µM | 3.13 fM | [78] |
| Hg2+ | Ce-ZnO hybrid nanofibers | 0.1–100 μM | 267 nM | [80] |
| Hg2+ | PANFs-CANFs | 30–450 nM | 9.98 nM | [81] |
| Cd2+ | Co/Zn-ZIF nanofibers | 100 nM–1 mM | 27.27 nM | [88] |
| Malathion | Nafion/ENCF-67@Au/CQDs | 1.0 × 10−12–1.0 × 10−7 M | 3.3 × 10−13 M | [99] |
| Diazinon | Poly(ε-caprolactone)/CHIT nanofibers | 3–100 nM | 2.888 nM | [104] |
| Atrazine | SnO2 nanofibers | 1 zM–1 μM | 0.9 zM | [106] |
| Chlorpyrifos | Fe-PyDA/CNF | 1.0–150 μM | 15.1 nM | [108] |
| Methyl parathion | BCL@MOF nanofiber/CHIT | 0.1–38 µM | 0.067 µM | [110] |
| Diphenylamine | TiO2/Au nanofibers | 0.05–60 µM | 0.009 µM | [111] |
| Benomyl | EuVO4/CNF | 0.125–23.875 µM | 0.00612 µM | [114] |
| Tetracycline | AgZFO/CHIT-CNF | 0.2–53.2 μM | 1 nM | [117] |
| Nitrofurazone | TiO2/Au-NFs/O-C3N4 | 0.008–105 μM | 0.001 μM | [118] |
| Metronidazole | CNF/Fe2WO6 composite | 0.01–1792 μM | 0.013 μM | [120] |
| Acetaminophen | PANI-CoPc-fur-f-MWCNTs | 10–200 μM | 0.094 μM | [123] |
| Nitrite | Bi/HCNF | 0.1–800 μM | 19 nM | [127] |
| Nitrite | Au WNWs/CNFs-Gr hybrid | 1.98 µM–3.77 mM | 1.24 µM | [128] |
| Rutin | MXene-FeWO4 nanofibers | 4–147 nM | 0.42 nM | [129] |
| Sunset yellow | RuO2 nanofibers/PSSA | 0.0005–9.0 μM | 0.38 nM | [130] |
| Luteolin | SWCNHs/CNF | 0.01–50 μM | 3.714 nM | [131] |
| Vanillin | LCO@CNF hybrid composites | 0.01–1670 μM | 4.67 nM | [132] |
| Vanillin | La2NiO4-CNF | 5 nM–3035 µM | 6 nM | [133] |
| Tartrazine | Ni2P@f-CNF | 0.01–1875 µM | 0.011 µM | [134] |
| Tartrazine | NdVO4@F-CNF | 0.05–271.6 μM | 0.0011 μM | [136] |
| Escherichia coli | Bacteriophages/CNF | 102–106 CFU/mL | 36 CFU/mL | [141] |
| Escherichia coli | CANF-decorated PBSP | 1.5 × 102–106 CFU/mL | 30 CFU/mL | [144] |
| Zearalenone | Bi2S3@CNF | 0.125–375.5 μM 438–1951 μM | 0.61 μM | [146] |
| Aflatoxin M1 | ss-HSDNA/AuNPs/ECNF mat | 1–100 pg/mL | 0.6 pg/mL | [147] |
| Penicillin | AuNPs/ECNF mat | 1–400 ng/mL | 0.6 ng/mL | [148] |
| Ochratoxin A | Ti3C2Tx/PVDF nanofiber composites | 1 fg/mL–1 ng/mL | 2.15 fg/mL | [150] |
| Aflatoxin B1 | Zein/PPy nanofibers immobilized with anti-AFB1 antibodies | 0.25–10 ng/mL | 0.092 ng/mL | [151] |
| Trimethylamine | PVAc/CA nanofiber | 0.5–2.5 ppm | 19 ppb | [157] |
| Trimethylamine | PAN/PANI fiber membranes | 6 ppb–1.1 ppm | 6 ppb | [159] |
| Trimethylamine | ZnWO4/ZnO nanofibers | 0.5–50 ppm | 150 ppb | [162] |
| Ammonia | Maltodextrin coated PVAc nanofibers | 10–250 ppm | 1.92 ppm | [168] |
| Histamine | Mn-Co(2-MeIm)MOF@CNF mat | 10–1500 µM | 89.6 nM | [171] |
| H2S | CuO/Co3O4 nanofiber | 0.5–100 ppm | 500 ppb | [172] |
| Bisphenol A | AuNDs@CNFs | 0.01–50 μM | 5 nM | [174] |
| Bisphenol A | CuO-NiO/CA-PANI@nanofiber | 2–100 nM | 0.6 nM | [175] |
| Bisphenol A | Ni-Cu(PDA)MOF/CNF | 1–150 µM | 75 nM | [176] |
| Hydrogen peroxide | CuO/PANI nanocomposite fiber | 5 μm–9.255 mM | 0.110 × 10−6 M | [178] |
| Hydrogen peroxide | AgNPs-doped CNF | 0.01–50 mM | 3 μM | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Tan, J.; Li, Y. Application of Electrospun Nanofiber-Based Electrochemical Sensors in Food Safety. Molecules 2024, 29, 4412. https://doi.org/10.3390/molecules29184412
Xu C, Tan J, Li Y. Application of Electrospun Nanofiber-Based Electrochemical Sensors in Food Safety. Molecules. 2024; 29(18):4412. https://doi.org/10.3390/molecules29184412
Chicago/Turabian StyleXu, Changdong, Jianfeng Tan, and Yingru Li. 2024. "Application of Electrospun Nanofiber-Based Electrochemical Sensors in Food Safety" Molecules 29, no. 18: 4412. https://doi.org/10.3390/molecules29184412
APA StyleXu, C., Tan, J., & Li, Y. (2024). Application of Electrospun Nanofiber-Based Electrochemical Sensors in Food Safety. Molecules, 29(18), 4412. https://doi.org/10.3390/molecules29184412








