C-C Bonding in Molecular Systems via Cross-Coupling-like Reactions Involving Noncovalently Bound Constituent Ions
Abstract
1. Introduction
2. Results and Discussion
2.1. CCl3-CCl4 → C2Cl6-Cl
2.2. NaCCl3
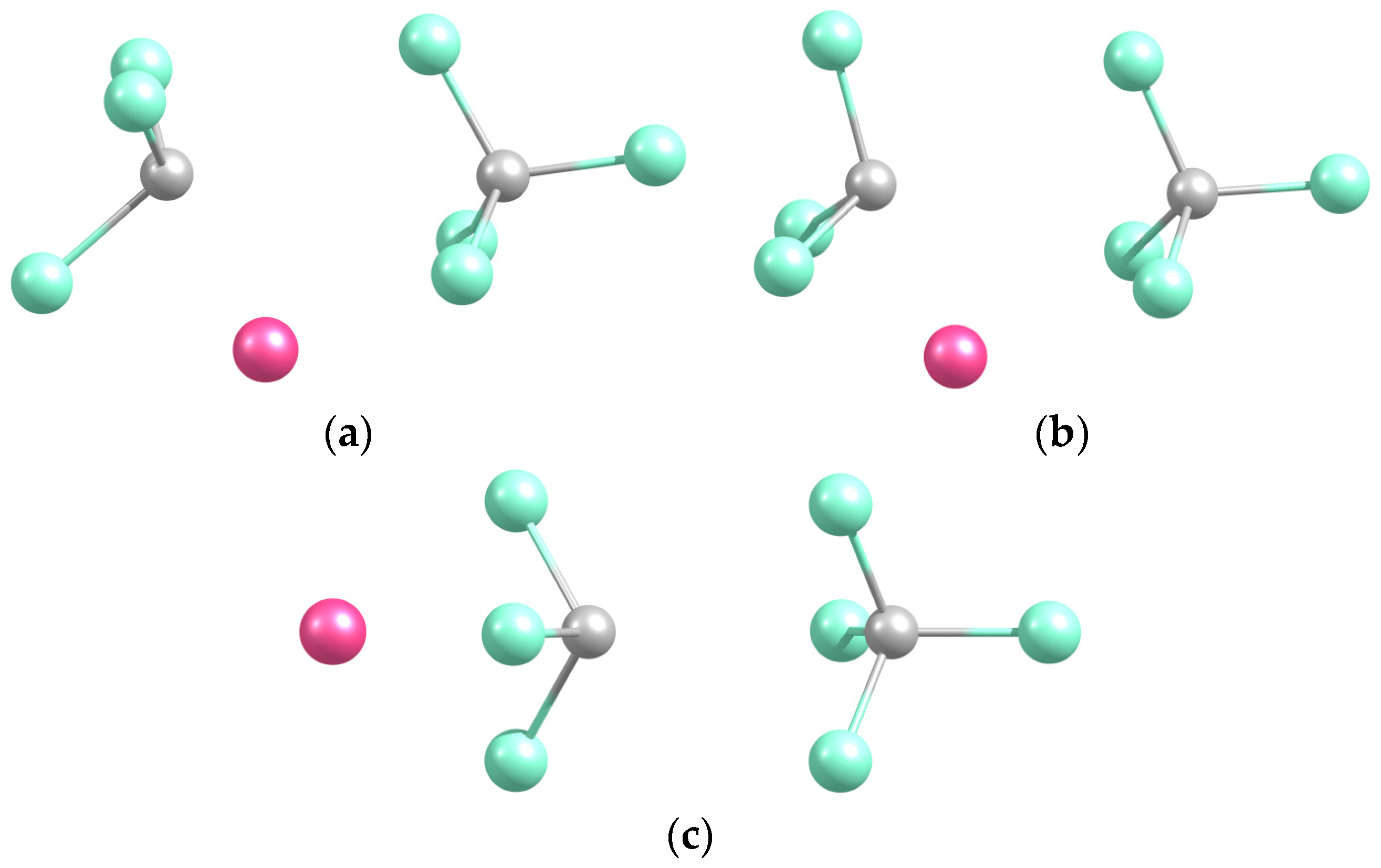
2.2.1. Structures and Stabilities
2.2.2. Charge Distributions
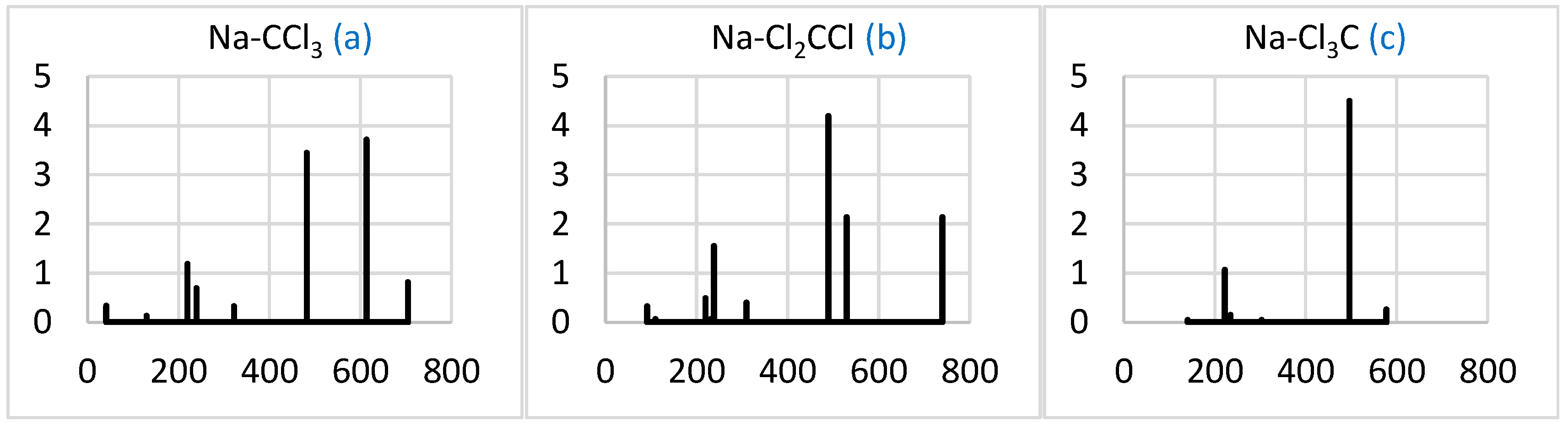
2.2.3. Simulated IR Spectra
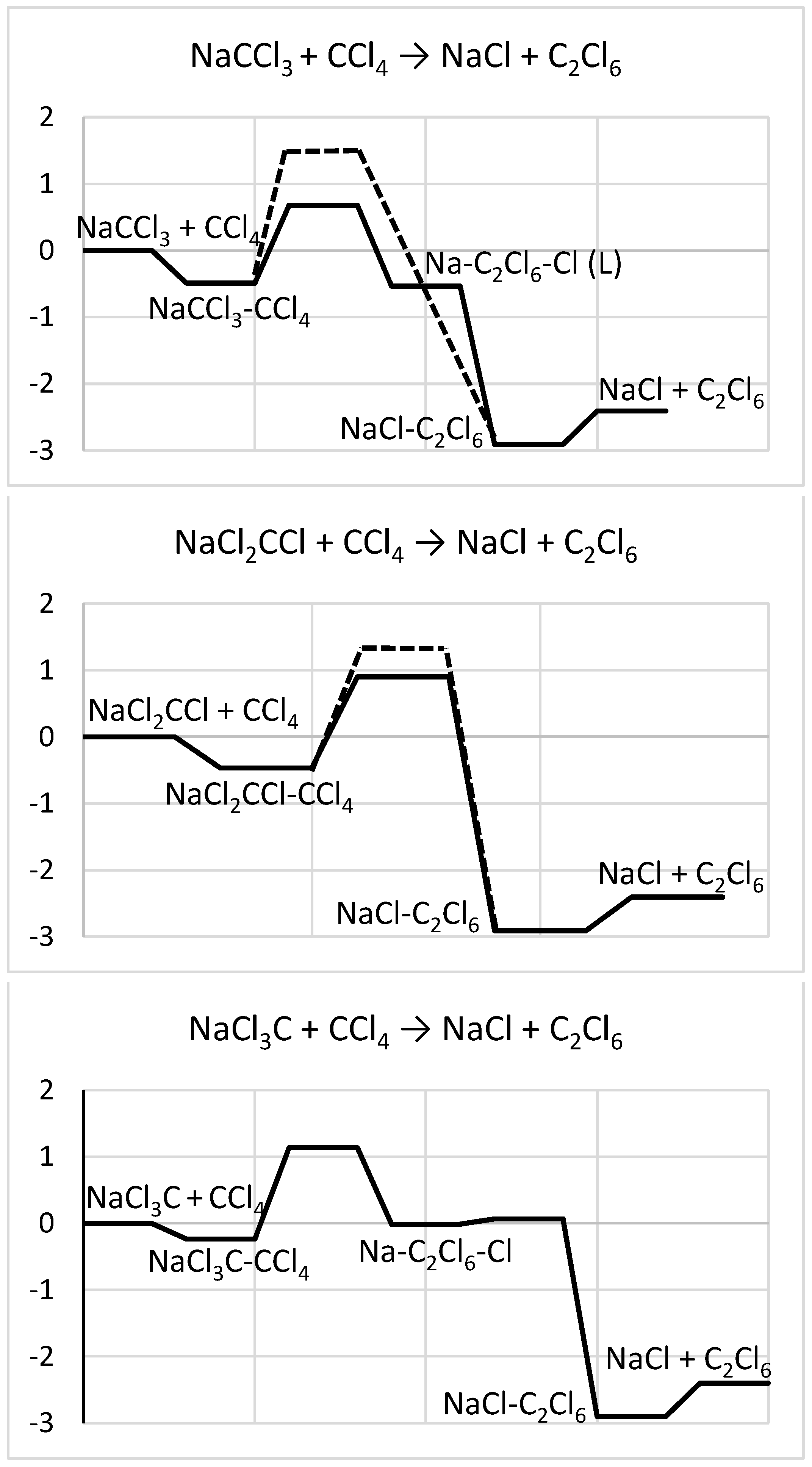
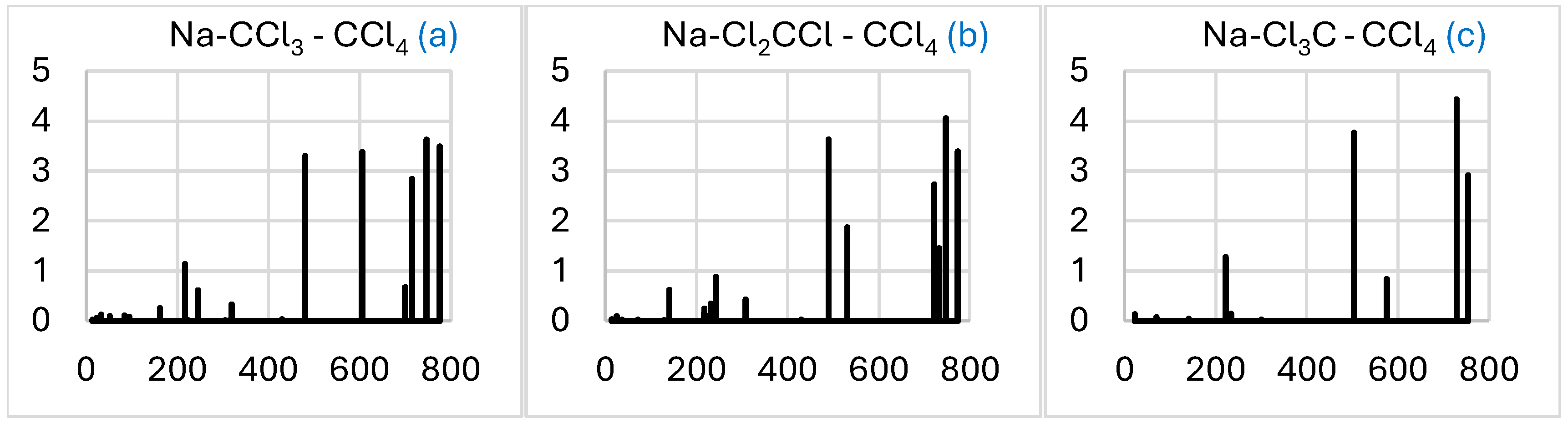
2.3. NaCCl3-CCl4 → C2Cl6-NaCl
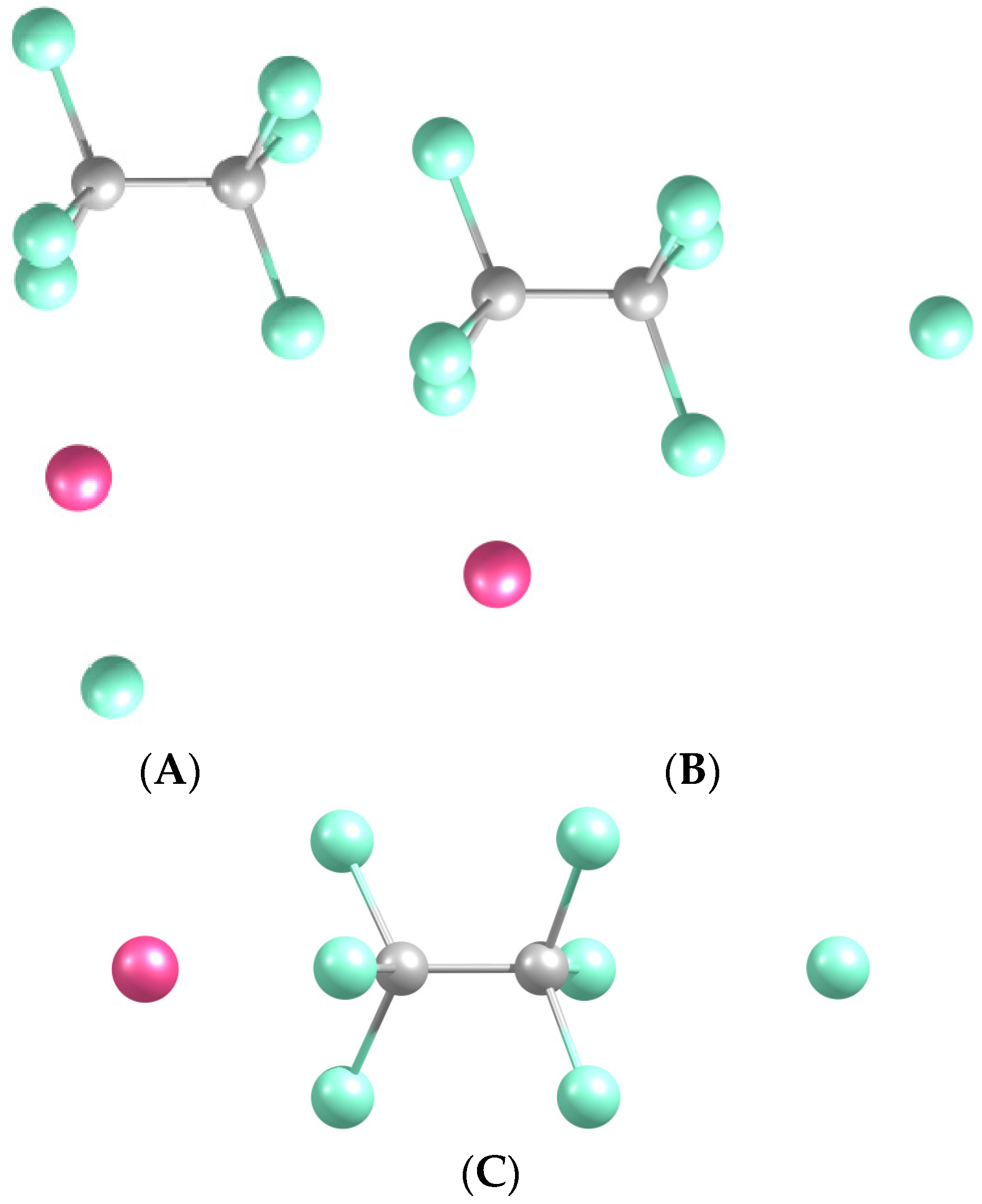
2.3.1. Structures and Stabilities
2.3.2. Charge Distributions
2.3.3. Simulated IR Spectra
2.4. CsCCl3-CCl4 → C2Cl6-CsCl
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seechurn, C.C.C.J.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Giammarco, M.; Naumkin, F.Y. Carbon-Carbon Bond Formation “Catalyzed” by Ion-Pair Constituents. ChemistrySelect 2023, 8, e202300057. [Google Scholar] [CrossRef]
- Cochrane, B.; Naumkin, F.Y. Reshaping and linking of molecules in ion-pair traps. Chem. Phys. Lett. 2016, 643, 137–141. [Google Scholar] [CrossRef]
- Kerr, S.; Naumkin, F.Y. Noncovalently bound complexes of polar molecules: Dipole-inside-of-dipole vs dipole-dipole systems. New J. Chem. 2017, 41, 13576–13584. [Google Scholar] [CrossRef]
- Sullivan, M.; Naumkin, F.Y. Highly polar insertion complexes with focused IR spectra and internal field-inhibited isomerization. ChemPlusChem 2020, 85, 2438–2445. [Google Scholar] [CrossRef] [PubMed]
- Naumkin, F.Y.; Wales, D.J. Counterion-trapped-molecules: From high polarity and enriched IR spectra to induced isomerization. ChemPhysChem 2020, 21, 348–355. [Google Scholar] [CrossRef]
- McDowell, S.A.C. A computational study of simultaneous cation/anion interactions in model clusters containing all-cis 1,2,3-trifluorocyclopropane (F3C3H3) and all-cis 1,2,3,4-tetrafluorobutane (F4C4H4). Chem. Phys. Lett. 2016, 665, 105–110. [Google Scholar] [CrossRef]
- McDowell, S.A.C. On the stability of clusters containing all-cis 1, 2, 3, 4, 5, 6-hexafluorocyclohexane. Comput. Theor. Chem. 2017, 1108, 18–22. [Google Scholar] [CrossRef]
- Naumkin, F.Y. Dipoles Inside of Dipoles: Insertion Complexes of Polar versus Nonpolar Molecules in Ion Pairs. J. Phys. Chem. A 2017, 121, 4545–4551. [Google Scholar] [CrossRef]
- Sullivan, M.; Naumkin, F.Y. Supramolecular complexes with insertion-enhanced polarity and tuned IR spectra. Int. J. Quantum Chem. 2021, 121, e26534. [Google Scholar] [CrossRef]
- Garau, C.; Quiñonero, D.; Frontera, A.; Ballester, P.; Costa, A.; Deyà, P.M. Anion–π interactions: Must the aromatic ring be electron deficient? New J. Chem. 2003, 27, 211–214. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J. Aromatic systems as charge insulators: Their simultaneous interaction with anions and cations. J. Phys. Chem. A 2003, 107, 9428–9433. [Google Scholar] [CrossRef]
- Alkorta, I.; Blanco, F.; Deya, P.M.; Elguero, J.; Estarellas, C.; Frontera, A.; Quinonero, D. Cooperativity in multiple unusual weak bonds. Theor. Chem. Acc. 2010, 126, 1. [Google Scholar] [CrossRef]
- Frontera, A.; Quinonero, D.; Deya, P.M. Cation–π and anion–π interactions. WIREs Comput. Mol. Sci. 2011, 1, 440–459. [Google Scholar] [CrossRef]
- Kochhar, G.; Naumkin, F.Y. Insertion complexes of an organic molecule trapped in ion-pairs. New J. Chem. 2010, 34, 2932–2936. [Google Scholar] [CrossRef]
- Trujillo, C.; Sanchez-Sanz, G.; Alkorta, I.; Elguero, J. Simultaneous interactions of anions and cations with cyclohexane and adamantane: Aliphatic cyclic hydrocarbons as charge insulators. J. Phys. Chem. A 2011, 115, 13124–13132. [Google Scholar] [CrossRef]
- He, Q.; Vargas-Zu, G.I.; Kim, S.H.; Kim, S.K.; Sessler, J.L. Macrocycles as ion pair receptors. Chem. Rev. 2019, 119, 9753–9835. [Google Scholar] [CrossRef]
- Aviles, J.R.; Berden, G.; Oomens, J.; Martın, B. Benchmark Ditopic Binding of Cl− and Cs+ by the Macrocycle Hexacyclen. ChemPhysChem 2017, 18, 1324–1332. [Google Scholar] [CrossRef]
- Saha, I.; Park, K.H.; Han, M.N.; Kim, S.K.; Lynch, V.M.; Sessler, J.L.; Lee, C.H. Calix [4] tetrahydrothiophenopyrrole: A Ditopic Receptor Displaying a Split Personality for Ion Recognition. Org. Lett. 2014, 16, 5414–5417. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A.; Dolak, J.A.; Waller, R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989, 43, 139–154. [Google Scholar] [CrossRef]
- Snedecor, G. Hexachloroethane. In Kirk-Othmer Concise Encyclopedia of Chemical Technology, 4th ed.; Kroschwitz, J.I., Ed.; John Wiley & Sons: New York, NY, USA, 1999; p. 428. [Google Scholar]
- Sowlati-Hashjin, S.; Šadek, V.; Sadjadi, S.A.; Karttunen, M.; Martín-Pendás, A.; Foroutan-Nejad, C. Collective interactions among organometallics are exotic bonds hidden on lab shelves. Nat. Commun. 2022, 13, 2069. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis set exchange: A community database for computational sciences. J. Chem. Inf. Model. 2007, 47, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Valiev, M.; Bylaska, E.J.; Govind, N.; Kowalski, K.; Straatsma, T.P.; van Dam, H.J.J.; Wang, D.; Nieplocha, J.; Apra, E.; Windus, T.L.; et al. NWChem: A comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 2010, 181, 1477–1489. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Ref. Database Number 69; NIST: Gaithersburg, MD, USA, 2023.
- Reed, A.E.; Robert, B.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Nikolaienko, T.Y.; Bulavin, L.A.; Hovorun, D.M. JANPA: An open source cross-platform implementation of the Natural Population Analysis on the Java platform. Comput. Theor. Chem. 2014, 1050, 15–22. [Google Scholar] [CrossRef]
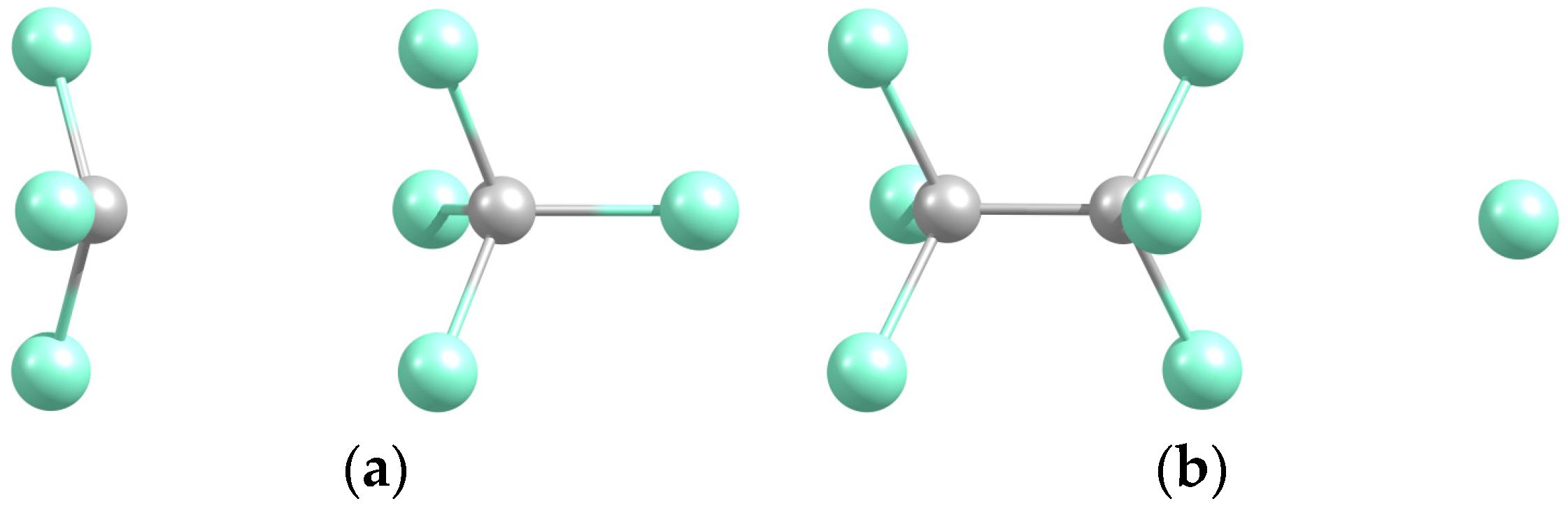
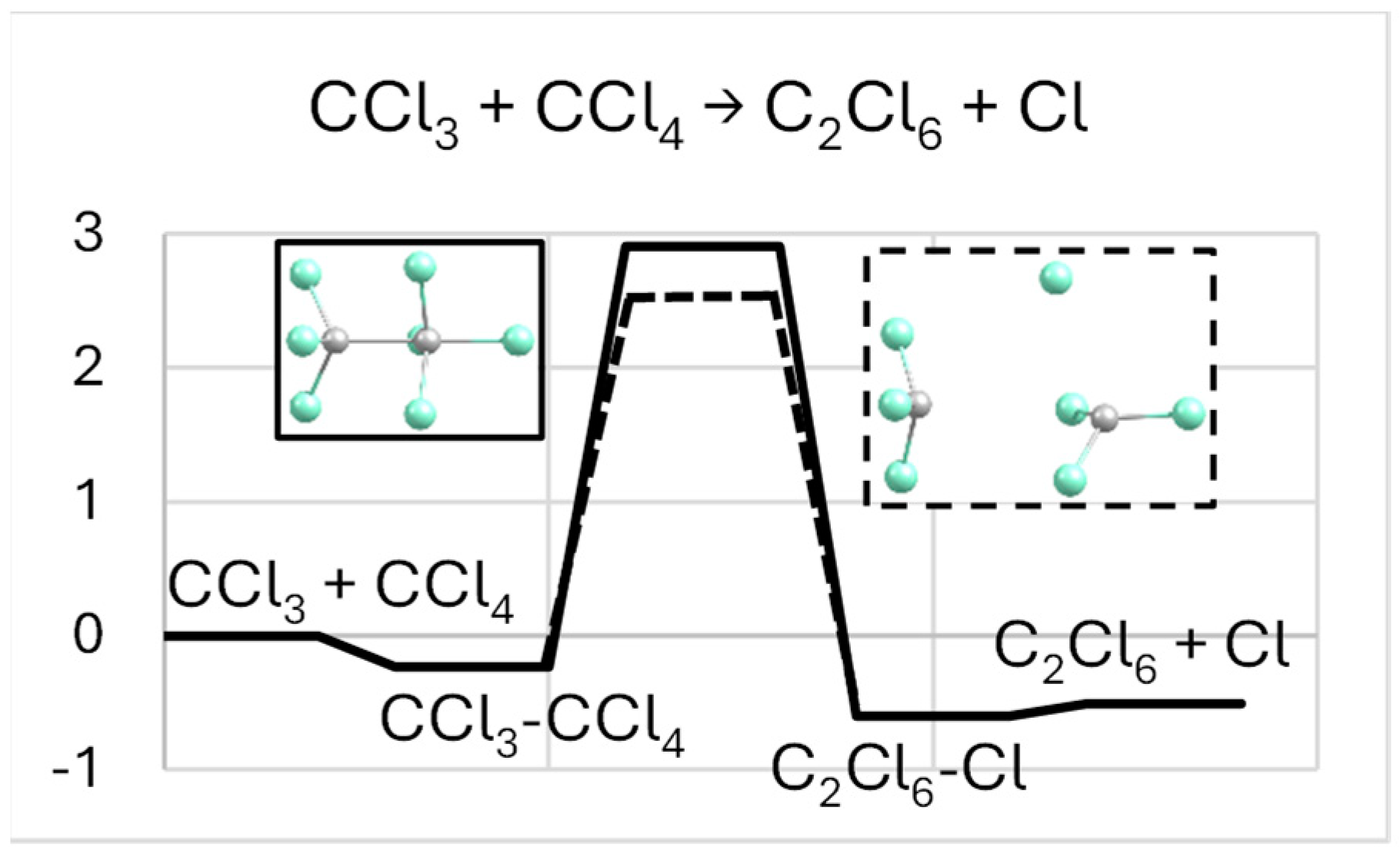
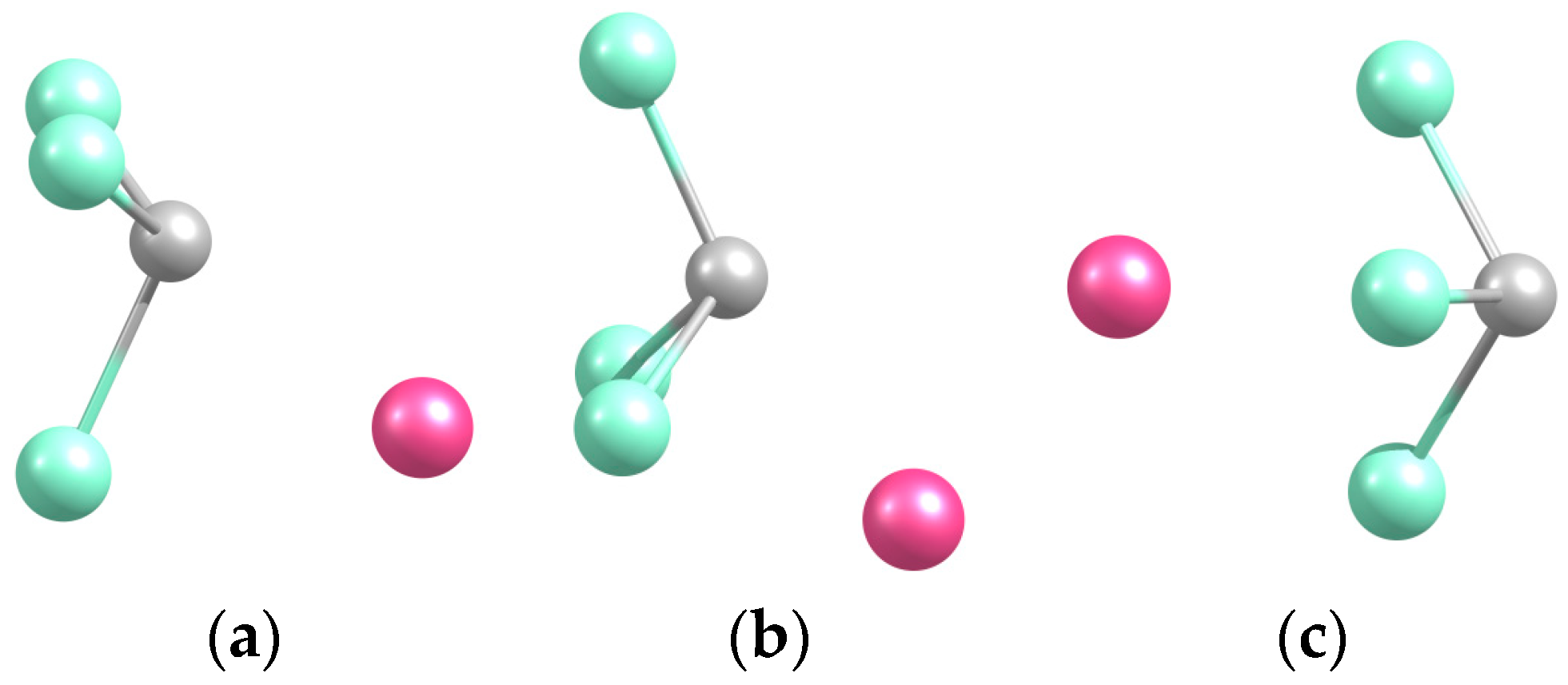
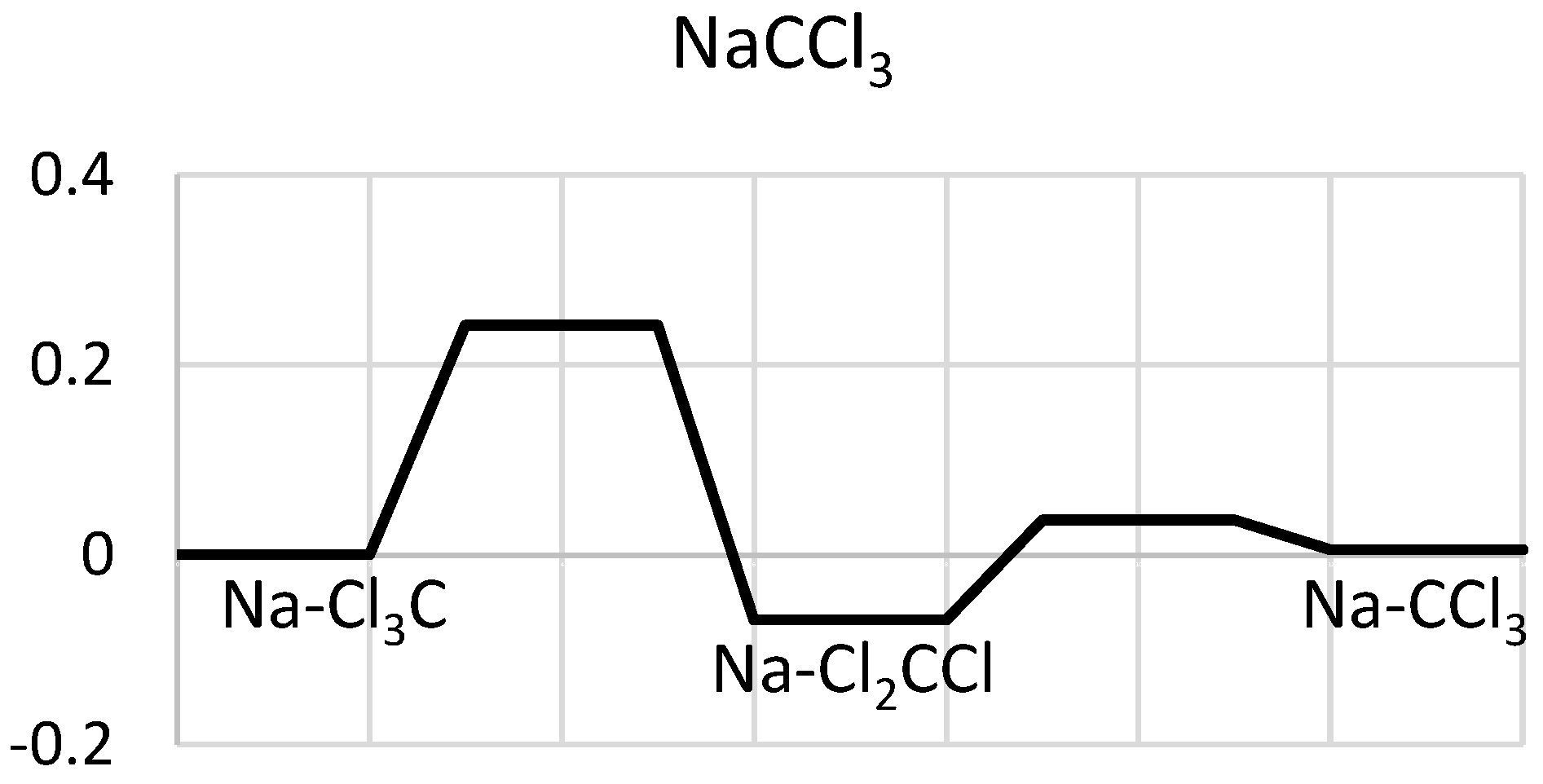

| System | De/eV | Re(C-C) | Re(C-Cl *) |
|---|---|---|---|
| CCl3-CCl4 (a) | 0.231 | 3.586 | 1.736 |
| C2Cl6-Cl (b) | 0.095 | 1.554 | 3.743 |
| System | De/eV | Re(Na-C)/Å | Re(Na-Cl *) |
|---|---|---|---|
| Na-CCl3 (a) | 2.334 | 2.297 | 2.638 |
| Na-Cl2CCl (b) | 2.408 | 2.225 | 2.697 |
| Na-Cl3C (c) | 2.339 | 2.831 | 2.613 |
| System | q(Na)/e | q(C) | q(Cl) |
|---|---|---|---|
| Na-CCl3 | 0.956 | −0.549 | −0.080, −0.247 |
| Na-Cl2CCl | 0.956 | −0.520 | −0.036, −0.200 |
| Na-Cl3C | 0.918 | −0.302 | −0.205 |
| System | De/eV | Re(Na-C #)/Å | Re(C-C) | Re(C-Cl *) | Re(Na-Cl &) |
|---|---|---|---|---|---|
| Na-CCl3-CCl4 (a) | 0.488 † | 2.286 | 3.797 | 1.729 | 2.635 |
| Na-Cl2CCl-CCl4 (b) | 0.465 † | 2.231 | 3.756 | 1.730 | 2.680 |
| Na-Cl3C-CCl4 (c) | 0.235 † | 2.825 | 3.349 | 1.745 | 2.615 |
| NaCl-C2Cl6 (A) | 0.502 ‡ | 3.309 | 1.555 | 2.384 | |
| Na-C2Cl6-Cl (L $) (B) | −1.869 ‡ | 3.101 | 1.557 | 3.334 | 2.579 |
| Na-C2Cl6-Cl (C) | −2.391 ‡ | 2.828 | 1.549 | 3.275 | 2.732 |
| System | q(Na)/e | q(CCl3) | q(CCl4) |
|---|---|---|---|
| Na-CCl3-CCl4 (a) | 0.927 | −0.957 | 0.030 |
| Na-Cl2CCl-CCl4 (b) | 0.920 | −0.950 | 0.030 |
| Na-Cl3C-CCl4 (c) | 0.919 | −0.929 | 0.010 |
| System | q(Na)/e | q(Cl) | q(C2Cl6) |
|---|---|---|---|
| NaCl-C2Cl6 (A) | 0.914 | −0.951 | 0.037 |
| Na-C2Cl6-Cl (L $) (B) | 0.949 | −0.983 | 0.034 |
| Na-C2Cl6-Cl (C) | 0.954 | −0.984 | 0.030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerr, S.; Naumkin, F.Y. C-C Bonding in Molecular Systems via Cross-Coupling-like Reactions Involving Noncovalently Bound Constituent Ions. Molecules 2024, 29, 4429. https://doi.org/10.3390/molecules29184429
Kerr S, Naumkin FY. C-C Bonding in Molecular Systems via Cross-Coupling-like Reactions Involving Noncovalently Bound Constituent Ions. Molecules. 2024; 29(18):4429. https://doi.org/10.3390/molecules29184429
Chicago/Turabian StyleKerr, Stephen, and Fedor Y. Naumkin. 2024. "C-C Bonding in Molecular Systems via Cross-Coupling-like Reactions Involving Noncovalently Bound Constituent Ions" Molecules 29, no. 18: 4429. https://doi.org/10.3390/molecules29184429
APA StyleKerr, S., & Naumkin, F. Y. (2024). C-C Bonding in Molecular Systems via Cross-Coupling-like Reactions Involving Noncovalently Bound Constituent Ions. Molecules, 29(18), 4429. https://doi.org/10.3390/molecules29184429









