Synthetic Approaches, Properties, and Applications of Acylals in Preparative and Medicinal Chemistry
Abstract
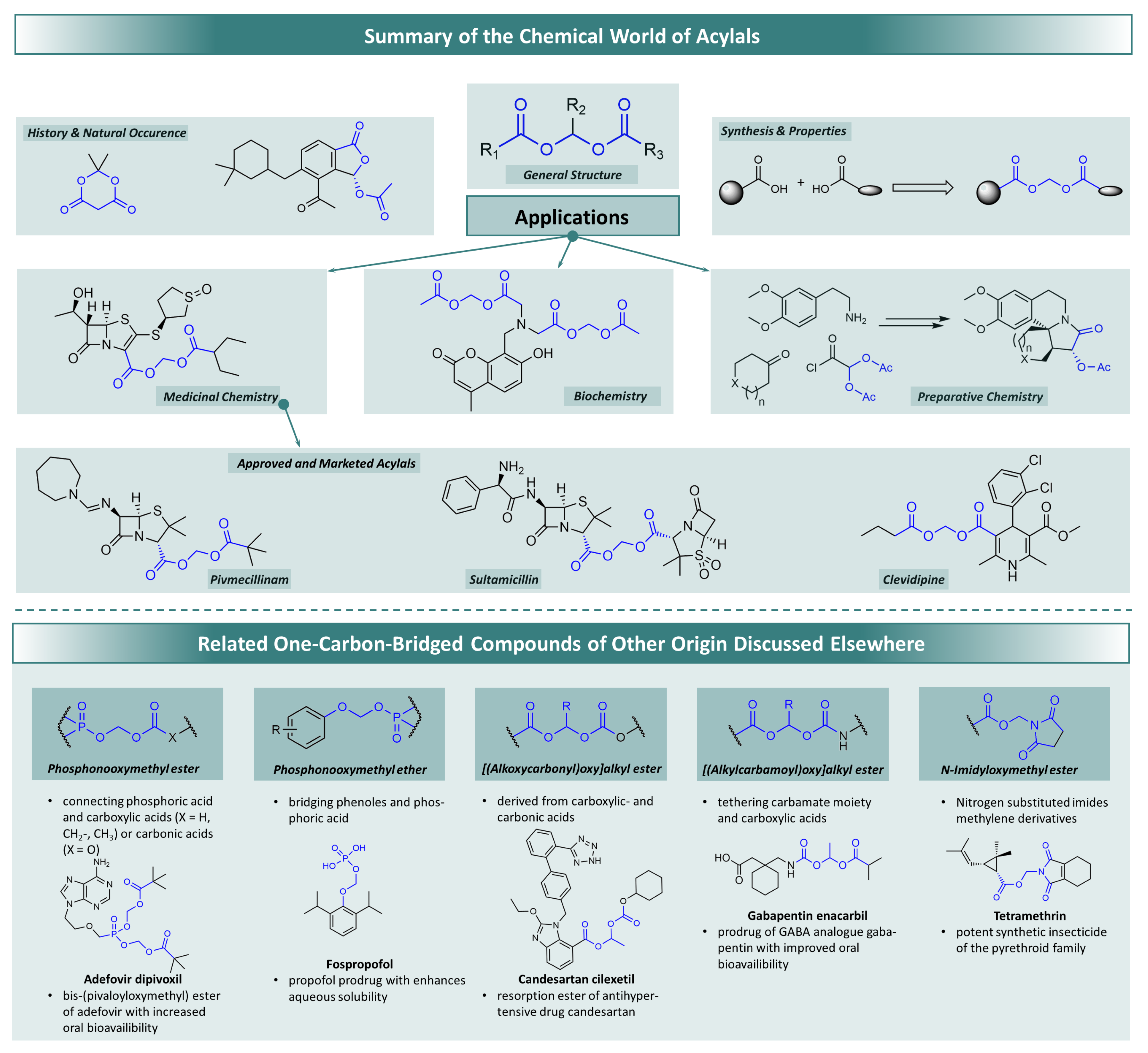
:1. Introduction
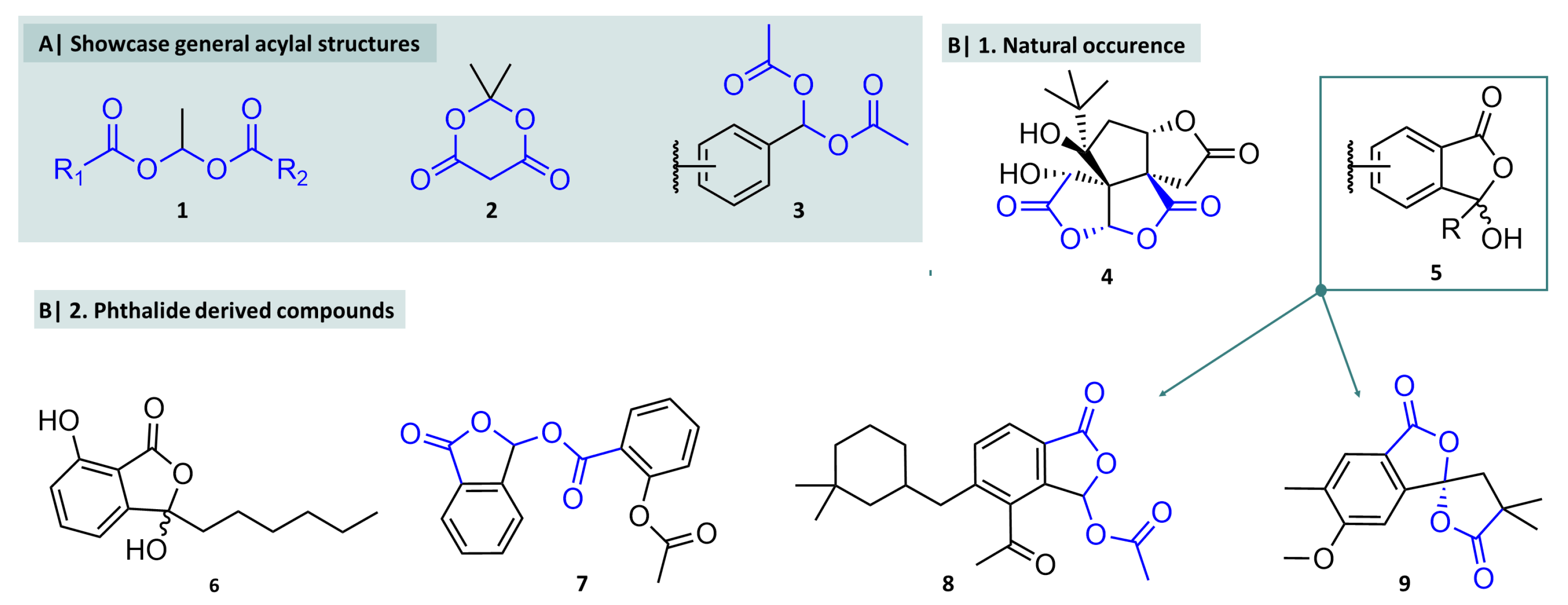
Historical Background and Natural Occurrence
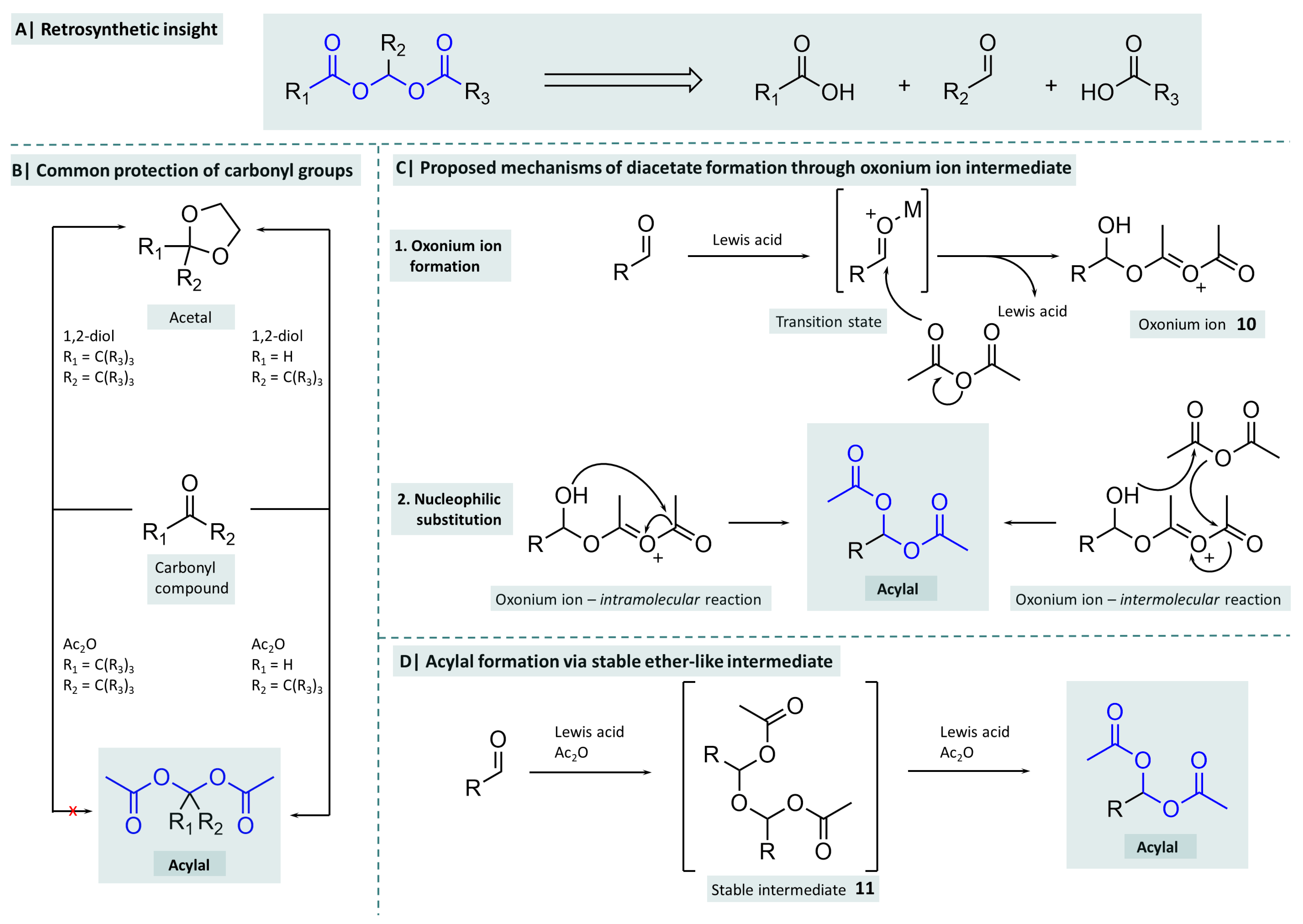
2. Chemistry of Acylals
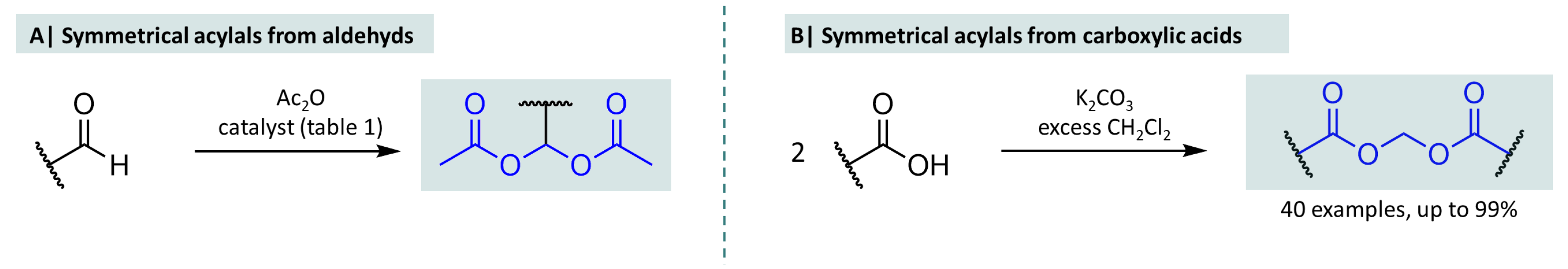
2.1. Synthesis of Symmetrical Acylals
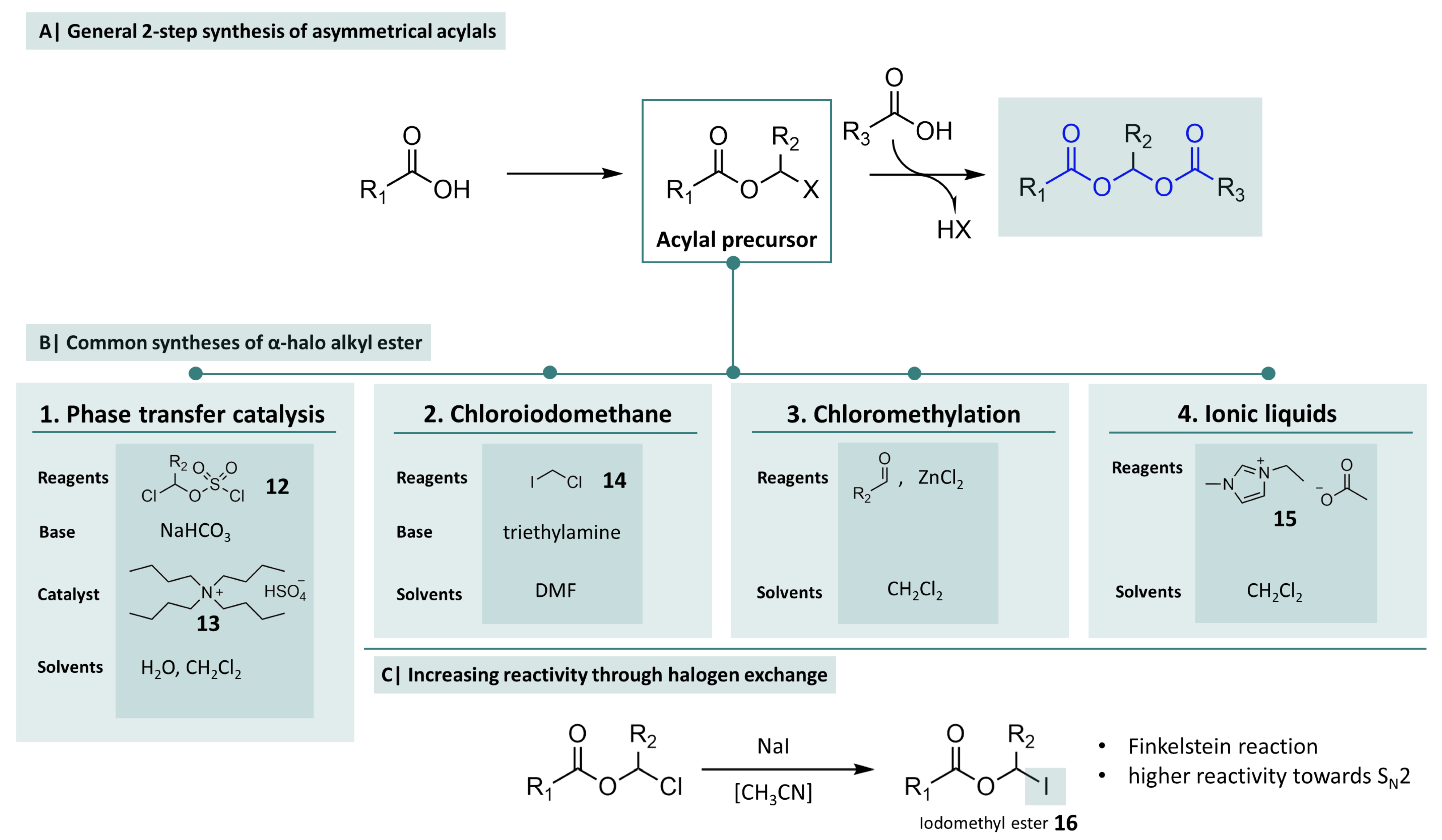
2.2. Synthesis of Asymmetrical Acylals
2.3. Chemical Properties of Acylals: Hydrolysis
3. Applications of Acylal Compounds
3.1. Acylals in Preparative Chemistry
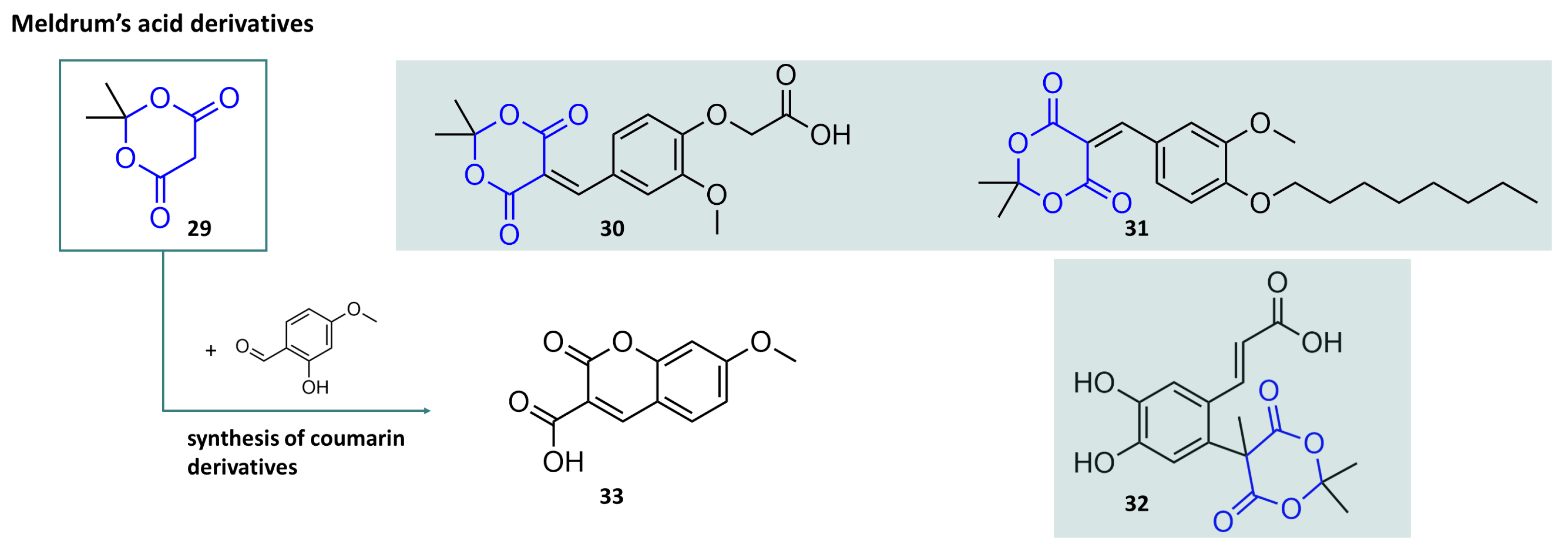
3.2. A Closer Look at Meldrum’s Acid
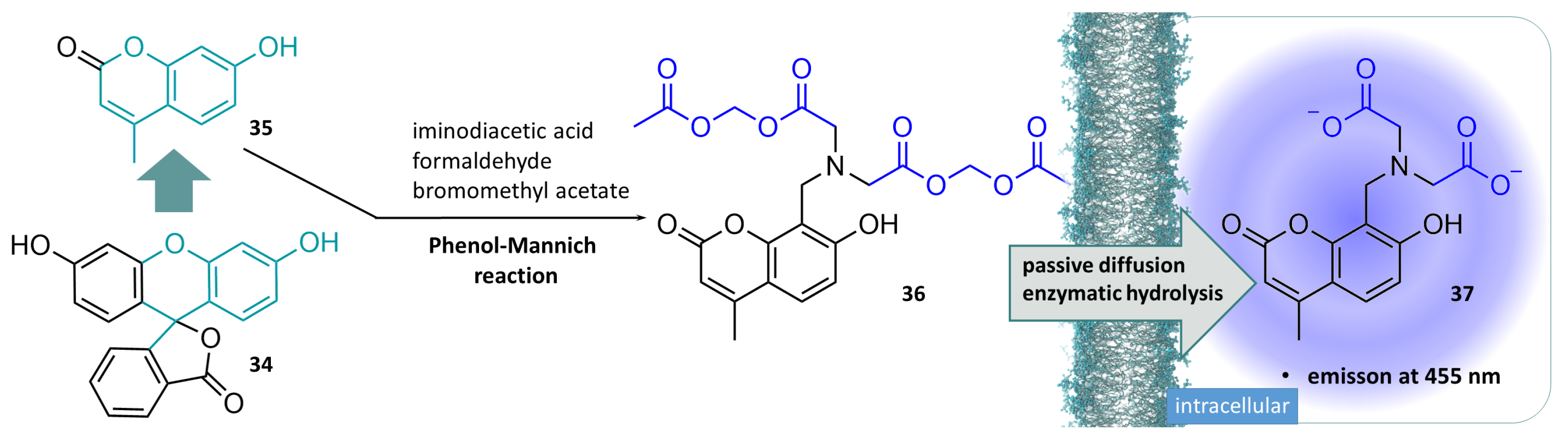
3.3. Acylal Compounds in Biochemistry Applications
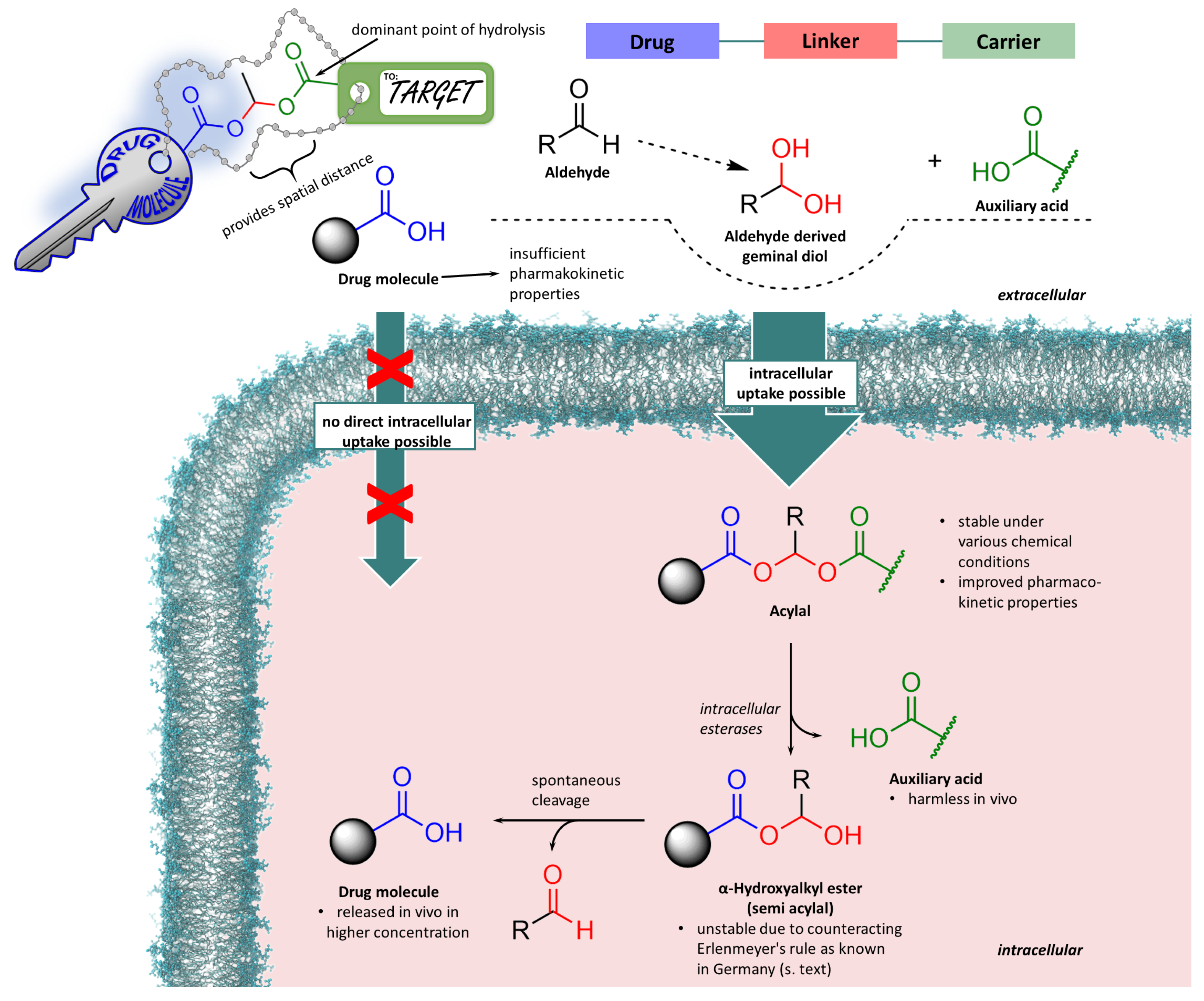
3.4. Acylals in Medicinal Chemistry Applications
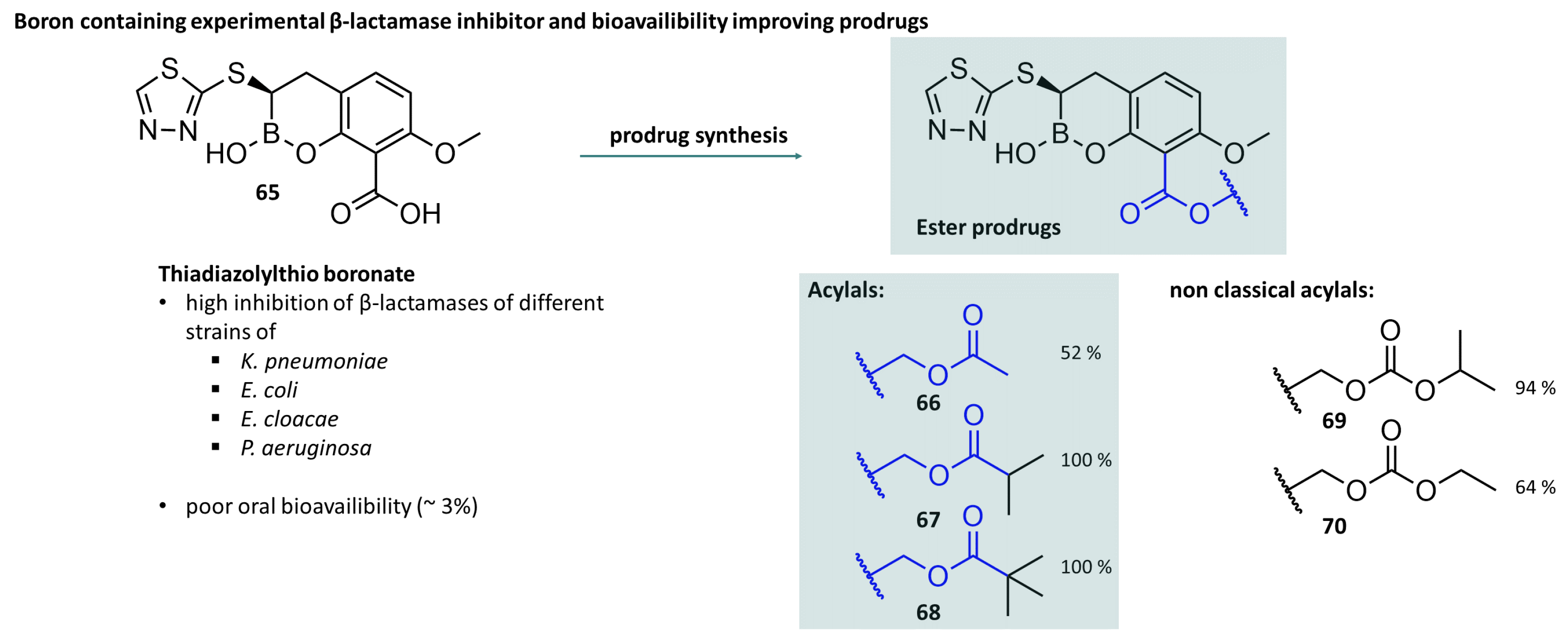
3.5. Acylal Prodrugs of -Lactam Antibiotics
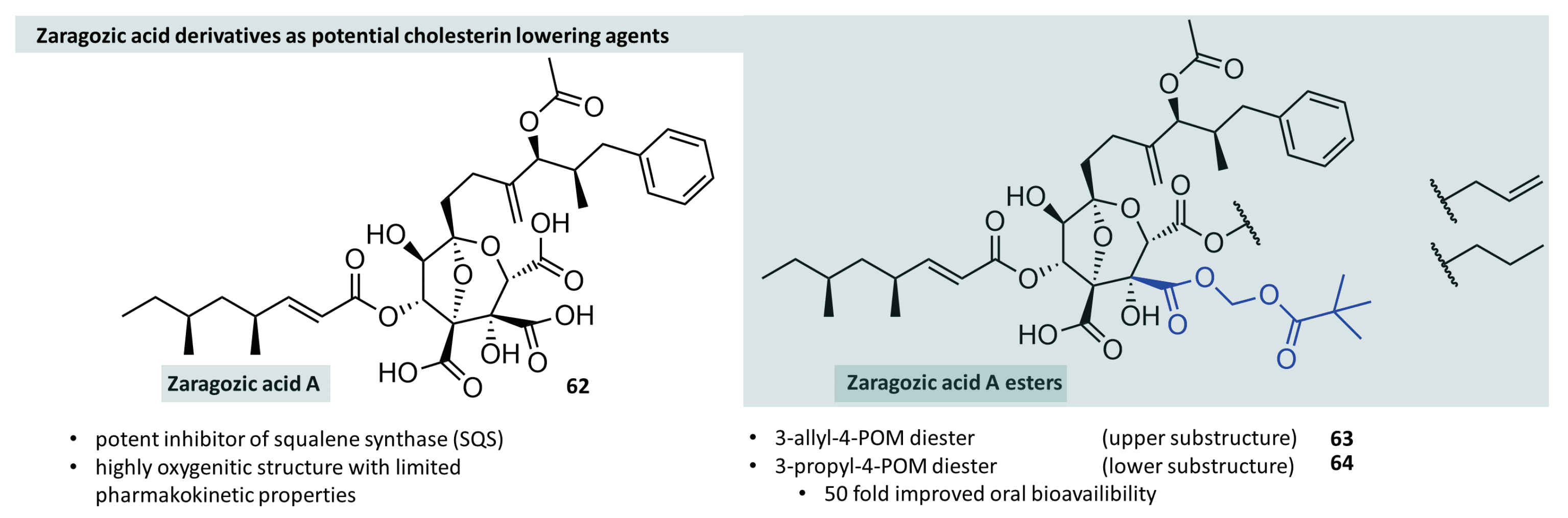
3.6. Further Utilisations in Drug Design
4. Summary
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Sertkaya, A.; Beleche, T.; Jessup, A.; Sommers, B.D. Costs of drug development and research and development intensity in the US, 2000–2018. JAMA Netw. Open 2024, 7, e2415445. [Google Scholar] [CrossRef]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Subbaiah, M.A.; Rautio, J.; Meanwell, N.A. Prodrugs as empowering tools in drug discovery and development: Recent strategic applications of drug delivery solutions to mitigate challenges associated with lead compounds and drug candidates. Chem. Soc. Rev. 2024, 53, 2099–2210. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Skonberg, C.; Olsen, J.; Madsen, K.G.; Hansen, S.H.; Grillo, M.P. Metabolic activation of carboxylic acids. Expert Opin. Drug Metab. Toxicol. 2008, 4, 425–438. [Google Scholar] [CrossRef]
- Elder, D.P.; Delaney, E.; Teasdale, A.; Eyley, S.; Reif, V.D.; Jacq, K.; Facchine, K.L.; Oestrich, R.S.; Sandra, P.; David, F. The utility of sulfonate salts in drug development. J. Pharm. Sci. 2010, 99, 2948–2961. [Google Scholar] [CrossRef]
- Yu, H.; Yang, H.; Shi, E.; Tang, W. Development and clinical application of phosphorus-containing drugs. Med. Drug Discov. 2020, 8, 100063. [Google Scholar] [CrossRef]
- Plescia, J.; Moitessier, N. Design and discovery of boronic acid drugs. Eur. J. Med. Chem. 2020, 195, 112270. [Google Scholar] [CrossRef]
- Holman, C.M. Recent Developments in FDA Regulatory Exclusivities. Biotechnol. Law Rep. 2023, 42, 118–131. [Google Scholar] [CrossRef]
- Ettmayer, P.; Amidon, G.L.; Clement, B.; Testa, B. Lessons learned from marketed and investigational prodrugs. J. Med. Chem. 2004, 47, 2393–2404. [Google Scholar] [CrossRef]
- Beaumont, K.; Webster, R.; Gardner, I.; Dack, K. Design of ester prodrugs to enhance oral absorption of poorly permeable compounds: Challenges to the discovery scientist. Curr. Drug Metab. 2003, 4, 461–485. [Google Scholar] [CrossRef]
- Dando, T.M.; Plosker, G.L. Adefovir dipivoxil: A review of its use in chronic hepatitis B. Drugs 2003, 63, 2215–2234. [Google Scholar] [CrossRef]
- Noble, S.; Goa, K.L. Adefovir dipivoxil. Drugs 1999, 58, 479–487. [Google Scholar] [CrossRef]
- McClellan, K.J.; Goa, K.L. Candesartan cilexetil: A review of its use in essential hypertension. Drugs 1998, 56, 847–869. [Google Scholar] [CrossRef]
- Cundy, K.C.; Branch, R.; Chernov-Rogan, T.; Dias, T.; Estrada, T.; Hold, K.; Koller, K.; Liu, X.; Mann, A.; Panuwat, M.; et al. XP13512 [(±)-1-([(α-Isobutanoyloxyethoxy) carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J. Pharmacol. Exp. Ther. 2004, 311, 315–323. [Google Scholar]
- Cundy, K.C.; Annamalai, T.; Bu, L.; De Vera, J.; Estrela, J.; Luo, W.; Shirsat, P.; Torneros, A.; Yao, F.; Zou, J.; et al. XP13512 [(±)-1-([(α-isobutanoyloxyethoxy) carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J. Pharmacol. Exp. Ther. 2004, 311, 324–333. [Google Scholar]
- Bockbrader, H.N.; Wesche, D.; Miller, R.; Chapel, S.; Janiczek, N.; Burger, P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin. Pharmacokinet. 2010, 49, 661–669. [Google Scholar] [CrossRef]
- Tatebayashi, H.; Narahashi, T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J. Pharmacol. Exp. Ther. 1994, 270, 595–603. [Google Scholar]
- Guo, Y.; Huang, Y.; Pang, S.; Zhou, T.; Lin, Z.; Yu, H.; Zhang, G.; Bhatt, P.; Chen, S. Novel mechanism and kinetics of tetramethrin degradation using an indigenous Gordonia cholesterolivorans A16. Int. J. Mol. Sci. 2021, 22, 9242. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X.; Li, C. Evaluation of seven chemical pesticides by mixed microbial culture (PCS-1): Degradation ability, microbial community, and Medicago sativa phytotoxicity. J. Hazard. Mater. 2020, 389, 121834. [Google Scholar] [CrossRef]
- Meldrum, A.N. LIV.—A β-lactonic acid from acetone and malonic acid. J. Chem. Soc. Trans. 1908, 93, 598–601. [Google Scholar] [CrossRef]
- McNab, H. Meldrum’s acid. Chem. Soc. Rev. 1978, 7, 345–358. [Google Scholar] [CrossRef]
- Pair, E.; Cadart, T.; Levacher, V.; Brière, J.F. Meldrum’s Acid: A Useful Platform in Asymmetric Organocatalysis. ChemCatChem 2016, 8, 1882–1890. [Google Scholar] [CrossRef]
- Fillion, E.; Fishlock, D.; Wilsily, A.; Goll, J.M. Meldrum’s acids as acylating agents in the catalytic intramolecular Friedel-Crafts reaction. J. Org. Chem. 2005, 70, 1316–1327. [Google Scholar] [CrossRef]
- Schneider, D.; Schneider, L.; Shulman, A.; Claussen, C.F.; Just, E.; Koltchev, C.; Kersebaum, M.; Dehler, R.; Goldstein, B.; Claussen, E. Gingko biloba (Rokan) therapy in tinnitus patients and measurable interactions between tinnitus and vestibular disturbances. Int. Tinnitus J. 2000, 6, 56–62. [Google Scholar]
- Sierpina, V.S.; Wollschlaeger, B.; Blumenthal, M. Ginkgo biloba. Am. Fam. Physician 2003, 68, 923–926. [Google Scholar]
- Ferrante, R.J.; Klein, A.M.; Dedeoglu, A.; Flint Beal, M. Therapeutic efficacy of EGb761 (Gingko biloba extract) in a transgenic mouse model of amyotrophic lateral sclerosis. J. Mol. Neurosci. 2001, 17, 89–96. [Google Scholar] [CrossRef]
- Seephonkai, P.; Pyne, S.G.; Willis, A.C.; Lie, W. Bioactive compounds from the roots of Strophioblachia fimbricalyx. J. Nat. Prod. 2013, 76, 1358–1364. [Google Scholar] [CrossRef]
- Cimino, G.; Crispino, A.; Gavagnin, M.; Sodano, G. Diterpenes from the nudibranch Chromodoris luteorosea. J. Nat. Prod. 1990, 53, 102–106. [Google Scholar] [CrossRef]
- Castaer, J.; Prous, J. Talosalate. Drugs Fut. 1986, 11, 394. [Google Scholar]
- Buddrus, J. Grundlagen der Organischen Chemie; Walter de Gruyter: Berlin, Germany, 2011. [Google Scholar]
- Kumar, R.; Kumar, D.; Chakraborti, A.K. Perchloric acid adsorbed on silica gel (HClO4-SiO2) as an inexpensive, extremely efficient, and reusable dual catalyst system for acetal/ketal formation and their deprotection to aldehydes/ketones. Synthesis 2007, 2007, 299–303. [Google Scholar] [CrossRef]
- Gopinath, R.; Haque, S.J.; Patel, B.K. Tetrabutylammonium tribromide (TBATB) as an efficient generator of HBr for an efficient chemoselective reagent for acetalization of carbonyl compounds. J. Org. Chem. 2002, 67, 5842–5845. [Google Scholar] [CrossRef]
- Mensah, E.A.; Green, S.D.; West, J.; Kindoll, T.; Lazaro-Martinez, B. Formation of acetals and ketals from carbonyl compounds: A new and highly efficient method inspired by cationic palladium. Synlett 2019, 30, 1810–1814. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Karimi, B. Zirconium tetrachloride (ZrCl4) catalyzed highly chemoselective and efficient acetalization of carbonyl compounds. Synlett 1999, 1999, 321–323. [Google Scholar] [CrossRef]
- Wuts, P.G.; Greene, T.W. Greene’s Protective Groups in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kochhar, K.S.; Bal, B.S.; Deshpande, R.; Rajadhyaksha, S.; Pinnick, H.W. Protecting groups in organic synthesis. Part 8. Conversion of aldehydes into geminal diacetates. J. Org. Chem. 1983, 48, 1765–1767. [Google Scholar] [CrossRef]
- Kavala, V.; Patel, B.K. Reinvestigation of the Mechanism of gem-Diacylation: Chemoselective Conversion of Aldehydes to Various gem-Diacylates and Their Cleavage under Acidic and Basic Conditions. Eur. J. Org. Chem. 2005, 2005, 441–451. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, Z.H.; Wang, Y.M. A stable intermediate: A new insight into the mechanism of Lewis acids-promoted formation of acylals from aldehydes. Tetrahedron Lett. 2007, 48, 3119–3122. [Google Scholar] [CrossRef]
- Rezayati, S.; Ramazani, A. Metal-based Lewis acid catalysts for conversion of a variety of aldehydes with acetic anhydride to gem 1,1-diacetates. Res. Chem. Intermed. 2020, 46, 3757–3799. [Google Scholar] [CrossRef]
- Jung, H.; Kang, J.; Chun, H.; Han, B. First principles computational study on hydrolysis of hazardous chemicals phosphorus trichloride and oxychloride (PCl3 and POCl3) catalyzed by molecular water clusters. J. Hazard. Mater. 2018, 341, 457–463. [Google Scholar] [CrossRef]
- Schroeder, H.A.; Mitchener, M.; Nason, A.P. Zirconium, niobium, antimony, vanadium and lead in rats: Life term studies. J. Nutr. 1970, 100, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J. Antimony toxicity. Int. J. Environ. Res. Public Health 2010, 7, 4267–4277. [Google Scholar] [CrossRef] [PubMed]
- Carrigan, M.D.; Eash, K.J.; Oswald, M.C.; Mohan, R.S. An efficient method for the chemoselective synthesis of acylals from aromatic aldehydes using bismuth triflate. Tetrahedron Lett. 2001, 42, 8133–8135. [Google Scholar] [CrossRef]
- Aggen, D.H.; Arnold, J.N.; Hayes, P.D.; Smoter, N.; Mohan, R.S. Bismuth compounds in organic synthesis. Bismuth nitrate catalyzed chemoselective synthesis of acylals from aromatic aldehydes. Tetrahedron 2004, 60, 3675–3679. [Google Scholar] [CrossRef]
- Heravi, M.M.; Bakhtiari, K.; Bamoharram, F.F. An efficient and chemoselective synthesis of acylals from aromatic aldehydes and their regeneration, catalyzed by 12-molybdophosphoric acid. Catal. Commun. 2006, 7, 499–501. [Google Scholar] [CrossRef]
- Roy, S.C.; Banerjee, B. A Mild and Efficient Method for the Chemoselective Synthesis of Acylals from Aldehydes and their Deprotections Catalysed by Ceric Ammonium Nitrate. Synlett 2002, 14, 1677–1678. [Google Scholar] [CrossRef]
- Negrón, G.E.; Palacios, L.N.; Angeles, D.; Lomas, L.; Gaviño, R. A mild and efficient method for the chemoselective synthesis of acylals from aromatic aldehydes and their deprotections catalyzed by sulfated zirconia. J. Braz. Chem. Soc. 2005, 16, 490–494. [Google Scholar] [CrossRef]
- Deka, N.; Borah, R.; Kalita, D.J.; Sarma, J.C. Synthesis of 1, 1-diacetates from aldehydes using trimethylchlorosilane and sodium iodide as catalyst. J. Chem. Res. Synopses 1998, 94–95. [Google Scholar] [CrossRef]
- Kamble, V.T.; Jamode, V.S.; Joshi, N.S.; Biradar, A.V.; Deshmukh, R.Y. An efficient method for the synthesis of acylals from aldehydes using silica-supported perchloric acid (HClO4–SiO2). Tetrahedron Lett. 2006, 47, 5573–5576. [Google Scholar] [CrossRef]
- Li, T.S.; Zhang, Z.H.; Gao, Y.J. A Rapid Preparation of Acylals of Aldehydes Catalysed by Fe3+ -Montmorillonite. Synth. Commun. 1998, 28, 4665–4671. [Google Scholar] [CrossRef]
- Yadav, J.S.; Subba Reddy, B.V.; Srinivas, C. Indium Trichloride Catalyzed Chemoselective Conversion of Aldehydes to Gem -Diacetates*. Synth. Commun. 2002, 32, 2149–2153. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Nowrouzi, F.; Amani, K. Aluminum dodecatungstophosphate (AlPW12O40) as an efficient heterogeneous inorganic catalyst for the chemoselective synthesis of geminal diacetates (acylals) under solvent-free conditions. Tetrahedron Lett. 2003, 44, 3951–3954. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, Z.H.; Wang, Y.M.; Pang, M.L. Indium Tribromide as a Highly Efficient and Versatile Catalyst for Chemoselective Synthesis of Acylals from Aldehydes under Solvent-Free Conditions. Synlett 2004, 2004, 1727–1730. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, P.; Maulik, P.R.; Misra, A.K. HClO4-SiO2 catalyzed chemoselective synthesis of acylals from aldehydes under solvent-free conditions. J. Mol. Catal. A Chem. 2006, 247, 27–30. [Google Scholar] [CrossRef]
- Wang, M.; Gong, H.; Jiang, H.; Wang, Z. Acetic Acid-Assisted Copper Methanesulfonate Catalyst for Chemoselective Conversion of Aldehydes to Acylals. Synth. Commun. 2006, 36, 1953–1960. [Google Scholar] [CrossRef]
- Niknam, K.; Saberi, D.; Sefat, M.N. Silica-bonded S-sulfonic acid as a recyclable catalyst for chemoselective synthesis of 1,1-diacetates. Tetrahedron Lett. 2009, 50, 4058–4062. [Google Scholar] [CrossRef]
- Fan, D.H.; Wang, H.; Mao, X.X.; Shen, Y.M. An efficient and chemoselective procedure for acylal synthesis. Molecules 2010, 15, 6493–6501. [Google Scholar] [CrossRef]
- Borikar, S.P.; Daniel, T. A convenient and efficient protocol for the synthesis of acylals catalyzed by Brønsted acidic ionic liquids under ultrasonic irradiation. Ultrason. Sonochem. 2011, 18, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Paul, S. Amorphous carbon-silica composites bearing sulfonic acid as solid acid catalysts for the chemoselective protection of aldehydes as 1,1-diacetates and for N-, O- and S-acylations. Green Chem. 2011, 13, 2365. [Google Scholar] [CrossRef]
- Kalla, R.M.N.; Park, H.; Hoang, T.T.K.; Kim, I. Phospho sulfonic acid as an efficient and recyclable solid acid catalyst for the solvent-free preparation of acylals. Tetrahedron Lett. 2014, 55, 5373–5376. [Google Scholar] [CrossRef]
- Bazi, F.; Mounir, B.; Hamza, M.; Sebti, S. A Mild and Efficient Method for the Synthesis of Acylals from Aromatic Aldehydes and Their Deprotections Catalyzed by Synthetic Phosphates under Solvent-Free Conditions. Green Sustain. Chem. 2018, 8, 334–344. [Google Scholar] [CrossRef]
- Curini, M.; Epifano, F.; Marcotullio, M.C.; Rosati, O.; Nocchetti, M. Preparation and deprotection of 1,1-diacetates (acylals) using zirconium sulfophenyl phosphonate as catalyst. Tetrahedron Lett. 2002, 43, 2709–2711. [Google Scholar] [CrossRef]
- Rezayati, S.; Hajinasiri, R.; Erfani, Z. Microwave-assisted green synthesis of 1,1-diacetates (acylals) using selectfluor™ as an environmental-friendly catalyst under solvent-free conditions. Res. Chem. Intermed. 2016, 42, 2567–2576. [Google Scholar] [CrossRef]
- Wang, S.; Fu, Z.; Huang, Z.; Jiang, Y.; Guo, S.; Cai, H. Dichloromethane as a methylene synthon for regioselective linkage of diverse carboxylic acids: Direct access to methylene diesters under metal-free conditions. Chin. Chem. Lett. 2019, 30, 1173–1177. [Google Scholar] [CrossRef]
- Scheeren, J.; Tax, W.; Schijf, R. Mixed Acylals-Synthesis of Alkylidene Carboxylate Formates. Synth. Int. J. Methods Synth. Org. 1973, 3, 151–153. [Google Scholar] [CrossRef]
- Harada, N.; Hongu, M.; Tanaka, T.; Kawaguchi, T.; Hashiyama, T.; Tsujihara, K. A simple preparation of chloromethyl esters of the blocked amino acids. Synth. Commun. 1994, 24, 767–772. [Google Scholar] [CrossRef]
- Gao, R.D.; Maeda, M.; Tallon, C.; Feinberg, A.P.; Slusher, B.S.; Tsukamoto, T. Effects of 6-aminonicotinic acid esters on the reprogrammed epigenetic state of distant metastatic pancreatic carcinoma. ACS Med. Chem. Lett. 2022, 13, 1892–1897. [Google Scholar] [CrossRef]
- Dalaijargal, B.; Mi, L.; Wu, Z.; Yin, Y.; Liang, H.; Qiu, Y.; Yan, Y.J.; Jin, H.; Chen, Z.L. Synthesis and evaluation of new sartan derivatives. Med. Chem. Res. 2022, 31, 1003–1010. [Google Scholar] [CrossRef]
- Koszelewski, D.; Ostaszewski, R. The studies on chemoselective promiscuous activity of hydrolases on acylals transformations. Bioorg. Chem. 2019, 93, 102825. [Google Scholar] [CrossRef]
- Coltescu, A.R.; Butnariu, M.; Sarac, I. The importance of solubility for new drug molecules. Biomed. Pharmacol. J. 2020, 13, 577–583. [Google Scholar] [CrossRef]
- Müller, C.E. Prodrug approaches for enhancing the bioavailability of drugs with low solubility. Chem. Biodivers. 2009, 6, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Mørk, N.; Bundgaard, H.; Shalmi, M.; Christensen, S. Furosemide prodrugs: Synthesis, enzymatic hydrolysis and solubility of various furosemide esters. Int. J. Pharm. 1990, 60, 163–169. [Google Scholar] [CrossRef]
- Azéma, J.; Guidetti, B.; Malet-Martino, M.; Martino, R.; Roques, C. Efficient approach to acyloxymethyl esters of nalidixic acid and in vitro evaluation as intra-ocular prodrugs. Bioorg. Med. Chem. 2006, 14, 2569–2580. [Google Scholar] [CrossRef]
- Li, J.; Gao, K.; Bian, M.; Ding, H. Recent advances in the total synthesis of cyclobutane-containing natural products. Org. Chem. Front. 2020, 7, 136–154. [Google Scholar] [CrossRef]
- Kim, A.N.; Ngamnithiporn, A.; Du, E.; Stoltz, B.M. Recent Advances in the Total Synthesis of the Tetrahydroisoquinoline Alkaloids (2002–2020). Chem. Rev. 2023, 123, 9447–9496. [Google Scholar] [CrossRef]
- Senge, M.O.; Sergeeva, N.N.; Hale, K.J. Classic highlights in porphyrin and porphyrinoid total synthesis and biosynthesis. Chem. Soc. Rev. 2021, 50, 4730–4789. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Singh, M.; Rathee, G.; Chandra, R. Recent advancements in synthetic methodologies of 3-substituted phthalides and their application in the total synthesis of biologically active natural products. RSC Adv. 2020, 10, 12626–12652. [Google Scholar] [CrossRef]
- Parenty, A.; Moreau, X.; Campagne, J.M. Macrolactonizations in the total synthesis of natural products. Chem. Rev. 2006, 106, 911–939. [Google Scholar] [CrossRef]
- Drazic, A.; Myklebust, L.M.; Ree, R.; Arnesen, T. The world of protein acetylation. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 1372–1401. [Google Scholar] [CrossRef]
- Koprinarova, M.; Schnekenburger, M.; Diederich, M. Role of histone acetylation in cell cycle regulation. Curr. Top. Med. Chem. 2016, 16, 732–744. [Google Scholar] [CrossRef]
- Ganesan, M.; Nagaraaj, P. Recent developments in dehydration of primary amides to nitriles. Org. Chem. Front. 2020, 7, 3792–3814. [Google Scholar] [CrossRef]
- Waibel, K.; Nickisch, R.; Möhl, N.; Seim, R.; Meier, M. A more sustainable and highly practicable synthesis of aliphatic isocyanides. Green Chem. 2020, 22, 933–941. [Google Scholar] [CrossRef]
- Downie, I.M.; Earle, M.J.; Heaney, H.; Shuhaibar, K.F. Vilsmeier formylation and glyoxylation reactions of nucleophilic aromatic compounds using pyrophosphoryl chloride. Tetrahedron 1993, 49, 4015–4034. [Google Scholar] [CrossRef]
- Hurd, C.D.; Cantor, S.M. The analytical separation of various classes of sugars. J. Am. Chem. Soc. 1938, 60, 2677–2687. [Google Scholar] [CrossRef]
- Hurd, C.D.; Green, F.O. Acylals. J. Am. Chem. Soc. 1941, 63, 2201–2204. [Google Scholar] [CrossRef]
- Chapman, R.S.; Francis, M.; Lawrence, R.; Tibbetts, J.D.; Bull, S.D. Formyloxyacetoxyphenylmethane and 1, 1-diacylals as versatile O-formylating and O-acylating reagents for alcohols. Tetrahedron 2018, 74, 6442–6452. [Google Scholar] [CrossRef]
- Chapman, R.S.; Tibbetts, J.D.; Bull, S.D. 1, 1-Diacyloxy-1-phenylmethanes as versatile N-acylating agents for amines. Tetrahedron 2018, 74, 5330–5339. [Google Scholar] [CrossRef]
- Pourmousavi, S.A.; Hadavankhani, M.; Zinati, Z. An Efficient Method for the Transthioacetalization of Acylals and Acetals under Mild Conditions. J. Chem. 2011, 8, S495–S501. [Google Scholar] [CrossRef]
- Wu, Y.J.; Meanwell, N.A. Geminal diheteroatomic motifs: Some applications of acetals, ketals, and their sulfur and nitrogen homologues in medicinal chemistry and drug design. J. Med. Chem. 2021, 64, 9786–9874. [Google Scholar] [CrossRef]
- Trost, B.M.; Vercauteran, J. Allylic geminal diacetates. Unusual carbonyl substitutes via metal catalyzed reactions. Tetrahedron Lett. 1985, 26, 131–134. [Google Scholar] [CrossRef]
- Trost, B.M.; Kalnmals, C.A. Annulative allylic alkylation reactions between dual electrophiles and dual nucleophiles: Applications in complex molecule synthesis. Chem. Eur. J. 2020, 26, 1906–1921. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, P.; Venkataramani, S.; Ramasastry, S. Pd-Catalyzed formal [3 + 3] heteroannulation of allylic gem-diacetates: Synthesis of chromene-based natural products and exploration of photochromic properties. ACS Catal. 2021, 12, 963–970. [Google Scholar]
- Ogiwara, Y.; Takahashi, K.; Kitazawa, T.; Sakai, N. Indium (III)-catalyzed knoevenagel condensation of aldehydes and activated methylenes using acetic anhydride as a promoter. J. Org. Chem. 2015, 80, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Reddy, B.S.; Reddy, G.K.K. Indium-mediated allylation of gem-diacetates to homoallylic acetates in aqueous media. Tetrahedron Lett. 2000, 41, 2695–2697. [Google Scholar] [CrossRef]
- Jeon, H.; Choi, S.W.; Park, S.; Lee, S.; Kim, S. Synthesis of Bridged Oxabicycles via Cascade Reactions involving O-Acyloxocarbenium Ion Intermediates. Org. Lett. 2021, 23, 8312–8316. [Google Scholar] [CrossRef]
- Monaco, A.; Aliev, A.E.; Hilton, S.T. Intramolecular acylal cyclisation (IAC) as an efficient synthetic strategy towards the total synthesis of erythrina alkaloid derivatives. Chem. Eur. J. 2015, 21, 13909–13912. [Google Scholar] [CrossRef]
- Monaco, A.; Szulc, B.R.; Rao, Z.X.; Barniol-Xicota, M.; Sehailia, M.; Borges, B.M.A.; Hilton, S.T. Short Total Synthesis of ±-g-Lycorane by a Sequential Intramolecular Acylal Cyclisation (IAC) and Intramolecular Heck Addition Reaction. Chem. Eur. J. 2017, 23, 4750–4755. [Google Scholar] [CrossRef]
- Ivanov, A.S. Meldrum’s acid and related compounds in the synthesis of natural products and analogs. Chem. Soc. Rev. 2008, 37, 789–811. [Google Scholar] [CrossRef]
- Chen, B.C. Meldrum’s acid in organic synthesis. Heterocycles 1991, 32, 529–597. [Google Scholar] [CrossRef]
- Nakamura, S.; Hirao, H.; Ohwada, T. Rationale for the acidity of Meldrum’s acid. Consistent relation of C-H acidities to the properties of localized reactive orbital. J. Org. Chem. 2004, 69, 4309–4316. [Google Scholar] [CrossRef]
- Bonifácio, V.D. Meldrum’s Acid. Synlett 2004, 2004, 1649–1650. [Google Scholar] [CrossRef]
- Ristovski, J.T.; Janković, N.; Borčić, V.; Jain, S.; Bugarčić, Z.; Mikov, M. Evaluation of antimicrobial activity and retention behavior of newly synthesized vanilidene derivatives of Meldrum’s acids using QSRR approach. J. Pharm. Biomed. Anal. 2018, 155, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A.; Abdelgawad, M.A.; Ahmed, N.; Amjad, M.W.; Hussain, M.A.; Elsherif, M.A.; Ejaz, H.; Alotaibi, N.H.; Filipović, I.; Janković, N. Synthesis, Characterization, and Biological Evaluation of Meldrum’s Acid Derivatives: Dual Activity and Molecular Docking Study. Pharmaceuticals 2023, 16, 281. [Google Scholar] [CrossRef]
- Pakravan, N.; Shayani-Jam, H.; Beiginejad, H.; Tavafi, H. A green and efficient synthesis of novel caffeic acid derivatives with Meldrum’s acid moieties as potential antibacterial agents. J. Iran. Chem. Soc. 2021, 18, 2679–2688. [Google Scholar] [CrossRef]
- Wichmann, H.; Vocke, F.; Brinkhoff, T.; Simon, M.; Richter-Landsberg, C. Cytotoxic effects of tropodithietic acid on mammalian clonal cell lines of neuronal and glial origin. Mar. Drugs 2015, 13, 7113–7123. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Lavis, L.D.; Chao, T.Y.; Raines, R.T. Synthesis and utility of fluorogenic acetoxymethyl ethers. Chem. Sci. 2011, 2, 521–530. [Google Scholar] [CrossRef]
- Boucher, D.; Laviéville, S.; Ladmiral, V.; Negrell, C.; Leclerc, E. Hemiacetal Esters: Synthesis, Properties, and Applications of a Versatile Functional Group. Macromolecules 2024, 57, 810–829. [Google Scholar] [CrossRef]
- Kao, J.P.; Harootunian, A.T.; Tsien, R.Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J. Biol. Chem. 1989, 264, 8179–8184. [Google Scholar] [CrossRef]
- Hausig-Punke, F.; Richter, F.; Hoernke, M.; Brendel, J.C.; Traeger, A. Tracking the Endosomal Escape: A Closer Look at Calcein and Related Reporters. Macromol. Biosci. 2022, 22, e2200167. [Google Scholar] [CrossRef]
- Bratosin, D.; Mitrofan, L.; Palii, C.; Estaquier, J.; Montreuil, J. Novel fluorescence assay using calcein-AM for the determination of human erythrocyte viability and aging. Cytom. Part A J. Int. Soc. Anal. Cytol. 2005, 66, 78–84. [Google Scholar] [CrossRef]
- Neri, S.; Mariani, E.; Meneghetti, A.; Cattini, L.; Facchini, A. Calcein-acetyoxymethyl cytotoxicity assay: Standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin. Diagn. Lab. Immunol. 2001, 8, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Bénard, M.; Gonzalez, B.J.; Schouft, M.T.; Falluel-Morel, A.; Vaudry, D.; Chan, P.; Vaudry, H.; Fontaine, M. Characterization of C3a and C5a receptors in rat cerebellar granule neurons during maturation: Neuroprotective effect of C5a against apoptotic cell death. J. Biol. Chem. 2004, 279, 43487–43496. [Google Scholar] [CrossRef] [PubMed]
- Izumi, S.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. A simple and effective strategy to increase the sensitivity of fluorescence probes in living cells. J. Am. Chem. Soc. 2009, 131, 10189–10200. [Google Scholar] [CrossRef]
- Mikesell, L.D.; Livinghouse, T. Base-Catalyzed Phenol-Mannich Condensation of Preformed Cesium Iminodiacetate. The Direct Synthesis of Calcein Blue AM and Related Acyloxymethyl Esters. J. Org. Chem. 2023, 88, 12064–12068. [Google Scholar] [CrossRef]
- Leroy, E.; Bensel, N.; Reymond, J.L. A low background high-throughput screening (HTS) fluorescence assay for lipases and esterases using acyloxymethylethers of umbelliferone. Bioorg. Med. Chem. Lett. 2003, 13, 2105–2108. [Google Scholar] [CrossRef]
- Burton, R.A.; Gidley, M.J.; Fincher, G.B. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 2010, 6, 724–732. [Google Scholar] [CrossRef]
- Micheli, F. Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001, 6, 414–419. [Google Scholar] [CrossRef]
- Zhang, W.H.; Rengel, Z.; Kuo, J. Determination of intracellular Ca2+ in cells of intact wheat roots: Loading of acetoxymethyl ester of Fluo–3 under low temperature. Plant J. 1998, 15, 147–151. [Google Scholar] [CrossRef]
- de Albuquerque Silva, A.T.; Chung, M.C.; Castro, L.F.; Carvalho Guido, R.V.; Ferreira, E.I. Advances in prodrug design. Mini Rev. Med. Chem. 2005, 5, 893–914. [Google Scholar] [CrossRef]
- Potlitz, F.; Link, A.; Schulig, L. Advances in the discovery of new chemotypes through ultra-large library docking. Expert Opin. Drug Discov. 2023, 18, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Wieler, L.H.; Ewers, C.; Guenther, S.; Walther, B.; Lübke-Becker, A. Methicillin-resistant staphylococci (MRS) and extended-spectrum beta-lactamases (ESBL)-producing Enterobacteriaceae in companion animals: Nosocomial infections as one reason for the rising prevalence of these potential zoonotic pathogens in clinical samples. Int. J. Med Microbiol. 2011, 301, 635–641. [Google Scholar]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, M.; Enke, T.; Chubukov, V.; Ricci, V.; Piddock, L.; Sauer, U. Metabolic constraints on the evolution of antibiotic resistance. Mol. Syst. Biol. 2017, 13, 917. [Google Scholar] [CrossRef]
- Webber, M.A.; Piddock, L.J. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef]
- Fontana, R.; Aldegheri, M.; Ligozzi, M.; Lopez, H.; Sucari, A.; Satta, G. Overproduction of a low-affinity penicillin-binding protein and high-level ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 1994, 38, 1980–1983. [Google Scholar] [CrossRef]
- Foster, T.J. Immune evasion by staphylococci. Nat. Rev. Microbiol. 2005, 3, 948–958. [Google Scholar] [CrossRef]
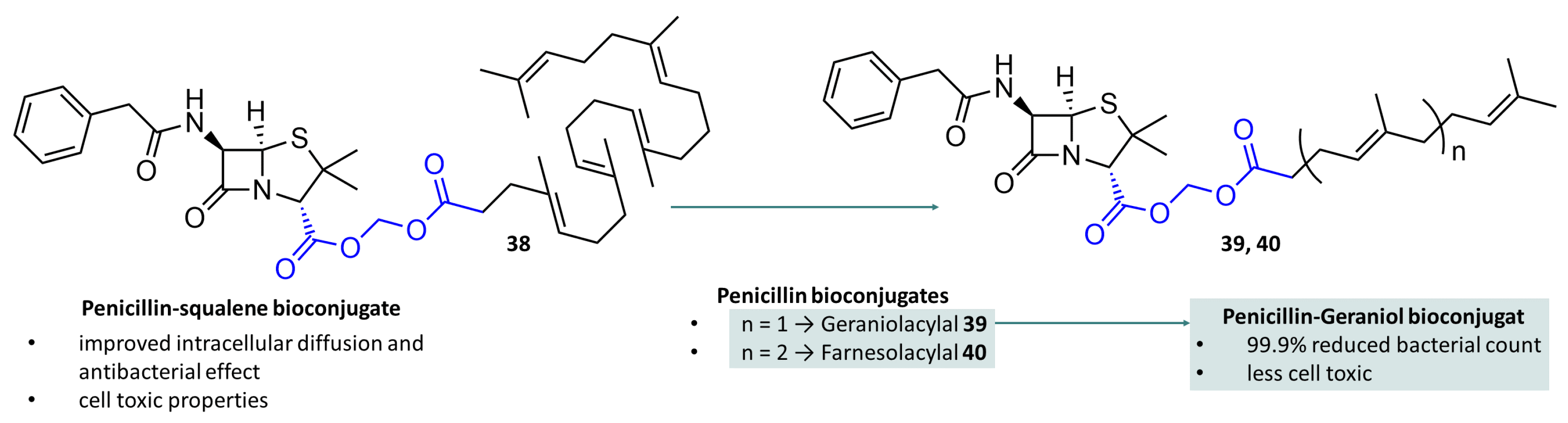
- Semiramoth, N.; Meo, C.D.; Zouhiri, F.; Said-Hassane, F.; Valetti, S.; Gorges, R.; Nicolas, V.; Poupaert, J.H.; Chollet-Martin, S.; Desmaele, D.; et al. Self-assembled squalenoylated penicillin bioconjugates: An original approach for the treatment of intracellular infections. ACS Nano 2012, 6, 3820–3831. [Google Scholar] [CrossRef]
- Abed, N.; Saïd-Hassane, F.; Zouhiri, F.; Mougin, J.; Nicolas, V.; Desmaële, D.; Gref, R.; Couvreur, P. An efficient system for intracellular delivery of beta-lactam antibiotics to overcome bacterial resistance. Sci. Rep. 2015, 5, 13500. [Google Scholar] [CrossRef]
- Couvreur, P.; Lepetre-Mouelhi, S.; Garbayo, E.; Blanco-Prieto, M.J. Self-assembled lipid–prodrug nanoparticles. Nat. Rev. Bioeng. 2023, 1, 749–768. [Google Scholar] [CrossRef]
- Feng, J.; Lepetre-Mouelhi, S.; Gautier, A.; Mura, S.; Cailleau, C.; Coudore, F.; Hamon, M.; Couvreur, P. A new painkiller nanomedicine to bypass the blood-brain barrier and the use of morphine. Sci. Adv. 2019, 5, eaau5148. [Google Scholar] [CrossRef] [PubMed]
- Lepetre-Mouelhi, S.; Gobeaux, F.; Da Silva, A.; Prades, L.; Feng, J.; Wien, F.; Couvreur, P.; Testard, F. Leu-Enkephalin Lipid Prodrug Nanoparticles: Relationship between Nanoparticles’ Structure, Interaction with Bovine Serum Albumin, and Analgesic Activity. Chem. Mater. 2024, 36, 694–707. [Google Scholar] [CrossRef]
- Bak, A.; Gudmundsson, O.; Gangwar, S.; Friis, G.; Siahaan, T.; Borchardt, R. Synthesis and evaluation of the physicochemical properties of esterase-sensitive cyclic prodrugs of opioid peptides using an (acyloxy) alkoxy linker. J. Pept. Res. 1999, 53, 393–402. [Google Scholar] [CrossRef]
- Bak, A.; Gudmundsson, O.S.; Friis, G.J.; Siahaan, T.J.; Borchardt, R.T. Acyloxyalkoxy-based cyclic prodrugs of opioid peptides: Evaluation of the chemical and enzymatic stability as well as their transport properties across Caco-2 cell monolayers. Pharm. Res. 1999, 16, 24–29. [Google Scholar] [CrossRef]
- Chen, W.; Yang, J.Z.; Andersen, R.; Nielsen, L.H.; Borchardt, R.T. Evaluation of the permeation characteristics of a model opioid peptide, H-Tyr-D-Ala-Gly-Phe-D-Leu-OH (DADLE), and its cyclic prodrugs across the blood-brain barrier using an in situ perfused rat brain model. J. Pharmacol. Exp. Ther. 2002, 303, 849–857. [Google Scholar] [CrossRef]
- Acred, P.; Brown, D.; Turner, D.; Wilson, M. Pharmacology and chemotherapy of ampicillin—A new broad-spectrum penicillin. Br. J. Pharmacol. Chemother. 1962, 18, 356–369. [Google Scholar] [CrossRef]
- Jusko, W.J.; Lewis, G.P. Comparison of ampicillin and hetacillin pharmacokinetics in man. J. Pharm. Sci. 1973, 62, 69–76. [Google Scholar] [CrossRef]
- Dhareshwar, S.S.; Stella, V.J. Your prodrug releases formaldehyde: Should you be concerned? No! J. Pharm. Sci. 2008, 97, 4184–4193. [Google Scholar] [CrossRef]
- Mushtaq, S.; Sarkar, R. Sulfasalazine in dermatology: A lesser explored drug with broad therapeutic potential. Int. J. Women’s Dermatol. 2020, 6, 191–198. [Google Scholar] [CrossRef]
- Friedel, H.A.; Campoli-Richards, D.M.; Goa, K.L. Sultamicillin: A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1989, 37, 491–522. [Google Scholar] [CrossRef]
- Lode, H. Role of sultamicillin and ampicillin/sulbactam in the treatment of upper and lower bacterial respiratory tract infections. Int. J. Antimicrob. Agents 2001, 18, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Paternotte, I.; Fan, H.J.; Scrève, P.; Claesen, M.; Tulkens, P.M.; Sonveaux, E. Syntheses and hydrolysis of basic and dibasic ampicillin esters tailored for intracellular accumulation. Bioorg. Med. Chem. 2001, 9, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Zaffiri, L.; Gardner, J.; Toledo-Pereyra, L.H. History of antibiotics. From salvarsan to cephalosporins. J. Investig. Surg. 2012, 25, 67–77. [Google Scholar] [CrossRef]
- Wise, R. The pharmacokinetics of the oral cephalosporins—A review. J. Antimicrob. Chemother. 1990, 26, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Stoeckel, K.; Hofheinz, W.; Laneury, J.P.; Duchene, P.; Shedlofsky, S.; Blouin, R.A. Stability of cephalosporin prodrug esters in human intestinal juice: Implications for oral bioavailability. Antimicrob. Agents Chemother. 1998, 42, 2602–2606. [Google Scholar] [CrossRef]
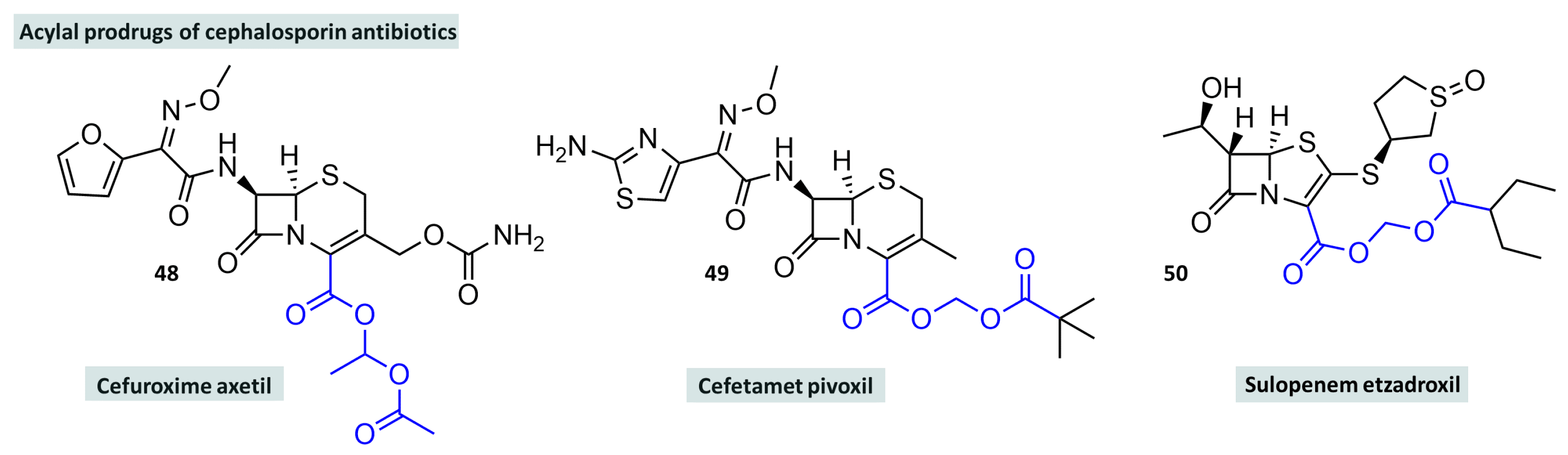
- Scott, L.J.; Ormrod, D.; Goa, K.L. Cefuroxime axetil: An updated review of its use in the management of bacterial infections. Drugs 2001, 61, 1455–1500. [Google Scholar] [CrossRef]
- Bryson, H.M.; Brogden, R.N. Cefetamet pivoxil: A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1993, 45, 589–621. [Google Scholar] [CrossRef]
- Mangum, B.R.; Pogue, J.M.; Barber, K.E. Tebipenem and Sulopenem: Dynamic Duo or Double Trouble? Curr. Infect. Dis. Rep. 2024, 26, 1–12. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Impalli, I.; Rangarajan, R.; Cohn, J.; Ramjeet, K.; Trainor, B.W.; Strathdee, S.; Sumpradit, N.; Berman, D.; Wertheim, H.; et al. Expanding antibiotic, vaccine, and diagnostics development and access to tackle antimicrobial resistance. Lancet 2024, 403, 2534–2550. [Google Scholar] [CrossRef]
- Todd, W.M. Cefpodoxime proxetil: A comprehensive review. Int. J. Antimicrob. Agents 1994, 4, 37–62. [Google Scholar] [CrossRef]
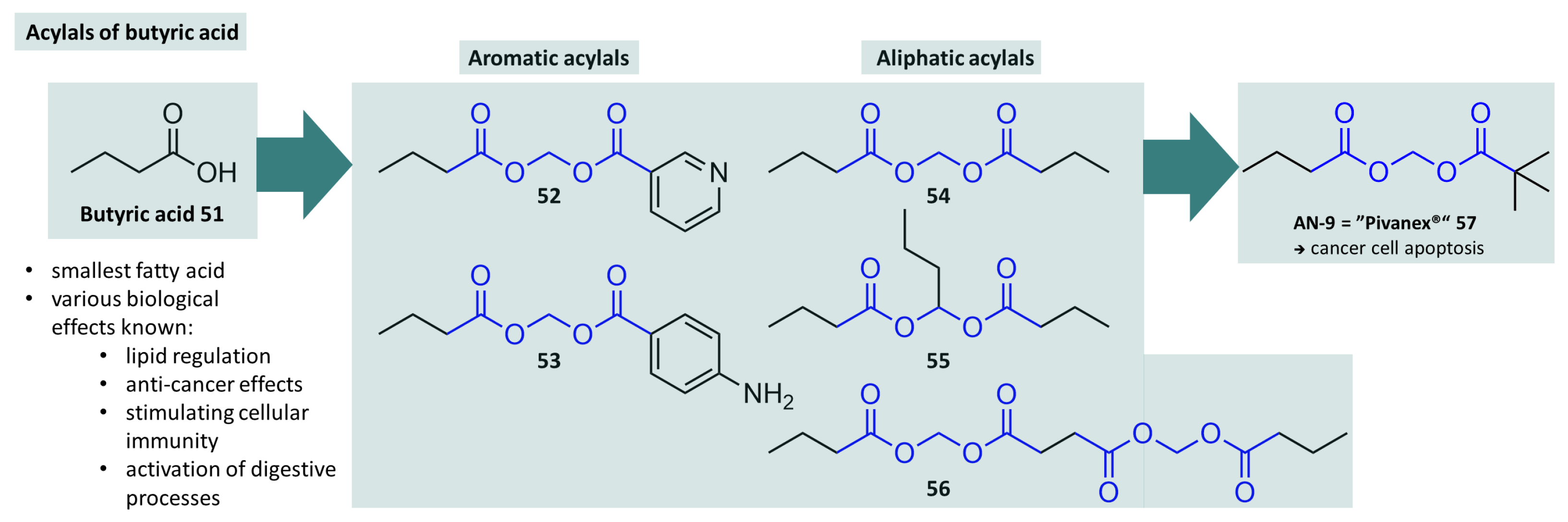
- Rephaeli, A.; Rabizadeh, E.; Aviram, A.; Shaklai, M.; Ruse, M.; Nudelman, A. Derivatives of butyric acid as potential anti-neoplastic agents. Int. J. Cancer 1991, 49, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Rephaeli, A.; Entin-Meer, M.; Angel, D.; Tarasenko, N.; Gruss-Fischer, T.; Bruachman, I.; Phillips, D.R.; Cutts, S.M.; Haas-Kogan, D.; Nudelman, A. The selectivty and anti-metastatic activity of oral bioavailable butyric acid prodrugs. Investig. New Drugs 2006, 24, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, A.; Gnizi, E.; Katz, Y.; Azulai, R.; Cohen-Ohana, M.; Zhuk, R.; Sampson, S.R.; Langzam, L.; Fibach, E.; Prus, E.; et al. Prodrugs of butyric acid. Novel derivatives possessing increased aqueous solubility and potential for treating cancer and blood diseases. Eur. J. Med. Chem. 2001, 36, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Reid, T.; Valone, F.; Lipera, W.; Irwin, D.; Paroly, W.; Natale, R.; Sreedharan, S.; Keer, H.; Lum, B.; Scappaticci, F.; et al. Phase II trial of the histone deacetylase inhibitor pivaloyloxymethyl butyrate (Pivanex, AN-9) in advanced non-small cell lung cancer. Lung Cancer 2004, 45, 381–386. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Maione, P. The potential role of histone deacetylase inhibitors in the treatment of non-small-cell lung cancer. Crit. Rev. Oncol. 2008, 68, 29–36. [Google Scholar] [CrossRef]
- Reid, T.; Cosgriff, T.M.; Yen, K.; Scislowski, P.; Valone, F.H.; Bhatnagar, A.; Scappaticci, F.A. P-358 Dose escalation study of pivanex (a histone deacetylase inhibitor) in combination with docetaxel for advanced non-small cell lung cancer. Lung Cancer 2003, 1, S182. [Google Scholar] [CrossRef]
- Gerunova, L.K.; Gerunov, T.V.; P’yanova, L.G.; Lavrenov, A.V.; Sedanova, A.V.; Delyagina, M.S.; Fedorov, Y.N.; Kornienko, N.V.; Kryuchek, Y.O.; Tarasenko, A.A. Butyric acid and prospects for creation of new medicines based on its derivatives: A literature review. J. Vet. Sci. 2024, 25, e23. [Google Scholar] [CrossRef]
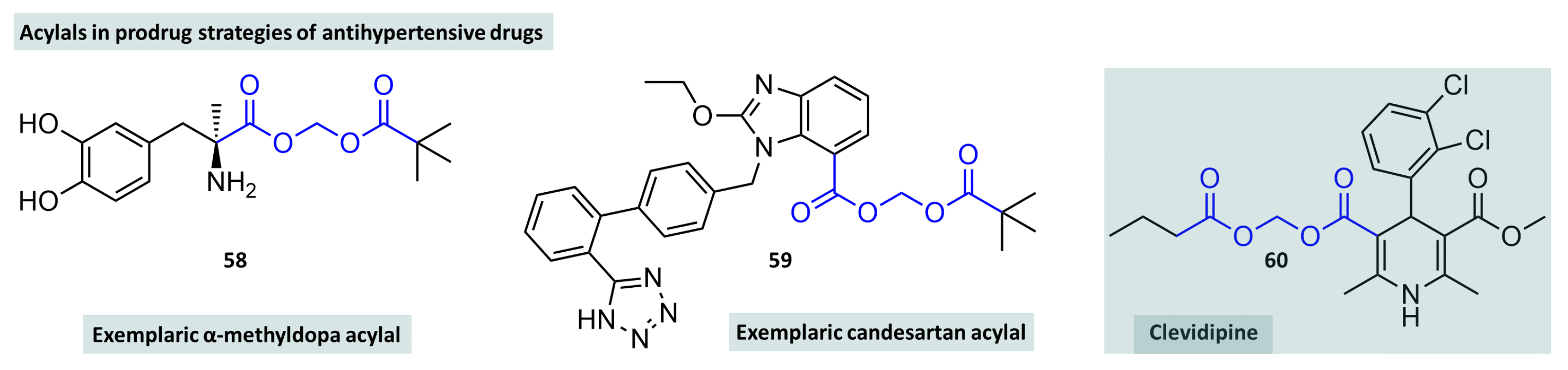
- Mah, G.T.; Tejani, A.M.; Musini, V.M. Methyldopa for primary hypertension. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Saari, W.S.; Freedman, M.B.; Hartman, R.D.; King, S.W.; Raab, A.W.; Randall, W.C.; Engelhardt, E.L.; Hirschmann, R.; Rosegay, A. Synthesis and antihypertensive activity of some ester progenitors of methyldopa. J. Med. Chem. 1978, 21, 746–753. [Google Scholar] [CrossRef]
- Kubo, K.; Kohara, Y.; Yoshimura, Y.; Inada, Y.; Shibouta, Y.; Furukawa, Y.; Kato, T.; Nishikawa, K.; Naka, T. Nonpeptide angiotensin II receptor antagonists. Synthesis and biological activity of potential prodrugs of benzimidazole-7-carboxylic acids. J. Med. Chem. 1993, 36, 2343–2349. [Google Scholar] [CrossRef]
- Lord, M.S.; Augoustides, J.G. Perioperative management of pheochromocytoma: Focus on magnesium, clevidipine, and vasopressin. J. Cardiothorac. Vasc. Anesth. 2012, 26, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Ripollés-Melchor, J.; Casans-Frances, R.; Abad-Gurumeta, A.; Bergese, S.D.; Zuleta-Alarcon, A.; Lopez-Timoneda, F.; Calvo-Vecino, J.M.; Group, E.A.R. Perioperative use of clevidipine: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0150625. [Google Scholar] [CrossRef] [PubMed]
- Shoup, T.M.; Griciuc, A.; Normandin, M.D.; Quinti, L.; Walsh, L.V.; Dhaynaut, M.; Moon, S.H.; Guehl, N.J.; Brugarolas, P.; Elmaleh, D.R.; et al. Evaluation of fluorinated cromolyn derivatives as potential therapeutics for Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 80, 775–786. [Google Scholar] [CrossRef]
- Kawamata, T.; Nagatomo, M.; Inoue, M. Total synthesis of zaragozic acid C: Implementation of photochemical C (sp3)–H acylation. J. Am. Chem. Soc. 2017, 139, 1814–1817. [Google Scholar] [CrossRef]
- Chiang, Y.C.P.; Kurtz, M.M.; Bergstrom, J.D.; Berger, G.D. 3, 4-Diesters of zaragozic acid A. Bioorg. Med. Chem. Lett. 1995, 5, 1643–1646. [Google Scholar] [CrossRef]
- Hecker, S.J.; Reddy, K.R.; Lomovskaya, O.; Griffith, D.C.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Sun, D.; Sabet, M.; Tarazi, Z.; et al. Discovery of cyclic boronic acid QPX7728, an ultrabroad-spectrum inhibitor of serine and metallo-β-lactamases. J. Med. Chem. 2020, 63, 7491–7507. [Google Scholar] [CrossRef]
- Rosa, L.C.S.; Argolo, C.O.; Nascimento, C.M.C.; Pimentel, A.S. Identifying Substructures That Facilitate Compounds to Penetrate the Blood–Brain Barrier via Passive Transport Using Machine Learning Explainer Models. ACS Chem. Neurosci. 2024, 15, 2144–2159. [Google Scholar] [CrossRef]
- Galetin, A.; Brouwer, K.L.; Tweedie, D.; Yoshida, K.; Sjöstedt, N.; Aleksunes, L.; Chu, X.; Evers, R.; Hafey, M.J.; Lai, Y.; et al. Membrane transporters in drug development and as determinants of precision medicine. Nat. Rev. Drug Discov. 2024, 23, 255–280. [Google Scholar] [CrossRef]





Catalysts Used for Protection Methods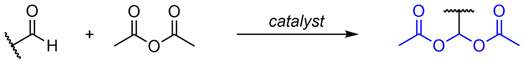 | |||||
| Year | Catalyst | Time | Yield (%) | Aldehyde | Source |
| 1998 | Fe3+-montmorillonite | 10–20 min | 81–98 | arom. | [52] |
| 2001 | Bi(OTf)3·xH2O | 20 min–5 h | 64–95 | arom./aliph. | [45] |
| 2003 | InCl3 | 0.5–6 h | 87–96 | arom./aliph. | [53] |
| 2004 | AlPW12O40 | 1–45 min | 88–96 | arom./aliph. | [54] |
| 2004 | Bi(NO3)3·5H2O | 1.5–19 h | 57–94 | arom./aliph. | [46] |
| 2006 | InBr3 | 3 min–14 h | 76–98 | arom./aliph. | [55] |
| 2006 | HClO4–SiO2 | 1–30 min | 90–99 | arom./aliph. | [51,56] |
| 2006 | C2H6CuO6S2/AcOH | 1.3–12 h | 66–95 | arom./aliph. | [57] |
| 2009 | SBSSA | 2–4 min | 72–91 | arom. | [58] |
| 2010 | see source | 2–35 min | 45–99 | arom./aliph. | [59] |
| 2011 | [bmpy]HSO4 | 5–25 min | 85–97 | arom./aliph. | [60] |
| 2011 | CSC-Star | 2–7 h | 94–96 | arom./aliph. | [61] |
| 2014 | PSA | 4–10 min | 93–99 | arom./aliph. | [62] |
| 2018 | F- or OH-apatites | 25 min–48 h a | 9–97 a | arom./aliph. | [63] |
| Catalysts Used for Protection (Prot.) and Deprotection (Deprot.) Methods | |||||
 | |||||
| Year | Catalyst | Time Prot./Deprot. | Yield (%) Prot./Deprot. | Aldehyde | Source |
| 2002 | Zr(CH3PO3)1.2− | 12 min–5 h/1–24 h | 21–91/32–95 | arom./aliph. | [64] |
| (O3PC6H4SO3H)0.8 | |||||
| 2002 | CAN | 1.5–32 h/4–7 h | 70–96/86–91 | arom./aliph. | [48] |
| 2006 | H3PMo12O40 | 20–120 min/12–45 min | 92–99/92–98 | arom. | [47] |
| 2016 | SelectfluorTM | 5–15/15 min | 84–98/98 | arom./aliph. | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keydel, T.; Link, A. Synthetic Approaches, Properties, and Applications of Acylals in Preparative and Medicinal Chemistry. Molecules 2024, 29, 4451. https://doi.org/10.3390/molecules29184451
Keydel T, Link A. Synthetic Approaches, Properties, and Applications of Acylals in Preparative and Medicinal Chemistry. Molecules. 2024; 29(18):4451. https://doi.org/10.3390/molecules29184451
Chicago/Turabian StyleKeydel, Tobias, and Andreas Link. 2024. "Synthetic Approaches, Properties, and Applications of Acylals in Preparative and Medicinal Chemistry" Molecules 29, no. 18: 4451. https://doi.org/10.3390/molecules29184451
APA StyleKeydel, T., & Link, A. (2024). Synthetic Approaches, Properties, and Applications of Acylals in Preparative and Medicinal Chemistry. Molecules, 29(18), 4451. https://doi.org/10.3390/molecules29184451







