The Push-Out Bond Strength, Surface Roughness, and Antimicrobial Properties of Endodontic Bioceramic Sealers Supplemented with Silver Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Synthesis of AgNPs
2.2. Push-Out Bond Strength Test
2.3. Type of Failure
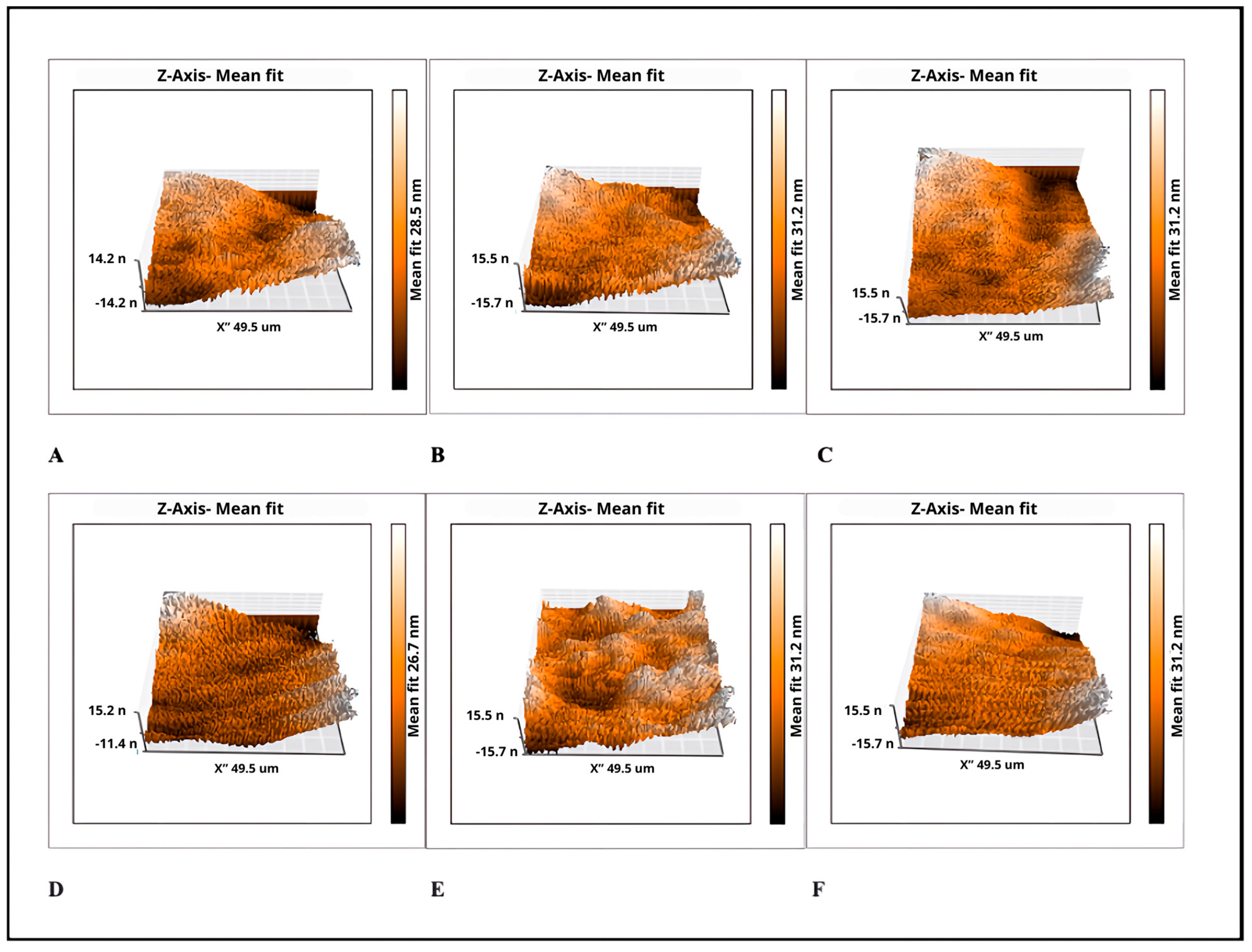
2.4. Surface Roughness Measurement
2.5. Disk Diffusion Test
2.6. Plate Microdilution Method
3. Discussion
4. Materials and Methods
4.1. Chemical Synthesis of NPs
4.2. Push-Out Bond Strength Test
4.3. Surface Roughness Measurement
4.4. Disk Diffusion Test
4.5. Plate Microdilution Method
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pirani, C.; Camilleri, J. Effectiveness of root canal filling materials and techniques for treatment of apical periodontitis: A systematic review. Int. Endod. J. 2023, 56 (Suppl. 3), 436–454. [Google Scholar] [CrossRef] [PubMed]
- Ordinola-Zapata, R.; Noblett, W.C.; Perez-Ron, A.; Ye, Z.; Vera, J. Present status and future directions of intracanal medicaments. Int. Endod. J. 2022, 55 (Suppl. 3), 613–636. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rôças, I.N. Present status and future directions: Microbiology of endodontic infections. Int. Endod. J. 2022, 55 (Suppl. 3), 512–530. [Google Scholar] [CrossRef] [PubMed]
- Gasner, N.S.; Brizuela, M. Endodontic Materials Used to Fill Root Canals. 2023 Mar 19. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Marconi, D.F.; da Silva, G.S.; Weissheimer, T.; Silva, I.A.; Só, G.B.; Jahnke, L.T.; Skupien, J.A.; Só, M.V.R.; da Rosa, R.A. Influence of the root canal filling technique on the success rate of primary endodontic treatments: A systematic review. Restor. Dent. Endod. 2022, 47, e40. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiyasat, A.S.; Alfirjani, S.A. The effect of obturation techniques on the push-out bond strength of a premixed bioceramic root canal sealer. J. Dent. 2019, 89, 103169. [Google Scholar] [CrossRef]
- Baras, B.H.; Melo, M.A.S.; Thumbigere-Math, V.; Tay, F.R.; Fouad, A.F.; Oates, T.W.; Weir, M.D.; Cheng, L.; Xu, H.H.K. Novel Bioactive and Therapeutic Root Canal Sealers with Antibacterial and Remineralization Properties. Materials 2020, 13, 1096. [Google Scholar] [CrossRef]
- Vishwanath, V.; Rao, H.M. Gutta-percha in endodontics—A comprehensive review of material science. J. Conserv. Dent. 2019, 22, 216–222. [Google Scholar] [CrossRef]
- Pandey, P.; Aggarwal, H.; Tikku, A.P.; Singh, A.; Bains, R.; Mishra, S. Comparative evaluation of sealing ability of gutta percha and resilon as root canal filling materials- a systematic review. J. Oral Biol. Craniofac. Res. 2020, 10, 220–226. [Google Scholar] [CrossRef]
- Komabayashi, T. Comprehensive review of current endodontic sealers. Dent. Mater. J. 2020, 39, 703–720. [Google Scholar] [CrossRef]
- Vo, K.; Daniel, J.; Ahn, C.; Primus, C.; Komabayashi, T. Coronal and apical leakage among five endodontic sealers. J. Oral Sci. 2022, 64, 95–98. [Google Scholar] [CrossRef]
- Al-Haddad, A.; Che Ab Aziz, Z.A. Bioceramic-Based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Kumar, A.; Badiyani, B.K.; Sukhtankar, L.; Madhumitha, M.; Kumar, A. Comparison of Sealing Ability of Bioceramic Sealer, AH Plus, and GuttaFlow in Conservatively Prepared Curved Root Canals Obturated with Single-Cone Technique: An In vitro Study. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. 1), S857–S860. [Google Scholar] [CrossRef] [PubMed]
- López-García, S.; Myong-Hyun, B.; Lozano, A.; García-Bernal, D.; Forner, L.; Llena, C.; Guerrero-Gironés, J.; Murcia, L.; Rodríguez-Lozano, F.J. Cytocompatibility, bioactivity potential, and ion release of three premixed calcium silicate-based sealers. Clin. Oral Investig. 2020, 24, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Liu, S.Y.; Wu, J.L.; Qiu, D.; Dong, Y.M. A novel bioactive glass-based root canal sealer in endodontics. J. Dent. Sci. 2022, 17, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Talabani, R.M.; Garib, B.T.; Masaeli, R. Bioactivity and Physicochemical Properties of Three Calcium Silicate-Based Cements: An In Vitro Study. Biomed. Res. Int. 2020, 2020, 9576930. [Google Scholar] [CrossRef]
- Motwani, N.; Ikhar, A.; Nikhade, P.; Chandak, M.; Rathi, S.; Dugar, M.; Rajnekar, R. Premixed bioceramics: A novel pulp capping agent. J. Conserv. Dent. 2021, 24, 124–129. [Google Scholar] [CrossRef]
- Dong, X.; Xu, X. Bioceramics in Endodontics: Updates and Future Perspectives. Bioengineering 2023, 10, 354. [Google Scholar] [CrossRef]
- Jafari, F.; Jafari, S. Composition and physicochemical properties of calcium silicate based sealers: A review article. J. Clin. Exp. Dent. 2017, 9, e1249–e1255. [Google Scholar] [CrossRef]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Martínez-Castañon, G.A.; Nino-Martinez, N.; Martinez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Ma, J.; Li, K.; Gu, S. Selective strategies for antibacterial regulation of nanomaterials. RSC Adv. 2022, 12, 4852–4864. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Milani, M.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A.; Tayefi Nasrabadi, H.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; et al. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2016, 42, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Nandi, A.; Simnani, F.Z.; Saha, U.; Ghosh, A.; Sinha, A.; Sahay, A.; Samal, S.K.; Panda, P.K.; Verma, S.K. Emerging Trends in Advanced Translational Applications of Silver Nanoparticles: A Progressing Dawn of Nanotechnology. J. Funct. Biomater. 2023, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Haghgoo, R.; Ahmadvand, M.; Nyakan, M.; Jafari, M. Antimicrobial Efficacy of Mixtures of Nanosilver and Zinc Oxide Eugenol against Enterococcus faecalis. J. Contemp. Dent. Pract. 2017, 18, 177–181. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef]
- Oncu, A.; Huang, Y.; Amasya, G.; Sevimay, F.S.; Orhan, K.; Celikten, B. Silver nanoparticles in endodontics: Recent developments and applications. Restor. Dent. Endod. 2021, 46, e38. [Google Scholar] [CrossRef]
- Alghamdi, F.; Shakir, M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus 2020, 12, e7257. [Google Scholar] [CrossRef]
- Prada, I.; Micó-Muñoz, P.; Giner-Lluesma, T.; Micó-Martínez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of microbiology on endodontic failure. Literature review. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e364–e372. [Google Scholar] [CrossRef]
- Kharouf, N.; Arntz, Y.; Eid, A.; Zghal, J.; Sauro, S.; Haikel, Y.; Mancino, D. Physicochemical and Antibacterial Properties of Novel, Premixed Calcium Silicate-Based Sealer Compared to Powder-Liquid Bioceramic Sealer. J. Clin. Med. 2020, 9, 3096. [Google Scholar] [CrossRef]
- Dalmia, S.; Gaikwad, A.; Samuel, R.; Aher, G.; Gulve, M.; Kolhe, S. Antimicrobial Efficacy of Different Endodontic Sealers against Enterococcus faecalis: An In vitro Study. J. Int. Soc. Prev. Community Dent. 2018, 8, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kaukab, A.; Gaur, S.; Agnihotri, R.; Taneja, V. Silver Nanoparticles as an Intracanal Medicament: A Scoping Review. Sci. World J. 2023, 2023, 9451685. [Google Scholar] [CrossRef] [PubMed]
- Francisco, P.A.; Fagundes, P.I.D.G.; Lemes-Junior, J.C.; Lima, A.R.; Passini, M.R.Z.; Gomes, B.P.F.A. Pathogenic potential of Enterococcus faecalis strains isolated from root canals after unsuccessful endodontic treatment. Clin. Oral Investig. 2021, 25, 5171–5179. [Google Scholar] [CrossRef] [PubMed]
- Samiei, M.; Aghazadeh, M.; Lotfi, M.; Shakoei, S.; Aghazadeh, Z.; Vahid Pakdel, S.M. Antimicrobial Efficacy of Mineral Trioxide Aggregate with and without Silver Nanoparticles. Iran. Endod. J. 2013, 8, 166–170. [Google Scholar]
- Jonaidi-Jafari, N.; Izadi, M.; Javidi, P. The effects of silver nanoparticles on antimicrobial activity of ProRoot mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM). J. Clin. Exp Dent. 2016, 8, e22–e26. [Google Scholar] [CrossRef]
- Teixeira, A.B.V.; Vidal, C.L.; de Castro, D.T.; de Oliveira-Santos, C.; Schiavon, M.A.; Dos Reis, A.C. Incorporating Antimicrobial Nanomaterial and its Effect on the Antimicrobial Activity, Flow and Radiopacity of Endodontic Sealers. Eur. Endod. J. 2017, 2, 1–6. [Google Scholar] [CrossRef]
- Seung, J.; Weir, M.D.; Melo, M.A.S.; Romberg, E.; Nosrat, A.; Xu, H.H.K.; Tordik, P.A. A Modified Resin Sealer: Physical and Antibacterial Properties. J. Endod. 2018, 44, 1553–1557. [Google Scholar] [CrossRef]
- Dem, K.; Wu, Y.; Kaminga, A.C.; Dai, Z.; Cao, X.; Zhu, B. The push out bond strength of polydimethylsiloxane endodontic sealers to dentin. BMC Oral Health 2019, 19, 181. [Google Scholar] [CrossRef]
- Nikhade, P.; Kela, S.; Chandak, M.; Chandwani, N.; Adwani, F. Comparative evaluation of push-out bond strength of calcium silicate-based materials: An ex-vivo study. J. Dent. Med. Sci. 2016, 15, 65–68. [Google Scholar]
- Reymus, M.; Fotiadou, C.; Kessler, A.; Heck, K.; Hickel, R.; Diegritz, C. 3D printed replicas for endodontic education. Int. Endod. J. 2019, 52, 123–130. [Google Scholar] [CrossRef]
- De-Deus, G.; Souza, E.M.; Silva, E.J.N.L.; Belladonna, F.G.; Simões-Carvalho, M.; Cavalcante, D.M.; Versiani, M.A. A critical analysis of research methods and experimental models to study root canal fillings. Int. Endod. J. 2022, 55 (Suppl. 2), 384–445. [Google Scholar] [CrossRef] [PubMed]
- Aminoshariae, A.; Primus, C.; Kulild, J.C. Tricalcium silicate cement sealers: Do the potential benefits of bioactivity justify the drawbacks? J. Am. Dent. Assoc. 2022, 153, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Frasquetti, K.S.; Piasecki, L.; Kowalczuck, A.; Carneiro, E.; Westphalen, V.P.D.; Neto, U.X.D.S. Effect of Different Root Canal Drying Protocols on the Bond Strength of Two Bioceramic Sealers. Eur. J. Dent. 2023, 17, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, S.A.L.; Alves Dos Santos, G.N.; Silva-Sousa, A.C.; Camargo, R.V.; Silva-Sousa, Y.T.; Lopes-Olhê, F.C.; Mazzi-Chaves, J.F.; Sousa-Neto, M.D. Influence of bioceramic cones on the quality of root canal filling relative to bond strength and adaptation of the adhesive interface. Clin. Oral Investig. 2023, 27, 7919–7933. [Google Scholar] [CrossRef] [PubMed]
- Retana-Lobo, C.; Tanomaru-Filho, M.; Guerreiro-Tanomaru, J.M.; Benavides-García, M.; Hernández-Meza, E.; Reyes-Carmona, J. Push-Out Bond Strength, Characterization, and Ion Release of Premixed and Powder-Liquid Bioceramic Sealers with or without Gutta-Percha. Scanning 2021, 2021, 6617930. [Google Scholar] [CrossRef]
- Do Prado, M.; De Assis, D.F.; Gomes, B.P.; Simão, R.A. Adhesion of resin-based sealers to dentine: An atomic force microscopy study. Int. Endod. J. 2014, 47, 1052–1057. [Google Scholar] [CrossRef]
- Kılıç, V.; Gök, A. Effect of different polishing systems on the surface roughness of various bulk-fill and nano-filled resin-based composites: An atomic force microscopy and scanning electron microscopy study. Microsc. Res. Tech. 2021, 84, 2058–2067. [Google Scholar] [CrossRef]
- Mühlemann, S.; Bernini, J.M.; Sener, B.; Hämmerle, C.H.; Özcan, M. Effect of Aging on Stained Monolithic Resin-Ceramic CAD/CAM Materials: Quantitative and Qualitative Analysis of Surface Roughness. J. Prosthodont. 2019, 28, e563–e571. [Google Scholar] [CrossRef]
- Dos Santos, P.H.; Pavan, S.; Suzuki, T.Y.; Briso, A.L.; Assunção, W.G.; Sinhoreti, M.A.; Correr-Sobrinho, L.; Consani, S. Effect of fluid resins on the surface roughness and topography of resin composite restorations analyzed by atomic force microscope. J. Mech. Behav. Biomed. Mater. 2011, 4, 433–439. [Google Scholar] [CrossRef]
- Kharouf, N.; Sauro, S.; Eid, A.; Zghal, J.; Jmal, H.; Seck, A.; Macaluso, V.; Addiego, F.; Inchingolo, F.; Affolter-Zbaraszczuk, C.; et al. Physicochemical and Mechanical Properties of Premixed Calcium Silicate and Resin Sealers. J. Funct. Biomater. 2022, 14, 9. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, Y.; Haapasalo, M. Antimicrobial and Antibiofilm Properties of Bioceramic Materials in Endodontics. Materials 2021, 14, 7594. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Mala, K.; Kamath, P.M. Biofilm models in endodontics-A narrative review. J. Conserv. Dent. 2021, 24, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Bhandi, S.; Mehta, D.; Mashyakhy, M.; Chohan, H.; Testarelli, L.; Thomas, J.; Dhillon, H.; Raj, A.T.; Madapusi Balaji, T.; Varadarajan, S.; et al. Antimicrobial Efficacy of Silver Nanoparticles as Root Canal Irrigant’s: A Systematic Review. J. Clin. Med. 2021, 10, 1152. [Google Scholar] [CrossRef]
- Capuano, N.; Amato, A.; Dell’Annunziata, F.; Giordano, F.; Folliero, V.; Di Spirito, F.; More, P.R.; De Filippis, A.; Martina, S.; Amato, M.; et al. Nanoparticles and Their Antibacterial Application in Endodontics. Antibiotics 2023, 12, 1690. [Google Scholar] [CrossRef]
- Haapasalo, M.; Orstavik, D. In vitro infection and disinfection of dentinal tubules. J. Dent. Res. 1987, 66, 1375–1379. [Google Scholar] [CrossRef]
- Badawy, R.E.; Mohamed, D.A. Evaluation of new bioceramic endodontic sealers: An in vitro study. Dent. Med. Probl. 2022, 59, 85–92. [Google Scholar] [CrossRef]
- Tonini, R.; Salvadori, M.; Audino, E.; Sauro, S.; Garo, M.L.; Salgarello, S. Irrigating Solutions and Activation Methods Used in Clinical Endodontics: A Systematic Review. Front. Oral Health 2022, 3, 838043, Erratum in Front. Oral Health 2022, 3, 876265. [Google Scholar] [CrossRef]
- Tosco, V.; Monterubbianesi, R.; Aranguren, J.; Memè, L.; Putignano, A.; Orsini, G. Evaluation of the Efficacy of Different Irrigation Systems on the Removal of Root Canal Smear Layer: A Scanning Electron Microscopic Study. Appl. Sci. 2023, 13, 149. [Google Scholar] [CrossRef]
- CLSI Document M100-S25; Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Fifth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015.


| Group | Cervical | Middle | Apical | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (MPa) | Std. Error | p-Value | Mean ± SD (MPa) | Std. Error | p-Value | Mean ± SD (MPa) | Std. Error | p-Value | |
| G1 CeraSeal | 8.5 ± 2.8 | 0.6 | 14.9 ± 4.7 | 1.0 | 45.3 ± 13.8 | 3.0 | |||
| G2 CeraSeal + AgNPs | 6.6 ± 3.6 | 0.8 | 0.128 | 14.1 ± 5.4 | 1.2 | 0.983 | 49.2 ± 14.2 | 3.1 | 0.96 |
| G3 EndoSeq | 4.7 ± 2.2 | 0.5 | 11.3 ± 3.6 | 0.8 | 40.4 ± 13.7 | 3.0 | |||
| G4EndoSeq | 3.6 ± 1.7 | 0.3 | 0.648 | 6.7 ± 2.8 | 0.6 | 0.002 | 34.3 ± 16.6 | 3.7 | 0.772 |
| +AgNPs | |||||||||
| G5 Bio-C Sealer | 2.3 ± 0.9 | 0.2 | 4.5 ± 2.4 | 0.5 | 13.7 ± 7.6 | 1.7 | |||
| G6 Bio-C Sealer + AgNPs | 2.6 ± 1.5 | 0.3 | 0.997 | 4.2 ± 2.0 | 0.4 | 0.998 | 14.2 ± 6.7 | 1.5 | 0.998 |
| Group | Sa | Sy | ||||
|---|---|---|---|---|---|---|
| Mean ± SD (nm) | Std. Error | p-Value | Mean ± SD (nm) | Std. Error | p-Value | |
| G1 CeraSeal | 4.1 ± 0.8 | 0.8 | 38.9 ± 5.6 | 3.2 | ||
| G2 CeraSeal + AgNPs | 3.4 ± 1.0 | 0.5 | 0.723 | 36.1 ± 4.4 | 2.5 | 0.599 |
| G3 EndoSeq | 4.8 ± 0.2 | 0.1 | 39.2 ± 1.9 | 1.1 | ||
| G4 EndoSeq + AgNPs | 4.6 ± 0.7 | 0.4 | 0.118 | 38.2 ± 2.6 | 1.5 | 0.556 |
| G5 Bio-C Sealer | 4.2 ± 0.3 | 0.1 | 40.9 ± 4.1 | 2.4 | ||
| G6 Bio-C Sealer + AgNPs | 4.5 ± 0.4 | 0.2 | 0.438 | 43.9 ± 8.7 | 5.0 | 0.332 |
| Group | Mean ± SD | Std. Error | p-Value |
|---|---|---|---|
| G1 CeraSeal | 17.7 ± 0.8 | 0.4 | |
| G2 CeraSeal + AgNPs | 16.5 ± 1.6 | 0.9 | 0.275 |
| G3 EndoSeq | 16.3 ± 1.4 | 0.8 | |
| G4 EndoSeq + AgNPs | 20.9 ± 3.6 | 2.1 | 0.127 |
| G5 Bio-C Sealer | 15.3 ± 2.1 | 1.1 | |
| G6 Bio-C Sealer + AgNPs | 17.0 ± 2.8 | 1.6 | 0.376 |
| AgNPs (535 μg/mL) † | 14.2 ± 1.3 | 0.7 |
| Group | Mean ± SD (μg/mL) | Std. Error | p-Value |
|---|---|---|---|
| G1 CeraSeal | 133.7 ± 0.0 | 0.0 | |
| G2 CeraSeal + AgNPs | 133.7 ± 0.0 | 0.0 | 1.000 |
| G3 EndoSeq | 267.5 ± 0.0 | 0.0 | |
| G4 EndoSeq + AgNPs | 8.3 ± 0.0 | 0.0 | 0.025 |
| G5 Bio-C Sealer | 535.0 ± 0.0 | 0.0 | |
| G6 Bio-C Sealer + AgNPs | 267.5 ± 0.0 | 0.0 | 0.025 |
| AgNPs (535 μg/mL) † | 267.5 ± 0.0 | 0.0 |
| Name | Manufacturer | Composition * |
|---|---|---|
| CeraSeal® | Meta Biomed Co., Ltd., Cheongju, Republic of Korea | Zirconium dioxide, tricalcium silicate, dicalcium silicate, tricalcium aluminate, and thickening agent |
| EndoSequence® BC SealerTM | Brasseler USA, Savannah, GA, USA (EE. UU.) | Zirconium oxide, calcium silicates, calcium phosphate monobasic, calcium hydroxide, filler, and thickening agents |
| Bio-C® Sealer | Angelus, Londrína, PR, Brazil | Calcium silicates, calcium aluminate, calcium oxide, zirconium oxide, iron oxide, silicon dioxide, and dispersing agent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarrete-Olvera, K.; Niño-Martínez, N.; De Alba-Montero, I.; Patiño-Marín, N.; Ruiz, F.; Bach, H.; Martínez-Castañón, G.-A. The Push-Out Bond Strength, Surface Roughness, and Antimicrobial Properties of Endodontic Bioceramic Sealers Supplemented with Silver Nanoparticles. Molecules 2024, 29, 4422. https://doi.org/10.3390/molecules29184422
Navarrete-Olvera K, Niño-Martínez N, De Alba-Montero I, Patiño-Marín N, Ruiz F, Bach H, Martínez-Castañón G-A. The Push-Out Bond Strength, Surface Roughness, and Antimicrobial Properties of Endodontic Bioceramic Sealers Supplemented with Silver Nanoparticles. Molecules. 2024; 29(18):4422. https://doi.org/10.3390/molecules29184422
Chicago/Turabian StyleNavarrete-Olvera, Karla, Nereyda Niño-Martínez, Idania De Alba-Montero, Nuria Patiño-Marín, Facundo Ruiz, Horacio Bach, and Gabriel-Alejandro Martínez-Castañón. 2024. "The Push-Out Bond Strength, Surface Roughness, and Antimicrobial Properties of Endodontic Bioceramic Sealers Supplemented with Silver Nanoparticles" Molecules 29, no. 18: 4422. https://doi.org/10.3390/molecules29184422
APA StyleNavarrete-Olvera, K., Niño-Martínez, N., De Alba-Montero, I., Patiño-Marín, N., Ruiz, F., Bach, H., & Martínez-Castañón, G.-A. (2024). The Push-Out Bond Strength, Surface Roughness, and Antimicrobial Properties of Endodontic Bioceramic Sealers Supplemented with Silver Nanoparticles. Molecules, 29(18), 4422. https://doi.org/10.3390/molecules29184422







